Thermosensitive Poloxamer-graft-Carboxymethyl Pullulan: A Potential Injectable Hydrogel for Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
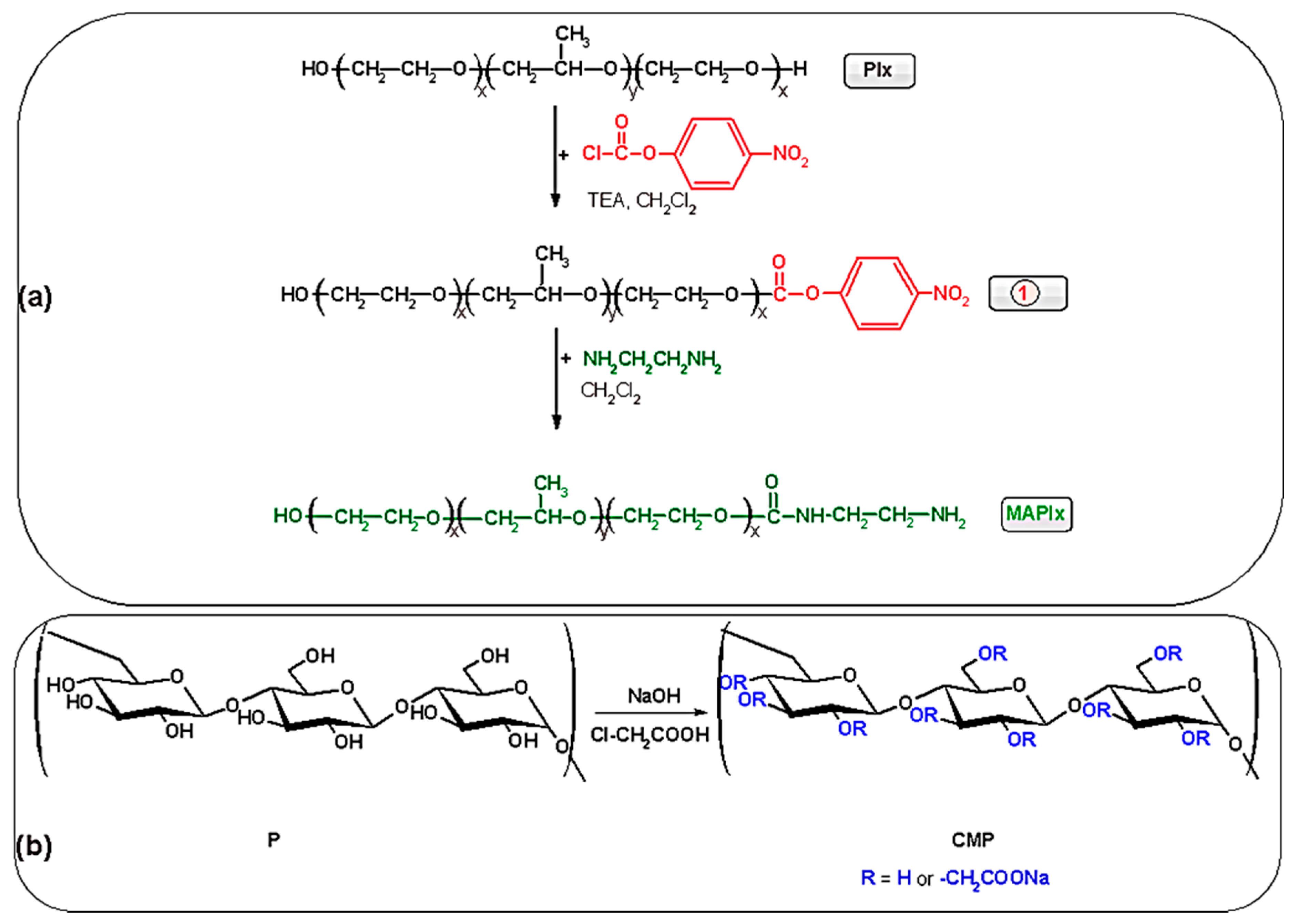
2.2. Synthesis of Monoamine Derivative of Poloxamer (MAPlx)
2.3. Synthesis of Carboxymethyl Pullulan (CMP)
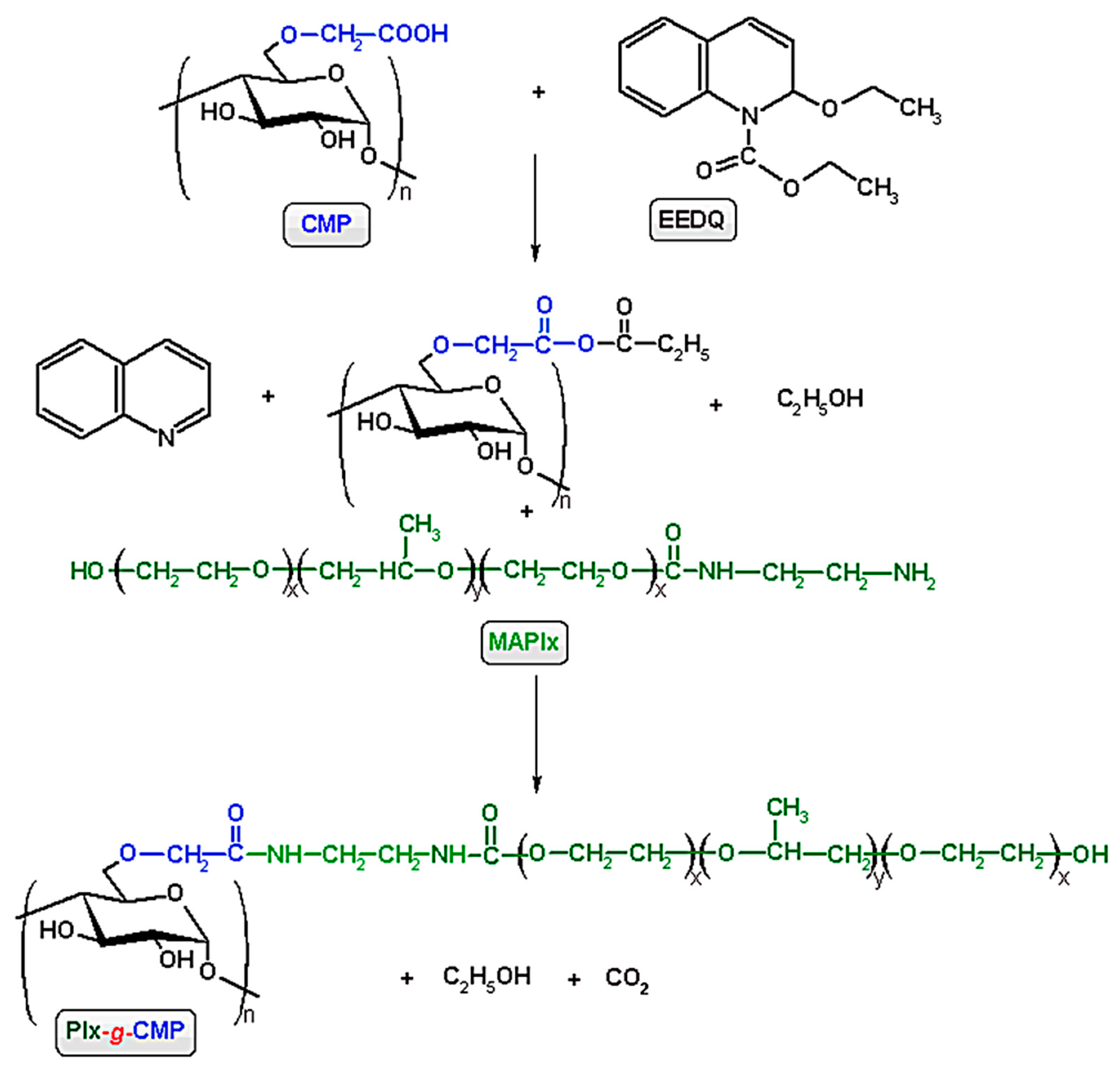
2.4. Synthesis of Grafted Copolymer (Plx-g-CMP)
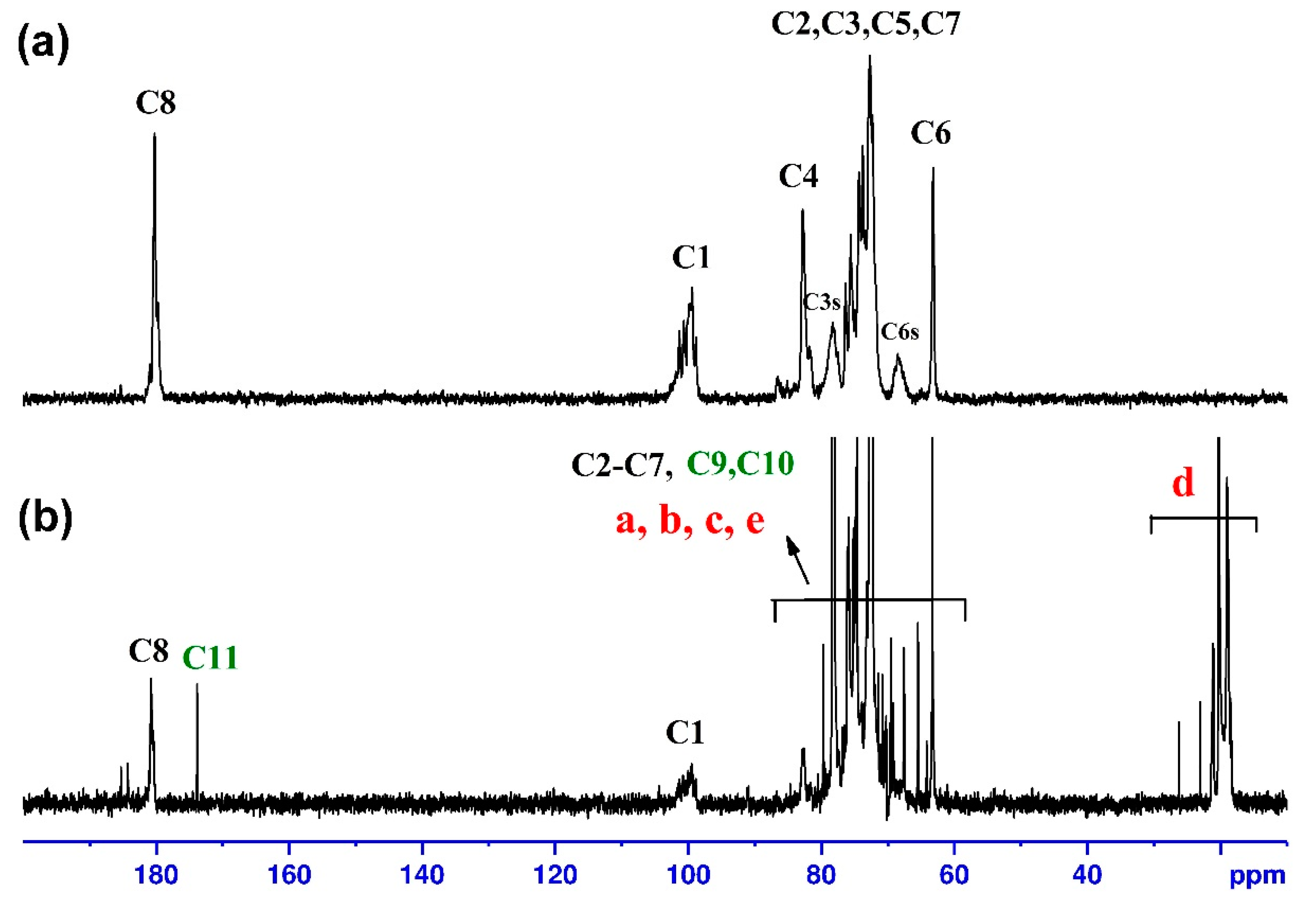
2.5. Structural Characterization
2.6. Hydrogel Characterization
2.6.1. Thermosensitive Sol–Gel Transition Behavior
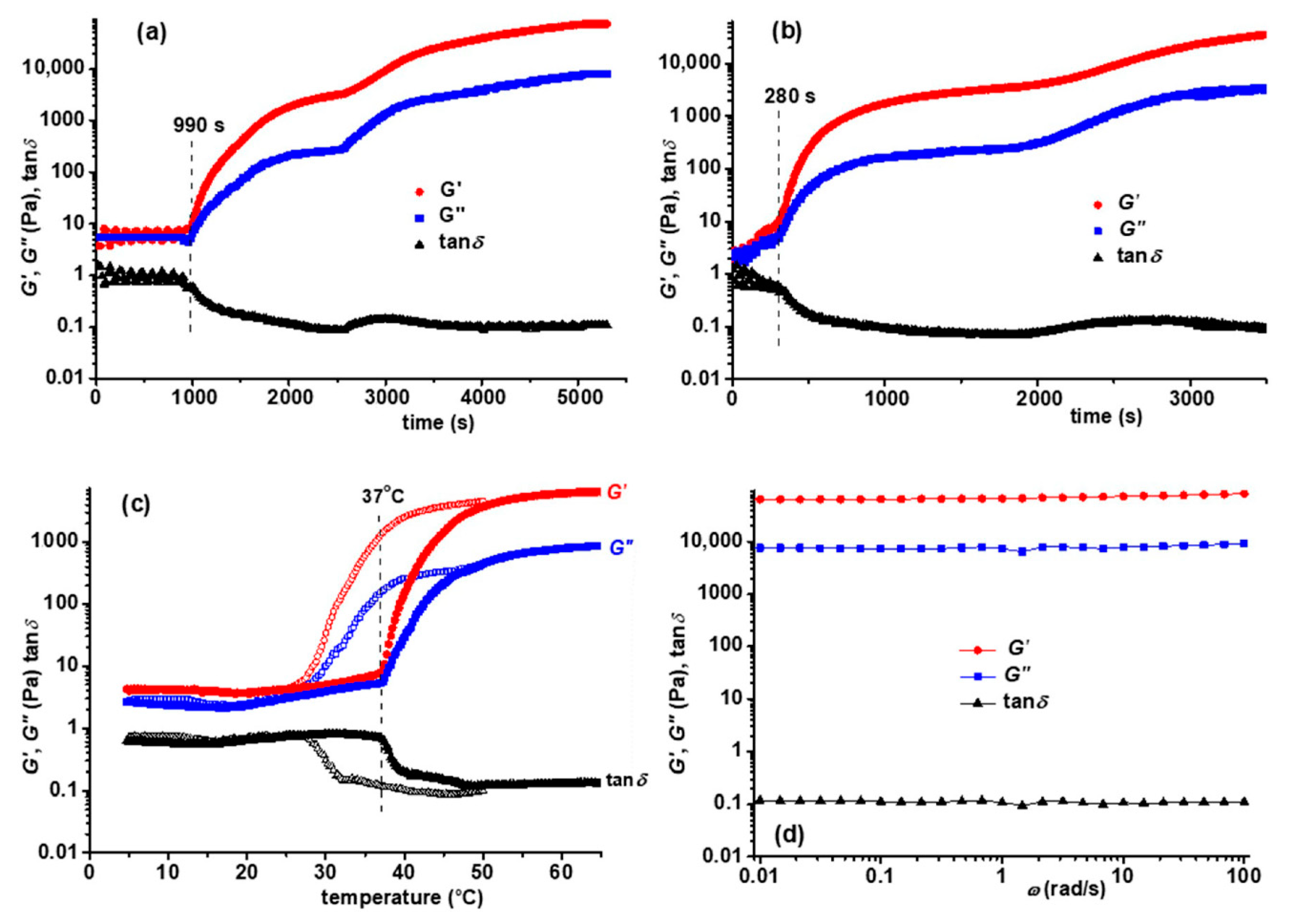
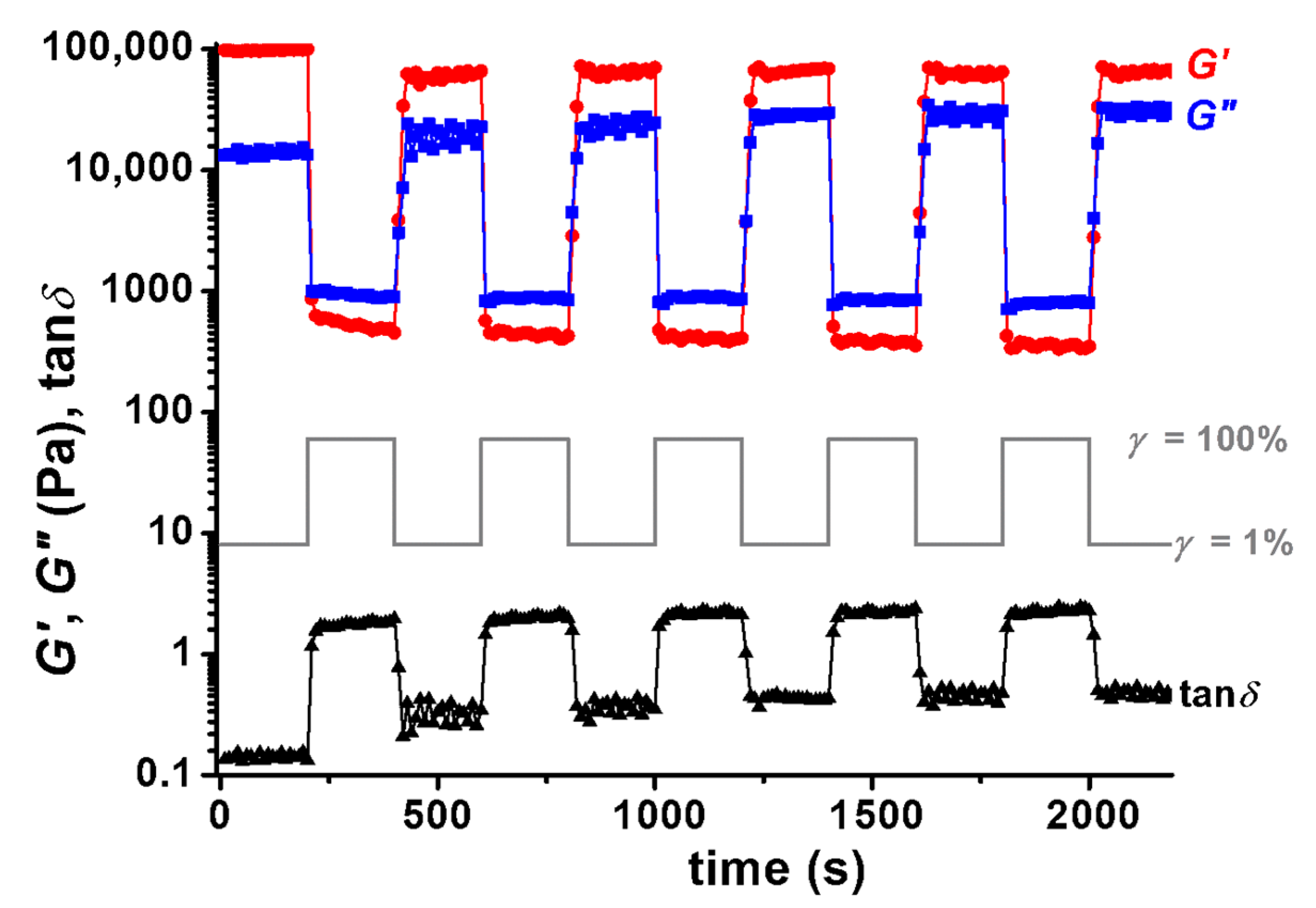
2.6.2. Rheological Analysis
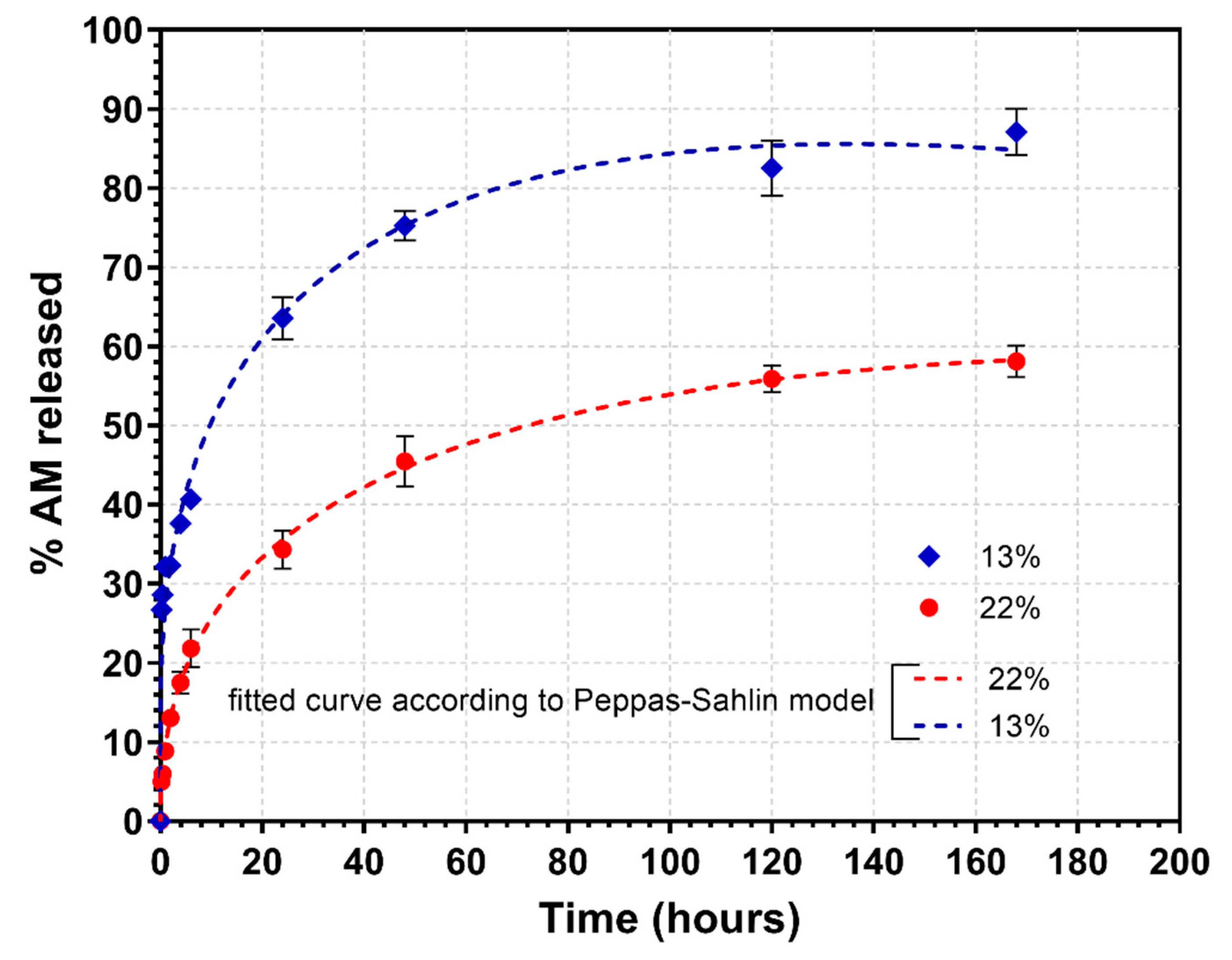
2.7. Drug Loading and In Vitro Release Studies
3. Results and Discussion
3.1. Synthesis and Characterization of Plx-g-CMP
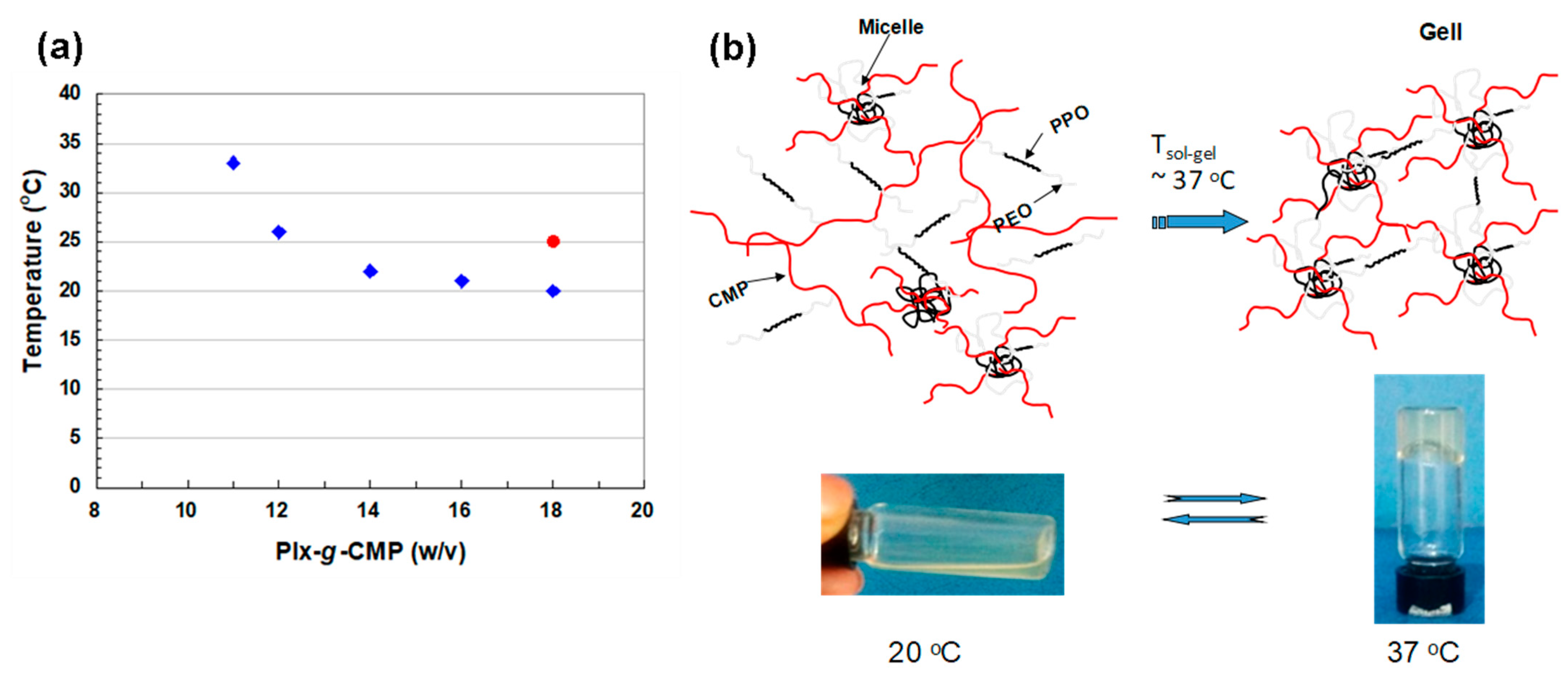
3.2. Gelation Temperature
3.3. Rheological Behavior
3.4. Drug Loading and In Vitro Release Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Y.; Nan, D.; Jin, H.; Qu, X. Recent advances of injectable hydrogels for drug delivery and tissue engineering applications. Polym. Test. 2020, 81, 106283. [Google Scholar] [CrossRef]
- Tan, H.; Marra, K.G. Injectable, biodegradable hydrogels for tissue engineering applications. Materials 2010, 3, 1746–1767. [Google Scholar] [CrossRef]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Han, Z. Injectable hydrogels for ophthalmic applications. J. Control. Release 2017, 268, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Ruel-Gariepy, E.; Leroux, J.C. In situ-forming hydrogels—Review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar] [CrossRef]
- Ta, H.T.; Dass, C.R.; Dunstan, D.E. Injectable chitosan hydrogels for localised cancer therapy. J. Control. Release 2008, 126, 205–216. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Yu, G.-E.; Deng, Y.; Dalton, S.; Wang, Q.G.; Attwood, D.; Price, C.; Booth, C. Micellisation and gelation of triblock copoly(oxyethylene/oxypropylene/oxyethylene), F127. J. Chem. Soc. Faraday Trans. 1992, 88, 2537–2544. [Google Scholar] [CrossRef]
