The Surface Characterisation of Fused Filament Fabricated (FFF) 3D Printed PEEK/Hydroxyapatite Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Processing Conditions
2.2. Physical and Chemical Characterisation of the 3D Printed Samples
2.2.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.2. X-ray Diffraction (XRD)
2.2.3. Scanning Electron Microscopy (SEM)
2.2.4. Stylus Profilometry
2.2.5. X-ray Photoelectron Spectroscopy (XPS)
2.2.6. Time-of-Flight Secondary Ion Mass Spectrometry (ToFSIMS)
2.2.7. Contact Angle
2.3. In Vitro Characterisation of the 3D Printed Samples
2.3.1. Scaffold Sterilisation
2.3.2. Cell Culture and Cell-Seeding
2.3.3. Measuring Cell Metabolic Activity
2.3.4. Measuring Deoxyribonucleic Acid (DNA)
2.3.5. Scanning Electron Microscope (SEM) of the Osteoblast-Like Cells
2.3.6. Statistical Analyses for the In Vitro Measurements
3. Results
3.1. Physical and Chemical Characterisation of the 3D Printed Samples
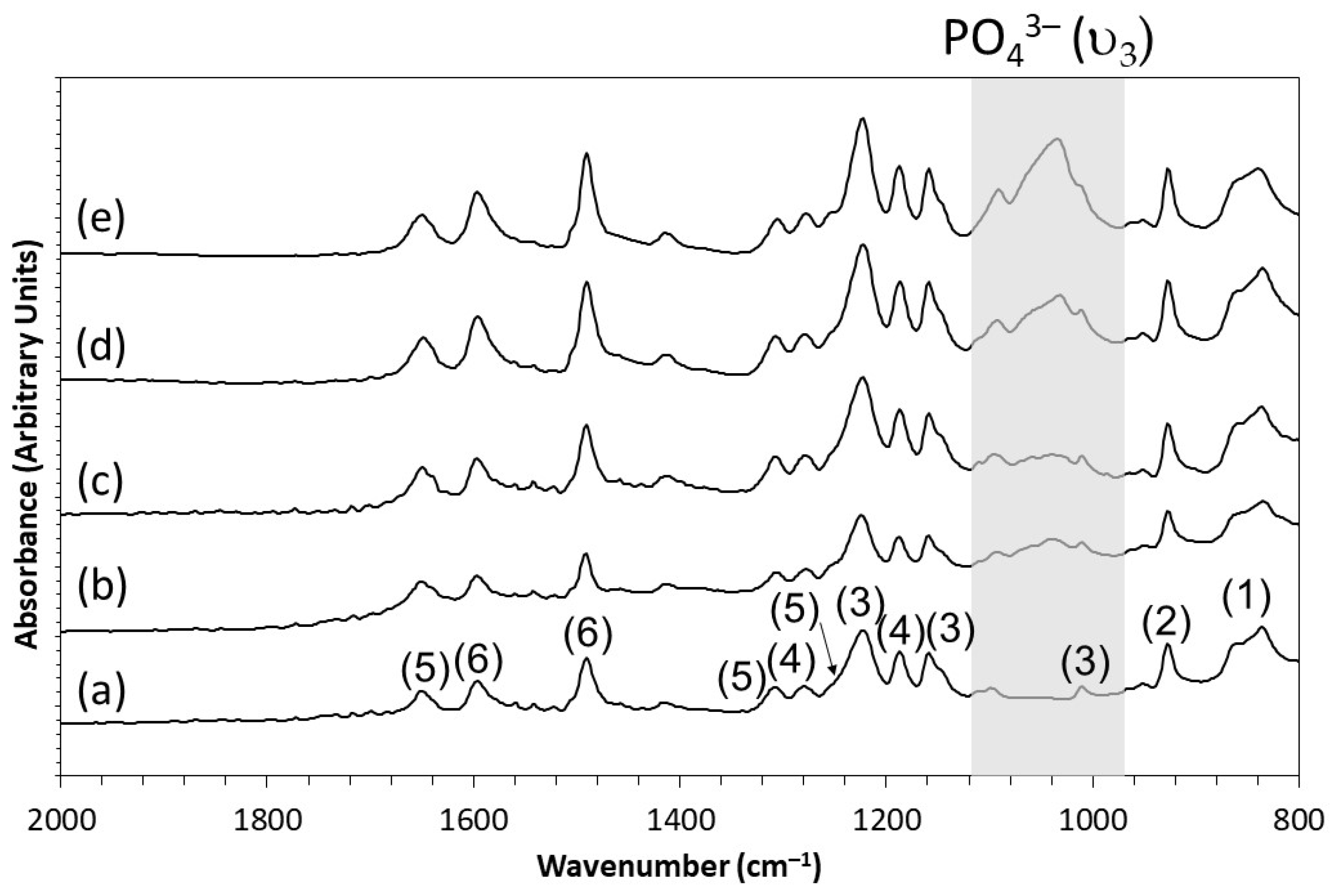
3.1.1. FTIR
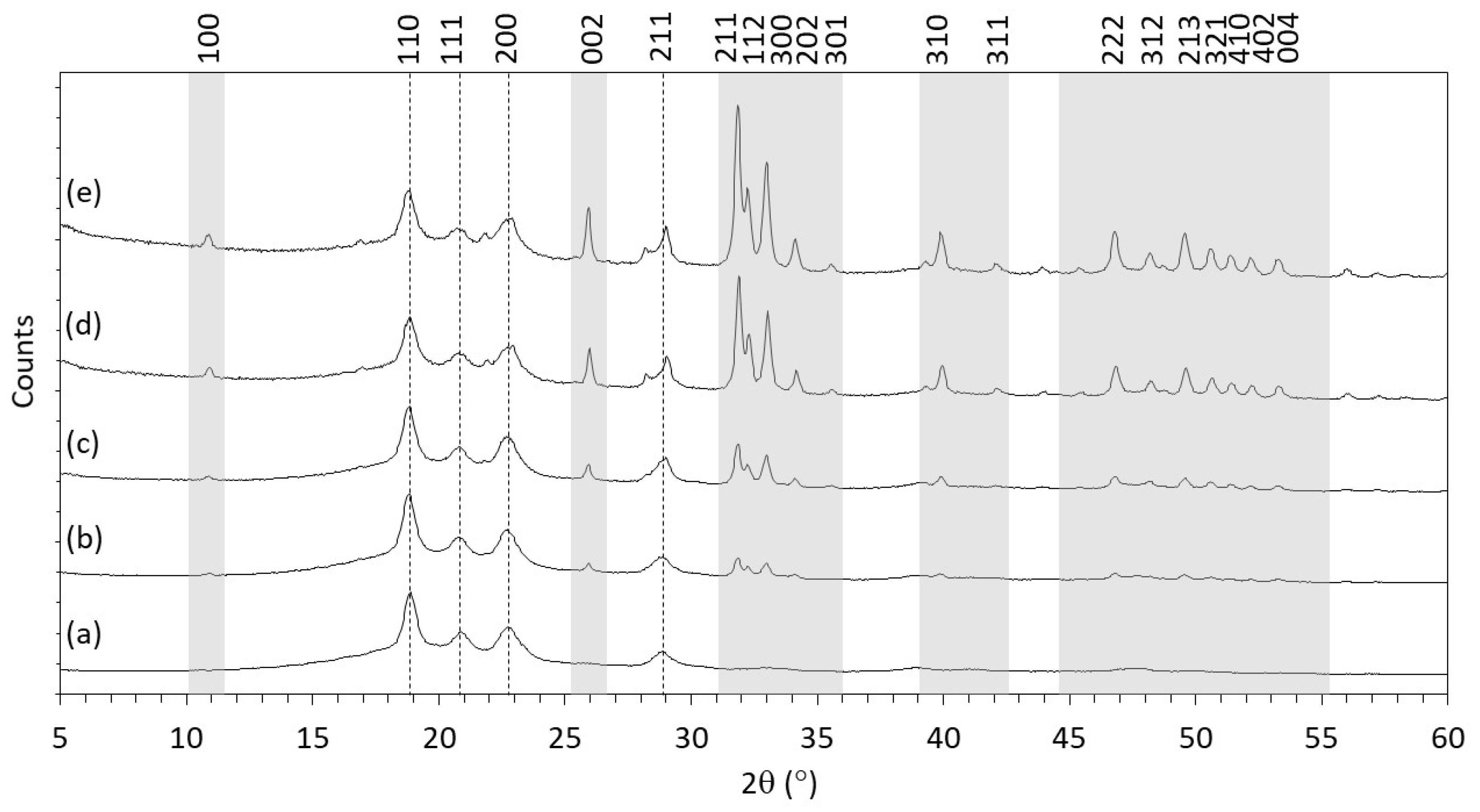
3.1.2. XRD
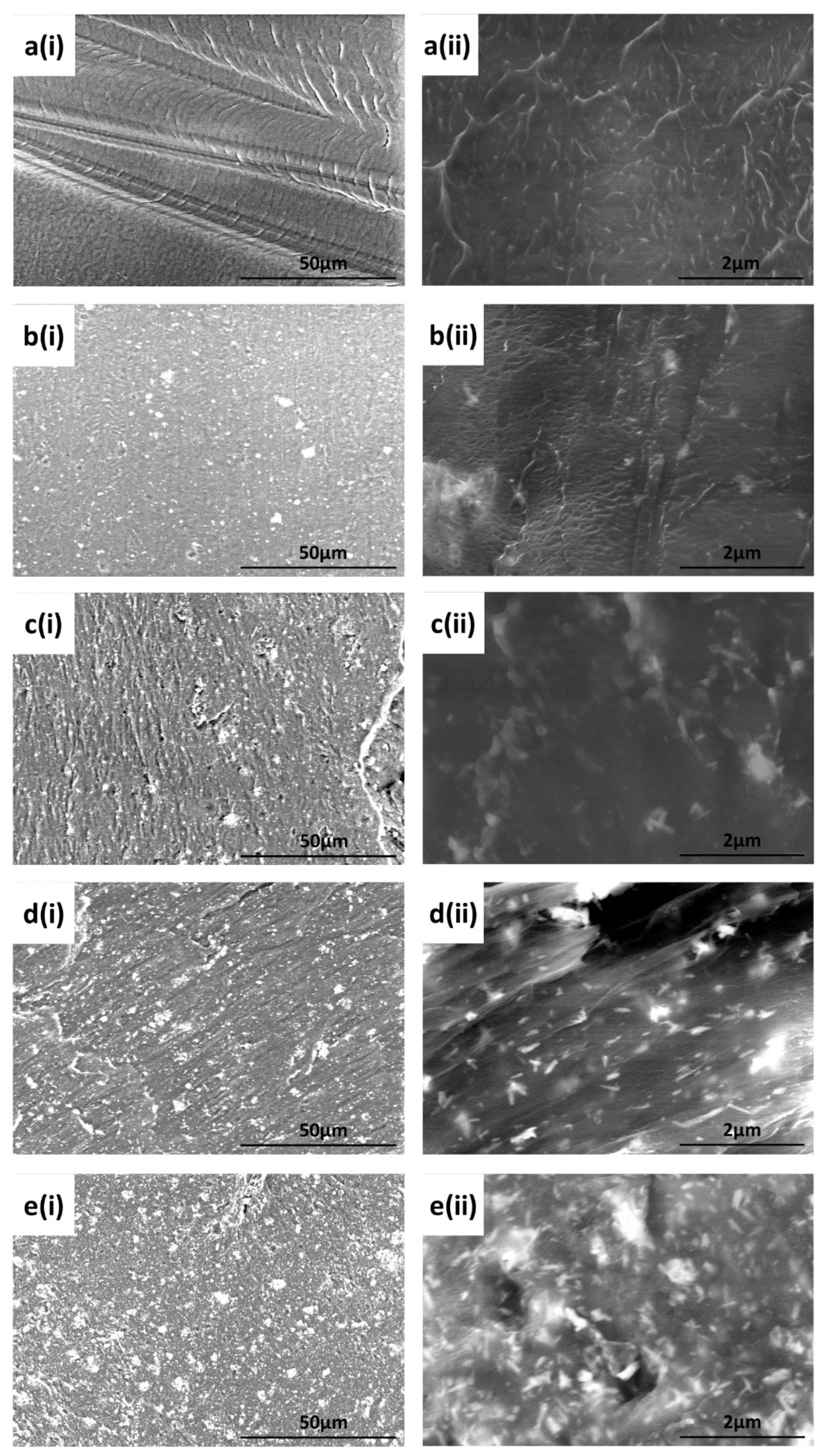
3.1.3. SEM
3.1.4. Stylus Profilometry
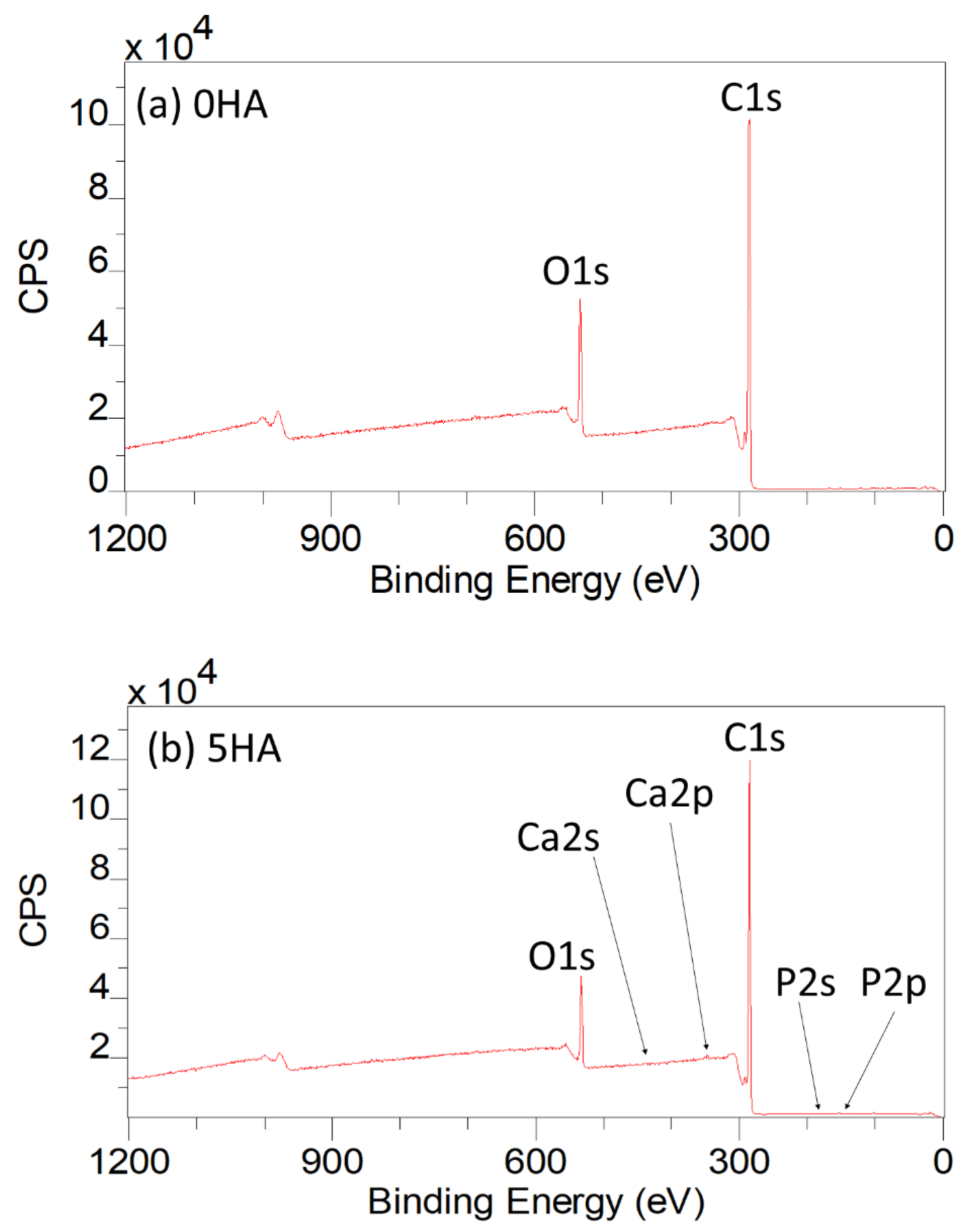
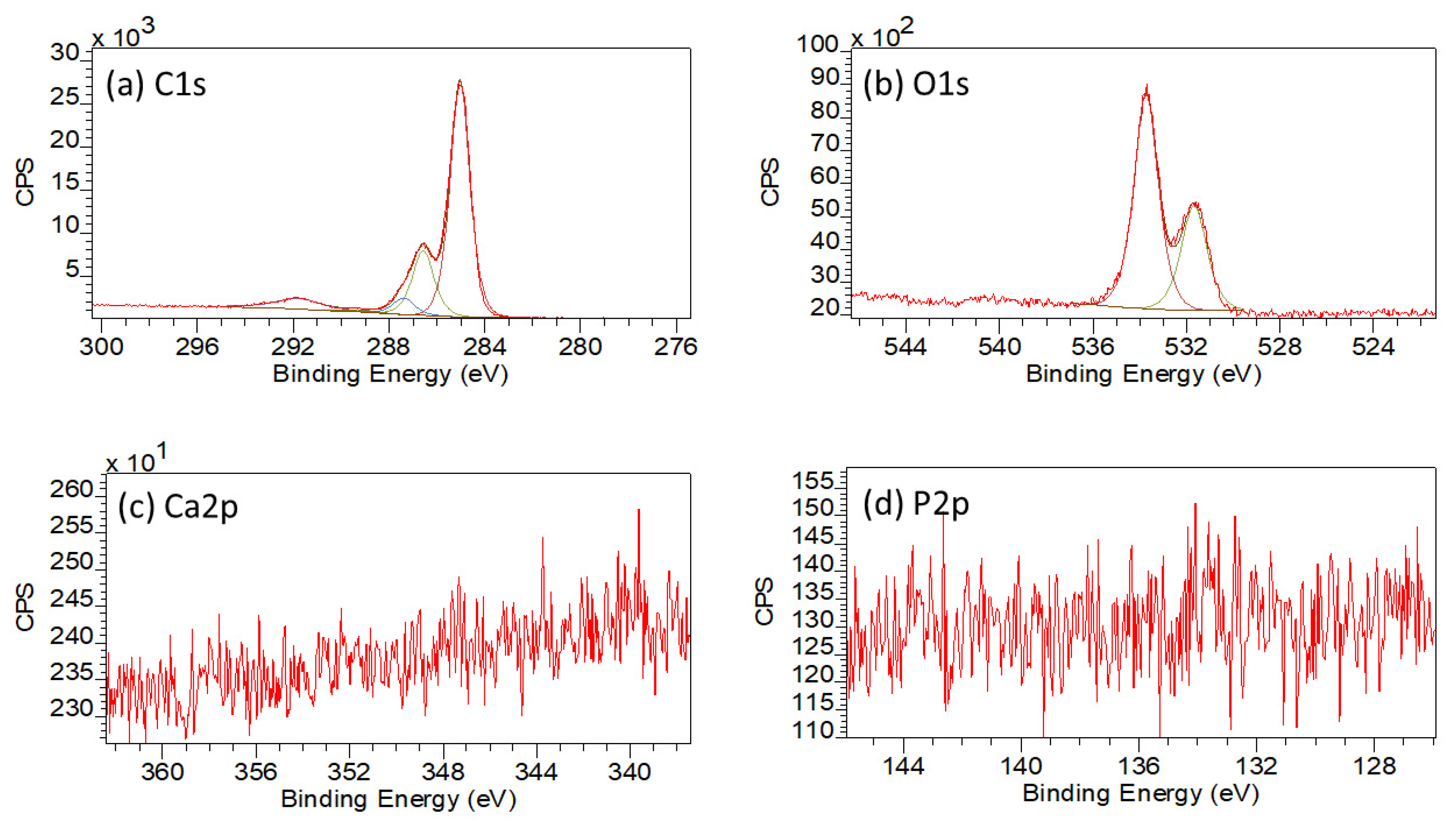
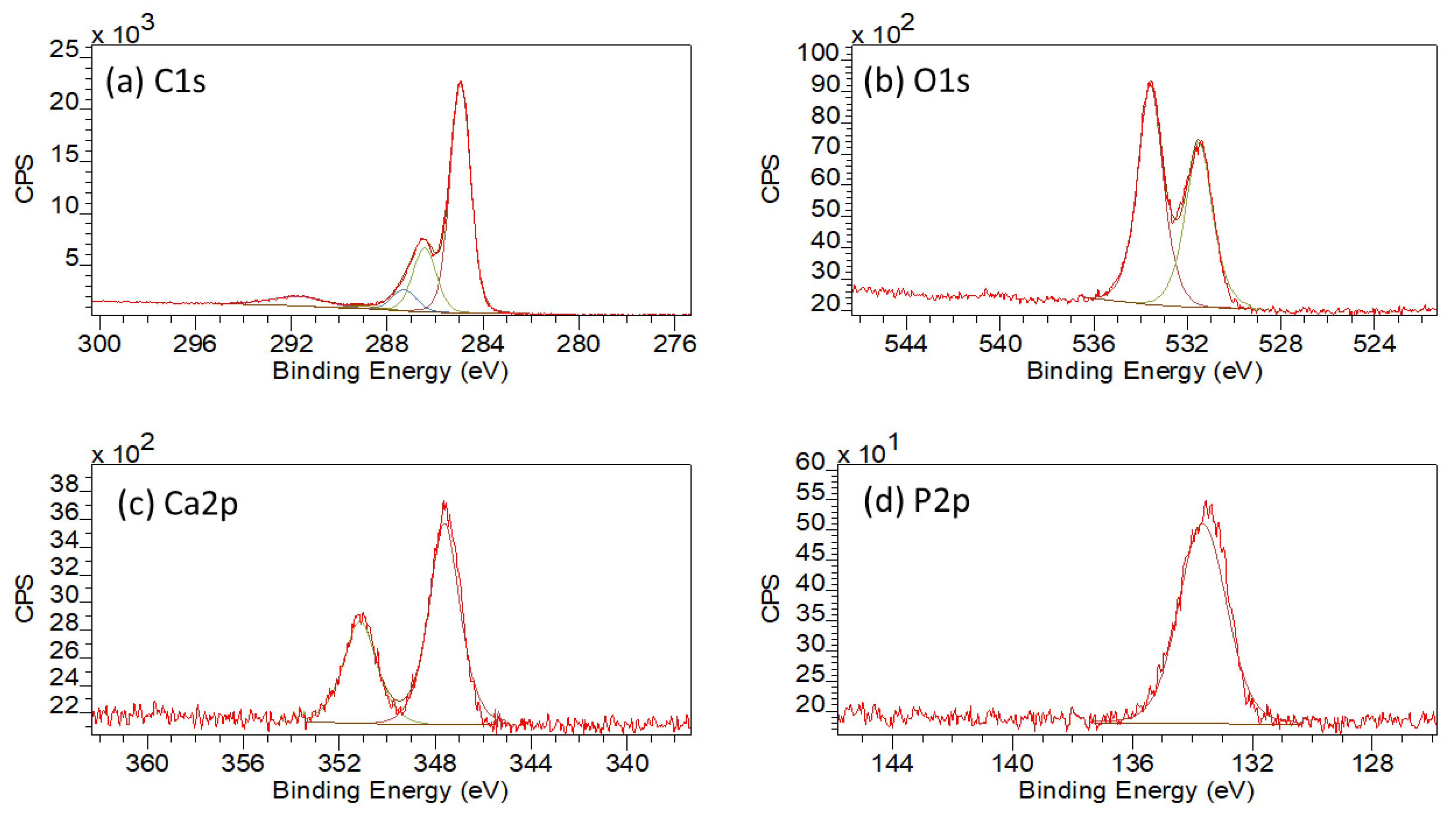
3.1.5. XPS
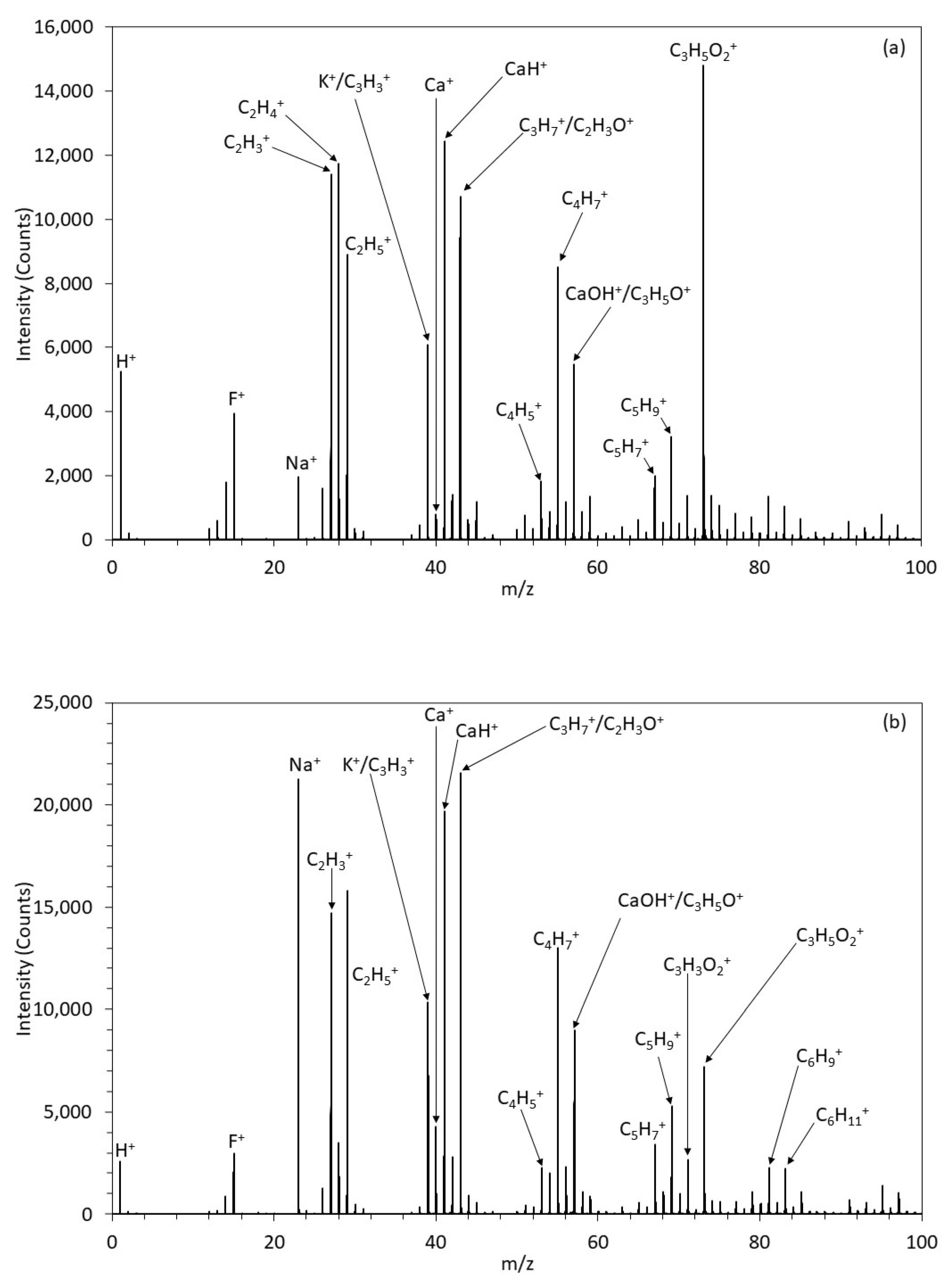
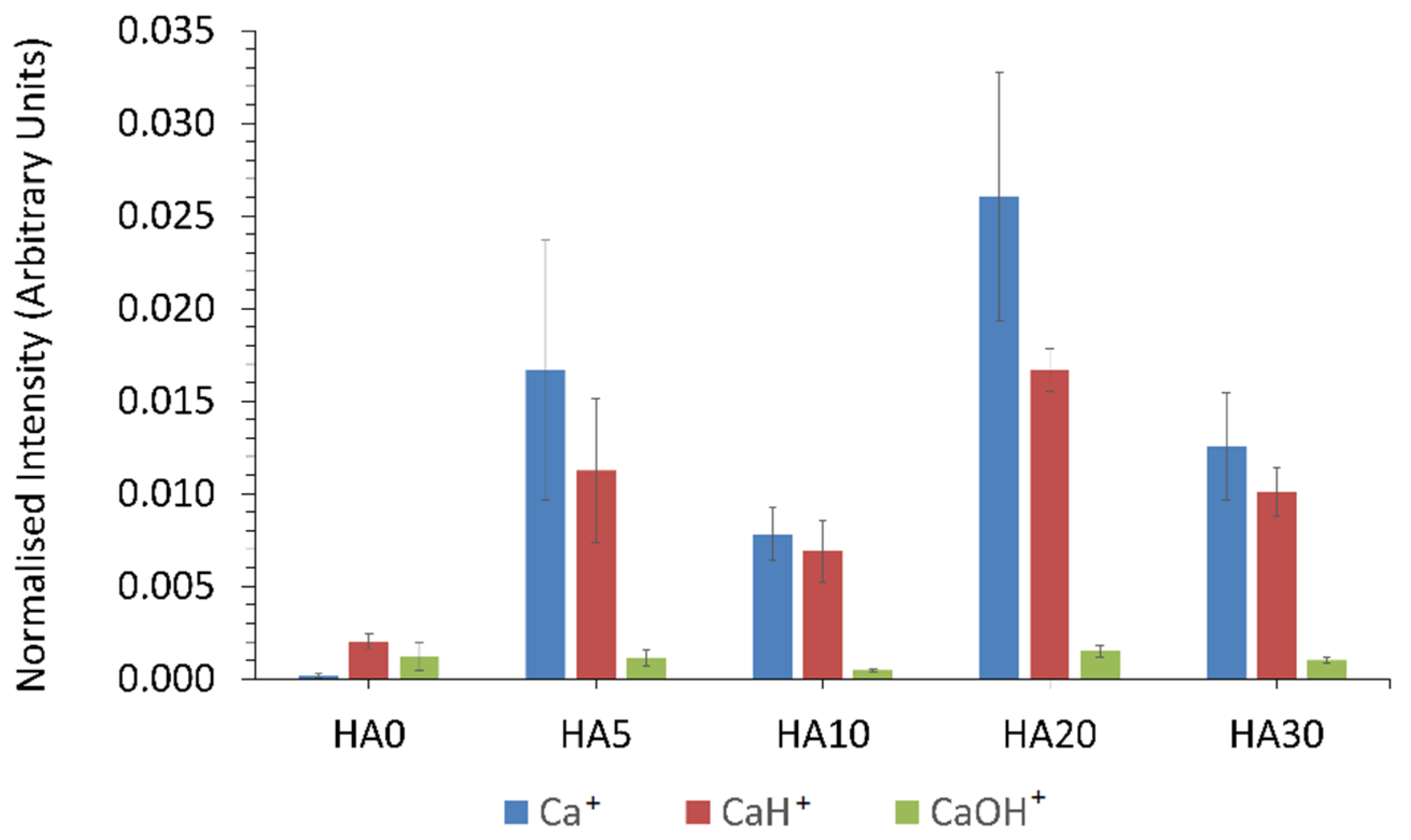
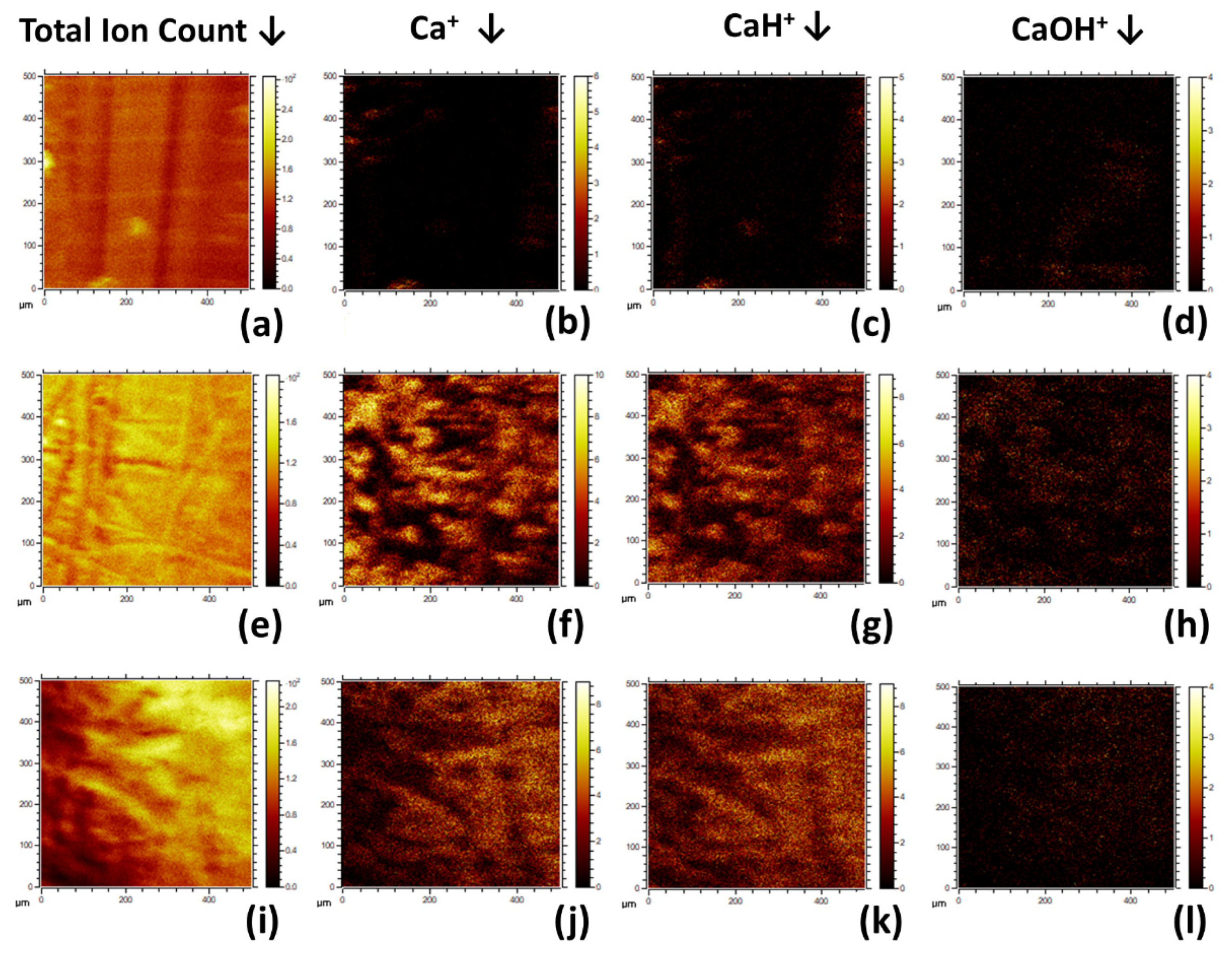
3.1.6. ToFSIMS
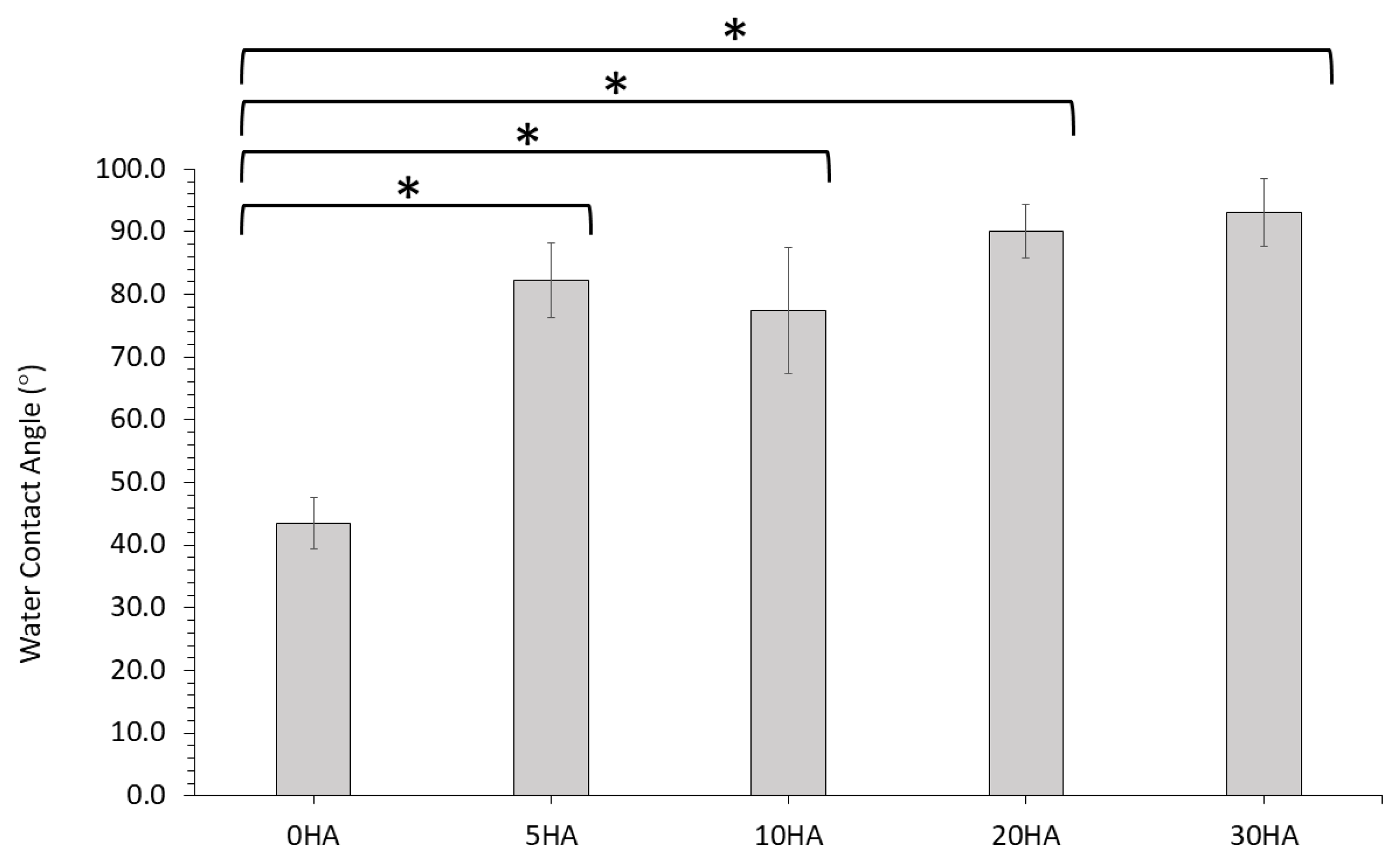
3.1.7. Water Contact Angle
3.2. In Vitro Characterisation of the 3D Printed Samples
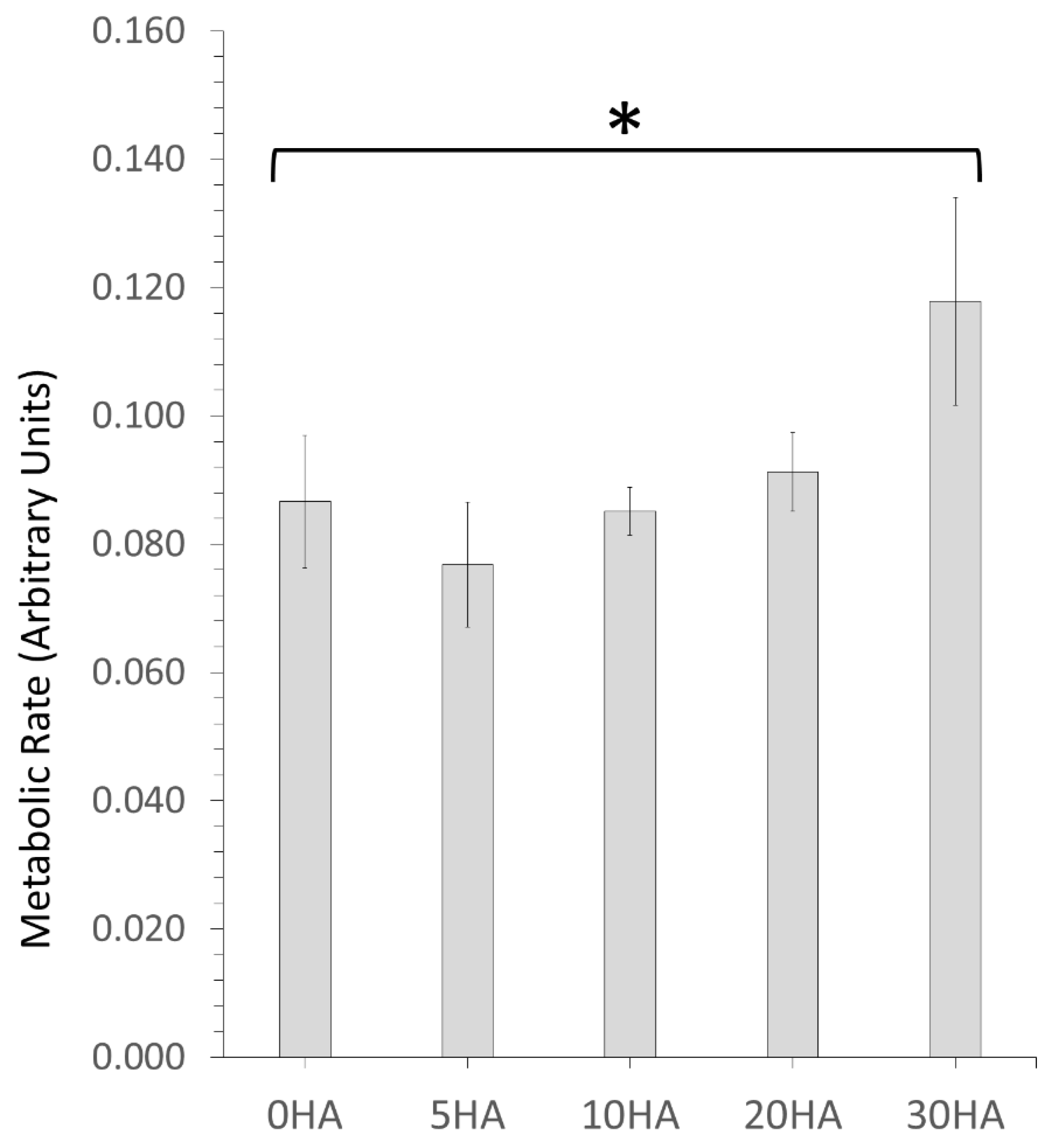
3.2.1. MTT Testing for Cell Metabolism
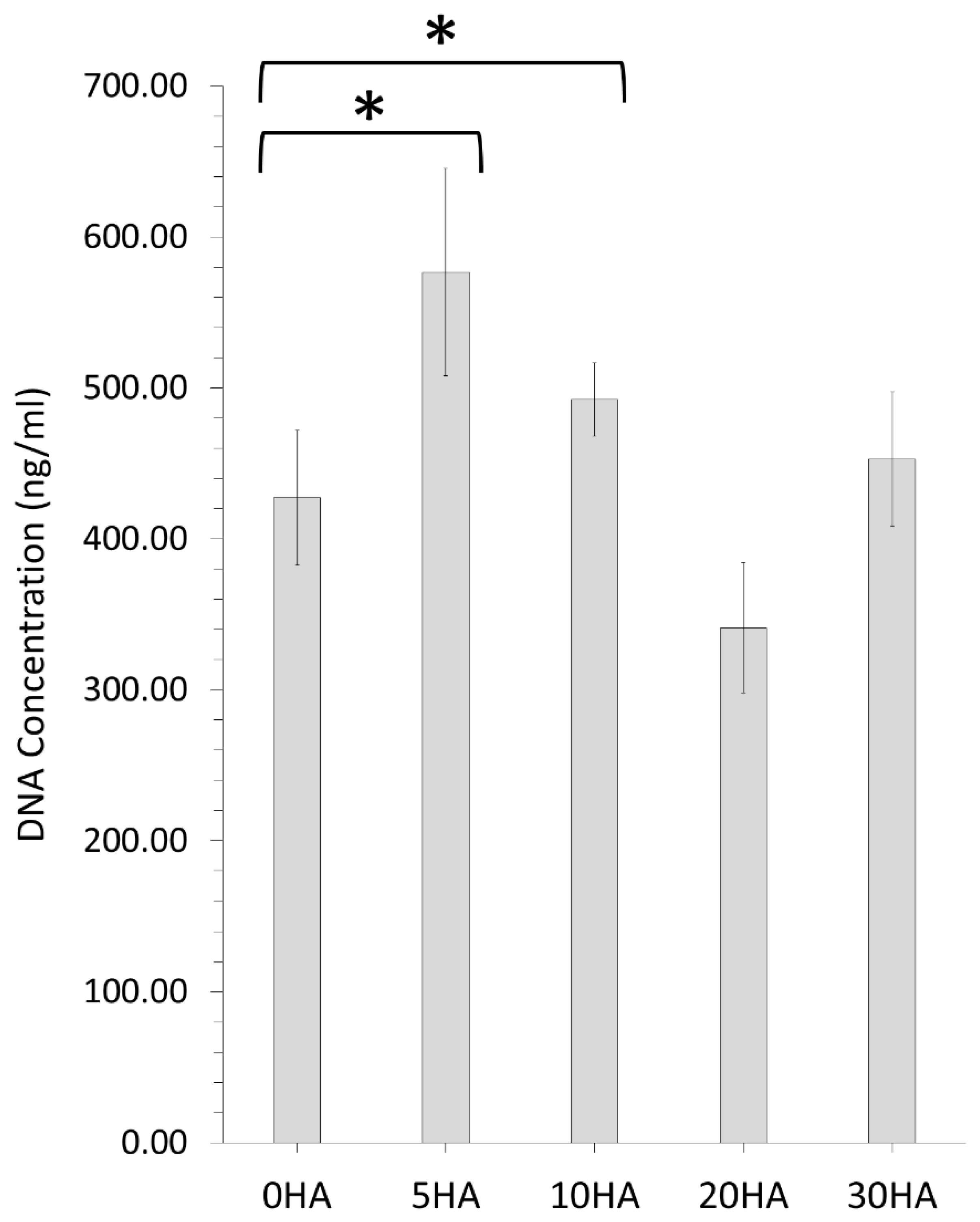
3.2.2. Pico Green™ Assay for Measuring DNA Concentration
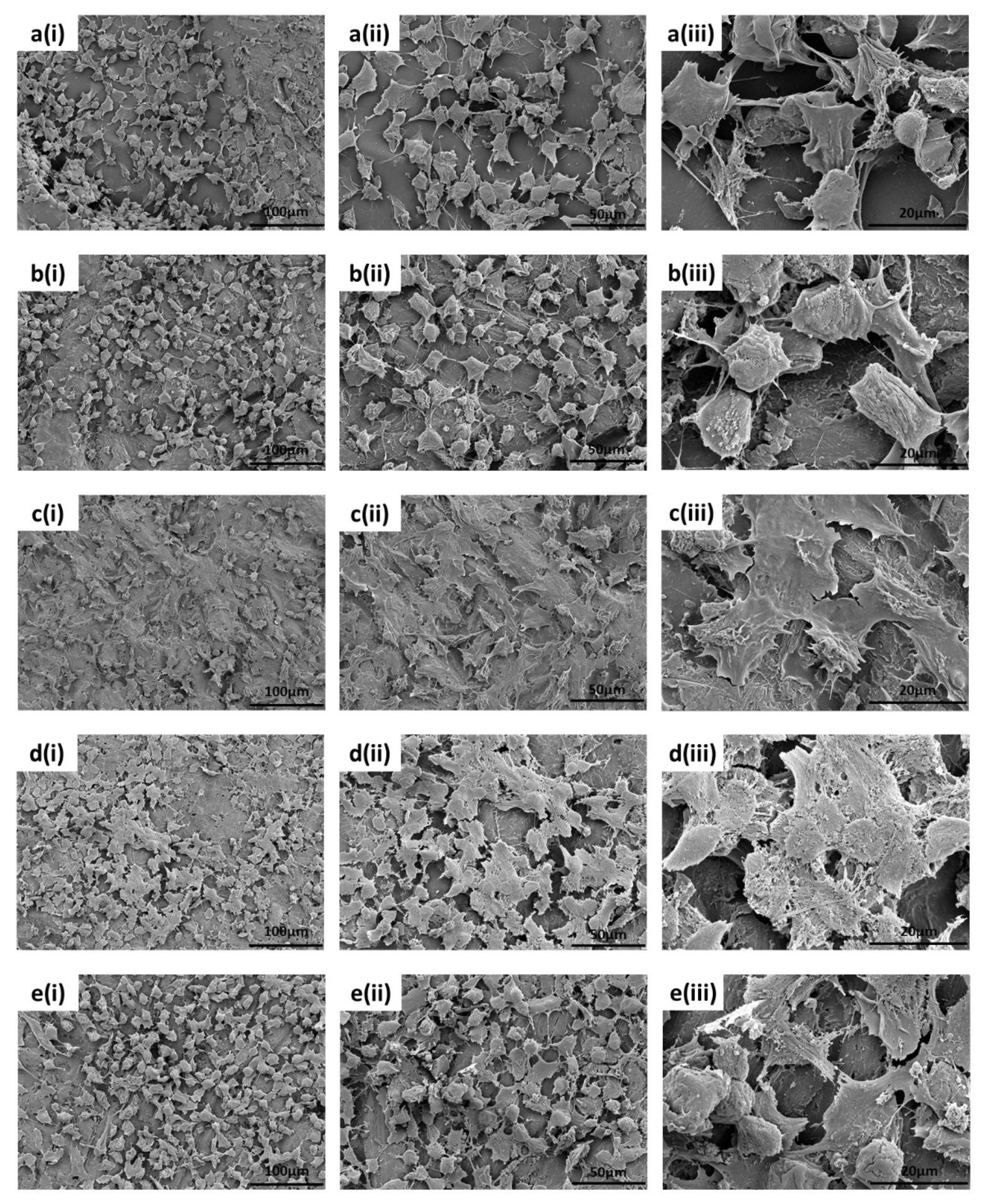
