Artificial Intelligence-Assisted Throat Sensor Using Ionic Polymer–Metal Composite (IPMC) Material
Abstract
:1. Introduction
2. Materials and Fabrication Methods
2.1. Chemicals and Materials
2.2. Fabrication of Sponge-like IPMC Sensor
2.3. Characterization
3. Results and Discussion
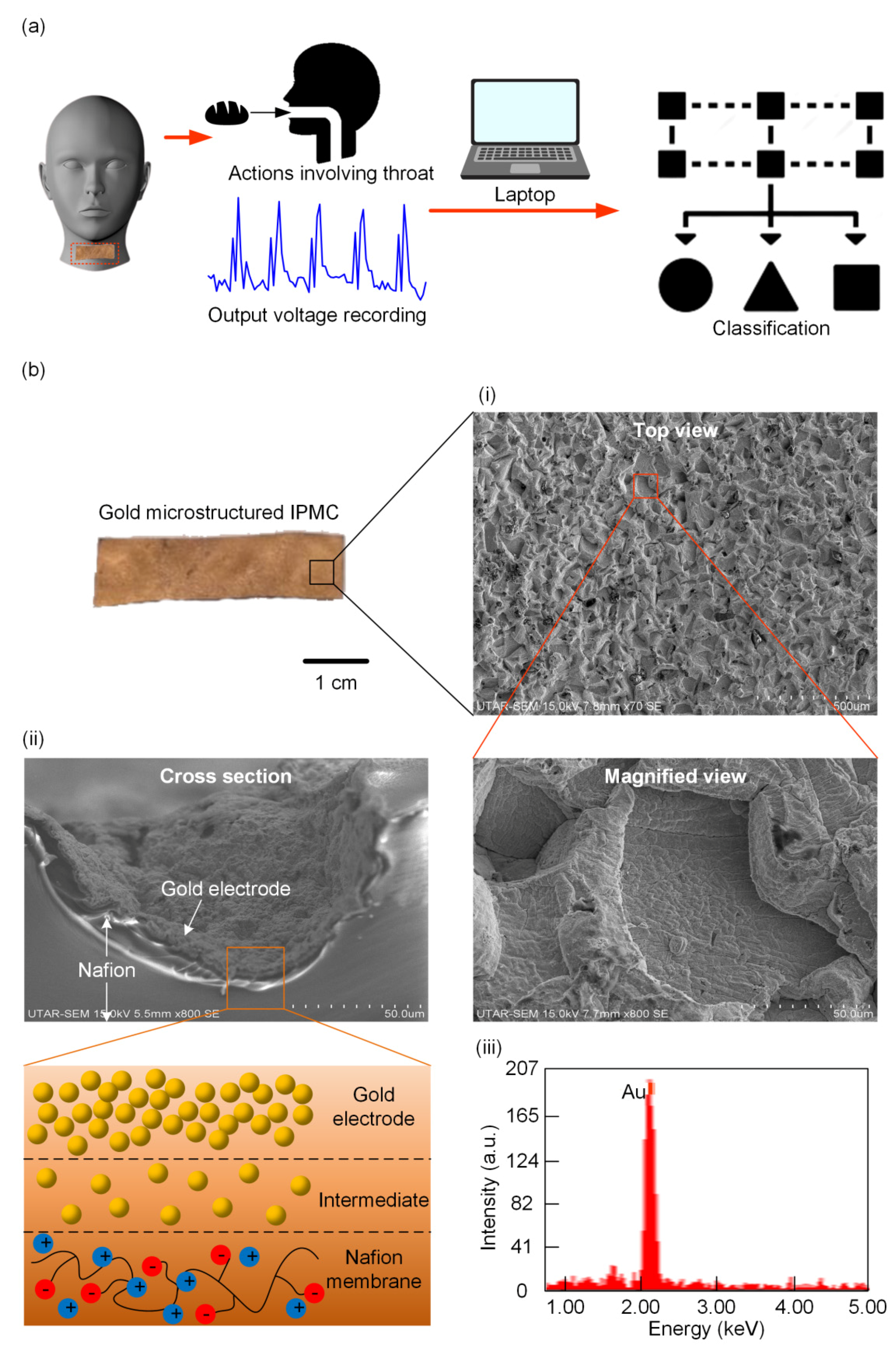
3.1. Design of the Self-Powered IPMC Sensor
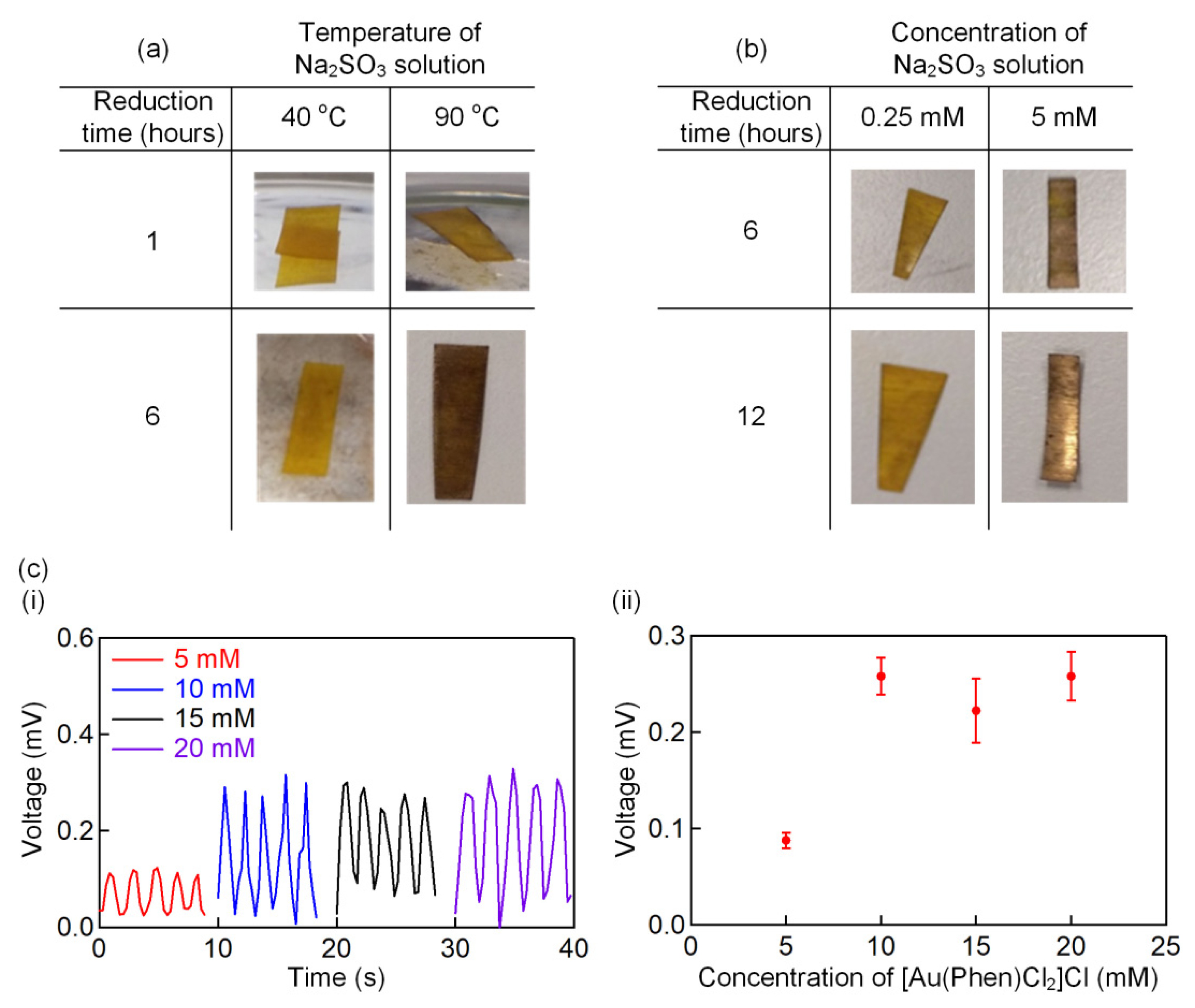
3.2. Effect of Synthesis Conditions on the Properties of the Self-Powered IPMC Sensor
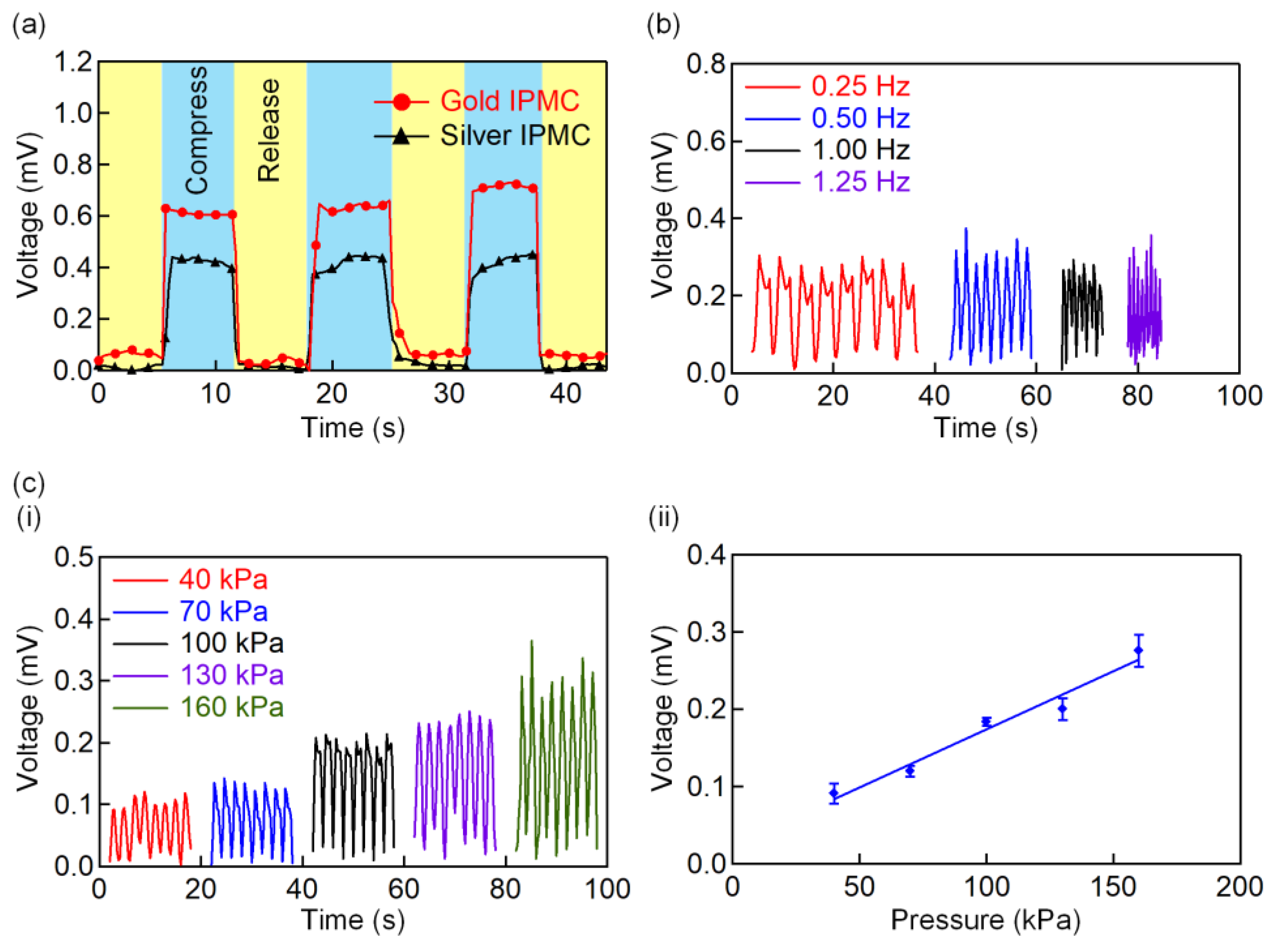
3.3. Characterization of the Self-Powered IPMC Sensor
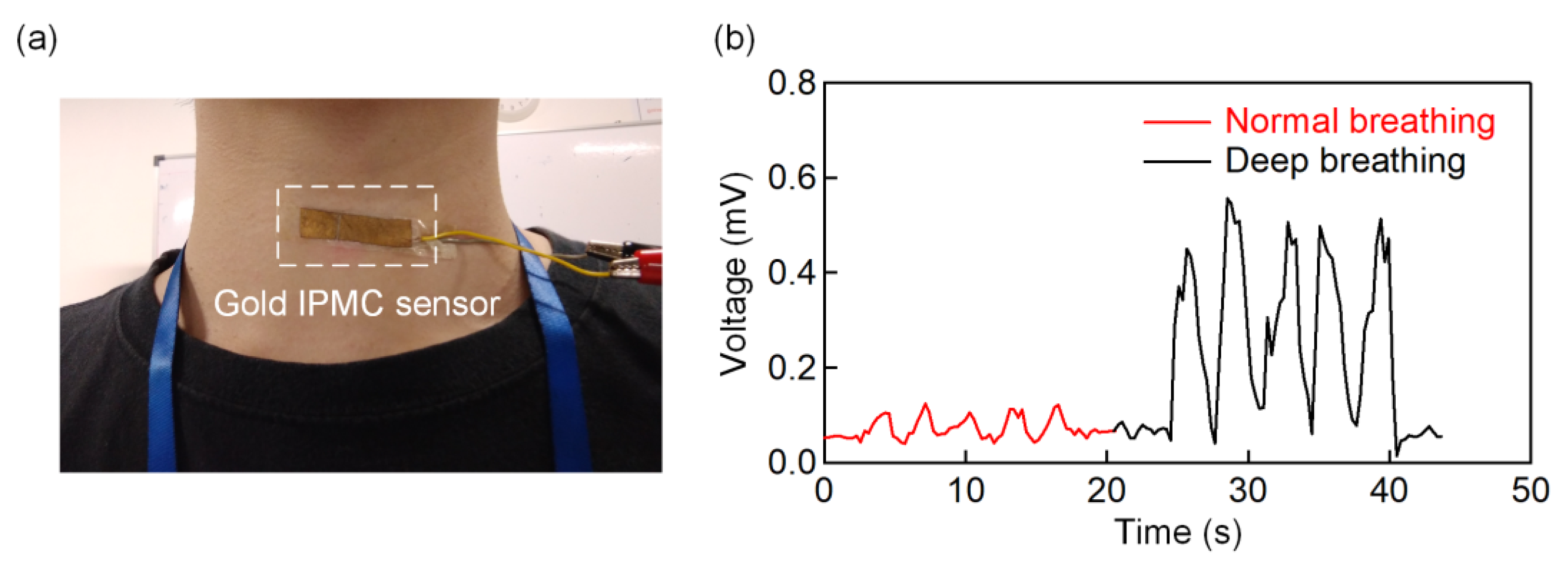
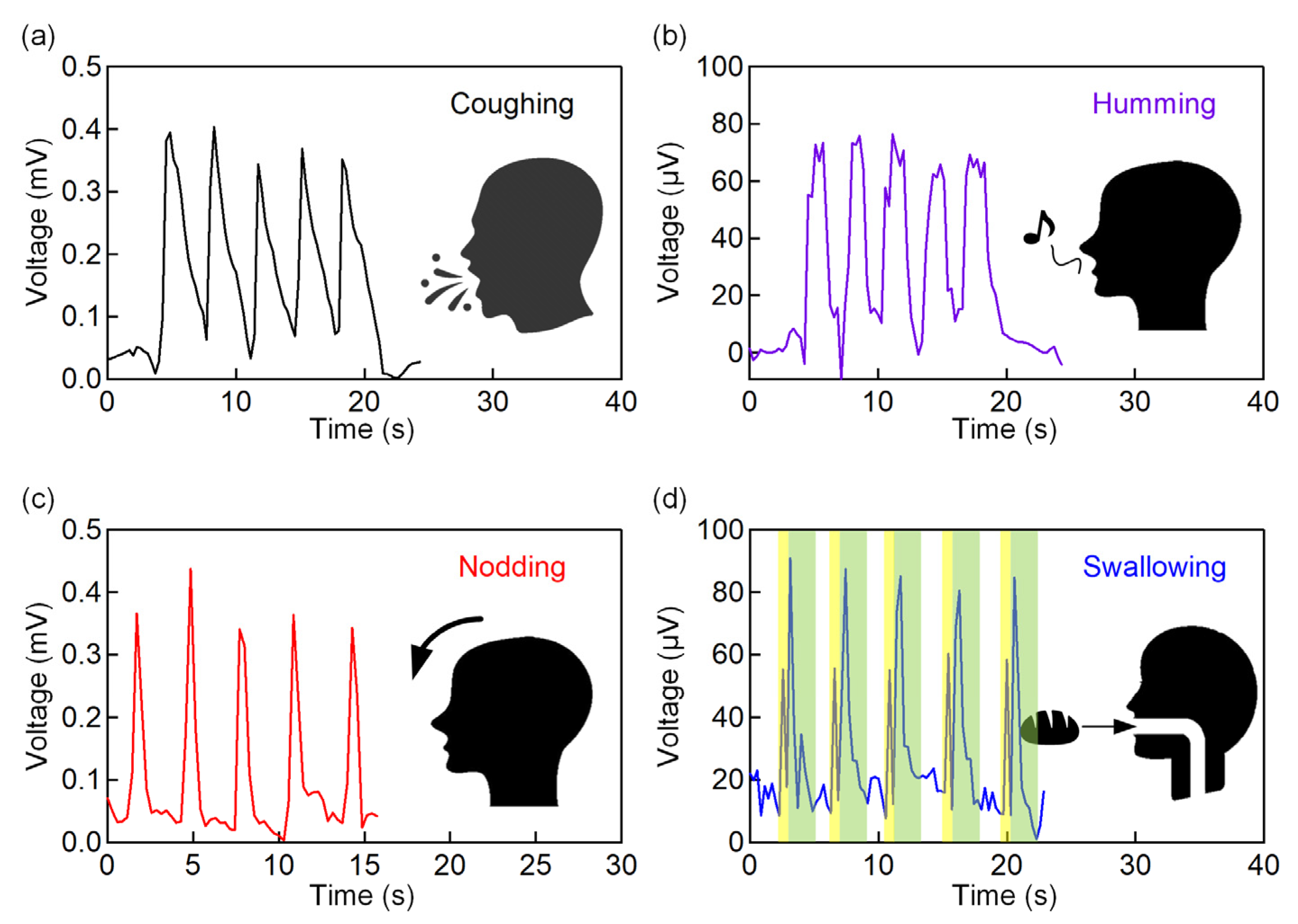
3.4. Application of the IPMC Sensor for Various Throat Movement Sensing
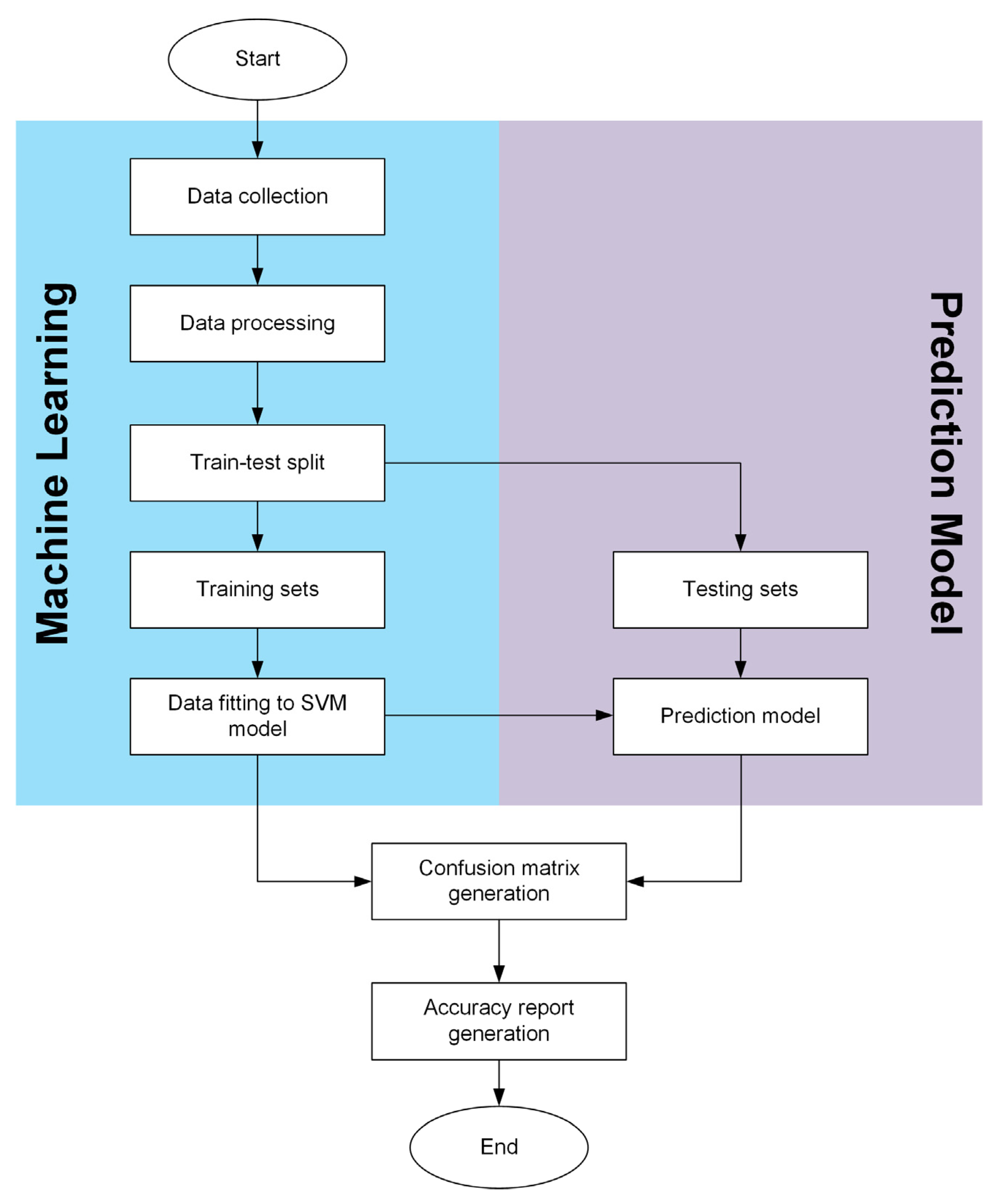
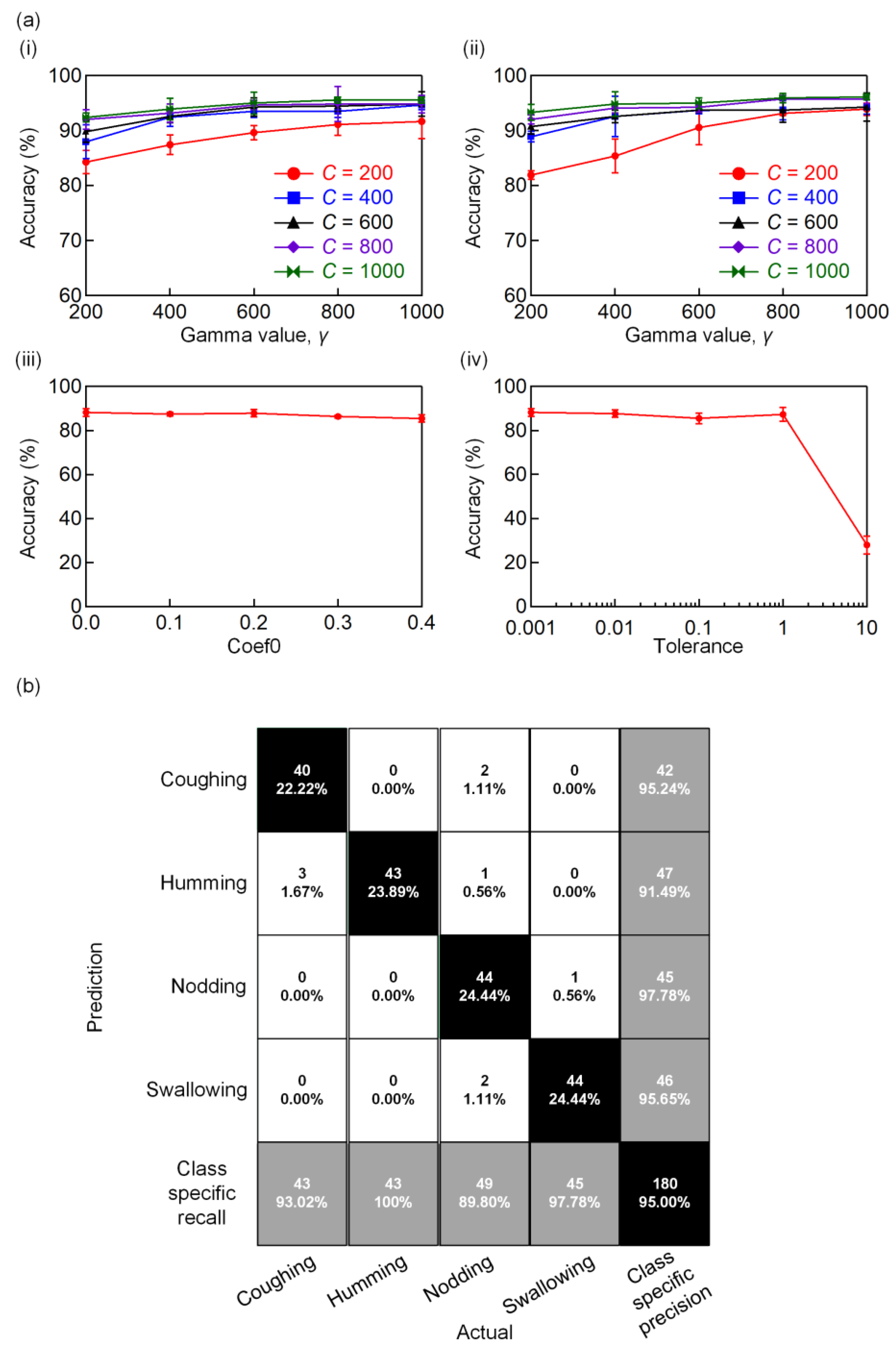
3.5. Data Processing via Machine Learning Technique
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iizuka, M.; Kobayashi, M.; Hasegawa, Y.; Tomita, K.; Takeshima, R.; Izumizaki, M. A new flexible piezoelectric pressure sensor array for the noninvasive detection of laryngeal movement during swallowing. J. Physiol. Sci. 2018, 68, 837–846. [Google Scholar] [CrossRef]
- Rommel, N.; Hamdy, S. Oropharyngeal dysphagia: Manifestations and diagnosis. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.; Leslie, P.; Beale, T.; Payten, C.; Drinnan, M. Fibreoptic endoscopic evaluation of swallowing and videofluoroscopy: Does examination type influence perception of pharyngeal residue severity? Clin. Otolaryngol. 