- Peretti, K.L.; Ajiro, H.; Cohen, C.T.; Lobkovsky, E.B.; Coates, G.W. A highly active, isospecific cobalt catalyst for propylene oxide polymerization. J. Am. Chem. Soc. 2005, 127, 11566–11567. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Stern, I.J. Disposition in rats of a polyoxypropylene-polyoxyethylene copolymer used in plasma fractionation. Drug. Metab. Dispos. 1975, 3, 536–542. [Google Scholar]
- Blonder, J.M.; Baird, L.; Fulfs, J.C.; Rosenthal, G.J. Dose-dependent hyperlipidemia in rabbits following administration of poloxamer 407 gel. Life Sci. 1999, 65, PL261–PL266. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K. Recent progress in biomedical applications of Pluronic (PF127): Pharmaceutical perspectives. J. Control. Release 2015, 209, 120–138. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Batrakova, E.V.; Alakhov, V.Y. Pluronic® block copolymers as novel polymer therapeutics for drug and gene delivery. J. Control. Release 2002, 82, 189–212. [Google Scholar] [CrossRef]
- Branco, M.C.; Schneider, J.P. Self-assembling materials for therapeutic delivery. Acta Biomater. 2009, 5, 817–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fathalla, Z.M.A.; Vangala, A.; Longman, M.; Khaled, K.A.; Hussein, A.K.; El-Garhy, O.H.; Alany, R.G. Poloxamer-based thermoresponsive ketorolac tromethamine in situ gel preparations: Design, characterization, toxicity and transcorneal permeation studies. Eur. J. Pharm. Biopharm. 2017, 114, 119–134. [Google Scholar] [CrossRef] [Green Version]
- Fakhari, A.; Corcoran, M.; Schwarz, A. Thermogelling properties of purified poloxamer 407. Heliyon 2017, 3, 200390. [Google Scholar] [CrossRef]