3.2.3. SEM Analysis of the Cell Morphology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vaezi, M.; Black, C.; Gibbs, D.M.R.; Oreffo, R.O.C.; Brady, M.; Moshrefi-Torbati, M.; Yang, S. Characterization of New PEEK/HA Composites with 3D HA Network Fabricated by Extrusion Freeforming. Molecules 2016, 21, 687. [Google Scholar] [CrossRef] [Green Version]
- Almasi, D.; Iqbal, N.; Sadeghi, M.; Sudin, I.; Kadir, M.; Kamarul, T. Preparation Methods for Improving PEEK’s Bioactivity for Orthopedic and Dental Application: A Review. Int. J. Biomater. 2016, 2016, 8202653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monich, P.R.; Henriques, B.; Novaes de Oliveira, P.A.; Souza, J.C.M.; Fredel, M.C. Mechanical and biological behavior of biomedical PEEK matrix composites: A focused review. Mater Lett. 2016, 185, 593–597. [Google Scholar] [CrossRef]
- Yuan, B.; Cheng, Q.; Zhao, R.; Zhu, X.; Yang, X.; Yang, X.; Zhang, K.; Song, Y.; Zhang, X. Comparison of osteointegration property between PEKK and PEEK: Effects of surface structure and chemistry. Biomaterials 2018, 170, 116–126. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Zahedi, S.A.; Ismail, S.O.; Omigbodun, F.T.; Bowoto, O.K.; Olawumi, M.A.; Muhammad, M.A. 3D printing of PEEK–cHAp scaffold for medical bone implant. Bio-Des. Manuf. 2020, 4, 44–59. [Google Scholar] [CrossRef]
- Zhao, Y.; Wong, H.M.; Lui, S.C.; Chong, E.Y.; Wu, G.; Zhao, X.; Wang, C.; Pan, H.; Cheung, K.M.; Wu, S.; et al. Plasma Surface Functionalized Polyetheretherketone for Enhanced Osseo-Integration at Bone-Implant Interface. ACS Appl. Mater. Interfaces 2016, 8, 3901–3911. [Google Scholar] [CrossRef]
- Lee, J.H.; Jang, H.L.; Lee, K.M.; Baek, H.R.; Jin, K.; Hong, K.S.; Noh, J.H.; Lee, H.K. In vitro and in vivo evaluation of the bioactivity of hydroxyapatite-coated polyetheretherketone biocomposites created by cold spray technology. Acta Biomater. 2013, 9, 6177–6187. [Google Scholar] [CrossRef]
