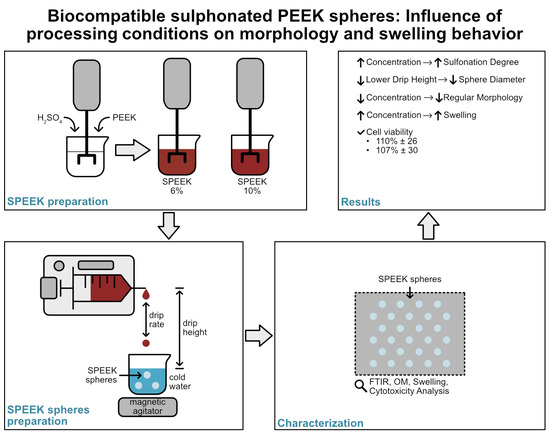
Biocompatible Sulphonated PEEK Spheres: Influence of Processing Conditions on Morphology and Swelling Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
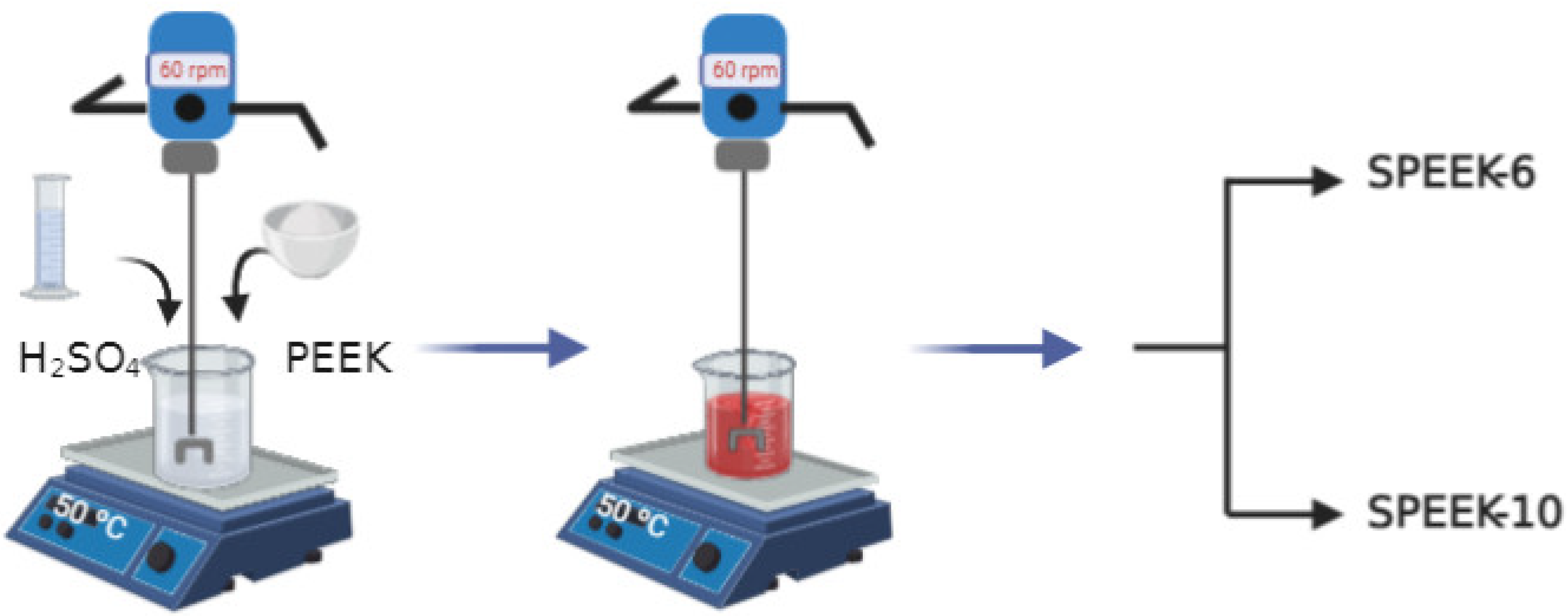
2.2. Preparation of SPEEK
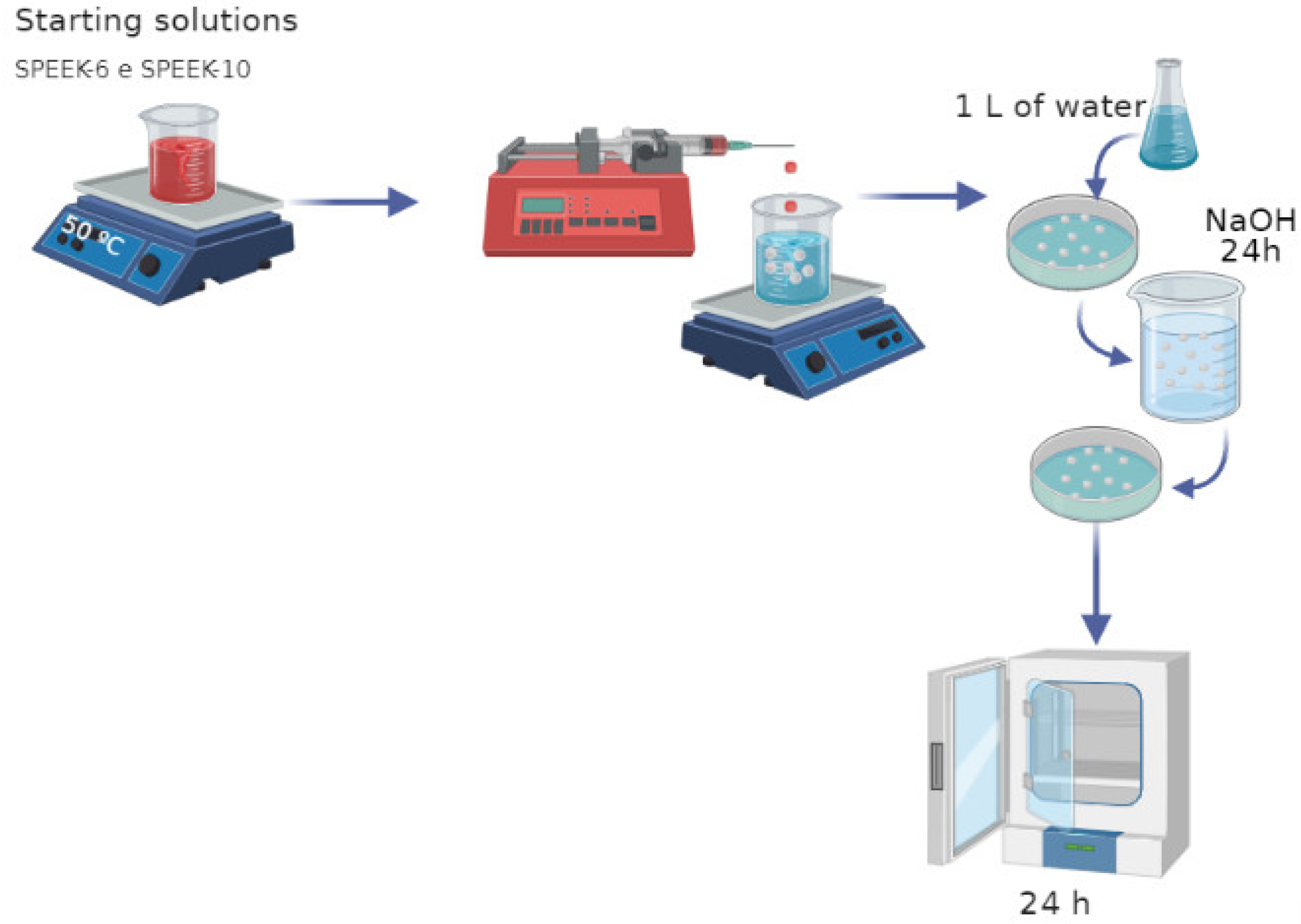
2.3. Preparation of SPEEK Spheres
2.4. Fourier-Transform Infrared Spectroscopy (FTIR)
2.5. Optical Microscopy (OM)
2.6. Swelling (%)
2.7. DOE
2.8. Cytotoxicity Study
3. Results and Discussion
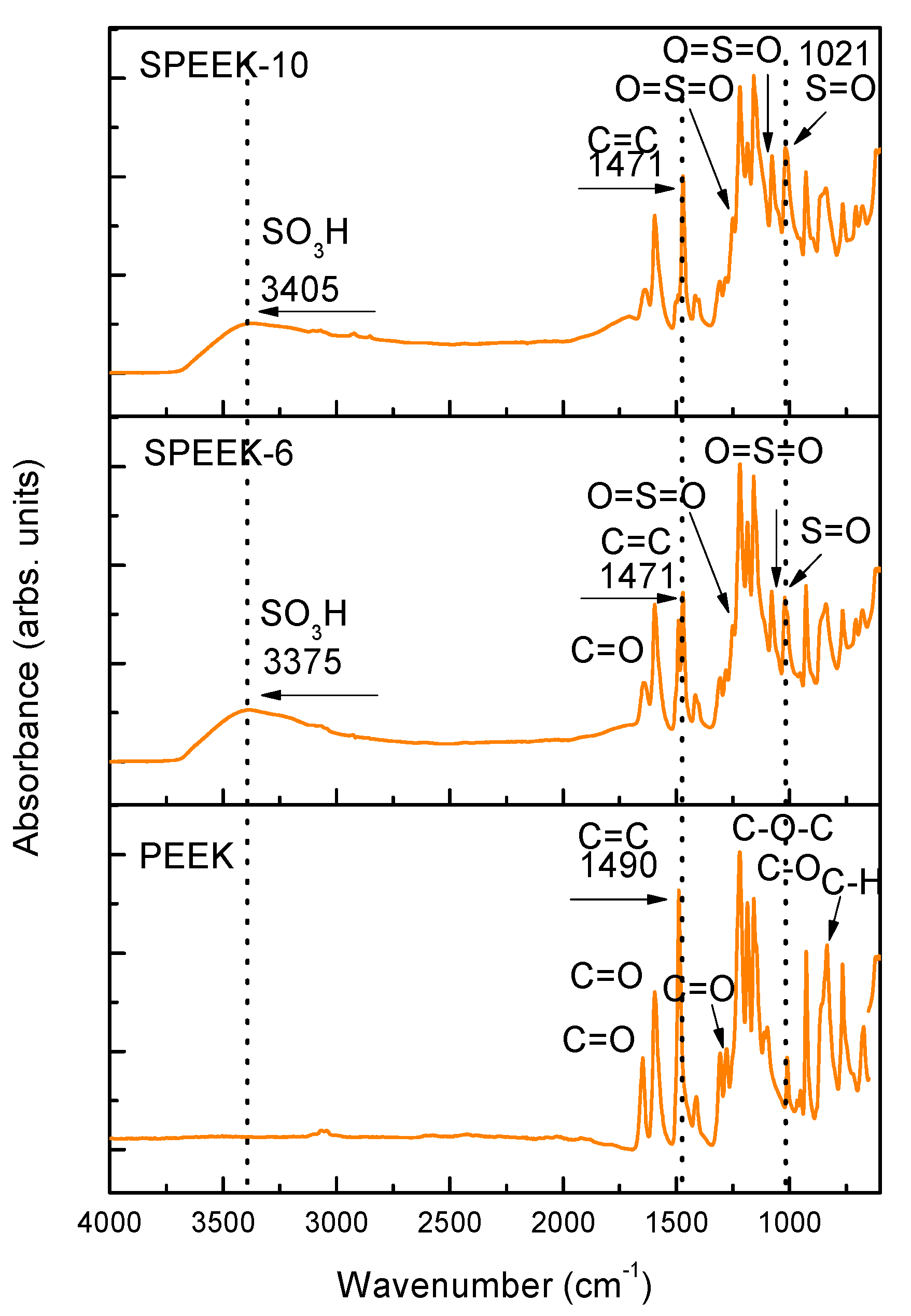
3.1. FTIR
3.2. OM
3.3. Swelling (%)
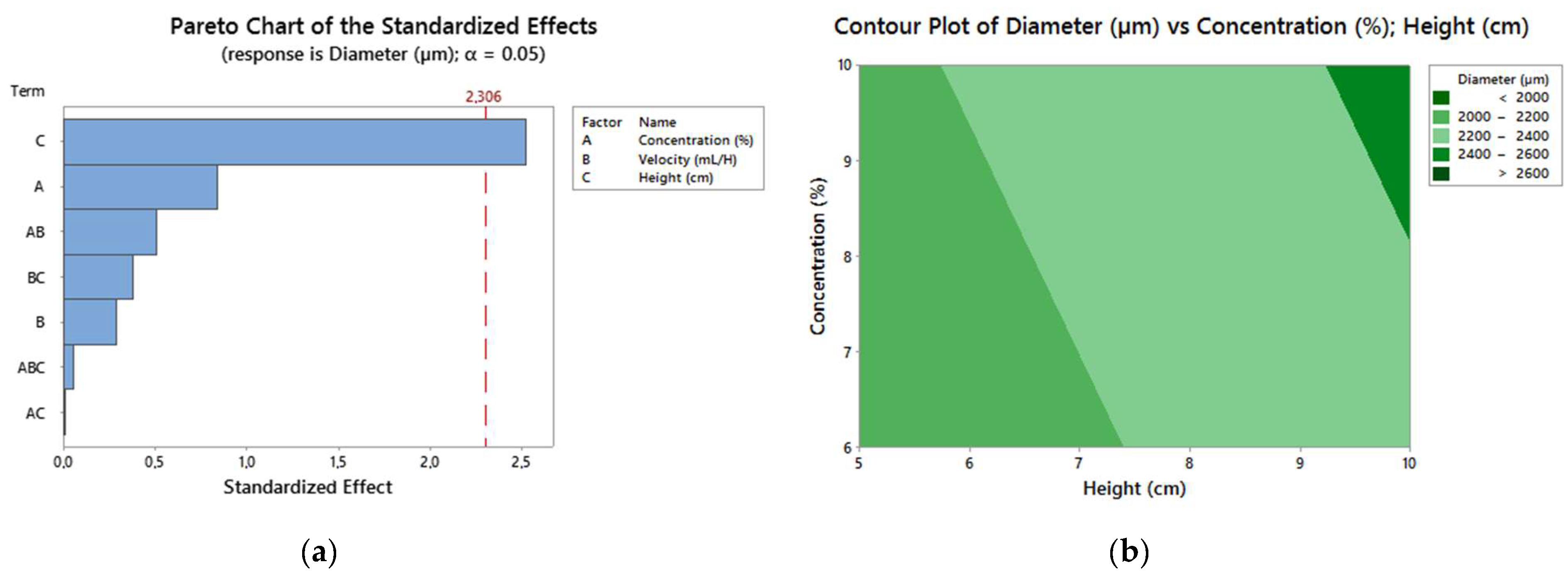
3.4. DOE Results
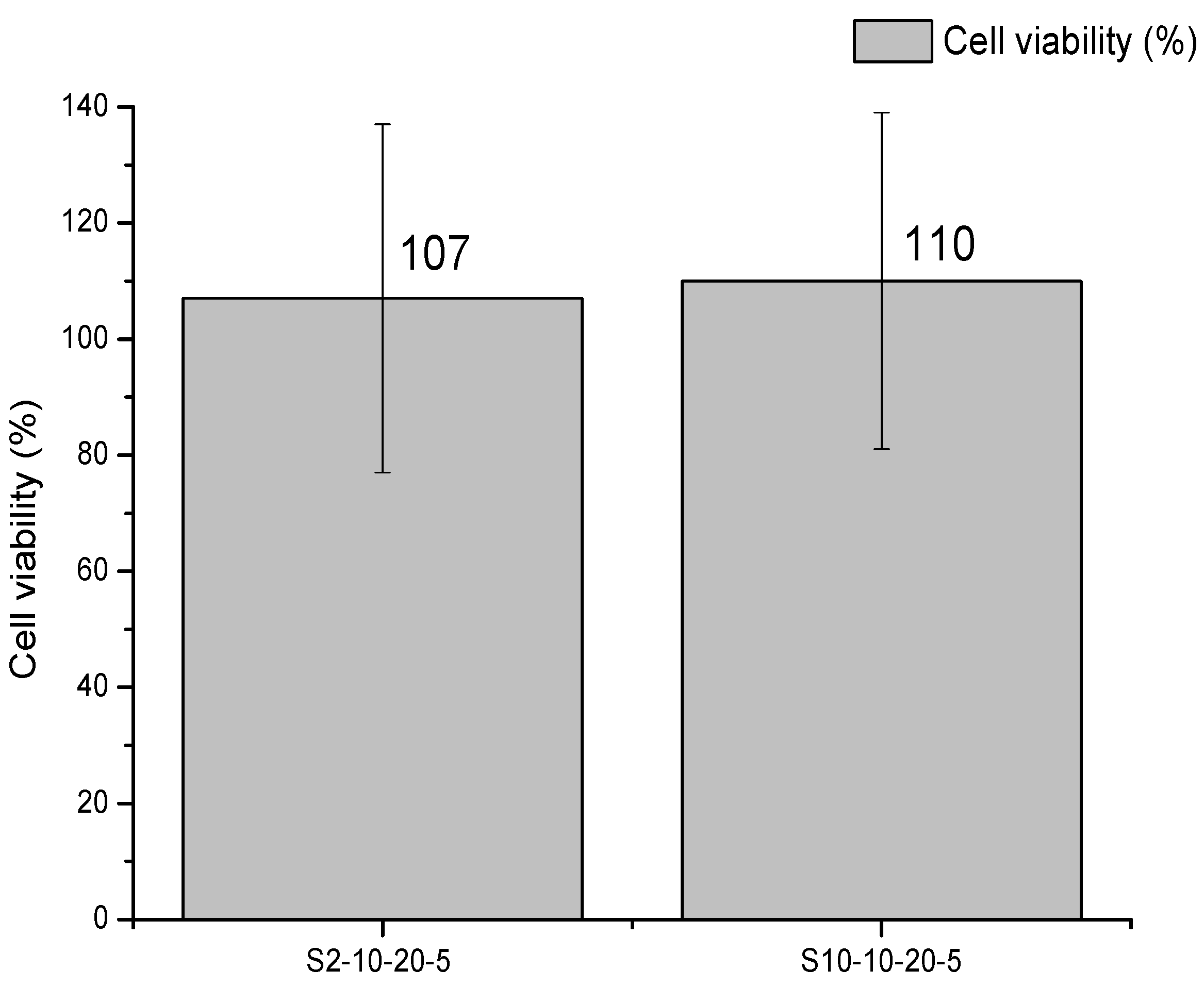
3.5. Cytotoxicity Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurtz, S.M. PEEK Biomaterials Handbook; William Andrew: Cambridge, MA, USA, 2019; p. 465. [Google Scholar]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef]
- Mazur, R.L.; Botelho, E.C.; Costa, M.L.; Rezende, M.C.J.P. Avaliações térmica e reológica da matriz termoplástica PEKK utilizada em compósitos aeronáuticos. Polímeros 2008, 18, 237–243. [Google Scholar] [CrossRef]
- Han, C.-M.; Lee, E.-J.; Kim, H.-E.; Koh, Y.-H.; Kim, K.N.; Ha, Y.; Kuh, S. The electron beam deposition of titanium on polyetheretherketone (PEEK) and the resulting enhanced biological properties. Biomaterials 2010, 31, 3465–3470. [Google Scholar] [CrossRef]
