Polysaccharides as Stabilizers for Polymeric Microcarriers Fabrication
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Microparticle Fabrication and Characterization
3. Results
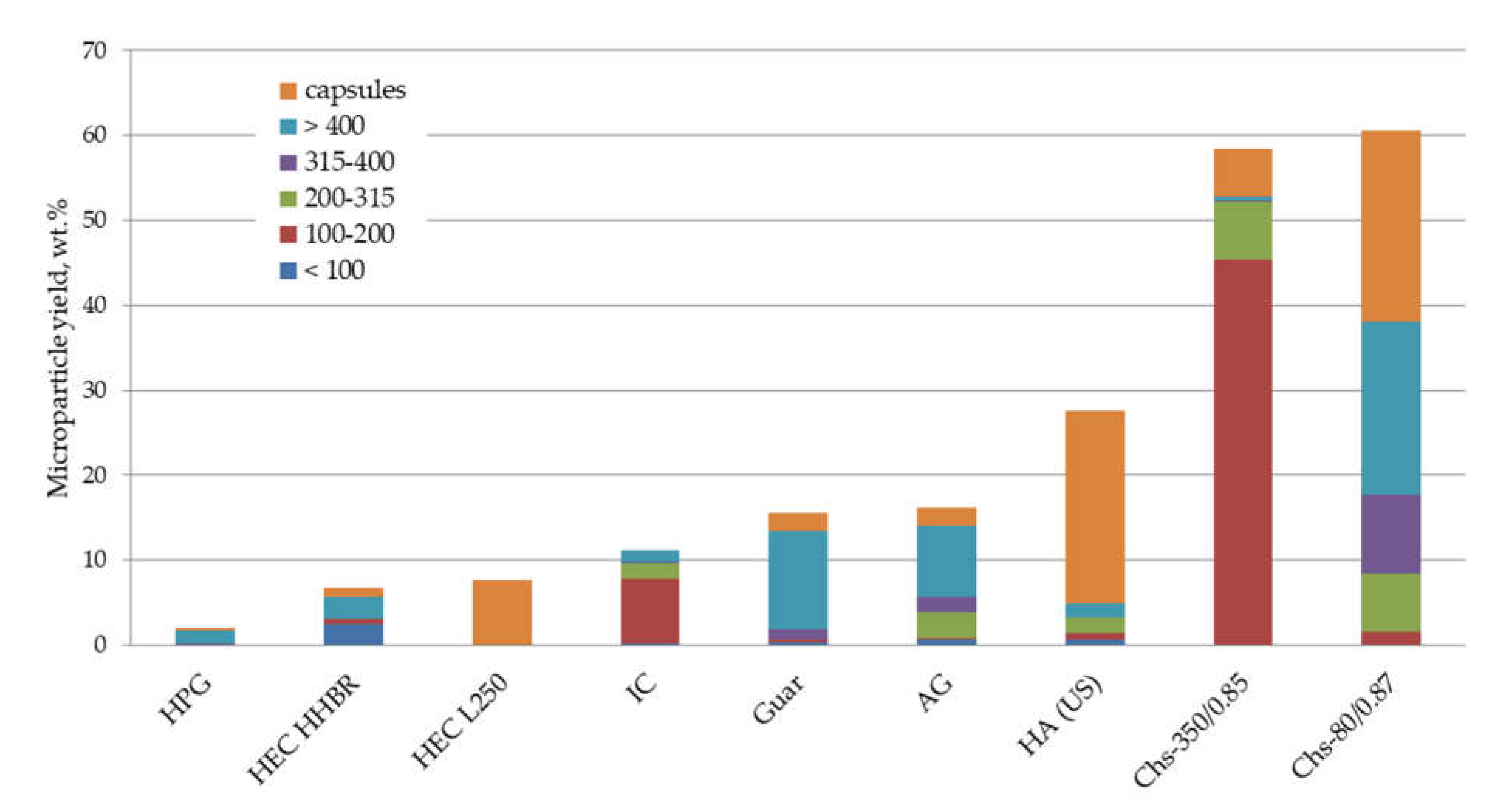
3.1. Non-Modified Biomacromolecules as Emulsifiers for PLA Microparticle Fabrication
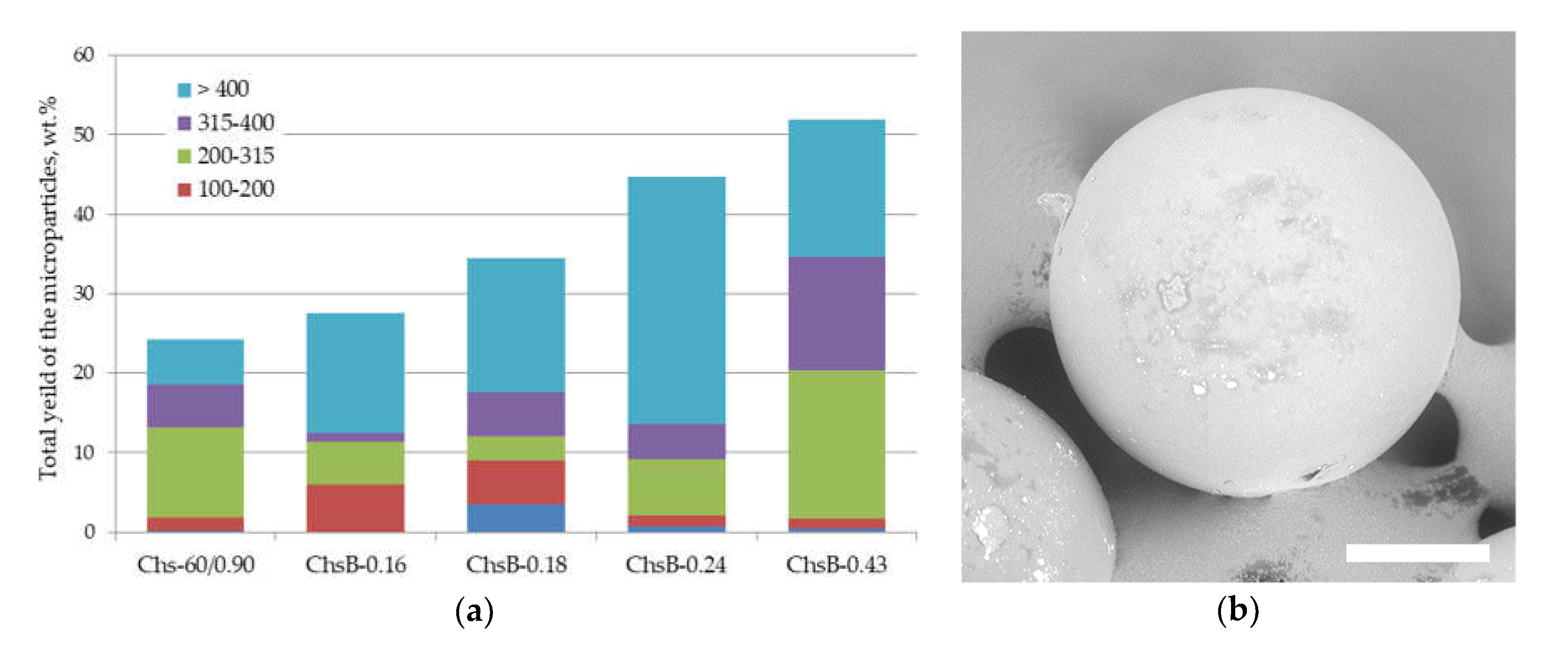
3.2. Effect of Chitosan Chemical Structure on Its Emulsification Ability
3.3. Core/Shell Microparticles Stabilized by Biopolymer Nanoparticles
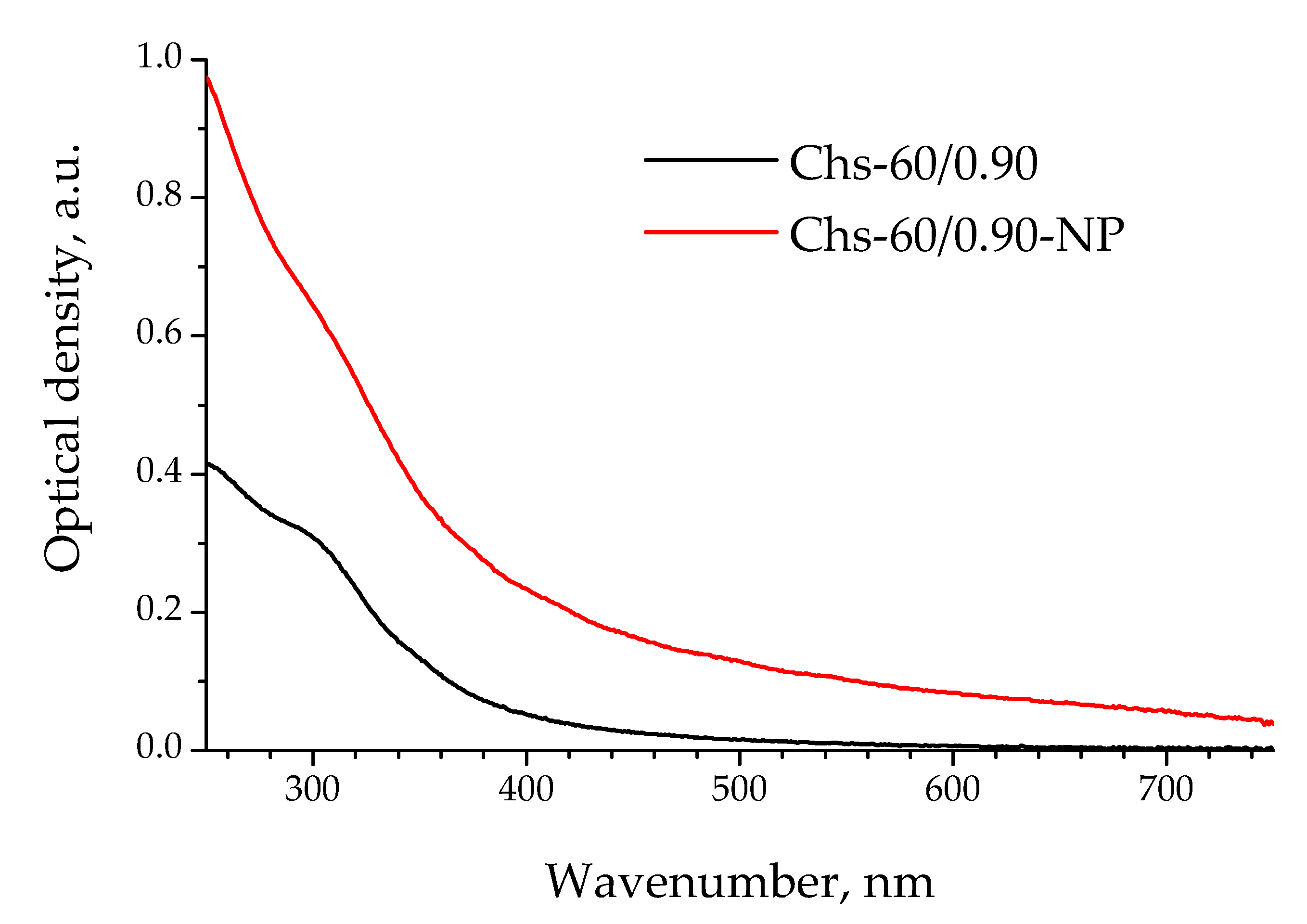
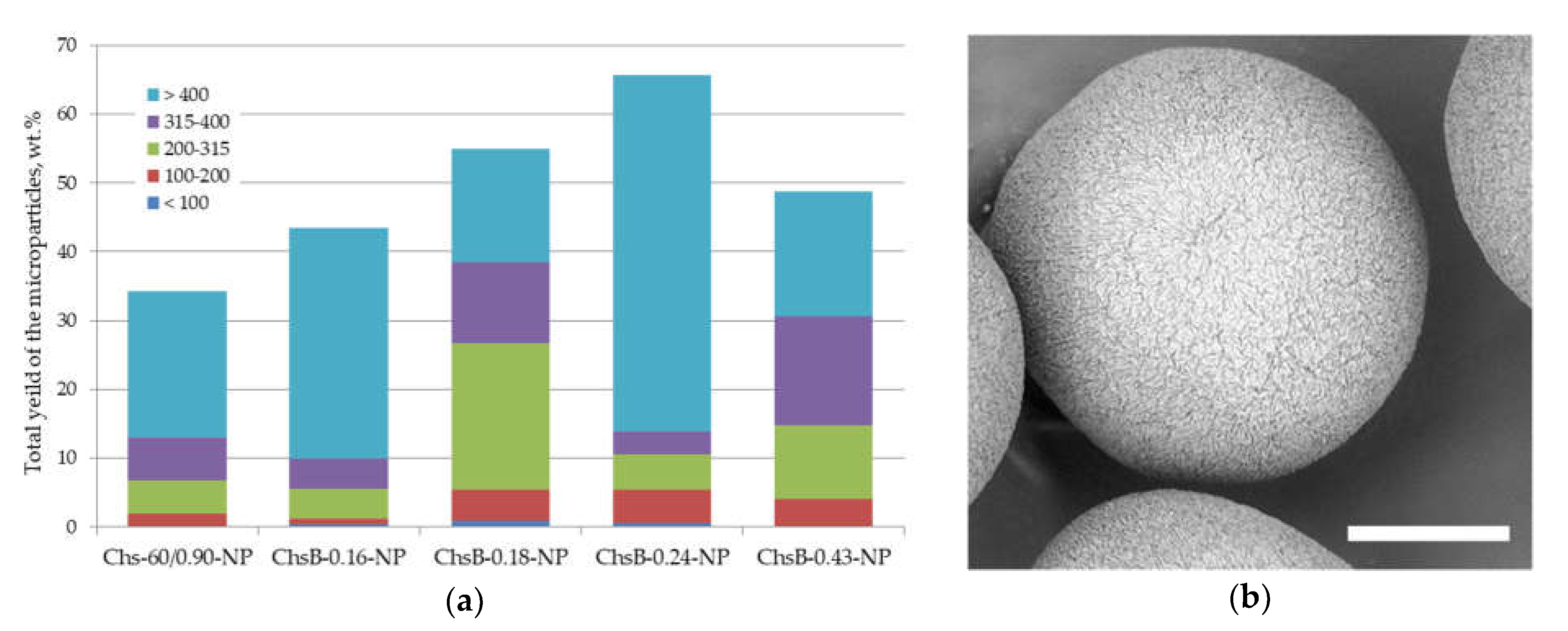
3.3.1. Microparticles Stabilized by Chitosan Nanoparticles Obtained by Polysaccharide Precipitation
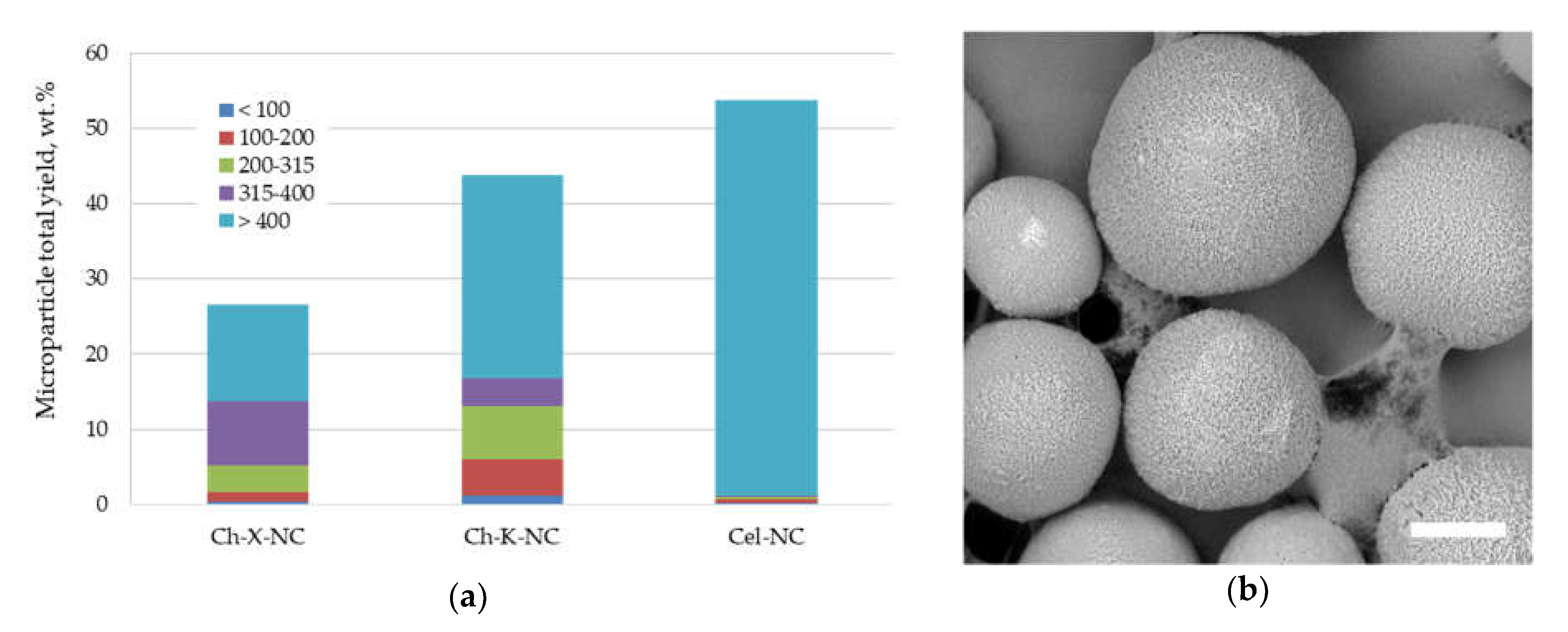
3.3.2. Application of Polysaccharide Nanocrystals Fabricated via Top-Down Approach
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bouyer, E.; Mekhloufi, G.; Rosilio, V.; Grossiord, J.-L.; Agnely, F.P. Polysaccharides, and their complexes used as stabilizers for emulsions: Alternatives to synthetic surfactants in the pharmaceutical field? Int. J. Pharm. 2012, 436, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Y.; Chen, J.-Y.; Tong, X.-M.; Mei, J.-G.; Chen, Y.-F.; Mou, X.-Z. Recent advances in the use of microcarriers for cell cultures and their ex vivo and in vivo applications. Biotechnol. Lett. 2020, 42, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surfaces B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.M.; Orive, G.; Murua, A.; Pedraz, J.L. Microcapsules and microcarriers for in situ cell delivery. Adv. Drug Deliv. Rev. 2010, 62, 711–730. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.; Branquinho, J.; Carreira, A.S.; Carvalho, A.; Coimbra, P.; Ferreira, P.; Gil, M.H. Designing polymeric microparticles for biomedical and industrial applications Eur. Polym. J. 2013, 49, 2005–2021. [Google Scholar]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Molavi, F.; Barzegar-Jalali, M.; Hamishehkar, H. Polyester based polymeric nano and microparticles for pharmaceutical purposes: A review on formulation approaches J. Control. Release 2020, 320, 265–282. [Google Scholar] [CrossRef]
- Sawalha, H.; Schroën, K.; Boom, R. Biodegradable polymeric microcapsules: Preparation and properties Chem. Eng. J. 2011, 169, 1–10. [Google Scholar] [CrossRef]
- Rosca, I.D.; Watari, F.; Uo, M. Microparticle formation and its mechanism in single and double emulsion solvent evaporation J. Control. Release 2004, 99, 271–280. [Google Scholar] [CrossRef]
- O’Donnell, P.B.; McGinity, J.W. Preparation of microspheres by the solvent evaporation technique Adv. Drug Deliv. Rev. 1997, 28, 25–42. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Panyam, J.; Prabha, S.; Labhasetwar, V. Residual polyvinyl alcohol associated with poly (D,L-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake J. Control. Release 2002, 82, 105–114. [Google Scholar] [CrossRef]