- Hussain, S.; Rutledge, L.; Acheson, J.G.; Meenan, B.J.; Boyd, A.R. The Surface Characterisation of Polyetheretherketone (PEEK) Modified via the Direct Sputter Deposition of Calcium Phosphate Thin Films. Coatings 2020, 10, 1088. [Google Scholar] [CrossRef]
- Ozeki, K.; Masuzawa, T.; Aoki, H. Fabrication of hydroxyapatite thin films on polyetheretherketone substrates using a sputtering technique. Mater. Sci. Eng. C 2017, 72, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, H.; Liu, X.; Wei, J.; Ji, J.; Yang, S.; Hu, Z.; Wei, S. Response of Human Osteoblast to n-HA/PEEK—Quantitative Proteomic Study of Bio-effects of Nano-Hydroxyapatite Composite. Sci. Rep. 2016, 6, 22832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodzeń, K.; Sharma, P.K.; McIlhagger, A.; Mokhtari, M.; Dave, F.; Tormey, D.; Sherlock, R.; Meenan, B.J.; Boyd, A. The Direct 3D Printing of Functional PEEK/Hydroxyapatite Composites via a Fused Filament Fabrication Approach. Polymers 2021, 13, 545. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, F.; Golbang, A.; Jindal, S.; Dixon, D.; McIlhagger, A.; Harkin-Jones, E.; Crawford, D.; Mancuso, E. 3D printed PEEK/HA composites for bone tissue engineering applications: Effect of material formulation on mechanical performance and bioactive potential. J. Mech. Behav. Biomed. Mater. 2021, 121, 104601. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Guo, D. Evaluating the bioactivity of a hydroxyapatite-incorporated polyetheretherketone biocomposite. J. Orthop. Surg. Res. 2019, 14, 32. [Google Scholar] [CrossRef]
- Wong, K.L.; Wong, C.T.; Liu, W.C.; Pan, H.B.; Fong, M.K.; Lam, W.M.; Cheung, W.L.; Tang, W.M.; Chiu, K.Y.; Luk, K.D.; et al. Mechanical properties and in vitro response of strontium-containing hydroxyapatite/polyetheretherketone composites. Biomaterials 2009, 30, 3810–3817. [Google Scholar] [CrossRef]