- Baldwin, A.D.; Kiick, K.L. Polysaccharide-modified synthetic polymeric biomaterials. Biopolymers 2010, 94, 128–140. [Google Scholar] [CrossRef] [Green Version]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef]
- Yamaoka, T.; Tabata, Y.; Ikada, Y. Distribution and tissue uptake of poly(ethylene glycol) with different molecular weights after intravenous administration to mice. J. Pharm. Sci. 1994, 83, 601–606. [Google Scholar] [CrossRef]
- Yamaoka, T.; Tabata, Y.; Ikada, Y. Comparison of body distribution of poly(vinyl alcohol) with other water-soluble polymers after intravenous administration. J. Pharm. Pharmacol. 1995, 47, 479–486. [Google Scholar] [CrossRef]
- Hoffman, A.S.; Chen, G. Block and Graft Copolymers and Methods Relating Thereto. PCT. International Patent Application No. WO 95/24430, 14 September 1995. [Google Scholar]
- Bromberg, L. Polyether-modified poly(acrylic acid): Synthesis and applications. Ind. Eng. Chem. Res. 1998, 37, 4267–4274. [Google Scholar] [CrossRef]
- Bromberg, L. Novel family of thermogelling materials via C\C bonding between poly(acrylic acid) and poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide). J. Phys. Chem. B 1998, 102, 1956–1963. [Google Scholar] [CrossRef]
- Bromberg, L. Properties of aqueous solutions and gels of poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide)-g-poly(acrylic acid). J. Phys. Chem. B 1998, 102, 10736–10744. [Google Scholar] [CrossRef]
- Lee, Y.; Park, S.Y.; Chung, H.J.; Park, T.G. New sol-gel transition hydrogels based on pluronic-mimicking copolymers grafted with oligo(lactic acids). Macromol. Symp. 2007, 249–250, 130–136. [Google Scholar] [CrossRef]
- Yoo, M.K.; Cho, K.Y.; Song, H.H.; Choi, Y.J.; Kwon, J.W.; Kim, M.K.; Lee, J.H.; Wee, W.R.; Cho, C.S. Release of ciprofloxacin from chondroitin 6-sulfate-graftpoloxamer hydrogel in vitro for ophthalmic drug delivery. Drug Dev. Ind. Pharm. 2005, 31, 455–463. [Google Scholar] [CrossRef]
