Chitosan-Based Glycolipid Conjugated siRNA Delivery System for Improving Radiosensitivity of Laryngocarcinoma
Abstract
:1. Introduction
2. Results and Discussion
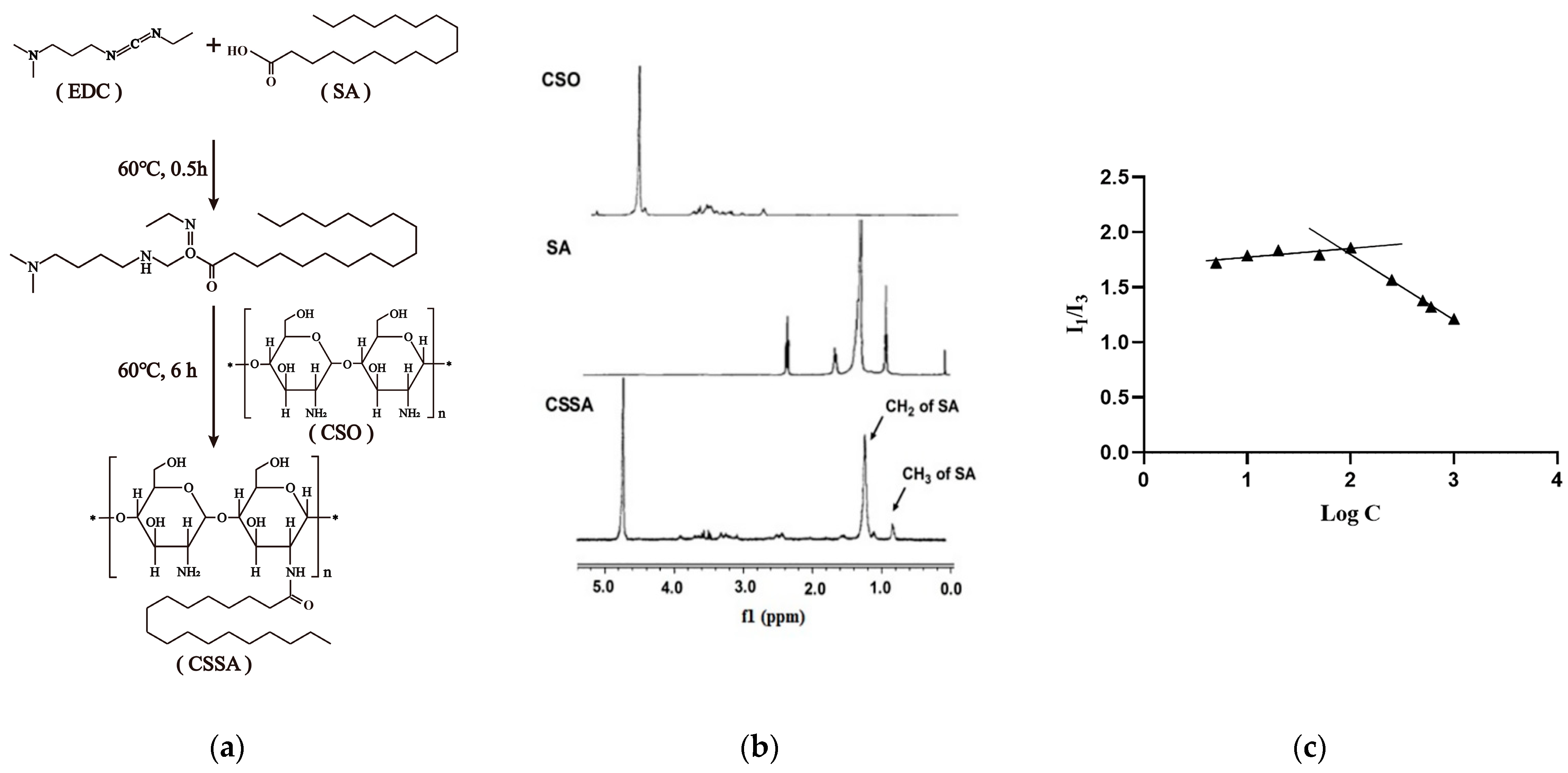
2.1. Synthesis and Characteristics of CSSA
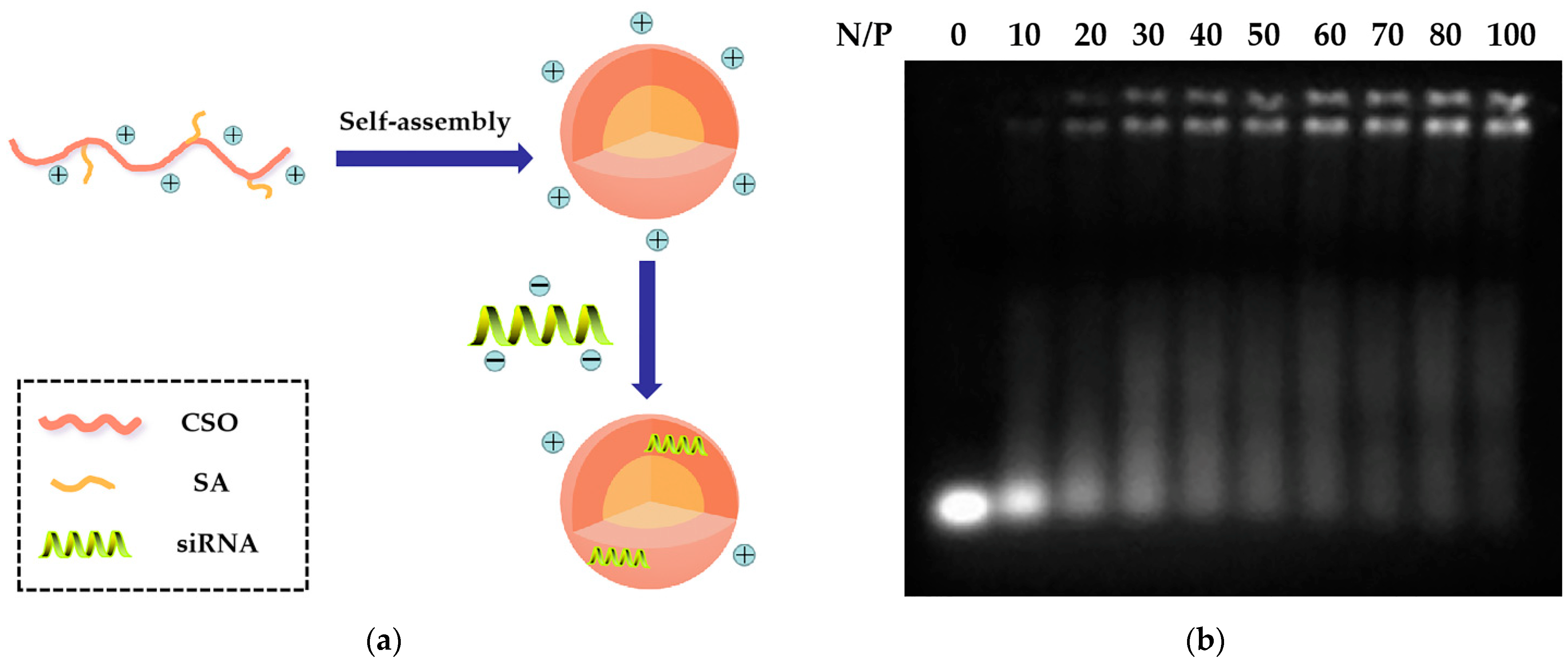
2.2. Preparation and Characteristics of CSSA/siRNA
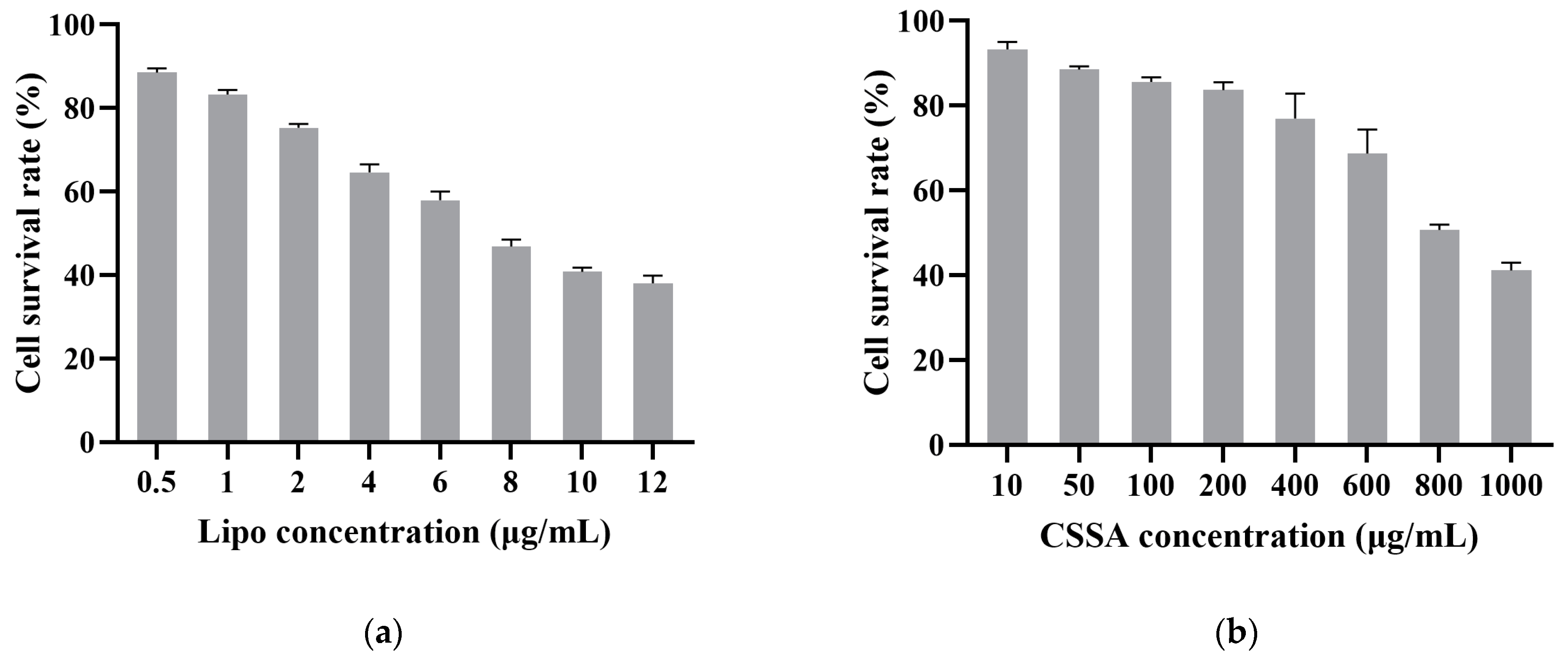
2.3. Cytotoxicity
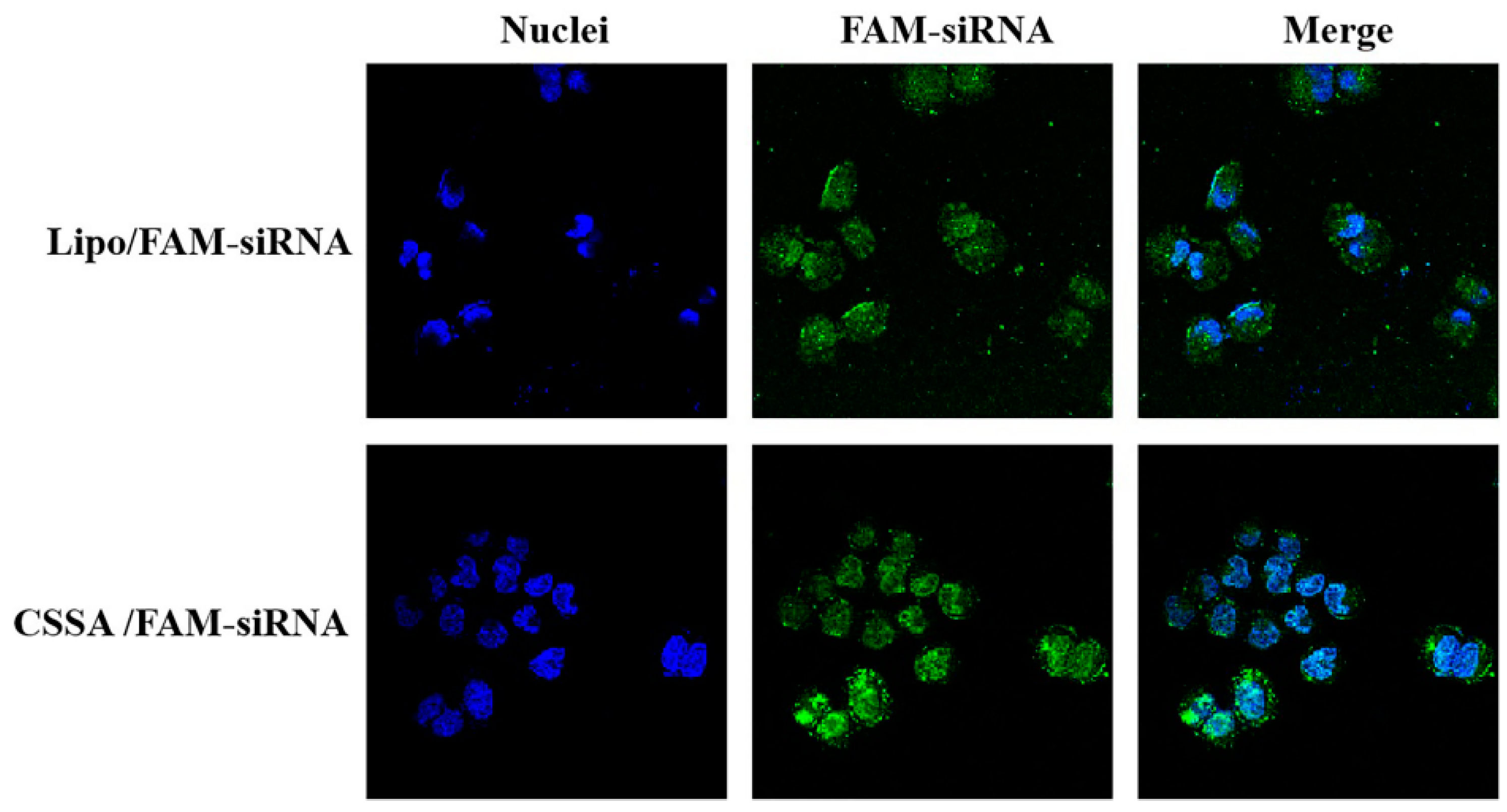
2.4. Internalization of FAM-siRNA
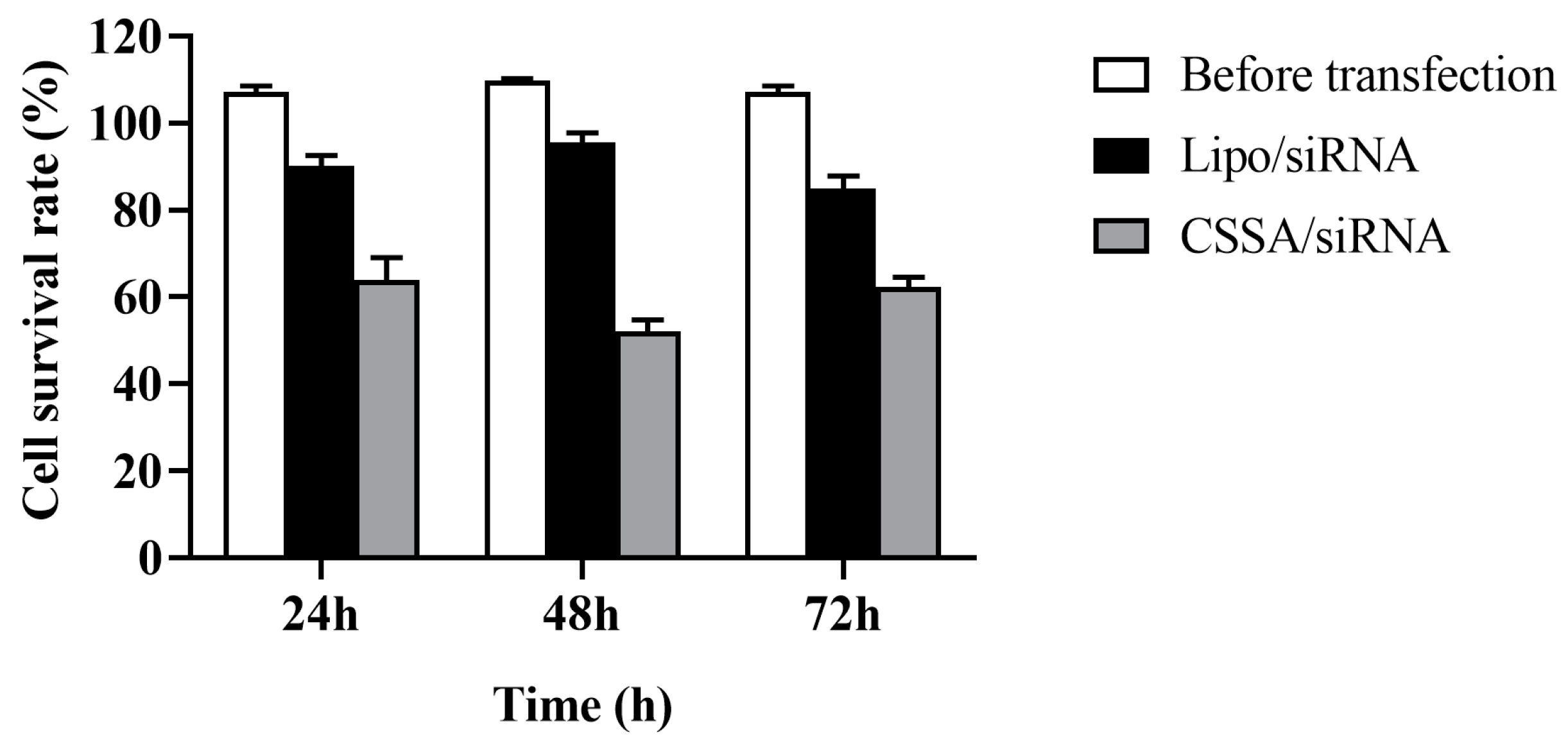
2.5. Cell Survival Rate with X-ray Radiation
3. Materials and Methods
3.1. Materials
3.2. Cell Culture
3.3. Design and Synthesis of siRNA
- sense, 5′-GGAAUUCAAUGCUGAUGAUTT-3′;
- antisense, 5′-AUCAUCAGCAUUGAAUUCCTT-3′.
3.4. Synthesis of CSSA
3.5. Preparation of CSSA/siRNA Complex and Gel Retardation Assay
3.6. Characterization of CSSA and CSSA/siRNA
3.6.1. Size and Zeta Potential
3.6.2. Substitution Degree of Amino Groups
3.6.3. Critical Micelle Concentration
3.7. Cytotoxicity Assay
3.8. Internalization of CSSA Micelles
3.9. Determination of Cell Survival Rate with X-Ray Radiation
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mirzoeva, S.; Franzen, C.A.; Pelling, J.C. Apigenin inhibits TGF-beta-induced VEGF expression in human prostate carcinoma cells via a Smad2/3- and Src-dependent mechanism. Mol. Carcinog. 2014, 53, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Cui, L.; Chen, M.; Soto, L.A.; Graves, E.E.; Rao, J. A Near-Infrared Phosphorescent Nanoprobe Enables Quantitative, Longitudinal Imaging of Tumor Hypoxia Dynamics during Radiotherapy. Cancer Res. 2019, 79, 4787–4797. [Google Scholar] [CrossRef]
