Curing Behavior and Thermomechanical Performance of Bioepoxy Resin Synthesized from Vanillyl Alcohol: Effects of the Curing Agent
Abstract
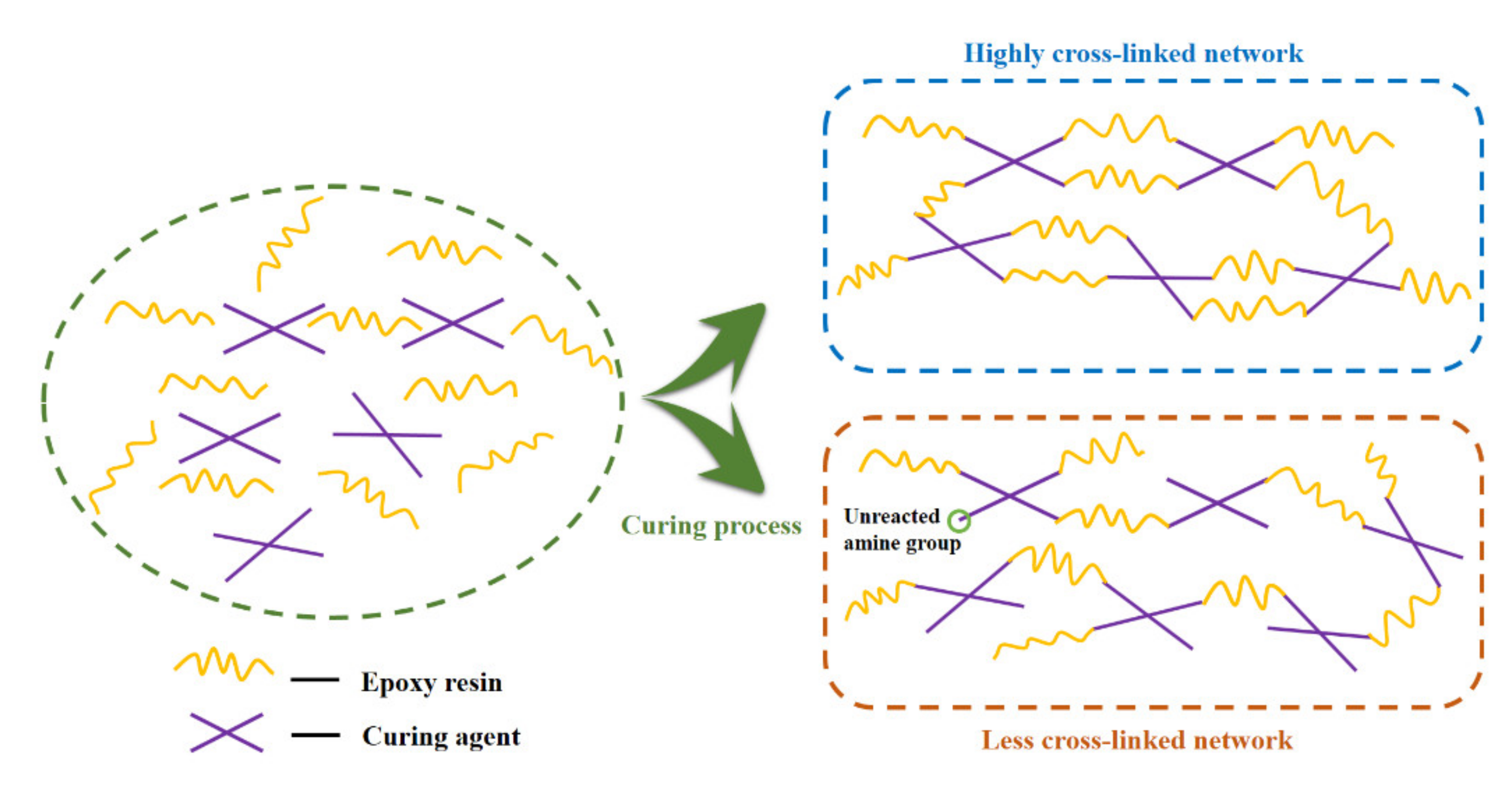
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Vanillyl Alcohol–Based Bioepoxy Resin
2.3. Curing Process of the Bioepoxy Resin
2.4. DSC Analysis
2.5. ATR–FTIR Analysis
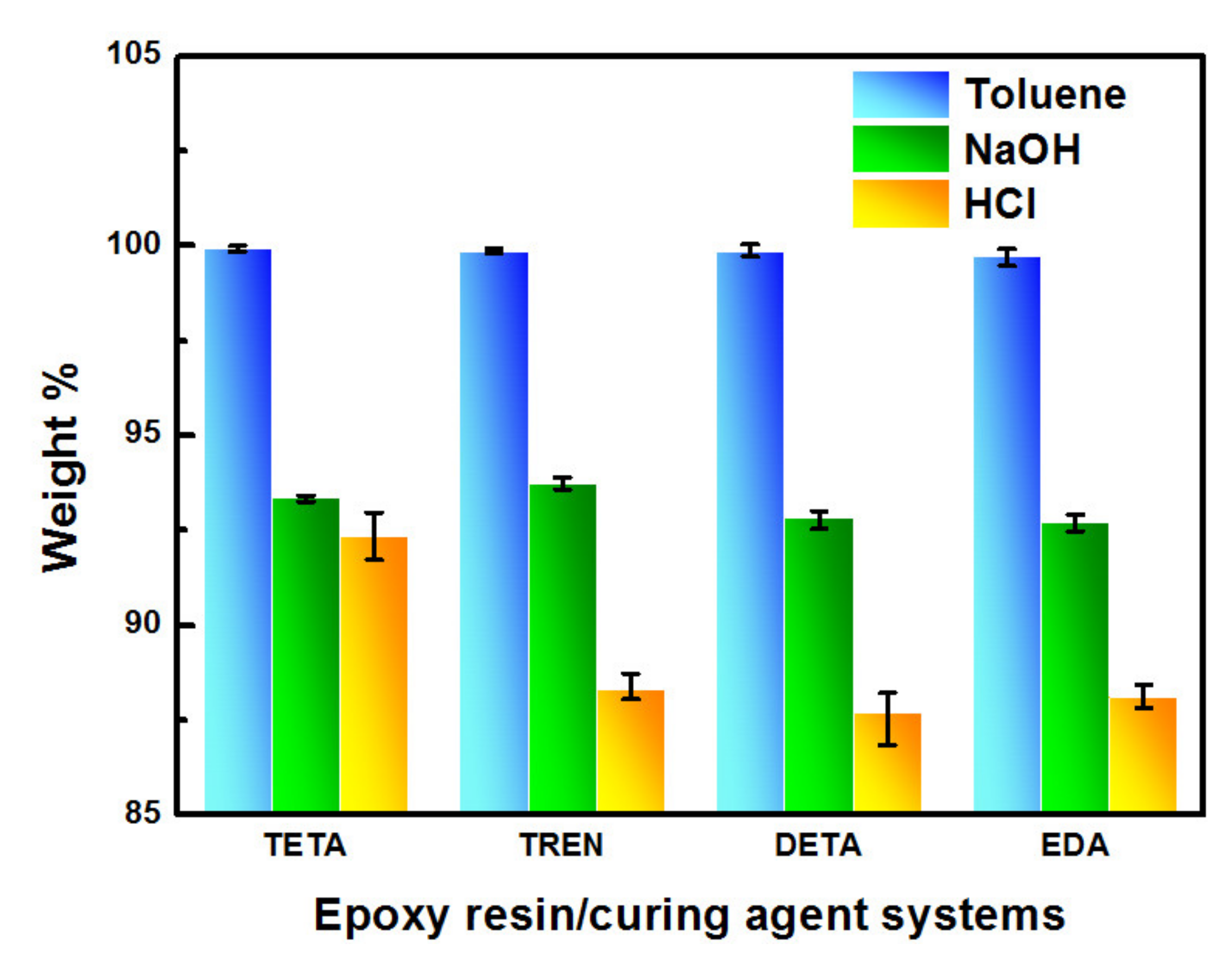
2.6. Swelling and Chemical Resistance Testing
2.7. Thermogravimetric Analysis
2.8. Dynamic Mechanical Analysis
2.9. Tensile Testing
3. Results and Discussion
3.1. Curing Behavior of the Synthesized Bioepoxies
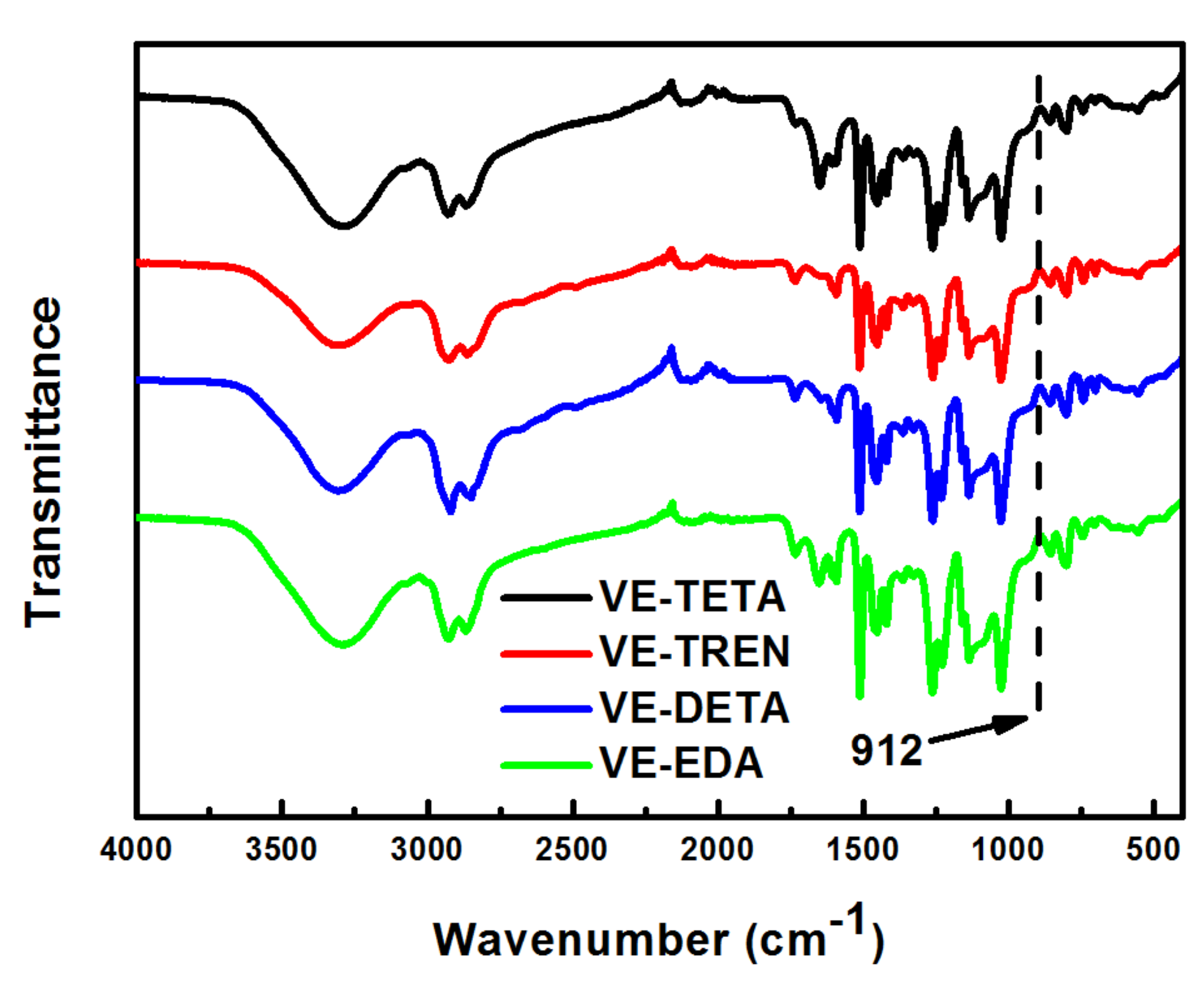
3.2. Chemical Structure and Chemical Resistance of the Cured Bioepoxies
3.3. Thermal Properties of the Cured Bioepoxies
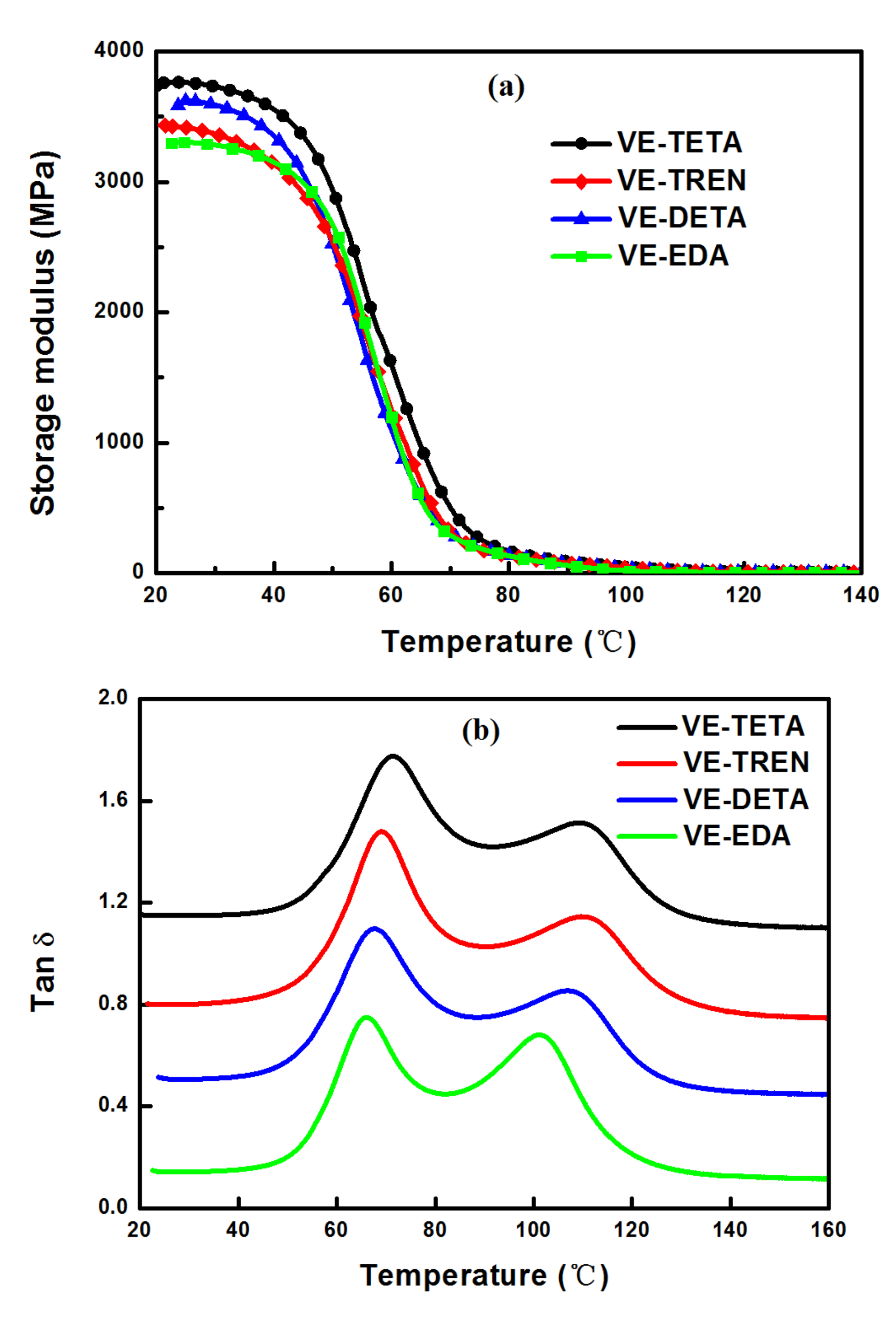
3.4. Dynamic Mechanical Analysis of the Cured Bioepoxies
3.5. Mechanical Properties of the Cured Bioepoxies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuo, P.; Sain, M.; Yan, N. Synthesis and characterization of an extractive-based bio-epoxy resin from beetle infested pinus contorta bark. Green Chem. 2014, 16, 3483–3493. [Google Scholar] [CrossRef]
- Mashouf Roudsari, G.; Mohanty, A.K.; Misra, M. Green approaches to engineer tough biobased epoxies: A review. Acs Sustain. Chem. Eng. 2017, 5, 9528–9541. [Google Scholar] [CrossRef]
- Baroncini, E.A.; Kumar Yadav, S.; Palmese, G.R.; Stanzione Iii, J.F. Recent advances in bio-based epoxy resins and bio-based epoxy curing agents. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Fourcade, D.; Ritter, B.S.; Walter, P.; Schönfeld, R.; Mülhaupt, R. Renewable resource-based epoxy resins derived from multifunctional poly(4-hydroxybenzoates). Green Chem. 2013, 15, 910–918. [Google Scholar] [CrossRef]
