In Situ Grafting of Silica Nanoparticle Precursors with Covalently Attached Bioactive Agents to Form PVA-Based Materials for Sustainable Active Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
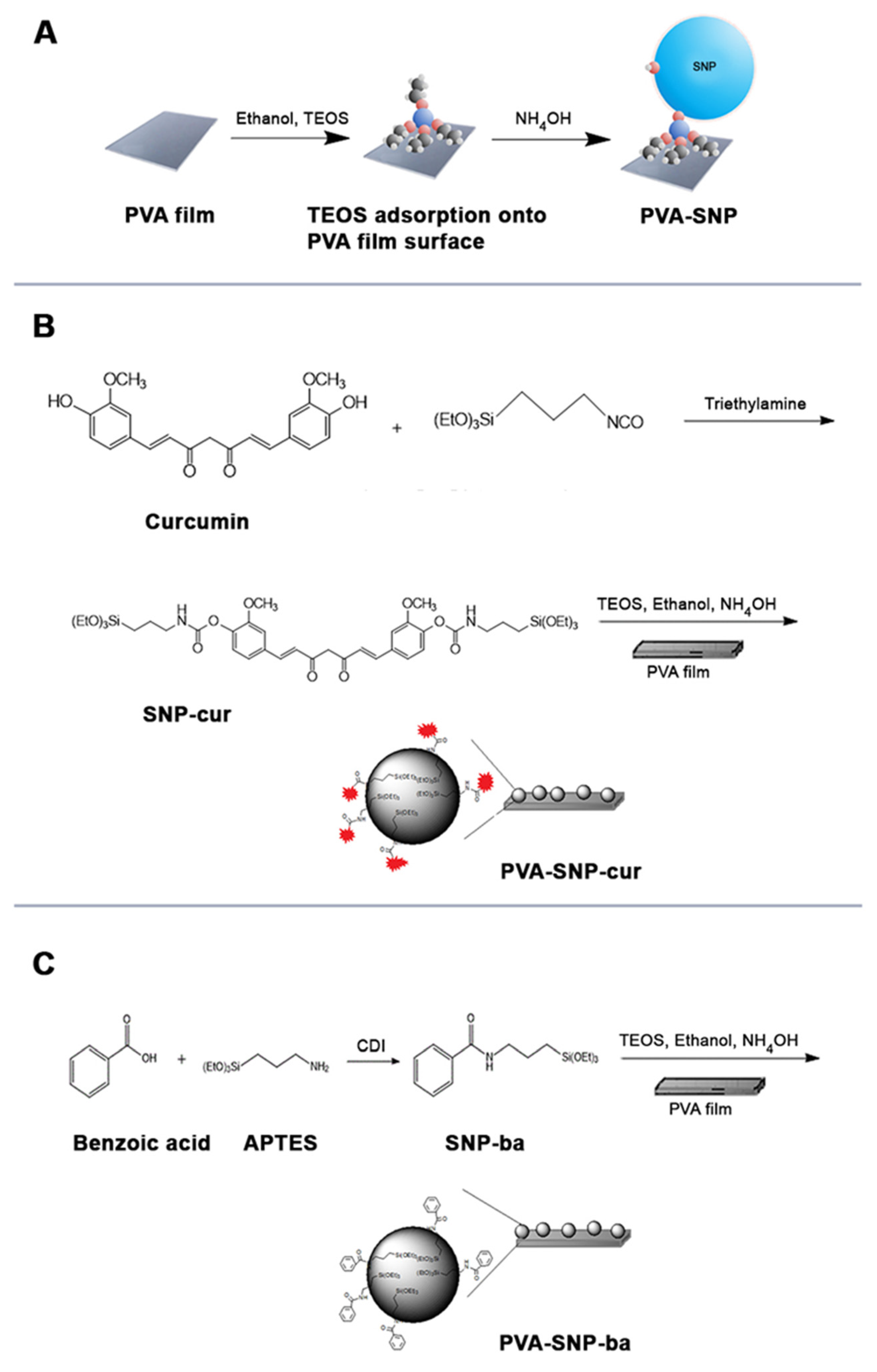
2.2. Synthesis
2.2.1. PVA-SNP-cur
2.2.2. PVA-SNP-ba
2.2.3. PVA-SNP
2.3. Characterization