- Wang, L.; He, S.; Wu, X.; Liang, S.; Mu, Z.; Wie, J.; Deng, F.; Deng, Y.; Wie, S. Polyetheretherketone/nano-fluorohydroxyapatite composite with antimicrobial activity and osseointegration properties. Biomaterials 2014, 35, 6758–6775. [Google Scholar] [CrossRef]
- Von Wilmonsky, C.; Lutz, R.; Meisel, U.; Srour, S.; Rupprecht, S.; Toyoshima, T.; Nkenke, E.; Schlegel, K.A.; Pohle, D.; Münstedt, H.; et al. In Vivo Evaluation of ß-TCP Containing 3D Laser Sintered Poly(ether ketone) Composites in Pigs. J. Bioact. Compat. Polym. 2009, 24, 69–184. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.Y.; Sugino, A.; Kikuta, K.; Ohtsuki, C.; Cho, S.B. Bioactive Composites Consisting of PEEK And Calcium Silicate Powders. J. Biomater. Appl. 2009, 24, 105–118. [Google Scholar] [PubMed]
- Ma, R.; Tang, S.; Tan, H.; Qian, J.; Lin, W.; Wang, Y.; Liu, C.; Wei, J.; Tang, T. Preparation, Characterization, In Vitro Bioactivity, and Cellular Responses to a Polyetheretherketone Bioactive Composite Containing Nanocalcium Silicate for Bone Repair. ACS Appl. Mater. Interfaces 2014, 6, 12214–12225. [Google Scholar] [CrossRef] [PubMed]
- von Wilmowsky, C.; Vairaktaris, E.; Pohle, D.; Rechtenwald, T.; Lutz, R.; Münstedt, H.; Koller, G.; Schmidt, M.; Neukam, F.W.; Schlegel, K.A.; et al. Effects of bioactive glass and β-TCP containing three-dimensional laser sintered polyetheretherketone composites on osteoblasts in vitro. J. Biomed. Mater. Res. 2008, 87A, 896–902. [Google Scholar] [CrossRef]
- Wu, X.; Liu, X.; Wei, J.; Ma, J.; Deng, F.; Wei, S. Nano-TiO2/PEEK bioactive composite as a bone substitute material: In vitro and in vivo studies. Int. J. Nanomed. 2012, 7, 1215–1225. [Google Scholar]