- Gnanasekar, P.; Chen, H.; Tratnik, N.; Feng, M.; Yan, N. Enhancing performance of phosphorus containing vanillin-based epoxy resins by P-N non-covalently functionalized graphene oxide nanofillers. Compos. B Eng. 2021, 207, 108585. [Google Scholar] [CrossRef]
- Kuo, P.Y.; de Assis Barros, L.; Sain, M.; Tjong, J.S.Y.; Yan, N. Effects of reaction parameters on the glycidyl etherification of bark extractives during bioepoxy resin synthesis. ACS Sustain. Chem. Eng. 2016, 4, 1016–1024. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, X.; Zhang, R.; Zhu, J.; Jiang, Y. Synthesis and properties of full bio-based thermosetting resins from rosin acid and soybean oil: The role of rosin acid derivatives. Green Chem. 2013, 15, 1300–1310. [Google Scholar] [CrossRef]
- Shou, Z.; Abu-Omar, M.M. Biobased epoxy nanocomposites derived from lignin-based monomers. Biomacromolecules 2015, 16, 2025–2031. [Google Scholar] [CrossRef]
- Niu, H.; Nabipour, H.; Wang, X.; Song, L.; Hu, Y. Phosphorus-free vanillin-derived intrinsically flame-retardant epoxy thermoset with extremely low heat release rate and smoke emission. ACS Sustain. Chem. Eng. 2021, 9, 5268–5277. [Google Scholar] [CrossRef]
- Brazinha, C.; Barbosa, D.S.; Crespo, J.G. Sustainable recovery of pure natural vanillin from fermentation media in a single pervaporation step. Green Chem. 2011, 13, 2197–2203. [Google Scholar] [CrossRef]
- Fache, M.; Darroman, E.; Besse, V.; Auvergne, R.; Caillol, S.; Boutevin, B. Vanillin, a promising biobased building-block for monomer synthesis. Green Chem. 2014, 16, 1987–1998. [Google Scholar] [CrossRef]
- Savonnet, E.; Grau, E.; Grelier, S.; Defoort, B.; Cramail, H. Divanillin-based epoxy precursors as dgeba substitutes for biobased epoxy thermosets. ACS Sustain. Chem. Eng. 2018, 6, 11008–11017. [Google Scholar] [CrossRef]
