Color Stability and Staining Susceptibility of Direct Resin-Based Composites after Light-Activated In-Office Bleaching
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Bleaching Procedure
2.3. Staining Procedure
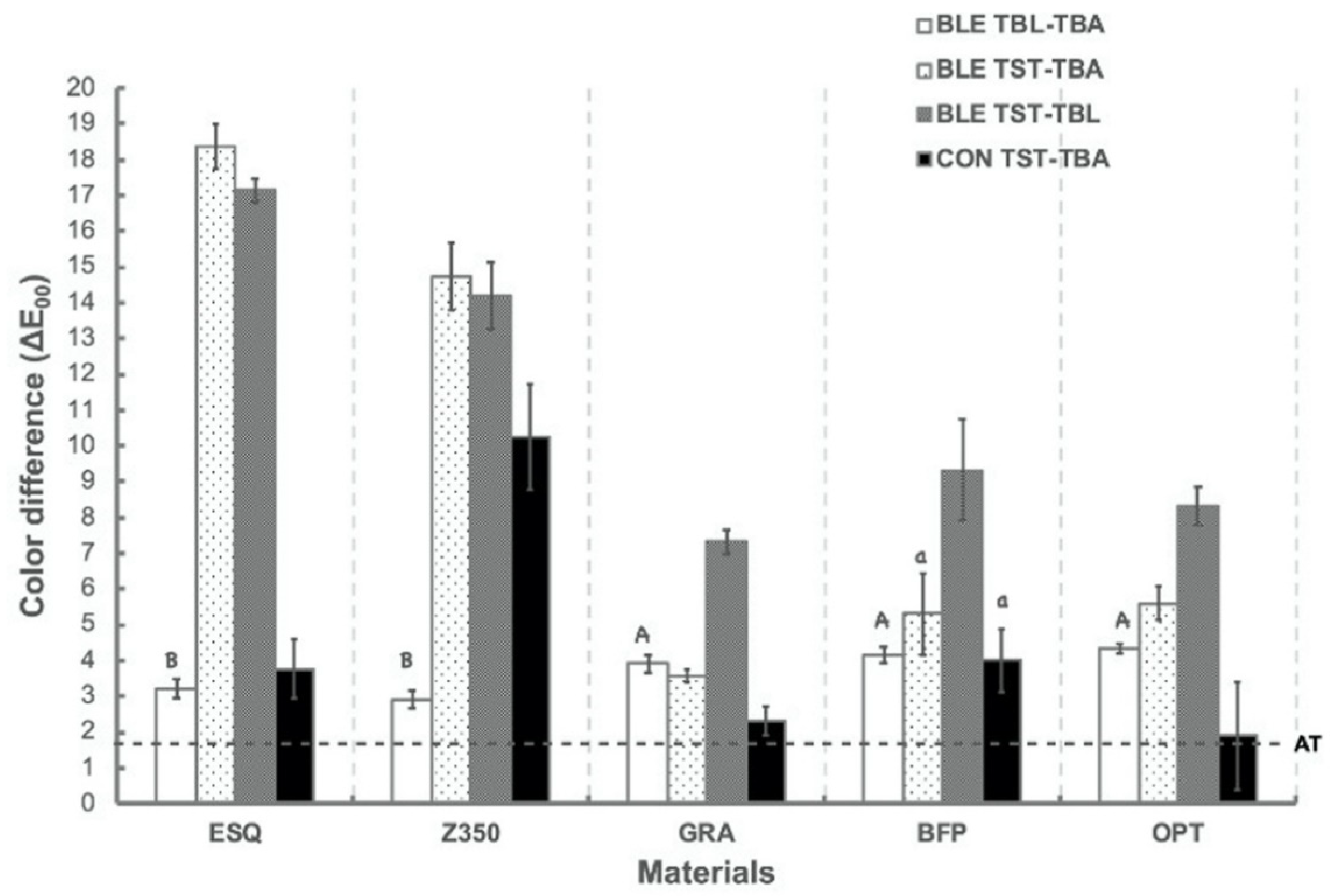
2.4. Color Measurements
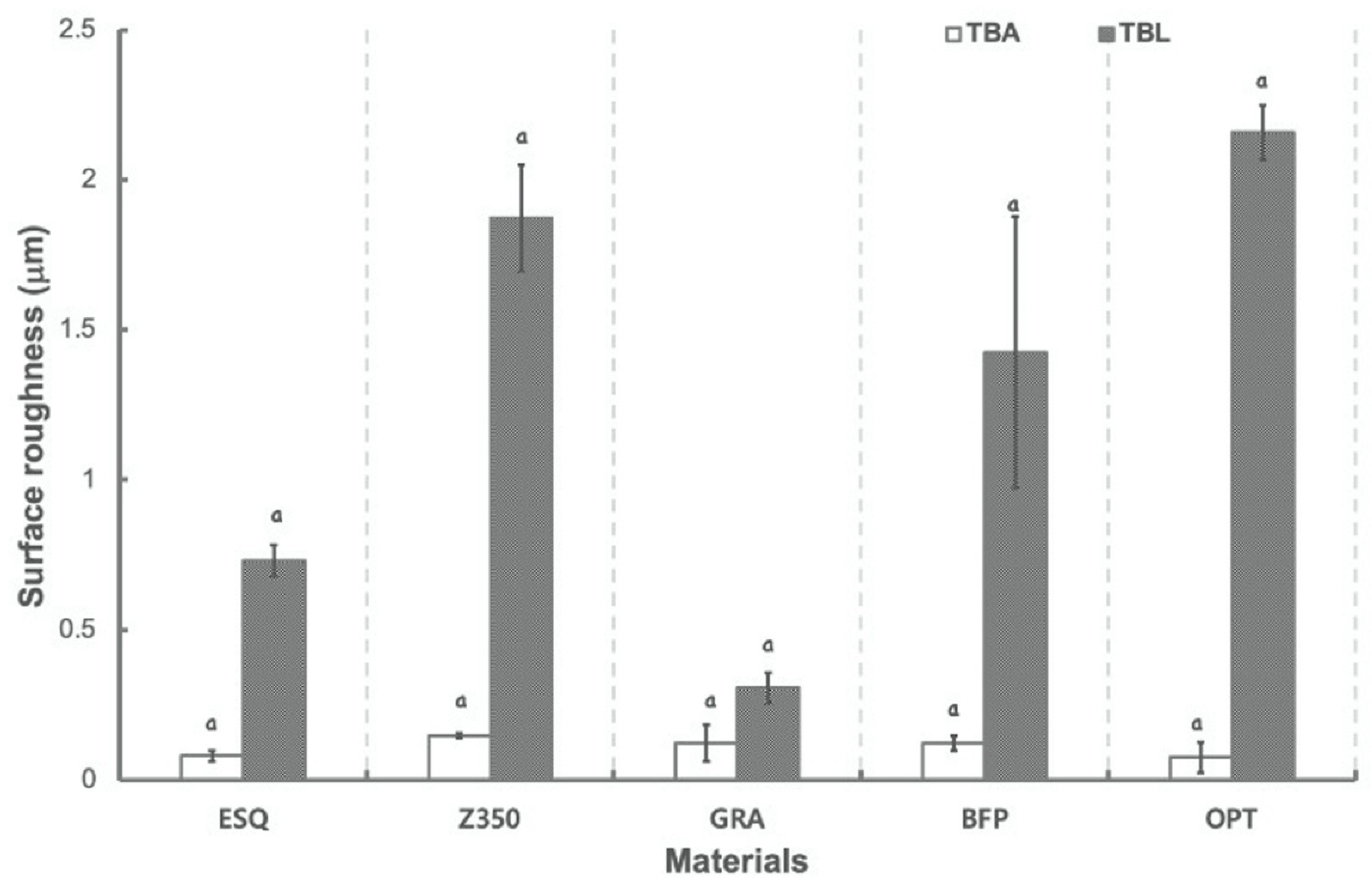
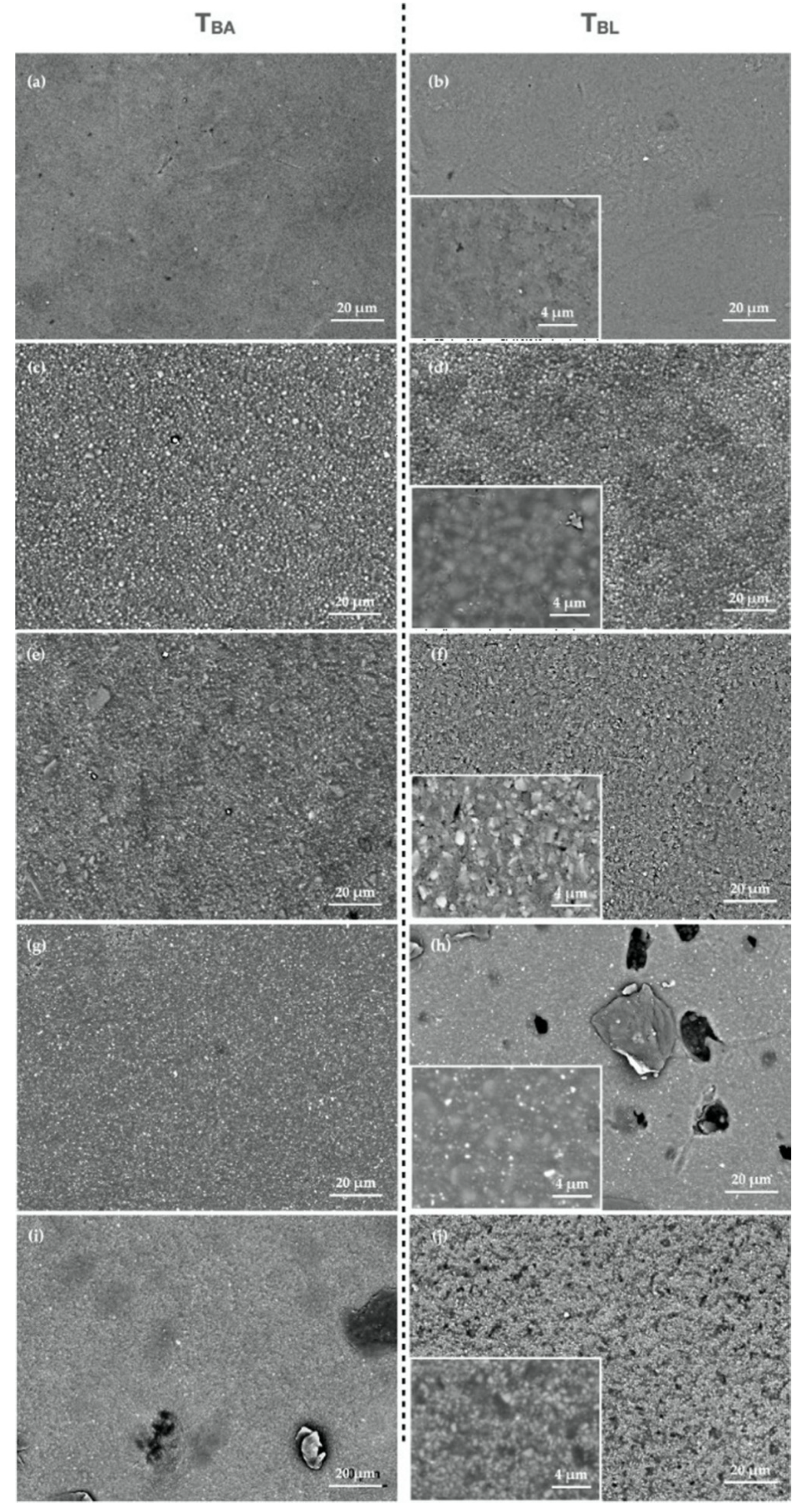
2.5. Evaluation of Surface Roughness
2.6. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dos Santos, D.M.; da Silva, E.V.; Mendonça, J.B.; Cetrangolo, D.; Caxias, F.P.d.; Goiato, M.C. Influence of a Bleaching Agent on the Color Stability of Indirect Composite Resins Immersed in Dyes. Ceramics 2019, 2, 19. [Google Scholar] [CrossRef]
- Randolph, L.D.; Palin, W.M.; Leloup, G.; Leprince, J.G. Filler characteristics of modern dental resin composites and their influence on physico-mechanical properties. Dent. Mater. 2016, 32, 1586–1599. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zanetti, F.; Wang, L.; Pan, J.; Majeed, S.; Malmstrom, H.; Peitsch, M.C.; Hoeng, J.; Ren, Y. Effects of different discoloration challenges and whitening treatments on dental hard tissues and composite resin restorations. J. Dent. 2019, 89, 103182. [Google Scholar] [CrossRef] [PubMed]
- Karadas, M.; Alkurt, M.; Duymus, Z.Y. Effects of hydrogen peroxide-based mouthwashes on color changes of stained direct composite resins. J. Restor. Dent. 2016, 4, 11. [Google Scholar] [CrossRef]
- Lauvahutanon, S.; Shiozawa, M.; Takahashi, H.; Iwasaki, N.; Oki, M.; Finger, W.J.; Arksornnukit, M. Discoloration of various CAD/CAM blocks after immersion in coffee. Restor. Dent. Endod. 2017, 42, 9–18. [Google Scholar] [CrossRef]