- Tian, J.-L.; Zhao, Y.-Z.; Jin, Z.; Lu, C.-T.; Tang, Q.-Q.; Xiang, Q.; Sun, C.-Z.; Zhang, L.; Xu, Y.-Y.; Gao, H.-S.; et al. Synthesis and characterization of new poloxamer conjugate, synthesis and characterization of poloxamer 188-grafted heparin copolymer. Drug Dev. Ind. Pharm. 2010, 36, 832–838. [Google Scholar] [CrossRef]
- Zhao, Y.-Z.; Lv, H.-F.; Lu, C.-T.; Chen, L.-J.; Lin, M.; Zhang, M.; Jiang, X.; Shen, X.T.; Jin, R.-R.; Cai, J.; et al. Evaluation of a novel thermosenitive heparin-poloxamer hydrogel for improving vascular anastomosis quality and safety in a rabbit model. PLoS ONE 2013, 8, e73178. [Google Scholar]
- Chen, C.C.; Fang, C.L.; Al-Suwayeh, S.A.; Leu, Y.L.; Fang, J.Y. Transdermal delivery of selegiline from alginate-pluronic composite thermogels. Int. J. Pharm. 2011, 415, 119–128. [Google Scholar] [CrossRef]
- Fang, J.Y.; Hsu, S.H.; Leu, Y.L.; Hu, J.W. Delivery of cisplatin from pluronic co-polymer systems: Liposome inclusion and alginate coupling. J. Biomater. Sci. Polym. Ed. 2009, 20, 1031–1047. [Google Scholar] [CrossRef]
- Liu, J.; Fang, Q.; Lin, H.; Yu, X.; Zheng, H.; Wan, Y. Alginate-poloxamer/silk fibroin hydrogels with covalently and physically cross-linked networks for cartilage tissue engineering. Carbohydr. Polym. 2020, 247, 116593. [Google Scholar] [CrossRef]
- Bobbala, S.; Gibson, B.; Gamble, A.B.; McDowell, A.; Hook, S. Poloxamer 407-chitosan grafted thermoresponsive hydrogels achieve synchronous and sustained release of antigen and adjuvant from single-shot vaccines. Immunol. Cell. Biol. 2018, 96, 656–665. [Google Scholar] [CrossRef]
- Park, K.M.; Lee, S.Y.; Joung, K.J.; Na, J.S.; Lee, M.C.; Park, K.D. Thermosensitive chitosan-pluronic hydrogel as an injectable cell delivery carrier for cartilage regeneration. Acta Biomater. 2009, 5, 1956–1965. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.Y.; Chung, T.W.; Kim, B.C.; Kim, M.K.; Lee, J.H.; Wee, W.R.; Cho, C.S. Release of ciprofloxacin from poloxamer-graft-hyaluronic acid hydrogels in vitro. Int. J. Pharm. 2003, 260, 83–91. [Google Scholar] [CrossRef]