- Lora, J.H.; Glasser, W.G. Recent industrial applications of lignin: A sustainable alternative to nonrenewable materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Aouf, C.; Le Guernevé, C.; Caillol, S.; Fulcrand, H. Study of the o-glycidylation of natural phenolic compounds. The relationship between the phenolic structure and the reaction mechanism. Tetrahedron 2013, 69, 1345–1353. [Google Scholar] [CrossRef]
- Bjørsvik, H.R.; Minisci, F. Fine chemicals from lignosulfonates. 1. Synthesis of vanillin by oxidation of lignosulfonates. Org. Process Res. Dev. 1999, 3, 330–340. [Google Scholar] [CrossRef]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin production from lignin and its use as a renewable chemical. ACS Sustain. Chem. Eng. 2016, 4, 6379–6401. [Google Scholar] [CrossRef]
- Gnanasekar, P.; Feng, M.; Yan, N. Facile synthesis of a phosphorus-containing sustainable biomolecular platform from vanillin for the production of mechanically strong and highly flame-retardant resins. ACS Sustain. Chem. Eng. 2020, 8, 17417–17426. [Google Scholar] [CrossRef]
- Silva, E.A.B.D.; Zabkova, M.; Araújo, J.D.; Cateto, C.A.; Barreiro, M.F.; Belgacem, M.N.; Rodrigues, A.E. An integrated process to produce vanillin and lignin-based polyurethanes from kraft lignin. Chem. Eng. Res. Des. 2009, 87, 1276–1292. [Google Scholar] [CrossRef]
- Fache, M.; Auvergne, R.; Boutevin, B.; Caillol, S. New vanillin-derived diepoxy monomers for the synthesis of biobased thermosets. Eur. Polym. J. 2015, 67, 527–538. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, F.; Wong, C.P. Novel, environmentally friendly crosslinking system of an epoxy using an amino acid: Tryptophan-cured diglycidyl ether of bisphenol a epoxy. J. Polym. Sci. A Polym. Chem. 2007, 45, 181–190. [Google Scholar] [CrossRef]
- Jin, F.L.; Li, X.; Park, S.J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, K.; Liu, Z.; Yuan, Y.; Zhou, C.; Lei, J. Aliphatic diamide as novel asphalt-modified epoxy curing agent for enhanced performance. Adv. Polym. Technol. 2018, 37, 830–836. [Google Scholar] [CrossRef]