- Villalta, P.; Lu, H.; Okte, Z.; Garcia-Godoy, F.; Powers, J.M. Effects of staining and bleaching on color change of dental composite resins. J. Prosthet. Dent. 2006, 95, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, J.W.; Balbierz, M.M.; Schultz, C.M.; Simetich, B.; Beatty, M.W. Effect of tooth-whitening procedures on stained composite resins. Oper. Dent. 2019, 44, 65–75. [Google Scholar] [CrossRef]
- Paravina, R.D.; Ghinea, R.; Herrera, L.J.; Bona, A.D.; Igiel, C.; Linninger, M.; Sakai, M.; Takahashi, H.; Tashkandi, E.; Mar Perez, M.d. Color difference thresholds in dentistry. J. Esthet. Restor. Dent. 2015, 27, S1–S9. [Google Scholar] [CrossRef]
- Poggio, C.; Ceci, M.; Beltrami, R.; Mirando, M.; Wassim, J.; Colombo, M. Color stability of esthetic restorative materials: A spectrophotometric analysis. Acta Biomater. Odontol. Scand. 2016, 2, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Poggio, C.; Vialba, L.; Berardengo, A.; Federico, R.; Colombo, M.; Beltrami, R.; Scribante, A. Color stability of new esthetic restorative materials: A spectrophotometric analysis. J. Funct. Biomater. 2017, 8, 26. [Google Scholar] [CrossRef]
- Pruthi, G.; Jain, V.; Kandpal, H.; Mathur, V.P.; Shah, N. Effect of bleaching on color change and surface topography of composite restorations. Int. J. Dent. 2010, 2010, 695748. [Google Scholar] [CrossRef][Green Version]
- Joiner, A.; Luo, W. Tooth colour and whiteness: A review. J. Dent. 2017, 67, S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.M.; Herrera, L.J.; Carrillo, F.; Pecho, O.E.; Dudea, D.; Gasparik, C.; Ghinea, R.; Della Bona, A. Whiteness difference thresholds in dentistry. Dent. Mater. 2019, 35, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, A.; Ardu, S.; Bortolotto, T.; Krejci, I. In-office bleaching efficacy on stain removal from CAD/CAM and direct resin composite materials. J. Esthet. Restor. Dent. 2018, 30, 51–58. [Google Scholar] [CrossRef]