- Torstrick, F.B.; Lin, A.S.; Potter, D.; Safranski, D.L.; Sulchek, T.A.; Gall, K.; Guldberg, R.E. Porous PEEK improves the bone-implant interface compared to plasma-sprayed titanium coating on PEEK. Biomaterials 2018, 185, 106–116. [Google Scholar] [CrossRef]
- Doumeng, M.; Ferry, F.; Delbé, K.; Mérian, T.; Chabert, F.; Berthet, F.; Marsan, O.; Nassiet, V.; Denape, J. Evolution of crystallinity of PEEK and glass-fibre reinforced PEEK under tribological conditions using Raman spectroscopy. Wear 2019, 426–427, 1040–1046. [Google Scholar] [CrossRef]
- Pimentel, C.A.; Souza, J.W.D.L.; Dos Santos, F.S.F.; De Sá, M.D.; Ferreira, V.P.; Barreto, G.B.D.C.; Rodrigues, J.F.B.; De Sousa, W.J.B.; Filho, C.O.B.; De Sousa, F.K.A.; et al. Sulfonated poly(ether ether ketone)/hydroxyapatite membrane as biomaterials: Process evaluation. Polímeros 2019, 29, 1. [Google Scholar] [CrossRef]
- Aguiar, K.R.; Batalha, G.P.; Peixoto, M.; Ramos, A.; Pezzin, S.H. Produção de membranas híbridas zirconizadas de SPEEK/Copolissilsesquioxano para aplicação em células a combustível do tipo PEM. Polímeros 2012, 22, 453–459. [Google Scholar] [CrossRef][Green Version]
- Dai, W.; Yu, L.; Li, Z.; Yan, J.; Liu, L.; Xi, J.; Qiu, X. Sulfonated Poly(Ether Ether Ketone)/Graphene composite membrane for vanadium redox flow battery. Electrochim. Acta 2014, 132, 200–207. [Google Scholar] [CrossRef]