- Wan, J.; Li, C.; Bu, Z.-Y.; Xu, C.-J.; Li, B.-G.; Fan, H. A comparative study of epoxy resin cured with a linear diamine and a branched polyamine. Chem. Eng. J. 2012, 188, 160–172. [Google Scholar] [CrossRef]
- Cai, H.; Peng, L.; Gang, S.; Yu, Y.; Gang, L.; Yang, X.; Ryu, S. Curing kinetics study of epoxy resin/flexible amine toughness systems by dynamic and isothermal dsc. Thermochim. Acta 2008, 473, 101–105. [Google Scholar] [CrossRef]
- Chen, W.; Peng, L.; Yu, Y.; Yang, X. Curing kinetics study of an epoxy resin system for T800 carbon fiber filament wound composites by dynamic and isothermal dsc. J. Appl. Polym. Sci. 2010, 107, 1493–1499. [Google Scholar] [CrossRef]
- Montserrat, S.; Málek, J. A kinetic analysis of the curing reaction of an epoxy resin. Thermochim. Acta 1993, 228, 47–60. [Google Scholar] [CrossRef]
- Jin, F.L.; Park, S.J. Thermal properties of epoxy resin/filler hybrid composites. Polym. Degrad. Stabil. 2012, 97, 2148–2153. [Google Scholar] [CrossRef]
- Tucker, S.J.; Fu, B.; Kar, S.; Heinz, S.; Wiggins, J.S. Ambient cure poss–epoxy matrices for marine composites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1441–1446. [Google Scholar] [CrossRef]
- Ferdosian, F.; Yuan, Z.; Anderson, M.; Xu, C.C. Sustainable lignin-based epoxy resins cured with aromatic and aliphatic amine curing agents: Curing kinetics and thermal properties. Thermochim. Acta 2015, 618, 48–55. [Google Scholar] [CrossRef]
- Balabanovich, A.I.; Hornung, A.; Merz, D.; Seifert, H. The effect of a curing agent on the thermal degradation of fire retardant brominated epoxy resins. Polym. Degrad. Stabil. 2004, 85, 713–723. [Google Scholar] [CrossRef]
- Kornmann, X.; Lindberg, H.; Berglund, L.A. Synthesis of epoxy–clay nanocomposites. Influence of the nature of the curing agent on structure. Polymer 2001, 42, 4493–4499. [Google Scholar] [CrossRef]