- Hashemikamangar, S.S.; Hoseinpour, F.; Kiomarsi, N.; Dehaki, M.G.; Kharazifard, M.J. Effect of an Optical Whitening Toothpaste on Color Stability of Tooth-Colored Restorative Materials. Eur. J. Dent. 2020, 14, 85. [Google Scholar] [CrossRef]
- Vidal, M.L.; Pecho, O.E.; Xavier, J.; Della Bona, Á. Influence of the photoactivation distance on the color and whiteness stability of resin-based composite after bleaching and aging. J. Dent. 2020, 99, 103408. [Google Scholar] [CrossRef] [PubMed]
- Fekrazad, R.; Alimazandarani, S.; Kalhori, K.A.; Assadian, H.; Mirmohammadi, S.-M. Comparison of laser and power bleaching techniques in tooth color change. J. Clin. Exp. Dent. 2017, 9, e511. [Google Scholar] [CrossRef]
- Rodrigues, F.; Serro, A.; Polido, M.; Ramalho, A.; Figueiredo-Pina, C. Effect of bleaching teeth with hydrogen peroxide on the morphology, hydrophilicity, and mechanical and tribological properties of the enamel. Wear 2017, 374, 21–28. [Google Scholar] [CrossRef]
- Rodrigues, C.; Nora, B.D.; Mallmann, A.; May, L.; Jacques, L. Repolishing resin composites after bleaching treatments: Effects on color stability and smoothness. Oper. Dent. 2019, 44, 54–64. [Google Scholar] [CrossRef]
- Amengual-Lorenzo, J.; Montiel-Company, J.-M.; Bellot-Arcís, C.; Labaig-Rueda, C.; Solá-Ruiz, M.-F. Effect of two whitening agents on the color of composite dental restorations. J. Clin. Exp. Dent. 2019, 11, e15. [Google Scholar] [CrossRef]
- Elhoshy, A.Z.; Abouelenein, K.; Elbaz, M.A. Effect of 15% carbamide peroxide bleaching gel on color of Class V resin composite restoration. Future Dent. J. 2018, 4, 239–243. [Google Scholar] [CrossRef]