- Zhong, G.; Vaezi, M.; Mei, X.; Liu, P.; Yang, S. Strategy for Controlling the Properties of Bioactive Poly-EtherEther-Ketone/Hydroxyapatite Composites for Bone Tissue Engineering Scaffolds. ACS Omega 2019, 4, 19238–19245. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Hao, L.; Savalani, M.M.; Harris, R.A.; di Silvio, L.; Tanner, K.E. In vitro biocompatibility of hydroxyapatite-reinforced polymeric composites manufactured by selective laser sintering. J. Biomed. Mater. Res. A 2009, 91, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Hariram, P.K.; Kumar, R.; Cheang, P.; Aik, K.K. In vitro apatite formation and its growth kinetics on hydroxyapatite/polyetheretherketone biocomposites. Biomaterials 2005, 26, 2343–2352. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Fang, L.; Luo, Z. Mechanical performance, and in vivo bioactivity of functionally graded PEEK–HA biocomposite materials. J. Sol. Gel. Sci. Technol. 2014, 70, 339–345. [Google Scholar] [CrossRef] [Green Version]
- Baştan, F.E. Fabrication and characterization of an electrostatically bonded PEEK-hydroxyapatite composites for biomedical applications. J. Biomed. Mater. Res. 2020, 108, 2513–2527. [Google Scholar] [CrossRef]
- Dong, L.; Jun, F.; Hongbin, F.; Dichen, L.; Enchun, D.; Xin, X.; Ling, W.; Zheng, G. Application of 3D-printed PEEK scapula prosthesis in the treatment of scapular benign fibrous histiocytoma: A case report. J. Bone Oncol. 2018, 12, 78–82. [Google Scholar]
- Berretta, S.; Evans, K.; Ghita, O. Additive manufacture of PEEK cranial implants: Manufacturing considerations versus accuracy and mechanical performance. Mater. Des. 2018, 139, 141–152. [Google Scholar] [CrossRef]
- Yang, C.; Tian, X.; Li, D.; Cao, Y.; Zhao, F.; Shi, C. Influence of thermal processing conditions in 3D printing on the crystallinity and mechanical properties of PEEK material. J. Mater. Process. Tech. 2017, 248, 1–7. [Google Scholar] [CrossRef]