- Wang, Z.; Gnanasekar, P.; Sudhakaran Nair, S.; Farnood, R.; Yi, S.; Yan, N. Biobased epoxy synthesized from a vanillin derivative and its reinforcement using lignin-containing cellulose nanofibrils. ACS Sustain. Chem. Eng. 2020, 8, 11215–11223. [Google Scholar] [CrossRef]
- Ahn, B.U.; Su, K.L.; Sang, K.L.; Park, J.H.; Kim, B.K. Uv curable polyurethane dispersions from polyisocyanate and organosilane. Prog. Org. Coat. 2008, 62, 258–264. [Google Scholar] [CrossRef]
- Dusek, K.; Bleha, M.; Lunák, S. Curing of epoxide resins: Model reactions of curing with amines. J. Polym. Sci. A Polym. Chem. 2010, 15, 2393–2400. [Google Scholar] [CrossRef]
- Pham, H.Q.; Marks, M.J. Epoxy resins. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar] [CrossRef]
- Wang, X.; Gillham, J.K. Competitive primary amine/epoxy and secondary amine/epoxy reactions: Effect on the isothermal time-to-vitrify. J. Appl. Polym. Sci. 1991, 43, 2267–2277. [Google Scholar] [CrossRef]
- Jagtap, S.B.; Ratna, D. Effect of molecular weight of curing agents on properties of nanocomposites based on epoxy resin and organoclay with reactive modifier. J. Appl. Polym. Sci. 2017, 134, 44595. [Google Scholar] [CrossRef]
- Shibata, M.; Ohkita, T. Fully biobased epoxy resin systems composed of a vanillin-derived epoxy resin and renewable phenolic hardeners. Eur. Polym. J. 2017, 92, 165–173. [Google Scholar] [CrossRef]
- Mezzenga, R.; Boogh, L.; Månson, J.A.E.; Bo, P. Effects of the branching architecture on the reactivity of epoxy-amine groups. Macromolecules 2000, 33, 4373–4379. [Google Scholar] [CrossRef]
- Lakshmi, M.S.; Srividhya, M.; Reddy, B.S.R. New epoxy resins containing hard-soft segments: Synthesis, characterization and modification studies for high performance applications. J. Polym. Res. 2003, 10, 259–266. [Google Scholar] [CrossRef]