- Merry, H.E.; Phelan, P.; Doaks, M.; Zhao, M.; Mulligan, M.S. Functional roles of tumor necrosis factor-alpha and interleukin 1-Beta in hypoxia and reoxygenation. Ann. Thorac. Surg. 2015, 99, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Tan, Y.; Yan, L.; Zhang, C.; Xu, M.; Guo, H.; Zhuang, B.; Zhou, L.; Xie, X. Modulation of Tumor Hypoxia by pH-Responsive Liposomes to Inhibit Mitochondrial Respiration for Enhancing Sonodynamic Therapy. Int. J. Nanomed. 2020, 15, 5687–5700. [Google Scholar] [CrossRef] [PubMed]
- Song, C.W.; Griffin, R.J.; Lee, Y.J.; Cho, H.; Seo, J.; Park, I.; Kim, H.K.; Kim, D.H.; Kim, M.S.; Dusenbery, K.E.; et al. Reoxygenation and Repopulation of Tumor Cells after Ablative Hypofractionated Radiotherapy (SBRT and SRS) in Murine Tumors. Radiat Res. 2019, 192, 159–168. [Google Scholar] [CrossRef]
- Armitage, E.G.; Barbas, C. Metabolomics in cancer biomarker discovery: Current trends and future perspectives. J. Pharm. Biomed. Anal. 2014, 87, 1–11. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Wood, I.S.; Trayhurn, P. Glucose transporters (GLUT and SGLT): Expanded families of sugar transport proteins. Br. J. Nutr. 2003, 89, 3–9. [Google Scholar] [CrossRef]