- Cicala, G.; Latteri, A.; del Curto, B.; Russo, A.L.; Recca, G.; Farè, S. Engineering Thermoplastics for Additive Manufacturing: A Critical Perspective with Experimental Evidence to Support Functional Applications. J. Appl. Biomater. Funct. Mater. 2017, 15, 10–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Sharma, N.; Xu, Z.; Scheideler, L.; Geis-Gerstorfer, J.; Rupp, F.; Thieringer, F.M.; Spintzyk, S.J. Osteoblast Responses to Different Surface Morphology and Roughness of 3D-printed PEEK Implants and Prosthesis. J. Clin. Med. 2019, 8, 771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Prakash, C.; Ramakrishna, S. 3D printing of polyether-ether-ketone for biomedical applications. Eur. Polym. J. 2019, 114, 234–248. [Google Scholar] [CrossRef]
- O’Kane, C.; Duffy, H.; Meenan, B.J.; Boyd, A.R. The influence of target stoichiometry on the surface properties of sputter deposited calcium phosphate thin films. Surf. Coat. Technol. 2008, 203, 121–128. [Google Scholar] [CrossRef]
- Dawson, P.C.; Blundell, D.J. X-ray data for poly (aryl ether ketones). Polymer 1980, 21, 577–578. [Google Scholar] [CrossRef]
- Ha, S.W.; Hauert, R.; Ernst, K.H.; Wintermantel, E. Surface analysis of chemically etched and plasma-treated polyetheretherketone (PEEK) for biomedical applications. Surf. Coat. Technol. 1997, 96, 293–299. [Google Scholar] [CrossRef]
- Laurens, P.; Sadras, B.; Decobert, F.; Arefi-Khonsari, F.; Amouroux, J. Enhancement of the adhesive bonding properties of PEEK by excimer laser treatment. Int. J. Adhes. Adhes. 1998, 18, 19–27. [Google Scholar] [CrossRef]