2.3.1. Nuclear Magnetic Resonance (NMR) Spectroscopy
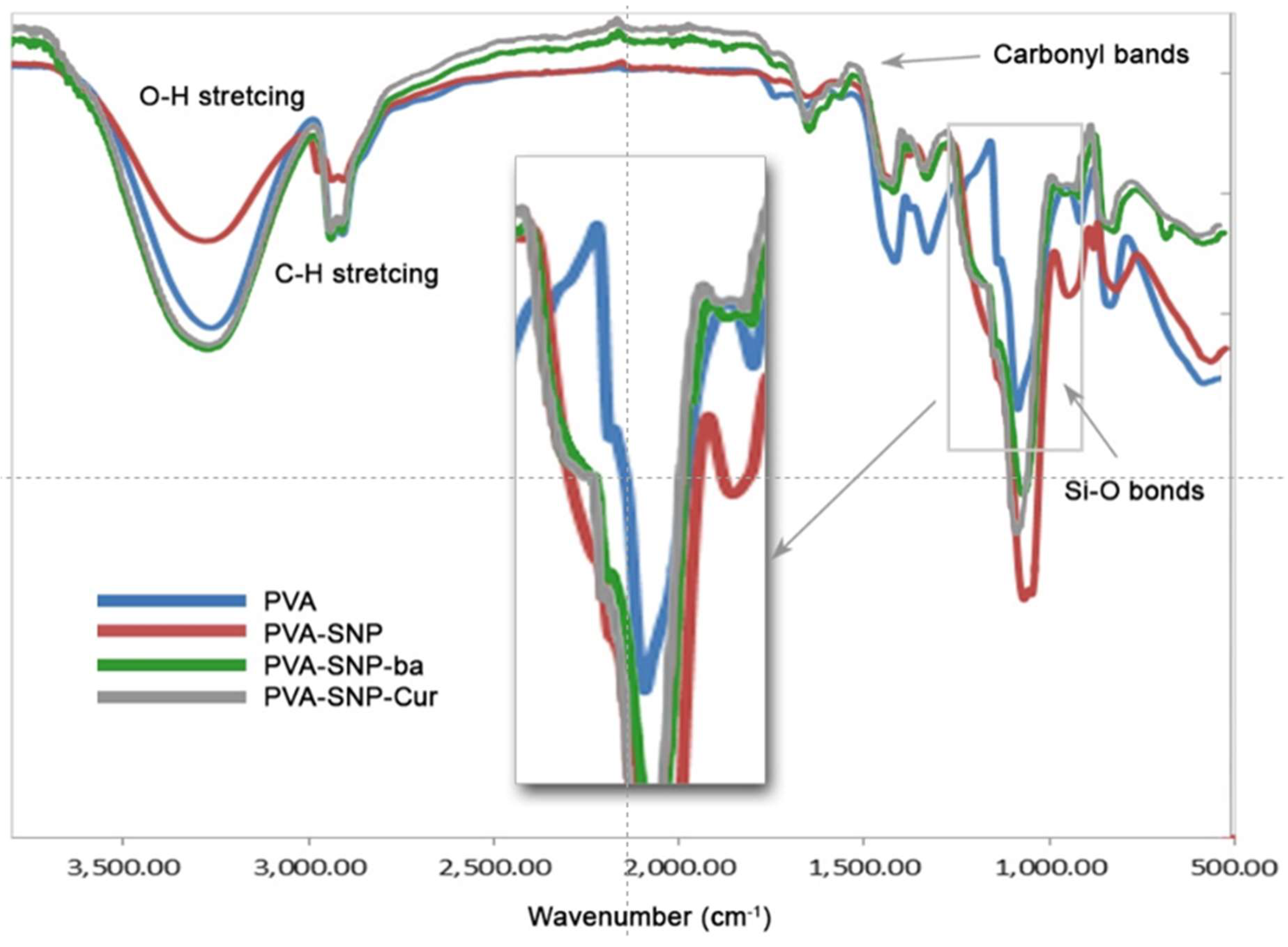
2.3.2. Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR)
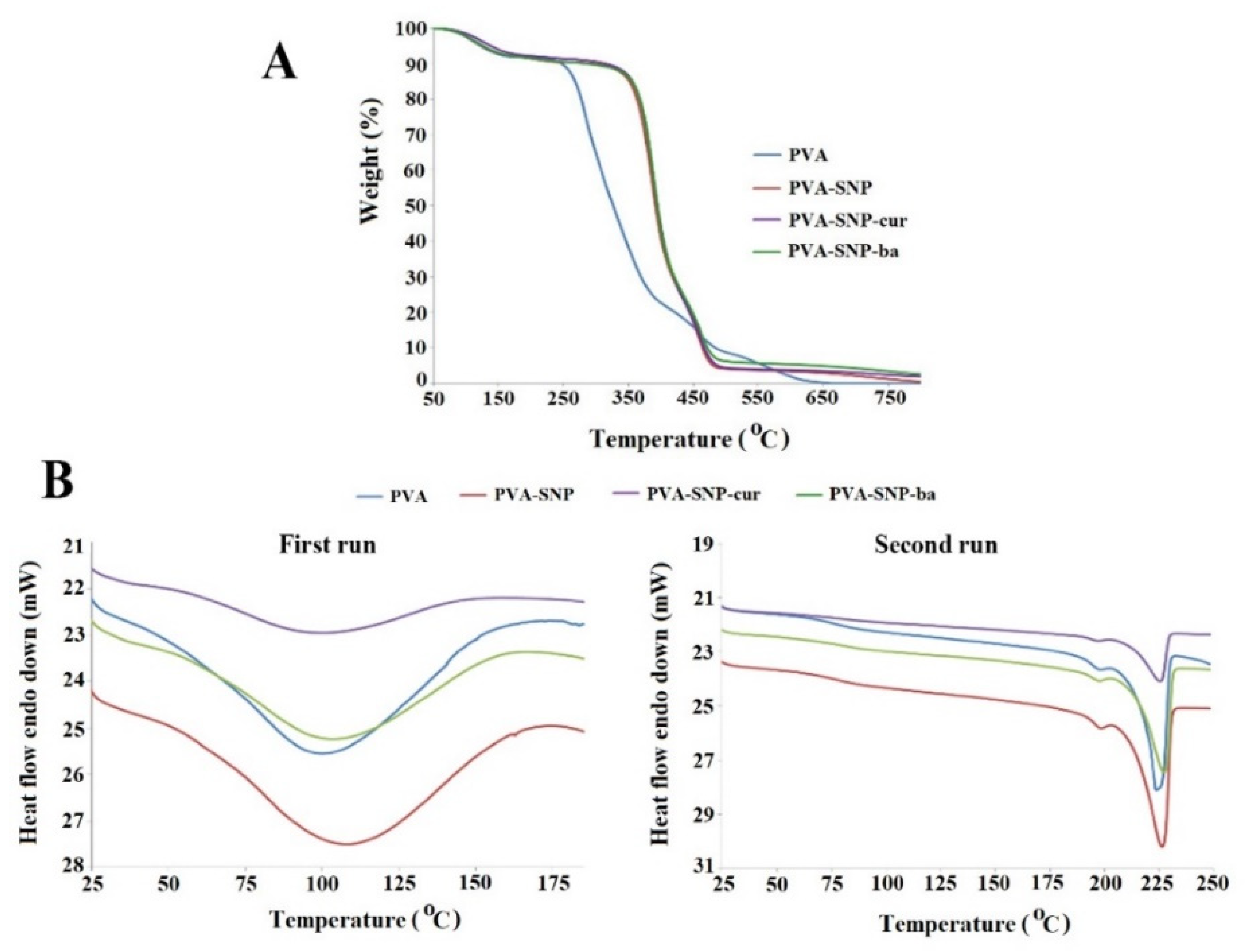
2.3.3. Thermal Analyses
2.3.4. High-Resolution Scanning Electron Microscopy (HR-SEM)
2.3.5. Contact-Angle Measurements