- Monich, P.R.; Henriques, B.; de Oliveira, A.P.N.; Souza, J.C.; Fredel, M.C. Mechanical and biological behavior of bio-medical PEEK matrix composites: A focused review. Mater. Lett. 2016, 185, 593–597. [Google Scholar] [CrossRef]
- Ekambaram, R.; Dharmalingam, S. Fabrication and evaluation of electrospun biomimetic sulphonated PEEK nanofibrous scaffold for human skin cell proliferation and wound regeneration potential. Mater. Sci. Eng. C 2020, 115, 111150. [Google Scholar] [CrossRef]
- Montero, J.F.; Tajiri, H.A.; Barra, G.M.; Fredel, M.C.; Benfatti, C.A.; Magini, R.S.; Pimenta, A.L.; Souza, J.C. Biofilm behavior on sulfonated poly(ether-ether-ketone) (sPEEK). Mater. Sci. Eng. C 2017, 70, 456–460. [Google Scholar] [CrossRef]
- Brum, R.S.; Monich, P.R.; Berti, F.; Fredel, M.C.; Porto, L.M.; Benfatti, C.A.; Souza, J.C. On the sulphonated PEEK for implant dentistry: Biological and physicochemical assessment. Mater. Chem. Phys. 2019, 223, 542–547. [Google Scholar] [CrossRef]
- Sundar, S.S.; Sangeetha, D. Investigation on sulphonated PEEK beads for drug delivery, bioactivity and tissue engineering applications. J. Mater. Sci. 2012, 47, 2736–2742. [Google Scholar] [CrossRef]
- Davarcı, F.; Turan, D.; Ozcelik, B.; Poncelet, D.J.F.H. The influence of solution viscosities and surface tension on calci-um-alginate microbead formation using dripping technique. Food Hydrocoll. 2017, 62, 119–127. [Google Scholar] [CrossRef]