2006, 31, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Logemann, J.A.; Rademaker, A.W.; Pauloski, B.R.; Ohmae, Y.; Kahrilas, P.J. Normal swallowing physiology as viewed by videofluoroscopy and videoendoscopy. Folia Phoniatr. Et Logop. 1998, 50, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Fattori, B.; Giusti, P.; Mancini, V.; Grosso, M.; Barillari, M.; Bastiani, L.; Molinaro, S.; Nacci, A. Comparison between videofluoroscopy, fiberoptic endoscopy and scintigraphy for diagnosis of oro-pharyngeal dysphagia. Acta Otorhinolaryngol. Ital. 2016, 36, 395. [Google Scholar] [CrossRef]
- Shieh, W.Y.; Wang, C.M.; Chang, C.S. Development of a portable non-invasive swallowing and respiration assessment device. Sensors 2015, 15, 12428–12453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinh, T.; Phan, H.P.; Dao, D.V.; Woodfield, P.; Qamar, A.; Nguyen, N.T. Graphite on paper as material for sensitive thermoresistive sensors. J. Mater. Chem. C 2015, 3, 8776–8779. [Google Scholar] [CrossRef] [Green Version]
- Dinh, T.; Phan, H.P.; Qamar, A.; Woodfield, P.; Nguyen, N.T.; Dao, D.V. Thermoresistive effect for advanced thermal sensors: Fundamentals, design considerations, and applications. J. Microelectromech. Syst. 2017, 26, 966–986. [Google Scholar] [CrossRef]