- Henneuse-Boxus, C.; Poleunis, C.; De-Ro, A.; Adriaensen, Y.; Bertrand, P.; Marchand-Brynaert, J. Surface functionalization of PEEK films studied by time-of-flight secondary ion mass spectrometry and X-ray photoelectron spectroscopy. Surf. Interface Anal. 1999, 27, 142–152. [Google Scholar] [CrossRef]
- Pawson, D.J.; Ameen, A.P.; Short, R.D.; Denison, P.; Jones, F.R. An investigation of the surface chemistry of poly (ether etherketone). I. The effect of oxygen plasma treatment on surface structure. Surf. Interface Anal. 1992, 18, 13–22. [Google Scholar] [CrossRef]
- Comyn, J.; Mascia, L.; Xiao, G.; Parker, B.M. Plasma-treatment of polyetheretherketone (PEEK) for adhesive bonding. Spec Issue honour Dr. K.W. Allen Occas his 70th Birthd. Int. J. Adhes. 1996, 16, 97–104. [Google Scholar] [CrossRef]
- Zheng, J.; Kang, J.; Sun, C.; Yang, C.; Wang, L.; Li, D. Effects of printing path and material components on mechanical properties of 3D-printed polyether-ether-ketone/hydroxyapatite composites. J. Mech. Behav. Biomed. Mater. 2021, 118, 104475. [Google Scholar] [CrossRef]
- Zanjanijam, A.R.; Major, I.; Lyons, J.G.; Lafont, U.; Devine, D.M. Fused Filament Fabrication of PEEK: A Review of Process-Structure-Property Relationships. Polymers 2020, 12, 1665. [Google Scholar] [CrossRef]
- Wu, W.Z.; Geng, P.; Zhao, J.; Zhang, Y.; Rosen, D.W.; Zhang, H.B. Manufacture and thermal deformation analysis of semicrystalline polymer polyether ether ketone by 3D printing. Mater. Res. Innov. 2014, 18, S5-12–S5-16. [Google Scholar] [CrossRef]
- Wang, P.; Zou, B.; Ding, S. Modeling of surface roughness based on heat transfer considering diffusion among deposition filaments for FDM 3D printing heat-resistant resin. Appl. Therm. Eng. 2019, 161, 114064. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Brimacombe, K., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004; Available online: https://www.ncbi.nlm.nih.gov/books/NBK144065/ (accessed on 1 July 2021).