- Weibel, M.I.; Mengatto, L.N.; Luna, J.A.; Rintoul, I. Accurate prediction of shape and size of polyvinyl alcohol beads produced by extrusion dripping. Iran. Polym. J. 2018, 27, 161–170. [Google Scholar] [CrossRef]
- Corvilain, M.; Klaysom, C.; Szymczyk, A.; Vankelecom, I.F. Formation mechanism of sPEEK hydrophilized PES supports for forward osmosis. Desalination 2017, 419, 29–38. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Yoo, D.J. Sulfonated-fluorinated copolymer blending membranes containing SPEEK for use as the electrolyte in polymer electrolyte fuel cells (PEFC). Int. J. Hydrogen Energy 2017, 42, 4349–4365. [Google Scholar] [CrossRef]
- Lakshmi, R.T.S.M.; Choudhary, V.; Varma, I.K. Sulphonated poly (ether ether ketone): Synthesis and characterisation. J. Mater. Sci. 2005, 40, 629–636. [Google Scholar] [CrossRef]
- Zorzin, J.; Maier, E.; Harre, S.; Fey, T.; Belli, R.; Lohbauer, U.; Petschelt, A.; Taschner, M. Bulk-fill resin composites: Polymerization properties and extended light curing. Dent. Mater. 2015, 31, 293–301. [Google Scholar] [CrossRef]
- Pimentel, C.A.; de Lima Souza, J.W.; Ferreira dos Santos, F.S.; Dantas de Sá, M.; Pereira Ferreira, V.; Pinto Borges, S.M.; Bezerra de Lima, D.; Alves de Sousa, F.K.; Lia Fook, M.V. Sulfonated poly (ether ether ketone)/hydroxyapatite membranes as bone graft materials. Mater. Res. Innov. 2019, 23, 270–275. [Google Scholar] [CrossRef]