- Chang, X.L.; Chee, P.S.; Lim, E.H.; Tan, R.C.C. A novel crenellated ionic polymer-metal composite (IPMC) actuator with enhanced electromechanical performances. Smart Mater. Struct. 2019, 28, 115011. [Google Scholar] [CrossRef]
- Chee, P.S.; Arsat, R.; Adam, T.; Hashim, U.; Rahim, R.A.; Leow, P.L. Modular architecture of a non-contact pinch actuation micropump. Sensors 2012, 12, 12572–12587. [Google Scholar] [CrossRef] [Green Version]
- Rusli, M.; Chee, P.S.; Arsat, R.; Lau, K.X.; Leow, P.L. Electromagnetic actuation dual-chamber bidirectional flow micropump. Sens. Actuators A 2018, 282, 17–27. [Google Scholar] [CrossRef]
- Chong, Y.S.; Yeoh, K.H.; Leow, P.L.; Chee, P.S. Piezoresistive strain sensor array using polydimethylsiloxane-based conducting nanocomposites for electronic skin application. Sens. Rev. 2018, 38, 494–500. [Google Scholar] [CrossRef]
- Low, J.H.; Chee, P.S.; Lim, E.H. Deformable liquid metal patch antenna for air pressure detection. IEEE Sens. J. 2019, 20, 3963–3970. [Google Scholar] [CrossRef]
- Low, J.H.; Chee, P.S.; Lim, E.H.; Lee, K.Y. Compact organic liquid dielectric resonator antenna for air pressure sensing using soft material. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Low, J.H.; Chee, P.S.; Lim, E.H.; Ganesan, V. Design of a wireless smart insole using stretchable microfluidic sensor for gait monitoring. Smart Mater. Struct. 2020, 29, 065003. [Google Scholar] [CrossRef]