- Bee, S.-L.; Hamid, Z.A.A.; Mariatti, M.; Yahaya, B.H.; Lim, K.; Bee, S.-T.; Sin, L.T. Approaches to improve therapeutic efficacy of biodegradable PLA/PLGA microspheres: A review Polym. Rev. 2018, 58, 495–536. [Google Scholar] [CrossRef]
- Rodriguez, M.S.; Albertengo, L.A.; Agullo, E. Emulsification capacity of chitosan Carbohydr. Polym. 2002, 48, 271–276. [Google Scholar]
- Satpute, S.K.; Banat, I.M.; Dhakephalkar, P.K.; Banpurkar, A.G.; Chopade, B.A. Biosurfactants, bioemulsifiers and exopolysaccharides from marine microorganisms Biotechnol. Adv. 2010, 28, 436–450. [Google Scholar]
- Bouyer, E.; Mekhloufi, G.; Le Potier, I.; Kerdaniel, T.D.F.; de Grossiord, J.-L.; Rosilio, V.; Agnely, F. Stabilization mechanism of oil-in-water emulsions by β-lactoglobulin and gum arabic. J. Colloid Interface Sci. 2011, 354, 467–477. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzinger, M.A. Emulsions stabilized with solid nanoparticles: Pickering emulsions Colloids Surfaces A Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An Overview of Pickering Emulsions: Solid-Particle Materials, Classification, Morphology, and Applications Front. Pharmacol. 2017, 8, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Ma, G.-H. Recent studies of Pickering emulsions: Particles make the difference. Small 2016, 12, 4633–4648. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Dufresne, A. Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: A review. Nanoscale 2012, 4, 3274–3294. [Google Scholar] [CrossRef]