- Singh, R.S.; Saini, G.K. Production, purification and characterization of pullulan from a novel strain of Aureobasidium pullulans FB-1. J. Biotechnol. 2008, 136, S506–S507. [Google Scholar] [CrossRef]
- Kumar, D.; Saini, N.; Pandit, V.; Ali, S. An insight to pullulan: A biopolymer in pharmaceutical approaches. Int. J. Basic Appl. Sci. 2012, 1, 202–219. [Google Scholar] [CrossRef]
- Oguzhan, P.; Yangilar, F. Pullulan: Production and usage in food industry. Afr. J. Food Sci. Technol. 2013, 4, 57–63. [Google Scholar]
- Singh, R.S.; Saini, G.K. Biosynthesis of pullulan and its applications in food and pharmaceutical industry. In Microorganisms in Sustainable Agriculture and Biotechnology, Part 2; Satyanarayana, T., Johri, B.N., Prakash, A., Eds.; Springer Science + Business Media B.V.: Berlin, Germany, 2012; Volume 24, pp. 509–553. [Google Scholar]
- Tiwari, S.; Patil, R.; Dubey, S.K.; Bahadur, K. Derivatization approaches and applications of pullulan. Adv. Coll. Interface Sci. 2019, 269, 296–308. [Google Scholar] [CrossRef]
- Panyamao, P.; Ruksiriwanich, W.; Sirisa-ard, P.; Charumanee, S. Injectable thermosensitive chitosan/pullulan-based hydrogels with improved mechanical properties and swelling capacity. Polymers 2020, 12, 2514. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Rana, V.; Kennedy, J.F. Recent insights on applications of pullulan in tissue engineering. Carbohydr. Polym. 2016, 153, 455–462. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N. Microbial biopolymers for edible film and coating applications. In Advances in Biotechnology; Nawani, N.N., Khetmalas, M., Razdan, P.N., Pandey, A., Eds.; IK International Publishing House Pvt. Ltd.: Delhi, India, 2015; Volume 12, pp. 187–216. [Google Scholar]