- Kim, M.K.; Kantarcigil, C.; Kim, B.; Baruah, R.K.; Maity, S.; Park, Y.; Kim, K.; Lee, S.; Malandraki, J.B.; Avlani, S. Flexible submental sensor patch with remote monitoring controls for management of oropharyngeal swallowing disorders. Sci. Adv. 2019, 5, eaay3210. [Google Scholar] [CrossRef] [Green Version]
- Ertekin, C.; Pehlivan, M.; Aydoǧdu, I.; Ertaşl, M.; Uludaǧ, B.; Çlelebi, G.; Çlolakoǧlu, Z.; Saǧduyu, A.; Yüceyar, N. An electrophysiological investigation of deglutition in man. Muscle Nerve 1995, 18, 1177–1186. [Google Scholar] [CrossRef]
- Ertekin, C.; Yüceyar, N.; Karasoy, H. Electrophysiological evaluation of oropharyngeal swallowing in myotonic dystrophy. J. Neurol. Neurosurg. Psychiatry 2001, 70, 363–371. [Google Scholar] [CrossRef] [Green Version]
- Tsuga, K.; Hayashi, R.; Sato, Y.; Akagawa, Y. Handy measurement for tongue motion and coordination with laryngeal elevation at swallowing. J. Oral Rehabil. 2003, 30, 985–989. [Google Scholar] [CrossRef]
- Abe, S.; Kaneko, H.; Nakamura, Y.; Watanabe, Y.; Shintani, M.; Hashimoto, M.; Yamane, G.; Ide, Y.; Shimono, M.; Ishikawa, T. Experimental device for detecting laryngeal movement during swallowing. Bull. Tokyo Dent. Coll. 2002, 43, 199–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, B.; Yu, X.; Ou, J. Self-sensing Concrete in Smart Structures; Butterworth-Heinemann: Oxford, UK, 2014. [Google Scholar]