- Juntavee, N.; Juntavee, A.; Saensutthawijit, P. Influences of light-emitting diode illumination bleaching technique on nanohardness of computer-aided design and computer-aided manufacturing ceramic restorative materials. J. Contemp. Dent. Pract. 2018, 19, 196–204. [Google Scholar] [CrossRef]
- Pecho, O.E.; Martos, J.; Pinto, K.V.; Pinto, K.V.; Baldissera, R.A. Effect of hydrogen peroxide on color and whiteness of resin-based composites. J. Esthet. Restor. Dent. 2019, 31, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Shahabi, S.; Assadian, H.; Nahavandi, A.M.; Nokhbatolfoghahaei, H. Comparison of tooth color change after bleaching with conventional and different light-activated methods. J. Lasers Med. Sci. 2018, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, C.Y.; Wang, Y.N.; Cheng, H. Hydrogen peroxide bleaching induces changes in the physical properties of dental restorative materials: Effects of study protocols. J. Esthet. Restor. Dent. 2018, 30, E52–E60. [Google Scholar] [CrossRef]
- Schanda, J. CIE colorimetry and colour displays. In Proceedings of the Color and Imaging Conference, Society for Imaging Science and Technology, Scottsdale, AZ, USA, 19–22 November 1996; Volume 1996, pp. 230–234. [Google Scholar]
- International Organization for Standardization. Dentistry: Guidance on Colour Measurement; International Organization for Standardization: Geneva, Switzerland, 2011. [Google Scholar]
- Del Mar Pérez, M.; Ghinea, R.; Rivas, M.J.; Yebra, A.; Ionescu, A.M.; Paravina, R.D.; Herrera, L.J. Development of a customized whiteness index for dentistry based on CIELAB color space. Dent. Mater. 2016, 32, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.M.; Pecho, O.E.; Ghinea, R.; Pulgar, R.; Della Bona, A. Recent advances in color and whiteness evaluations in dentistry. Curr. Dent. 2019, 1, 23–29. [Google Scholar] [CrossRef]

| Material (Code) | Composition | Manufacturer | |||
|---|---|---|---|---|---|