- T. de Carvalho, L.; da S. Paula, M.L.; M. de Moraes, R.; Alves, G.M.; M. Lacerda, T.; Santos, J.C.d.; M. dos Santos, A.; Medeiros, S.d.F. Chemical modification of pullulan exopolysaccharide by grafting poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBHV) via click chemistry. Polymers 2020, 12, 2527. [Google Scholar] [CrossRef]
- Li, H.; Yang, J.; Hu, X.; Liang, J.; Fan, Y.; Zhang, X. Superabsorbent polysaccharide hydrogels based on pullulan derivative as antibacterial release wound dressing. J. Biomed. Mater. Res. 2011, 98A, 31–39. [Google Scholar] [CrossRef]
- Dulong, V.; Mocanu, G.; Le Cerf, D. A novel amphiphilic pH-sensitive hydrogel based on pullulan. Colloid Polym. Sci. 2007, 285, 1085–1091. [Google Scholar] [CrossRef]
- Mocanu, G.; Mihai, D.; Dulong, V.; Picton, L.; Le Cerf, D. New anionic crosslinked multi-responsive pullulan hydrogels. Carbohydr. Polym. 2012, 87, 1440–1446. [Google Scholar] [CrossRef]
- Chen, F.; Yu, S.; Liu, B.; Ni, Y.; Yu, C.; Su, Y.; Zhu, X.; Yu, X.; Zhou, Y.; Ya, D. An injectable enzymatically crosslinked carboxymethylated pullulan/chondroitin sulfate hydrogel for cartilage tissue engineering. Sci. Rep. 2016, 6, 20014. [Google Scholar] [CrossRef] [PubMed]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable device. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems, 1st ed.; Bruschi, M.L., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 63–86. [Google Scholar]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Yamaoka, K.; Nakagawa, T.; Uno, T. Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J. Pharmacokinet. Biophacodyn. 1978, 6, 165–175. [Google Scholar] [CrossRef]
- Dugas, H.; Penney, C. Bioorganic Chemistry; A Chemical Approach to Enzyme Action; Springer: New York, NY, USA, 1981; p. 75. [Google Scholar]
- Asmarandei, I.; Fundueanu, G.; Cristea, M.; Harabagiu, V.; Constantin, M. Thermo-and pH-sensitive interpenetrating poly(N-isopropylacrylamide)/carboxymethyl pullulan network for drug delivery. J. Polym. Res. 2013, 20, 293–305. [Google Scholar] [CrossRef]