- El-Araby, R.; Attia, N.; Eldiwani, G.; Khafagi, M.; Sobhi, S.; Mostafa, T. Characterization and sulfonation degree of sulfonated poly ether ether ketone using Fourier transform infrared spectroscopy. World Appl. Sci. J. 2014, 32, 2239–2244. [Google Scholar]
- Zhao, Y.; Wong, H.M.; Wang, W.; Li, P.; Xu, Z.; Chong, E.Y.; Yan, C.H.; Yeung, K.W.; Chu, P.K. Cytocompatibility, osseointegration, and bioactivity of three-dimensional porous and nanostructured network on polyetheretherketone. Biomaterials 2013, 34, 9264–9277. [Google Scholar] [CrossRef]
- Jiang, R.; Kunz, H.R.; Fenton, J.M. Investigation of membrane property and fuel cell behavior with sulfonated poly (ether ether ketone) electrolyte: Temperature and relative humidity effects. J. Power Sources 2005, 150, 120–128. [Google Scholar] [CrossRef]
- Andrade, B.T.N.C.; Bezerra, A.C.; Calado, C.R. Adding value to polystyrene waste by chemically transforming it into sulfonated polystyrene. Matéria 2019, 24, 10. [Google Scholar] [CrossRef]
- Bowen, W.R.; Doneva, T.A.; Yin, H.B. Polysulfone—sulfonated poly (ether ether) ketone blend membranes: Systematic synthesis and characterisation. J. Membr. Sci. 2001, 181, 253–263. [Google Scholar] [CrossRef]
- Rubira, A.F.; Muniz, E.C.; Guilherme, M.R.; Paulino, A.T.; Tambourgi, E.B. Morfologia de hidrogéis-ipn termo-sensíveis e ph-responsivos para aplicação como biomaterial na cultura de células. Polímeros 2009, 19, 105–110. [Google Scholar] [CrossRef][Green Version]