| Type | Organic Matrix | Fillers | Filler Amount | ||
| Estelite Sigma Quick (ESQ) | Sub-micron | Bis-GMA, TEGDMA | monodispersing spherical SiO2 and ZrO2 (average 0.2 μm) | 82 wt% | Tokuyama Dental, Tokyo, Japan |
| Filtek Z350XT (Z350) | Nano-filled | Bis-GMA, UDMA, Bis- EMA, TEGDMA, and PEGDMA | Non-agglomerated nano-particles of silica (20 nm), nano-agglomerates formed of zirconia/silica particles (0.6–1.4 μm) | 72.5 wt% | 3M ESPE, Monrovia, CA, USA |
| Grandio (GRA) | Nano-hybrid | Bis-GMA, Bis-EMA, TEGDMA | glass ceramic (particle size 1 μm), silicon dioxide nanoparticles (20–40 nm) | 87 wt% | Voco GmbH, Cuxhaven, Germany |
| Bulk Fill Posterior (BFP) | Bluk-filled | AUDMA, AFM, DDDMA, UDMA | an aggregated zirconia/silica cluster (comprised of 20 nm silica and 4 to 11 nm zirconia particles) a ytterbium trifluoride filler consisting of agglomerate (100 nm) | 76.5 wt% | 3M ESPE, Monrovia, CA, USA |
| OptiComp LC (OPT) | Nano-hybrid | Bis-EMA, UDMA, TEGDMA | glass ceramic (particle size 0.7 μm), silica dioxide nanoparticles (20–40 nm), ytterbium trifluoride (100 nm) | 79.5 wt% | PacDent, Brea, CA, USA |
| TBA | TBL | TST | ||
|---|---|---|---|---|
| ESQ | L* | 90.38 (0.18) | 88.85 (0.37) | 76.63 (0.75) |
| a* | −6.45 (0.36) | −4.24 (0.47) | 2.47 (0.45) | |
| b* | 12.38 (0.59) | 10.71(0.73) | 20.63 (0.24) | |
| WID | 51.06 (0.83) | 46.93 (1.42) | 13.64 (1.58) | |
| Z350 | L* | 82.92 (0.27) | 81.97 (0.35) | 71.21 (0.86) |
| a* | −1.15 (0.10) | 0.46 (0.12) | 6.44 (0.46) | |
| b* | 38.88 (0.56) | 36.63 (0.26) | 43.88 (0.47) | |
| WID | 5.50 (0.73) | 3.70 (0.36) | −24.05 (2.05) | |
| GRA | L* | 62.94 (0.13) | 65.75 (0.14) | 60.33 (0.34) |
| a* | −1.27 (0.10) | −3.53 (0.33) | −0.01 (0.58) | |
| b* | 31.41 (0.33) | 29.94 (0.42) | 33.33 (0.76) | |