- Mitchell, G.; Wijnberg, A.C. Standardization of methodology for chemical functions in starch derivatives. Part 2. Starch/Starke 1997, 49, 485–488. [Google Scholar] [CrossRef]
- Singh, R.S.; Saini, G.K.; Kennedy, J.F. Downstream processing and characterization of pullulan from a novel colour variant strain of Aureobasidium pullulans FB-1. Carbohydr. Polym. 2009, 78, 89–94. [Google Scholar] [CrossRef]
- Sugumaran, K.R.; Gowthami, E.; Swathi, B.; Elakkiya, S.; Srivastava, S.N.; Ravikumar, R.; Gowdhaman, D.; Ponnusami, V. Production of pullulan by Aureobasidium pullulans from Asian palm kernel: A novel substrate. Carbohydr. Polym. 2013, 92, 697–703. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Demirci, A.; Catchmark, J.M. Evaluation of medium composition and fermentation parameters on pullulan production by Aureobasidium pullulans. Food Sci. Technol. Int. 2011, 17, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Karolewicz, B.; Gajda, M.; Gorniak, A.; Owczarek, A.; Mucha, I. Pluronic F127 as a suitable carrier for preparing the imatinib base solid dispersions and its potential in development of a modified release dosage forms. J. Therm. Anal. Calorim. 2017, 10, 383–390. [Google Scholar] [CrossRef]
- Morariu, S.; Eckelt, J.; Wolf, B.A. Dextran-based polycations: Thermodynamic interaction with water as compared with unsubstituted dextran, 1-volumetric properties and light scattering. Macromol. Chem. Phys. 2011, 212, 1925–1931. [Google Scholar] [CrossRef]
- Nita, L.; Chiriac, A.; Rusu, A.G.; Bercea, M.; Ghilan, A.; Dumitriu, R.; Mititelu-Tartau, L. New self-healing hydrogels based on reversible physical interactions and their potential applications. Eur. Polym. J. 2019, 118, 176–185. [Google Scholar] [CrossRef]
- Diaconu, A.; Nita, L.E.; Bercea, M.; Chiriac, A.; Rusu, A.G.; Rusu, D. Hyaluronic acid gels with tunable properties by conjugating with a synthetic copolymer. Biochem. Eng. J. 2017, 125, 135–143. [Google Scholar] [CrossRef]