- Ma, J.; Li, Z.; Xue YLiang, X.Y.; Tan, Z.Y.; Tang, B. Novel PEEK/nHA composites fabricated by hot-pressing of 3D braided PEEK matrix. Adv. Compos. Hybrid. Mater. 2020, 3, 156–166. [Google Scholar] [CrossRef]
- Xu, A.; Liu, X.; Gao, X.; Deng, F.; Deng, Y.; Wie, S. Enhancement of osteogenesis on micro/nano-topographical carbon fiber-reinforced polyetheretherketone–nanohydroxyapatite biocomposite. Mater. Sci. Eng. C 2015, 48, 592–598. [Google Scholar] [CrossRef]
- Baştan, F.E.; Rehman, M.A.U.; Avcu, Y.Y.; Avcu, E.; Üstel, F.; Boccaccini, A.R. Electrophoretic co-deposition of PEEK-hydroxyapatite composite coatings for biomedical applications. Colloids Surf. B Biointerfaces 2018, 169, 176–182. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, Z.; Zhou, J.; Zhao, H.; Chen, J.; Bai, R.; Lina, Q.; Alagarsamy, M. Preparation and biocompatibility of Fe50Ni50p/HAP/PEEK biocomposites with weak magnetic properties. RSC Adv. 2019, 9, 10081–10090. [Google Scholar] [CrossRef] [Green Version]
- Spece, H.; Yu, T.; Law, A.W.; Marcolongo, M.; Kurtz, S.M. 3D printed porous PEEK created via fused filament fabrication for osteoconductive orthopaedic surfaces. J. Mech. Behav. Biomed. Mater. 2020, 109, 103850. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M. Polyether ether ketone (PEEK) and its 3D printed implants applications in medical field: An overview. Clin. Epidemiol. Glob. Health 2019, 7, 571–577. [Google Scholar] [CrossRef] [Green Version]
- Guvendiren, M.; Molde, J.; Soares, R.M.D.; Kohn, J. Designing Biomaterials for 3D Printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef]
- Wilcox, B.; Mobbs, R.J.; Wu, A.M.; Phan, K. Systematic review of 3D printing in spinal surgery: The current state of play. J. Spine Surg. 2017, 3, 433–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
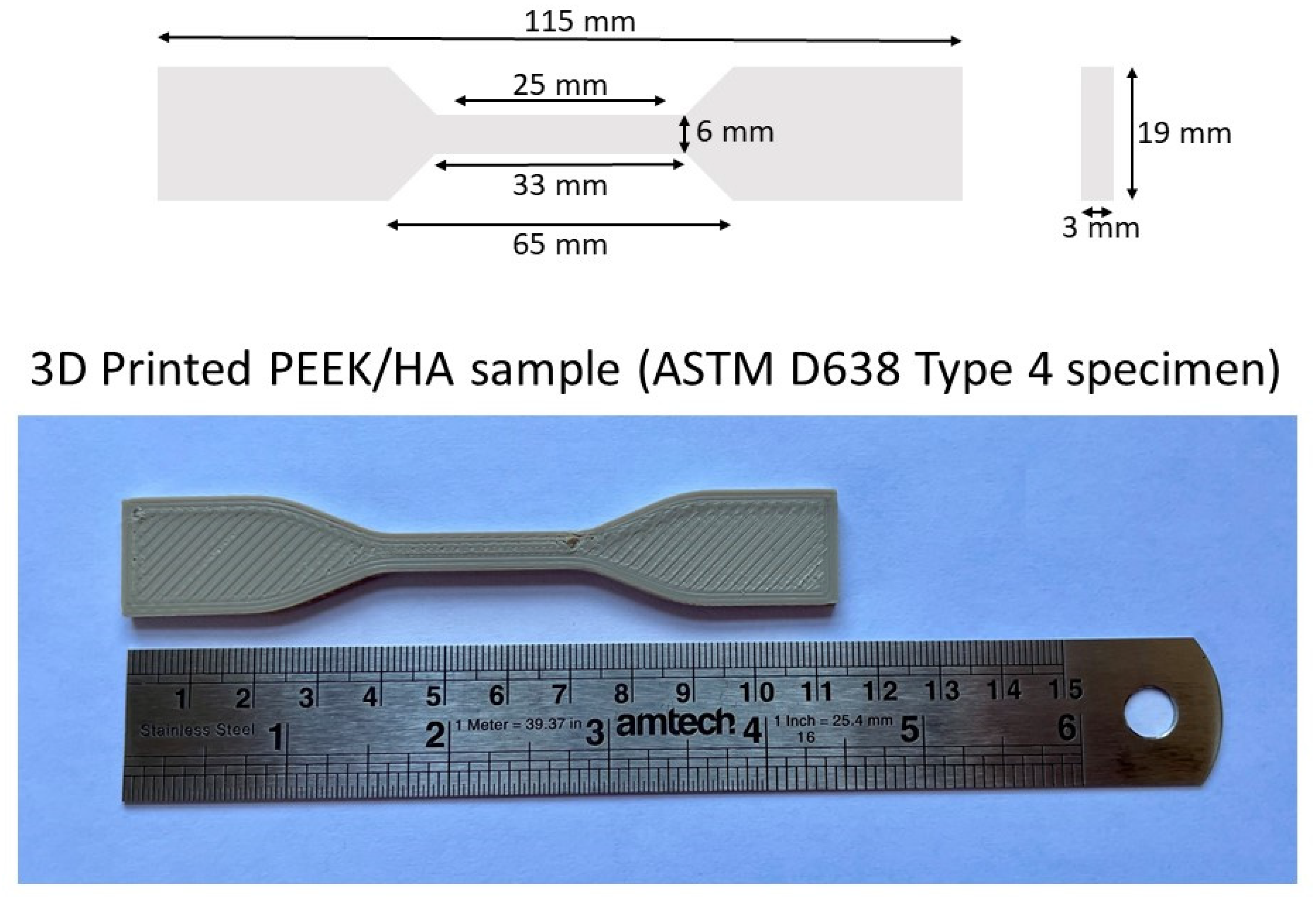
| Description | Value |
|---|---|
| Nozzle diameter | 1.0 mm |
| Layer Thickness | 0.1 mm |
| Nozzle temperature | 400 °C |
| Building plate temperature | 280 °C |
| Chamber temperature | 230 °C |
| Printing speed | 40 mm/s |
| Raster angle | XY 45°/−45° |
| Peak | Sample | ||||
|---|---|---|---|---|---|
| 0HA | 5HA | 10HA | 20HA | 30HA | |
| O1s | 531.6 | 531.7 | 531.9 | 531.9 | 531.5 |
| O1s | 533.8 | 533.7 | 533.8 | 533.6 | 533.6 |
| Ca2p3/2 | - | 347.6 | 347.6 | 347.6 | 347.6 |
| Ca2p1/2 | - | 350.9 | 351.1 | 351.1 | 351.1 |
| C1s | 285.0 | 285.0 | 285.0 | 285.0 | 285.0 |
| C1s | 286.6 | 286.7 | 286.5 | 286.6 | 286.4 |
| C1s | 287.4 | 287.3 | 287.2 | 287.1 | 287.3 |
| C1s | 289.2 | - | - | 288.8 | 288.9 |
| C1s | 291.8 | 291.8 | 291.9 | 291.7 | 291.7 |
| P2p | - | 133.6 | 133.6 | 133.7 | 133.7 |
| Sample | C | O | Ca | P | Ca/P | O/C |
|---|---|---|---|---|---|---|
| 0HA | 87.85 ± 0.76 | 12.15 ± 0.76 | - | - | - | 0.14 ± 0.01 |
| 5HA | 89.70 ± 0.10 | 9.92 ± 0.08 | 0.27 ± 0.03 | 0.11 ± 0.04 | 2.78 ± 0.87 | 0.11 ± 0.00 |
| 10HA | 89.50 ± 0.30 | 9.76 ± 0.27 | 0.49 ± 0.06 | 0.24 ± 0.05 | 2.22 ± 0.81 | 0.11 ± 0.00 |
| 20HA | 86.62 ± 0.50 | 12.29 ± 0.51 | 0.69 ± 0.07 | 0.40 ± 0.10 | 1.91 ± 0.69 | 0.14 ± 0.01 |
| 30HA | 83.49 ± 0.27 | 14.44 ± 0.25 | 1.29 ± 0.03 | 0.81 ± 0.03 | 1.59 ± 0.09 | 0.17 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodzeń, K.; McIvor, M.J.; Sharma, P.K.; Acheson, J.G.; McIlhagger, A.; Mokhtari, M.; McFerran, A.; Ward, J.; Meenan, B.J.; Boyd, A.R. The Surface Characterisation of Fused Filament Fabricated (FFF) 3D Printed PEEK/Hydroxyapatite Composites. Polymers 2021, 13, 3117. https://doi.org/10.3390/polym13183117
Rodzeń K, McIvor MJ, Sharma PK, Acheson JG, McIlhagger A, Mokhtari M, McFerran A, Ward J, Meenan BJ, Boyd AR. The Surface Characterisation of Fused Filament Fabricated (FFF) 3D Printed PEEK/Hydroxyapatite Composites. Polymers. 2021; 13(18):3117. https://doi.org/10.3390/polym13183117
Chicago/Turabian StyleRodzeń, Krzysztof, Mary Josephine McIvor, Preetam K. Sharma, Jonathan G. Acheson, Alistair McIlhagger, Mozaffar Mokhtari, Aoife McFerran, Joanna Ward, Brian J. Meenan, and Adrian R. Boyd. 2021. "The Surface Characterisation of Fused Filament Fabricated (FFF) 3D Printed PEEK/Hydroxyapatite Composites" Polymers 13, no. 18: 3117. https://doi.org/10.3390/polym13183117
APA StyleRodzeń, K., McIvor, M. J., Sharma, P. K., Acheson, J. G., McIlhagger, A., Mokhtari, M., McFerran, A., Ward, J., Meenan, B. J., & Boyd, A. R. (2021). The Surface Characterisation of Fused Filament Fabricated (FFF) 3D Printed PEEK/Hydroxyapatite Composites. Polymers, 13(18), 3117. https://doi.org/10.3390/polym13183117