- Deng, D.; Yan, N. GLUT, SGLT, and SWEET: Structural and mechanistic investigations of the glucose transporters. Protein Sci. 2016, 25, 546–558. [Google Scholar] [CrossRef] [Green Version]
- Ancey, P.B.; Contat, C.; Meylan, E. Glucose transporters in cancer—from tumor cells to the tumor microenvironment. FEBS J. 2018, 285, 2926–2943. [Google Scholar] [CrossRef]
- Meng, Y.; Xu, X.; Luan, H.; Li, L.; Dai, W.; Li, Z.; Bian, J. The progress and development of GLUT1 inhibitors targeting cancer energy metabolism. Future Med. Chem. 2019, 11, 2333–2352. [Google Scholar] [CrossRef]
- Wu, Q.; Ba-Alawi, W.; Deblois, G.; Cruickshank, J.; Duan, S.; Lima-Fernandes, E.; Haight, J.; Tonekaboni, S.; Fortier, A.M.; Kuasne, H.; et al. GLUT1 inhibition blocks growth of RB1-positive triple negative breast cancer. Nat. Commun. 2020, 11, 4205. [Google Scholar] [CrossRef]
- Zambrano, A.; Molt, M.; Uribe, E.; Salas, M. Glut 1 in Cancer Cells and the Inhibitory Action of Resveratrol as A Potential Therapeutic Strategy. Int. J. Mol. Sci. 2019, 20, 3374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Cao, L.; Li, Z.; Li, Y. SIRT1 promotes GLUT1 expression and bladder cancer progression via regulation of glucose uptake. Hum. Cell 2019, 32, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Wang, J.; Yan, W.; Cui, Y.; Chen, Z.; Gao, X.; Wen, X.; Chen, J. GLUT1 regulates cell glycolysis and proliferation in prostate cancer. Prostate 2018, 78, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Krzeslak, A.; Wojcik-Krowiranda, K.; Forma, E.; Jozwiak, P.; Romanowicz, H.; Bienkiewicz, A.; Brys, M. Expression of GLUT1 and GLUT3 glucose transporters in endometrial and breast cancers. Pathol. Oncol. Res. 2012, 18, 721–728. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, S.; Okada, M.; Takeda, H.; Kuramoto, K.; Sanomachi, T.; Togashi, K.; Seino, S.; Yamamoto, M.; Yoshioka, T.; Kitanaka, C. Involvement of GLUT1-mediated glucose transport and metabolism in gefitinib resistance of non-small-cell lung cancer cells. Oncotarget 2018, 9, 32667–32679. [Google Scholar] [CrossRef] [Green Version]
- Sztandera, K.; Dzialak, P.; Marcinkowska, M.; Stanczyk, M.; Gorzkiewicz, M.; Janaszewska, A.; Klajnert-Maculewicz, B. Sugar Modification Enhances Cytotoxic Activity of PAMAM-Doxorubicin Conjugate in Glucose-Deprived MCF-7 Cells—Possible Role of GLUT1 Transporter. Pharm. Res. 2019, 36, 140. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Menendez, P.; Hevia, D.; Mayo, J.C.; Sainz, R.M. The dark side of glucose transporters in prostate cancer: Are they a new feature to characterize carcinomas? Int. J. Cancer 2018, 142, 2414–2424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, L.B.; Zhong, J.T.; Shen, L.F.; Zhou, S.H.; Lu, Z.J.; Bao, Y.Y.; Fan, J. Radiosensitizing effects of curcumin alone or combined with GLUT1 siRNA on laryngeal carcinoma cells through AMPK pathway-induced autophagy. J. Cell Mol. Med. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Dai, L.B.; Yu, Q.; Zhou, S.H.; Bao, Y.Y.; Zhong, J.T.; Shen, L.F.; Lu, Z.J.; Fan, J.; Huang, Y.P. Effect of combination of curcumin and GLUT-1 AS-ODN on radiosensitivity of laryngeal carcinoma through regulating autophagy. Head Neck 2020, 42, 2287–2297. [Google Scholar] [CrossRef]
- Zhong, J.T.; Yu, Q.; Zhou, S.H.; Yu, E.; Bao, Y.Y.; Lu, Z.J.; Fan, J. GLUT-1 siRNA Enhances Radiosensitization Of Laryngeal Cancer Stem Cells Via Enhanced DNA Damage, Cell Cycle Redistribution, And Promotion of Apoptosis In Vitro And In Vivo. Oncol. Targets Ther. 2019, 12, 9129–9142. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.X.; Luo, X.M.; Zhou, S.H.; Bao, Y.Y.; Fan, J.; Lu, Z.J.; Liao, X.B.; Huang, Y.P.; Wu, T.T.; Wang, Q.Y. Effect of antisense oligodeoxynucleotides glucose transporter-1 on enhancement of radiosensitivity of laryngeal carcinoma. Int. J. Med. Sci. 2013, 10, 1375–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, C.E.; Ehrhardt, A.; Kay, M.A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003, 4, 346–358. [Google Scholar] [CrossRef]
- Morille, M.; Passirani, C.; Vonarbourg, A.; Clavreul, A.; Benoit, J.P. Progress in developing cationic vectors for non-viral systemic gene therapy against cancer. Biomaterials 2008, 29, 3477–3496. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.; Mao, D.; Wu, R.; Gao, Z.; Meng, T.; Wang, R.; Liu, L.; Miao, J. Hepatitis B virus S gene therapy with 10-23 DNAzyme delivered by chitosan-g-stearic acid micelles. RSC Adv. 2019, 9, 11524–15196. [Google Scholar] [CrossRef] [Green Version]