- Kanehashi, S.; Yokoyama, K.; Masuda, R.; Kidesaki, T.; Nagai, K.; Miyakoshi, T. Preparation and characterization of cardanol-based epoxy resin for coating at room temperature curing. J. Appl. Polym. Sci. 2013, 130, 2468–2478. [Google Scholar] [CrossRef]
- Lakshmi, B.; Shivananda, K.; Mahendra, K. Synthesis, characterization and curing studies of thermosetting epoxy resin with amines. Bull. Korean Chem. Soc. 2010, 31, 2272–2278. [Google Scholar] [CrossRef][Green Version]
- Wu, G.M.; Di, L.; Liu, G.F.; Jian, C.; Huo, S.P.; Kong, Z.W. Thermoset nanocomposites from waterborne bio-based epoxy resin and cellulose nanowhiskers. Carbohydr. Polym. 2015, 127, 229–235. [Google Scholar] [CrossRef]
- Koton, M.; Sazanov, Y.N. Thermal degradation of polypyromellitimides. Polym. Sci. USSR 1973, 15, 1857–1863. [Google Scholar] [CrossRef]
- He, X.; Xu, X.; Wan, Q.; Bo, G.; Yan, Y. Synthesis and characterization of dimmer-acid-based nonisocyanate polyurethane and epoxy resin composite. Polymers 2017, 9, 649. [Google Scholar] [CrossRef] [PubMed]
- Gowda, S.; Mahendra, K.N. Synthesis and reactivity studies of maleimide-epoxy resins with aliphatic amines. Iran. Polym. J. 2007, 16, 161–171. [Google Scholar]
- Madan, R.; Anand, R.; Varma, I. Thermal behaviour of polymers based on nadimides. J. Therm. Anal. Calorim. 2000, 59, 531–539. [Google Scholar] [CrossRef]
- Varma, I.; Gupta, V.; Sini, N. Thermosetting resin–properties. In Comprehensive Composite Materials II; Elsevier: Amsterdam, The Netherlands, 2018; pp. 401–468. [Google Scholar] [CrossRef]
- Shang, L.; Zhang, X.; Zhang, M.; Jin, L.; Liu, L.; Xiao, L.; Li, M.; Ao, Y. A highly active bio-based epoxy resin with multi-functional group: Synthesis, characterization, curing and properties. J. Mater. Sci. Technol. 2018, 53, 5402–5417. [Google Scholar] [CrossRef]