- Jin, H.; Chang, P.R.; Lin, N.; Dufresne, A. Polysaccharide-based nanocrystals: Chemistry and applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015. [Google Scholar]
- Tzoumaki, M.V.; Moschakis, T.; Kiosseoglou, V.; Biliaderis, C.G. Oil-in-water emulsions stabilized by chitin nanocrystal particles. Food Hydrocoll. 2011, 25, 1521–1529. [Google Scholar] [CrossRef]
- Cheikh, F.B.; Mabrouk, A.B.; Magnin, A.; Putaux, J.-L.; Boufi, S. Chitin nanocrystals as Pickering stabilizer for O/W emulsions: Effect of the oil chemical structure on the emulsion properties. Colloids Surfaces B Biointerfaces 2021, 200, 111604. [Google Scholar] [CrossRef] [PubMed]
- Sharkawy, A.; Barreiro, M.F.; Rodrigues, A.E. Chitosan-based Pickering emulsions and their applications: A review. Carbohydr. Polym. 2020, 250, 116885. [Google Scholar]
- Rescignano, N.; Fortunati, E.; Armentano, I.; Hernandez, R.; Mijangos, C.; Pasquino, R.; Kenny, J.M. Use of alginate, chitosan and cellulose nanocrystals as emulsion stabilizers in the synthesis of biodegradable polymeric nanoparticles J. Colloid Interface Sci. 2015, 445, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Demina, T.S.; Sotnikova, Y.S.; Istomin, A.V.; Grandfils, C.; Akopova, T.A.; Zelenetskii, A.N. Preparation of poly(L,L-lactide) microparticles via Pickering emulsions using chitin nanocrystals. Adv. Mater. Sci. Eng. 2018, 2018, 8518016. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Wang, C.; Zou, S.; Liu, H.; Tong, Z. Chitosan nanoparticles as particular emulsifier for preparation of novel pH-responsive Pickering emulsions and PLGA microcapsules. Polymer 2012, 53, 1229–1235. [Google Scholar] [CrossRef]
- Pan, H.M.; Yu, H.; Guigas, G.; Fery, A.; Weiss, M.; Patzel, V.; Trau, D. Engineering and design of polymeric shells: Inwards interweaving polymers as multilayer nanofilm, immobilization matrix, or chromatography resins ACS Appl. Mater. Interfaces 2017, 9, 5447–5456. [Google Scholar] [CrossRef]
- Akopova, T.A.; Zelenetskii, A.N.; Ozerin, A.N. Solid state synthesis and modification of chitosan. In Focus on Chitosan Research; Arthur, N.F., Amy, G.O., Eds.; Nova Science Publishers: New York, NY, USA, 2011; Chapter 8; pp. 223–253. [Google Scholar]
- Demina, T.S.; Akopova, T.A.; Vladimirov, L.V.; Shchegolikhin, A.N.; Kechek’yan, A.S.; Perov, N.S.; Chernyshenko, A.O.; Zelenetskii, A.N. The study of the interaction between chitosan and 2,2-bis(hydroxymethyl)propionic acid during solid-phase synthesis Polym. Sci. Ser. B 2011, 53, 358–370. [Google Scholar]
- Istomin, A.V.; Demina, T.S.; Subcheva, E.N.; Akopova, T.A.; Zelenetskii, A.N. Nanocrystalline cellulose from flax stalks: Preparation, structure, and use. Fibre Chem. 2016, 48, 199–201. [Google Scholar] [CrossRef]