| WID | 3.00 (0.46) | 11.43 (1.09) | −3.46 (1.17) | |
| BFP | L* | 64.25 (0.44) | 66.73 (0.52) | 60.49 (0.39) |
| a* | −3.31 (0.10) | −4.34 (0.15) | −1.48 (0.16) | |
| b* | 14.17 (0.28) | 11.01 (0.12) | 17.24 (1.78) | |
| WID | 27.44 (0.37) | 34.66 (0.54) | 17.75 (2.43) | |
| OPT | L* | 75.28 (0.43) | 77.60 (0.42) | 71.13 (0.47) |
| a* | −3.90 (0.12) | −5.54 (0.17) | −0.42 (0.37) | |
| b* | 24.71 (0.38) | 21.47 (0.31) | 23.04 (0.05) | |
| WID | 23.26 (0.13) | 31.92 (0.31) | 15.07 (0.86) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, P.-W.; Huang, C.-F.; Hsu, C.-Y.; Chen, A.; Ng, H.-H.; Cheng, M.-S.; Tsay, S.; Lai, J.-Y.; Yang, T.-S.; Lee, W.-F. Color Stability and Staining Susceptibility of Direct Resin-Based Composites after Light-Activated In-Office Bleaching. Polymers 2021, 13, 2941. https://doi.org/10.3390/polym13172941
Peng P-W, Huang C-F, Hsu C-Y, Chen A, Ng H-H, Cheng M-S, Tsay S, Lai J-Y, Yang T-S, Lee W-F. Color Stability and Staining Susceptibility of Direct Resin-Based Composites after Light-Activated In-Office Bleaching. Polymers. 2021; 13(17):2941. https://doi.org/10.3390/polym13172941
Chicago/Turabian StylePeng, Pei-Wen, Chiung-Fang Huang, Ching-Ying Hsu, Ann Chen, Ho-Him Ng, Man-Si Cheng, Shiang Tsay, Jia-Yi Lai, Tzu-Sen Yang, and Wei-Fang Lee. 2021. "Color Stability and Staining Susceptibility of Direct Resin-Based Composites after Light-Activated In-Office Bleaching" Polymers 13, no. 17: 2941. https://doi.org/10.3390/polym13172941
APA StylePeng, P.-W., Huang, C.-F., Hsu, C.-Y., Chen, A., Ng, H.-H., Cheng, M.-S., Tsay, S., Lai, J.-Y., Yang, T.-S., & Lee, W.-F. (2021). Color Stability and Staining Susceptibility of Direct Resin-Based Composites after Light-Activated In-Office Bleaching. Polymers, 13(17), 2941. https://doi.org/10.3390/polym13172941




_Chen.jpg)