- Tiwana, M.I.; Redmond, S.J.; Lovell, N.H. A review of tactile sensing technologies with applications in biomedical engineering. Sens. Actuators A 2012, 179, 17–31. [Google Scholar] [CrossRef]
- Lee, J.H.; Chee, P.S.; Lim, E.H. Development of a self-powered and stretchable sensor for wearable electronics. In Proceedings of the 2020 IEEE-EMBS Conference on Biomedical Engineering and Sciences (IECBES), Langkawi Island, Malaysia, 1–3 March 2021; pp. 162–165. [Google Scholar]
- Low, J.H.; Chee, P.S.; Lim, E.H.; Ganesan, V. Kirigami-structured and self-powered pressure sensor using electroactive polymer. In Proceedings of the 2021 IEEE 34th International Conference on Micro Electro Mechanical Systems (MEMS), Gainesville, FL, USA, 25–29 January 2021; pp. 748–751. [Google Scholar]
- Chang, X.L.; Chee, P.S.; Lim, E.H.; Chong, W.C. Radio-frequency enabled ionic polymer metal composite (IPMC) actuator for drug release application. Smart Mater. Struct. 2018, 28, 015024. [Google Scholar] [CrossRef]
- Cheong, H.R.; Teo, C.Y.; Leow, P.L.; Lai, K.C.; Chee, P.S. Wireless-powered electroactive soft microgripper. Smart Mater. Struct. 2018, 27, 055014. [Google Scholar] [CrossRef]
- Cheong, H.R.; Nguyen, N.T.; Khaw, M.K.; Teoh, B.Y.; Chee, P.S. Wirelessly activated device with an integrated ionic polymer metal composite (IPMC) cantilever valve for targeted drug delivery. Lab Chip 2018, 18, 3207–3215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Ma, W.; Zhong, J.; Zhang, Z. Comparative study of machine learning approaches for predicting creep behavior of polyurethane elastomer. Polymers 2021, 13, 1768. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Fan, X.; Liu, J.; Zhang, H. Moisture prediction of transformer oil-immersed polymer insulation by applying a support vector machine combined with a genetic algorithm. Polymers 2020, 12, 1579. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Y.; Liu, J.; Zheng, H.; Wang, K. A novel fault diagnosis system on polymer insulation of power transformers based on 3-stage GA–SA–SVM OFC selection and ABC–SVM classifier. Polymers 2018, 10, 1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Hu, Y.; Zhao, J.; Wu, G.; Tao, X.; Chen, W. Self-powered piezoionic strain sensor toward the monitoring of human activities. Small 2016, 12, 5074–5080. [Google Scholar] [CrossRef] [PubMed]
- Harris, C. 136. Nitrogenous chelate complexes of transition metals. Part I. The constitution and properties of the 1: 10-phenanthroline complexes of tervalent gold. J. Chem. Soc. 1959, 682–687. [Google Scholar] [CrossRef]
- Park, I.S.; Kim, S.M.; Kim, D.; Kim, K.J. The mechanical properties of ionic polymer-metal composites. In Proceedings of the Electroactive Polymer Actuators and Devices (EAPAD) 2007, San Diego, CA, USA, 18–22 March 2007; p. 65241R. [Google Scholar]
- Wang, J.; Wang, Y.; Zhu, Z.; Wang, J.; He, Q.; Luo, M. The effects of dimensions on the deformation sensing performance of ionic polymer-metal composites. Sensors 2019, 19, 2104. [Google Scholar] [CrossRef] [Green Version]
- Byun, J.M.; Hwang, T.; Kim, K.J. Formation of a gold nanoparticle layer for the electrodes of ionic polymer–metal composites by electroless deposition process. Appl. Surf. Sci. 2019, 470, 8–12. [Google Scholar] [CrossRef]
- Arrhenius, S. Über die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Säuren. Z. Phys. Chem. 1889, 4, 226–248. [Google Scholar] [CrossRef] [Green Version]
- Mickey, C.D. Chemical kinetics: Reaction rates. J. Chem. Educ. 1980, 57, 659. [Google Scholar] [CrossRef]
- Chung, C.K.; Fung, P.; Hong, Y.; Ju, M.S.; Lin, C.C.K.; Wu, T. A novel fabrication of ionic polymer-metal composites (IPMC) actuator with silver nano-powders. Sens. Actuators B Chem. 2006, 117, 367–375. [Google Scholar] [CrossRef]
- Tamagawa, H.; Okada, K.; Mulembo, T.; Sasaki, M.; Naito, K.; Nagai, G.; Nitta, T.; Yew, K.C.; Ikeda, K. Simultaneous enhancement of bending and blocking force of an ionic polymer-metal composite (IPMC) by the active use of its material characteristics change. Actuators 2019, 8, 29. [Google Scholar] [CrossRef] [Green Version]
- Kihara, H.; Fukushima, S.; Naemura, T. Analysis of human nodding behavior during group work for designing nodding robots. In Proceedings of the Proceedings of the 19th International Conference on Supporting Group Work, Sanibel Island, FL, USA, 13–16 November 2016; pp. 433–436. [Google Scholar]