- Sotnikova, Y.S.; Demina, T.S.; Istomin, A.V.; Goncharuk, G.P.; Grandfils, C.; Akopova, T.A.; Zelenetskii, A.N.; Babayevsky, P.G. Application of micro- and nanocrystalline cellulose IOP Conf. Ser. Mater. Sci. Eng. 2018, 347, 012006. [Google Scholar]
- Demina, T.S.; Akopova, T.A.; Vladimirov, L.V.; Zelenetskii, A.N.; Markvicheva, E.A.; Grandfils, C. Polylactide-based microspheres prepared using solid-state copolymerized chitosan and d, l -lactide Mater. Sci. Eng. C 2016, 59, 333–338. [Google Scholar] [CrossRef]
- Cheung, R.; Ng, T.; Wong, J.; Chan, W. Chitosan: An update on potential biomedical and pharmaceutical applications Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef]
- Anitha, A.; Sowmya, S.; Kumar, P.T.T.S.S.; Deepthi, S.; Chennazhi, K.P.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar]
- Li, X.; Xia, W. Effects of concentration, degree of deacetylation and molecular weight on emulsifying properties of chitosan Int. J. Biol. Macromol. 2011, 48, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Del Blanco, L.F.; Rodriguez, M.S.; Schulz, P.C.; Agulló, E. Influence of the deacetylation degree on chitosan emulsification properties Colloid Polym. Sci. 1999, 277, 1087–1092. [Google Scholar]
- Privalova, A.; Markvicheva, E.; Sevrin, C.; Drozdova, M.; Kottgen, C.; Gilbert, B.; Ortiz, M.; Grandfils, C. Biodegradable polyester-based microcarriers with modified surface tailored for tissue engineering J. Biomed. Mater. Res. Part. A 2015, 103, 939–948. [Google Scholar] [CrossRef]