- Park, G.-C.; Kim, D. Porous PTFE reinforced SPEEK proton exchange membranes for enhanced mechanical, dimensional, and electrochemical stability. Polymer 2021, 218, 123506. [Google Scholar] [CrossRef]
- Zeng, H.; Li, Y.; Wang, C.J.S.; Technology, P. Using zeolite imidazole frameworks as ionic cross-linkers to improve the per-formance of composite SPEEK membrane. Sep. Purif. Technol. 2020, 236, 116258. [Google Scholar] [CrossRef]
- Devi, M.P.; Sekar, M.; Chamundeswari, M.; Moorthy, A.; Krithiga, G.; Murugan, N.S.; Sastry, T.P. A novel wound dressing material—fibrin–chitosan–sodium alginate composite sheet. Bull. Mater. Sci. 2012, 35, 1157–1163. [Google Scholar] [CrossRef]

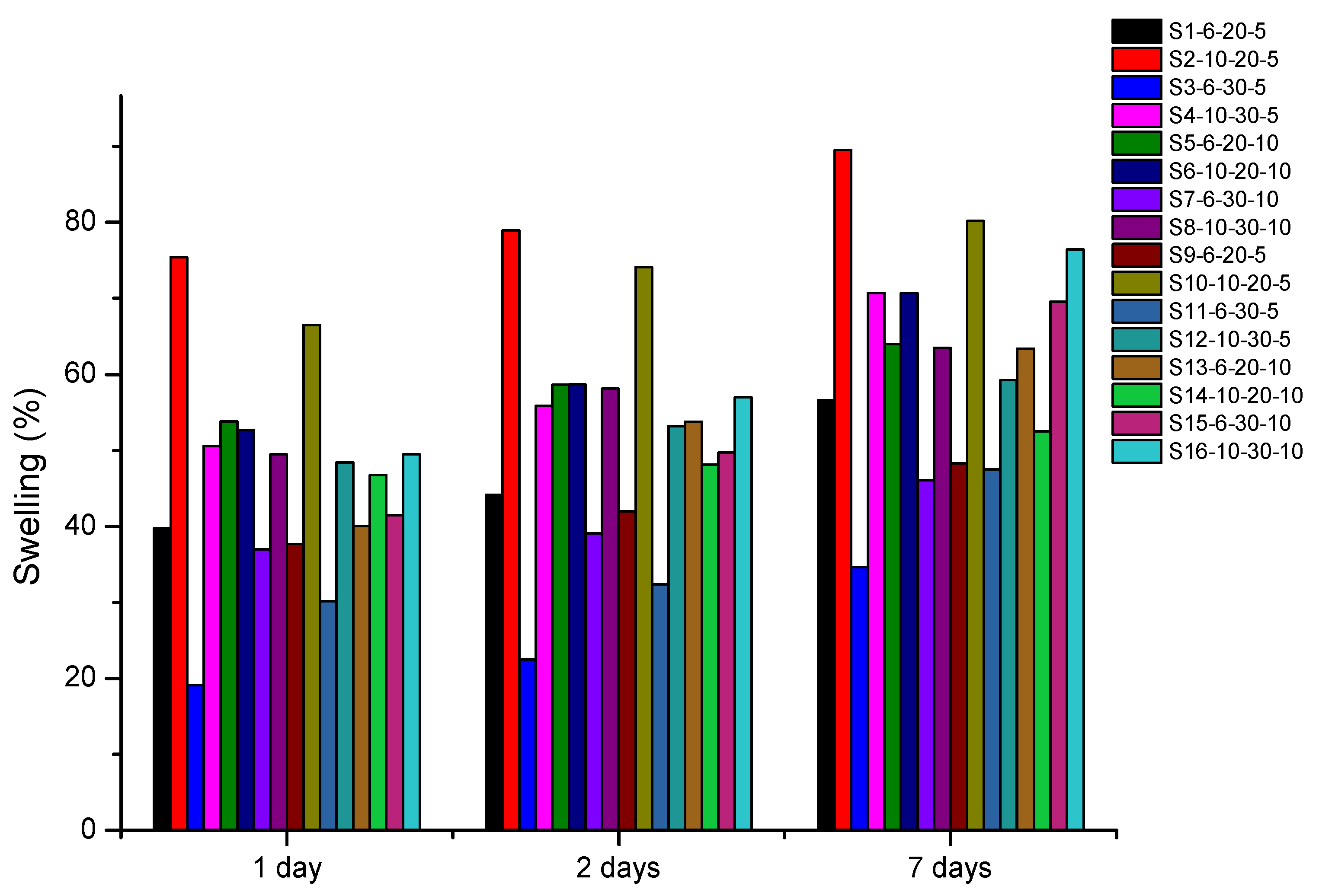

| Parameters | (−1 | +1) |
|---|---|---|
| Concentration (%) | 6 | 10 |
| Dripping rate (mL/h) | 20 | 30 |
| Dripping height (cm) | 5 | 10 |
| Experiment | Sample | Concentration (w/v; %) | Dripping Rate (mL/h) | Dripping Height (cm) |
|---|---|---|---|---|
| 01 | S1-6-20-5 | 6 | 20 | 5 |
| 02 | S2-10-20-5 | 10 | 20 | 5 |
| 03 | S3-6-30-5 | 6 | 30 | 5 |
| 04 | S4-10-30-5 | 10 | 30 | 5 |
| 05 | S5-6-20-10 | 6 | 20 | 10 |
| 06 | S6-10-20-10 | 10 | 20 | 10 |
| 07 | S7-6-30-10 | 6 | 30 | 10 |
| 08 | S8-10-30-10 | 10 | 30 | 10 |
| 09 | S9-6-20-5 | 6 | 20 | 5 |
| 10 | S10-10-20-5 | 10 | 20 | 5 |
| 11 | S11-6-30-5 | 6 | 30 | 5 |