- Gerard, J.; Galy, J.; Pascault, J.; Cukierman, S.; Halary, J. Viscoelastic response of model epoxy networks in the glass transition region. Polym. Eng. Sci. 1991, 31, 615–621. [Google Scholar] [CrossRef]
- Stanzione III, J.F.; Sadler, J.M.; La Scala, J.J.; Reno, K.H.; Wool, R.P. Vanillin-based resin for use in composite applications. Green Chem. 2012, 14, 2346–2352. [Google Scholar] [CrossRef]
- Feldman, D.; Banu, D.; Natansohn, A.; Wang, J. Structure–properties relations of thermally cured epoxy–lignin polyblends. J. Appl. Polym. Sci. 2010, 42, 1537–1550. [Google Scholar] [CrossRef]
- Poussard, L.; Mariage, J.; Grignard, B.; Detrembleur, C.; Jérôme, C.; Calberg, C.; Heinrichs, B.; De Winter, J.; Gerbaux, P.; Raquez, J.-M. Non-isocyanate polyurethanes from carbonated soybean oil using monomeric or oligomeric diamines to achieve thermosets or thermoplastics. Macromolecules 2016, 49, 2162–2171. [Google Scholar] [CrossRef]
- Yen, M.S.; Chen, P.Y.; Tsai, H.C. Synthesis, properties, and dyeing application of nonionic waterborne polyurethanes with different chain length of ethyldiamines as the chain extender. J. Appl. Polym. Sci. 2003, 90, 2824–2833. [Google Scholar] [CrossRef]
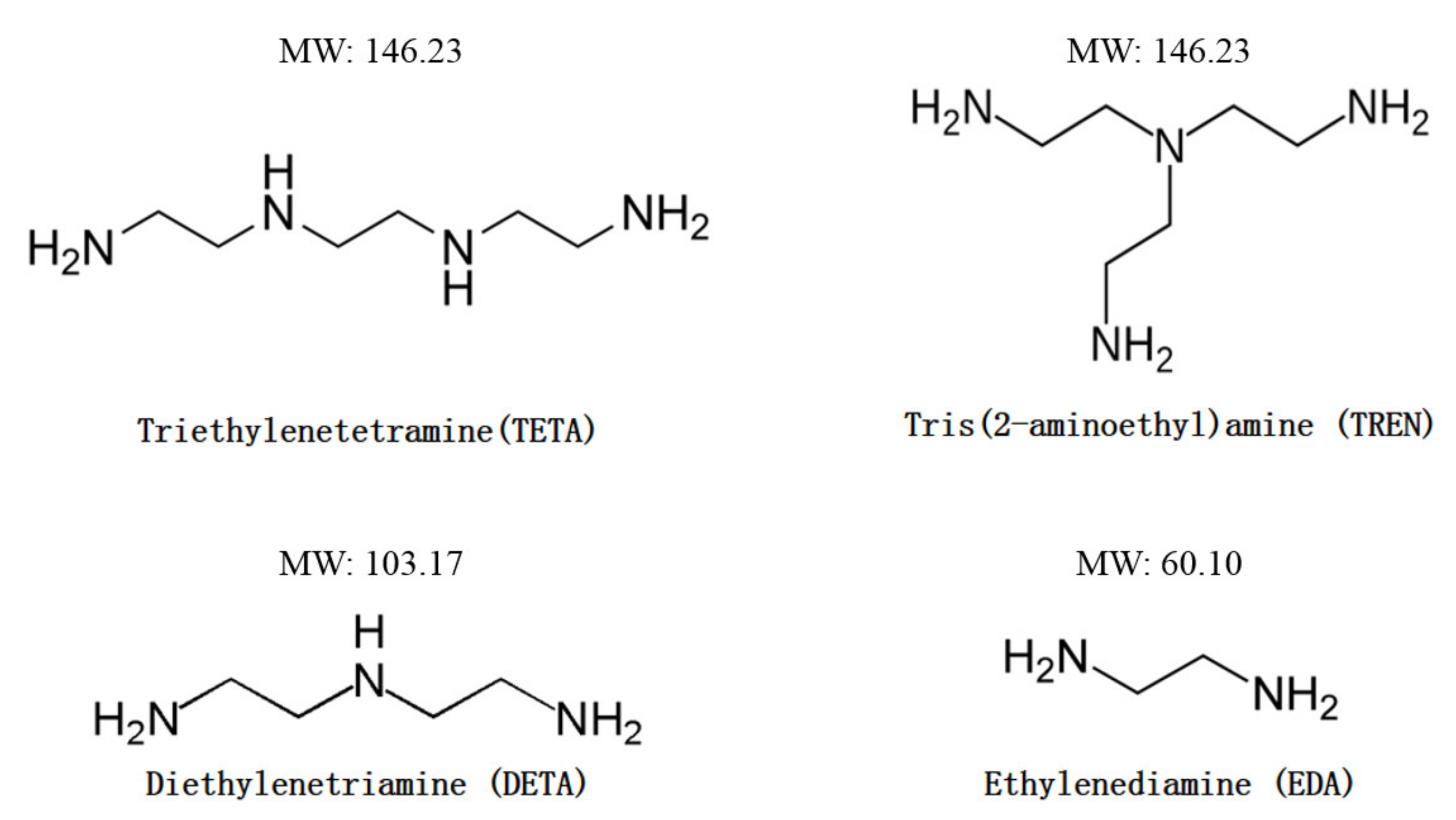




| Curing System | VE/Curing Agent Ratio |
|---|---|
| VE–TETA | 100.00/18.33 |
| VE–TREN | 100.00/18.33 |
| VE–DETA | 100.00/15.51 |
| VE–EDA | 100.00/11.30 |
| Ti (°C) | Tp1 (°C) | Tp2 (°C) | Tf (°C) | |
|---|---|---|---|---|
| VE–TETA | 64.42 | 112.71 | 167.30 | 214.60 |
| VE–TREN | 64.89 | 113.75 | 167.84 | 214.58 |
| VE–DETA | 61.81 | 112.18 | 165.20 | 214.04 |
| VE–EDA | 60.75 | 110.58 | 161.05 | 212.98 |
| T5% (°C) | T10% (°C) | T50% (°C) | Tp (°C) | Final Residue (%) at 600 °C | |
|---|---|---|---|---|---|
| VE–TETA | 233.16 | 267.05 | 330.17 | 309.13 | 16.04 |
| VE–TREN | 233.24 | 267.05 | 328.38 | 310.45 | 15.73 |