- Demina, T.S.; Birdibekova, A.V.; Svidchenko, E.A.; Ivanov, P.L.; Kuryanova, A.S.; Kurkin, T.S.; Khaibullin, Z.I.; Goncharuk, G.P.; Zharikova, T.M.; Bhuniya, S.; et al. Solid-State Synthesis of Water-Soluble Chitosan-g-Hydroxyethyl Cellulose Copolymers. Polymers 2020, 12, 611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demina, T.S.; Kilyashova, L.A.; Popyrina, T.N.; Svidchenko, E.A.; Bhuniya, S.; Akopova, T.A.; Grandfils, C. Polysaccharides as Stabilizers for Polymeric Microcarriers Fabrication. Polymers 2021, 13, 3045. https://doi.org/10.3390/polym13183045
Demina TS, Kilyashova LA, Popyrina TN, Svidchenko EA, Bhuniya S, Akopova TA, Grandfils C. Polysaccharides as Stabilizers for Polymeric Microcarriers Fabrication. Polymers. 2021; 13(18):3045. https://doi.org/10.3390/polym13183045
Chicago/Turabian StyleDemina, Tatiana S., Liubov A. Kilyashova, Tatiana N. Popyrina, Eugenia A. Svidchenko, Sankarprasad Bhuniya, Tatiana A. Akopova, and Christian Grandfils. 2021. "Polysaccharides as Stabilizers for Polymeric Microcarriers Fabrication" Polymers 13, no. 18: 3045. https://doi.org/10.3390/polym13183045
APA StyleDemina, T. S., Kilyashova, L. A., Popyrina, T. N., Svidchenko, E. A., Bhuniya, S., Akopova, T. A., & Grandfils, C. (2021). Polysaccharides as Stabilizers for Polymeric Microcarriers Fabrication. Polymers, 13(18), 3045. https://doi.org/10.3390/polym13183045