- Rusu, A.; Nita, L.; Bercea, M.; Tudorachi, N.; Diaconu, A.; Pamfil, D.; Rusu, D.; Ivan, F.E.; Chiriac, A. Interpenetrated polymer network with modified chitosan in composition and self-healing properties. Int. J. Biol. Macromol. 2019, 132, 374–384. [Google Scholar] [CrossRef] [PubMed]



| Conc. (%, w/v) | Zero-Order | Higuchi | Ritger–Peppas | Peppas–Sahlin | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k0 × 104 | R2 | AIC | kH × 102 | R2 | AIC | K × 102 | n | R2 | AIC | k1 × 102 | k2 × 104 | R2 | AIC | |
| 13 | 3.77 | 0.941 | −35.35 | 1.001 | 0.993 | −56.36 | 18.68 | 0.13 | 0.958 | −24.16 | 2.073 | −1.56 | 0.991 | −60.29 |
| 22 | 4.82 | 0.969 | −36.41 | 0.810 | 0.986 | −54.03 | 1.534 | 0.43 | 0.992 | −30.93 | 1.72 | −1.24 | 0.999 | −84.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantin, M.; Cosman, B.; Bercea, M.; Ailiesei, G.-L.; Fundueanu, G. Thermosensitive Poloxamer-graft-Carboxymethyl Pullulan: A Potential Injectable Hydrogel for Drug Delivery. Polymers 2021, 13, 3025. https://doi.org/10.3390/polym13183025
Constantin M, Cosman B, Bercea M, Ailiesei G-L, Fundueanu G. Thermosensitive Poloxamer-graft-Carboxymethyl Pullulan: A Potential Injectable Hydrogel for Drug Delivery. Polymers. 2021; 13(18):3025. https://doi.org/10.3390/polym13183025
Chicago/Turabian StyleConstantin, Marieta, Bogdan Cosman, Maria Bercea, Gabriela-Liliana Ailiesei, and Gheorghe Fundueanu. 2021. "Thermosensitive Poloxamer-graft-Carboxymethyl Pullulan: A Potential Injectable Hydrogel for Drug Delivery" Polymers 13, no. 18: 3025. https://doi.org/10.3390/polym13183025
APA StyleConstantin, M., Cosman, B., Bercea, M., Ailiesei, G.-L., & Fundueanu, G. (2021). Thermosensitive Poloxamer-graft-Carboxymethyl Pullulan: A Potential Injectable Hydrogel for Drug Delivery. Polymers, 13(18), 3025. https://doi.org/10.3390/polym13183025