| 12 | S12-10-30-5 | 10 | 30 | 5 |
| 13 | S13-6-20-10 | 6 | 20 | 10 |
| 14 | S14-10-20-10 | 10 | 20 | 10 |
| 15 | S15-6-30-10 | 6 | 30 | 10 |
| 16 | S16-10-30-10 | 10 | 30 | 10 |
| Response under Analysis | Unit | Characterization |
|---|---|---|
| Spheres diameter | µm | ImageJ |
| Swelling of spheres in PBS | % | Swelling |
| Sample | Diameter of Spheres (µm) | Swelling (%) |
|---|---|---|
| Response I | Response II | |
| S1-6-20-5 | 1846 ± 11 | 56 ± 9 |
| S2-10-20-5 | 1881 ± 20 | 89 ± 5 |
| S3-6-30-5 | 2025 ± 14 | 34 ± 11 |
| S4-10-30-5 | 2280 ± 44 | 70 ± 7 |
| S5-6-20-10 | 2388 ± 48 | 63 ± 6 |
| S6-10-20-10 | 2188 ± 37 | 70 ± 9 |
| S7-6-30-10 | 2279 ± 15 | 46 ± 7 |
| S8-10-30-10 | 2662 ± 18 | 63 ± 4 |
| S9-6-20-5 | 2353 ± 23 | 48 ± 4 |
| S10-10-20-5 | 2378 ± 41 | 80 ±10 |
| S11-6-30-5 | 2027 ± 13 | 47 ± 9 |
| S12-10-30-5 | 2087 ± 92 | 59 ± 3 |
| S13-6-20-10 | 2284 ± 17 | 63 ± 5 |
| S14-10-20-10 | 2573 ± 66 | 52 ± 3 |
| S15-6-30-10 | 2441 ± 15 | 69 ± 9 |
| S16-10-30-10 | 2353 ± 32 | 76 ± 9 |
| Solution | Concentration | Rate | Height | Diameter | Swelling | Compound Desirability |
|---|---|---|---|---|---|---|
| 1 | 10 | 20 | 5 | 2133 | 82 | 0.82 |
| Result | Adjustment | Standard Adjustment Error | 95% Confidence Interval |
|---|---|---|---|
| Diameter | 2133 | (1813; 2453) | (1553; 108.85) |