- Miao, J.; Zhang, X.; Hong, Y.; Rao, Y.F.; Li, Q.; Xie, X.; Wo, J.; Li, M. Inhibition on hepatitis B virus e-gene expression of 10–23 DNAzyme delivered by novel chitosan oligosaccharide–stearic acid micelles. Carbohyd. Polym. 2012, 87, 1342–1347. [Google Scholar] [CrossRef]
- Tahamtan, A.; Ghaemi, A.; Gorji, A.; Kalhor, H.R.; Sajadian, A.; Tabarraei, A.; Moradi, A.; Atyabi, F.; Kelishadi, M. Antitumor effect of therapeutic HPV DNA vaccines with chitosan-based nanodelivery systems. J. Biomed. Sci. 2014, 21, 69. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.K.; Zhang, M.; Lu, J.J.; Xu, F.; Chen, B.A.; Xing, L.; Jiang, H.L. PK11195-chitosan-graft-polyethylenimine-modified SPION as a mitochondria-targeting gene carrier. J. Drug Target. 2016, 24, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Yang, X.Q.; Gao, Z.; Li, Q.; Meng, T.T.; Wu, J.Y.; Yuan, H.; Hu, F.Q. Redox-responsive chitosan oligosaccharide-SS-Octadecylamine polymeric carrier for efficient anti-Hepatitis B Virus gene therapy. Carbohydr. Polym. 2019, 212, 215–221. [Google Scholar] [CrossRef]
- Miao, J.; Yang, X.; Shang, X.; Gao, Z.; Li, Q.; Hong, Y.; Wu, J.; Meng, T.; Yuan, H.; Hu, F.Q. Hepatocyte-targeting and microenvironmentally responsive glycolipid-like polymer micelles for gene therapy of hepatitis B. Mol. Ther. Nucleic Acids 2021, 24, 127–139. [Google Scholar] [CrossRef]