| VE–DETA | 231.59 | 262.66 | 325.80 | 305.06 | 16.95 |
| VE–EDA | 217.17 | 250.27 | 331.76 | 307.47 | 18.29 |
| E′ (MPa) | Tα1 (°C) | tan δ1 | Tα2 (°C) | tan δ2 | |
|---|---|---|---|---|---|
| VE–TETA | 3762.48 | 71.37 | 0.69 | 109.21 | 0.43 |
| VE–TREN | 3425.32 | 69.07 | 0.75 | 110.49 | 0.42 |
| VE–DETA | 3619.76 | 67.63 | 0.66 | 107.12 | 0.42 |
| VE–EDA | 3303.52 | 66.11 | 0.67 | 101.07 | 0.60 |
| Tensile Strength (MPa) | Tensile Modulus (GPa) | Strain at Break (%) | |
|---|---|---|---|
| VE–TETA | 32.94 (±3.96) | 2.47 (±0.30) | 1.33 (±0.00) |
| VE–TREN | 23.80(±3.83) | 1.51(±0.67) | 1.67(±0.47) |
| VE–DETA | 18.92(±1.89) | 2.84 (±0.28) | 0.99 (±0.47) |
| VE–EDA | 12.62 (±2.28) | 0.58 (±0.10) | 2.00 (±0.00) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Gnanasekar, P.; Nair, S.S.; Yi, S.; Yan, N. Curing Behavior and Thermomechanical Performance of Bioepoxy Resin Synthesized from Vanillyl Alcohol: Effects of the Curing Agent. Polymers 2021, 13, 2891. https://doi.org/10.3390/polym13172891
Wang Z, Gnanasekar P, Nair SS, Yi S, Yan N. Curing Behavior and Thermomechanical Performance of Bioepoxy Resin Synthesized from Vanillyl Alcohol: Effects of the Curing Agent. Polymers. 2021; 13(17):2891. https://doi.org/10.3390/polym13172891
Chicago/Turabian StyleWang, Zhenyu, Pitchaimari Gnanasekar, Sandeep Sudhakaran Nair, Songlin Yi, and Ning Yan. 2021. "Curing Behavior and Thermomechanical Performance of Bioepoxy Resin Synthesized from Vanillyl Alcohol: Effects of the Curing Agent" Polymers 13, no. 17: 2891. https://doi.org/10.3390/polym13172891
APA StyleWang, Z., Gnanasekar, P., Nair, S. S., Yi, S., & Yan, N. (2021). Curing Behavior and Thermomechanical Performance of Bioepoxy Resin Synthesized from Vanillyl Alcohol: Effects of the Curing Agent. Polymers, 13(17), 2891. https://doi.org/10.3390/polym13172891