| Swelling | 82 | (67.21; 96.81) | (55.17; 108.85) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sá, M.D.; de Lima Souza, J.W.; da Silva, H.N.; Torres, R.H.N.; Leite, M.D.R.; Barbosa, R.C.; Leite, I.F.; Pimentel, C.A.; Fook, M.V.L. Biocompatible Sulphonated PEEK Spheres: Influence of Processing Conditions on Morphology and Swelling Behavior. Polymers 2021, 13, 2920. https://doi.org/10.3390/polym13172920
de Sá MD, de Lima Souza JW, da Silva HN, Torres RHN, Leite MDR, Barbosa RC, Leite IF, Pimentel CA, Fook MVL. Biocompatible Sulphonated PEEK Spheres: Influence of Processing Conditions on Morphology and Swelling Behavior. Polymers. 2021; 13(17):2920. https://doi.org/10.3390/polym13172920
Chicago/Turabian Stylede Sá, Mayelli Dantas, José William de Lima Souza, Henrique Nunes da Silva, Rodolfo Henrique Nogueira Torres, Michele Dayane Rodrigues Leite, Rossemberg Cardoso Barbosa, Itamara Farias Leite, Cristiane Agra Pimentel, and Marcus Vinicius Lia Fook. 2021. "Biocompatible Sulphonated PEEK Spheres: Influence of Processing Conditions on Morphology and Swelling Behavior" Polymers 13, no. 17: 2920. https://doi.org/10.3390/polym13172920
APA Stylede Sá, M. D., de Lima Souza, J. W., da Silva, H. N., Torres, R. H. N., Leite, M. D. R., Barbosa, R. C., Leite, I. F., Pimentel, C. A., & Fook, M. V. L. (2021). Biocompatible Sulphonated PEEK Spheres: Influence of Processing Conditions on Morphology and Swelling Behavior. Polymers, 13(17), 2920. https://doi.org/10.3390/polym13172920