- Hu, F.Q.; Chen, W.W.; Zhao, M.D.; Yuan, H.; Du, Y.Z. Effective antitumor gene therapy delivered by polyethylenimine-conjugated stearic acid-g-chitosan oligosaccharide micelles. Gene Ther. 2013, 20, 597–606. [Google Scholar] [CrossRef] [Green Version]
- Meng, T.T.; Liu, J.; Wen, L.; Yuan, M.; Cheng, B.; Hu, Y.; Zhu, Y.; Liu, X.; Yuan, H.; Hu, F.Q. Multi-cycle chemotherapy with the glycolipid-like polymeric micelles evade cancer stem cell enrichment in breast cancer therapy. Oncotarget 2016, 7, 72978–72989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panja, S.; Nayak, S.; Ghosh, S.K.; Selvakumara, M.; Chattopadhyay, S. Self-assembly of a biodegradable branched PEPCL-b-PEC amphiphilic polymer: Synthesis, characterization and targeted delivery of doxorubicin to cancer cells. RSC Adv. 2014, 4, 51766–51775. [Google Scholar] [CrossRef]
- Hu, F.Q.; Zhao, M.D.; Yuan, H.; You, J.; Du, Y.Z.; Zeng, S. A novel chitosan oligosaccharide–stearic acid micelles for gene delivery: Properties and in vitro transfection studies. Int. J. Pharm. 2006, 315, 158–166. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Hu, F.Q.; Du, Y.Z.; Yuan, H. Polymeric micelles with glycolipid-like structure and multiple hydrophobic domains for mediating molecular target delivery of paclitaxel. Biomacromolecules 2007, 8, 2450–2456. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.Q.; Zhang, Y.Y.; You, J.; Yuan, H.; Du, Y.Z. pH triggered doxorubicin delivery of PEGylated glycolipid conjugate micelles for tumor targeting therapy. Mol. Pharm. 2012, 9, 2469–2478. [Google Scholar] [CrossRef]