- Li, Q.; Minagi, Y.; Hori, K.; Kondoh, J.; Fujiwara, S.; Tamine, K.; Inoue, M.; Maeda, Y.; Chen, Y.; Ono, T. Coordination in oro-pharyngeal biomechanics during human swallowing. Physiol. Behav. 2015, 147, 300–305. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, R.; Zhu, H. Recent progress in wearable tactile sensors combined with algorithms based on machine learning and signal processing. APL Mater. 2021, 9, 030906. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Chee, P.-S.; Lim, E.-H.; Tan, C.-H. Artificial Intelligence-Assisted Throat Sensor Using Ionic Polymer–Metal Composite (IPMC) Material. Polymers 2021, 13, 3041. https://doi.org/10.3390/polym13183041
Lee J-H, Chee P-S, Lim E-H, Tan C-H. Artificial Intelligence-Assisted Throat Sensor Using Ionic Polymer–Metal Composite (IPMC) Material. Polymers. 2021; 13(18):3041. https://doi.org/10.3390/polym13183041
Chicago/Turabian StyleLee, Jai-Hua, Pei-Song Chee, Eng-Hock Lim, and Chun-Hui Tan. 2021. "Artificial Intelligence-Assisted Throat Sensor Using Ionic Polymer–Metal Composite (IPMC) Material" Polymers 13, no. 18: 3041. https://doi.org/10.3390/polym13183041
APA StyleLee, J.-H., Chee, P.-S., Lim, E.-H., & Tan, C.-H. (2021). Artificial Intelligence-Assisted Throat Sensor Using Ionic Polymer–Metal Composite (IPMC) Material. Polymers, 13(18), 3041. https://doi.org/10.3390/polym13183041