| Micelles | dn (nm) | PDI | Zeta Potential (mV) | SD (%) | CMC (μg/mL) |
|---|---|---|---|---|---|
| CSSA | 144.7 ± 3.2 | 0.212 ± 0.031 | 39.7 ± 0.8 | 15.70 ± 1.02 | 75.02 ± 1.27 |
| CSSA/siRNA | 168.1 ± 2.9 | 0.136 ± 0.025 | 20.3 ± 0.5 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, J.; Zhang, L.; Gao, P.; Zhao, H.; Xie, X.; Wang, J. Chitosan-Based Glycolipid Conjugated siRNA Delivery System for Improving Radiosensitivity of Laryngocarcinoma. Polymers 2021, 13, 2929. https://doi.org/10.3390/polym13172929
Miao J, Zhang L, Gao P, Zhao H, Xie X, Wang J. Chitosan-Based Glycolipid Conjugated siRNA Delivery System for Improving Radiosensitivity of Laryngocarcinoma. Polymers. 2021; 13(17):2929. https://doi.org/10.3390/polym13172929
Chicago/Turabian StyleMiao, Jing, Liwen Zhang, Peng Gao, Huawei Zhao, Xianji Xie, and Junyan Wang. 2021. "Chitosan-Based Glycolipid Conjugated siRNA Delivery System for Improving Radiosensitivity of Laryngocarcinoma" Polymers 13, no. 17: 2929. https://doi.org/10.3390/polym13172929
APA StyleMiao, J., Zhang, L., Gao, P., Zhao, H., Xie, X., & Wang, J. (2021). Chitosan-Based Glycolipid Conjugated siRNA Delivery System for Improving Radiosensitivity of Laryngocarcinoma. Polymers, 13(17), 2929. https://doi.org/10.3390/polym13172929