2.3.6. Film Thickness
2.3.7. Water Vapor Permeability (WVP)
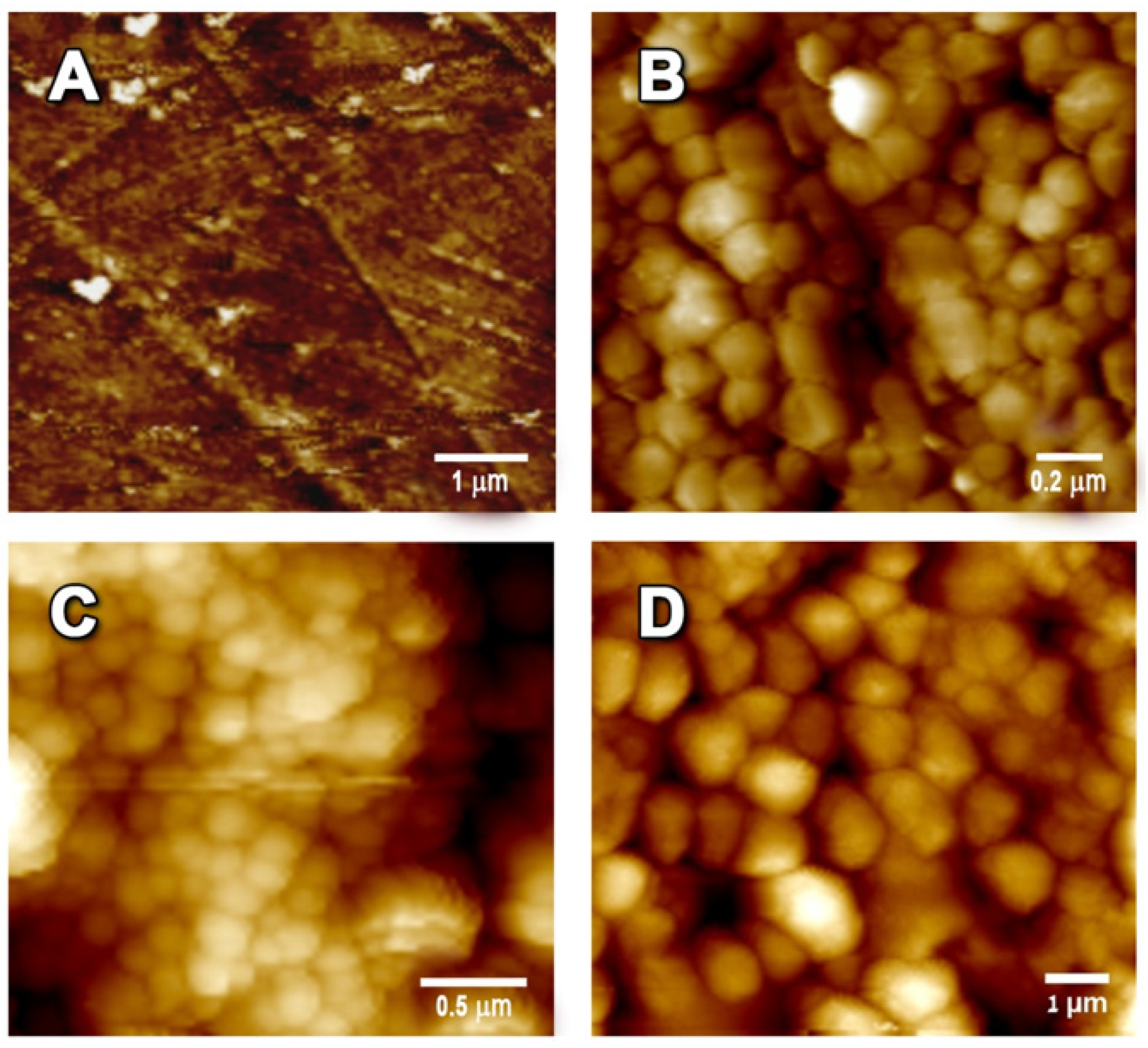
2.3.8. Atomic Force Microscopy (AFM)
2.4. Inhibition of Bacterial Growth
2.5. Antioxidant Activity
3. Results and Discussion
3.1. Synthesis, Characterization and Physicochemical Properties
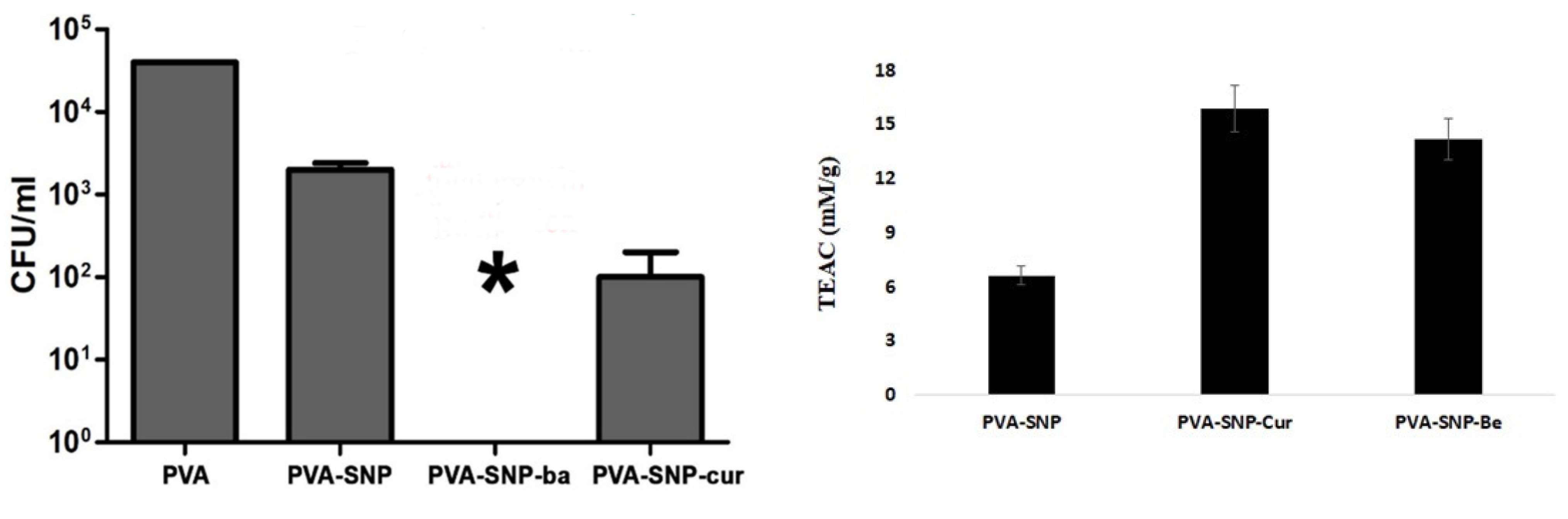
3.2. Antimicrobial and Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De la Franier, B.; Asker, D.; den Berg, D.; Hatton, B.; Thompson, M. Reduction of microbial adhesion on polyurethane by a sub-nanometer covalently-attached surface modifier. Colloids Surf. B Biointerfaces 2021, 200, 111579. [Google Scholar] [CrossRef]
- Han, J.W.; Ruiz-Garcia, L.; Qian, J.P.; Yang, X.T. Food Packaging: A Comprehensive Review and Future Trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860. [Google Scholar] [CrossRef]
- Shebis, Y.; Kumar, V.B.; Gedanken, A.; Poverenov, E. Cooperative crystallization effect in the formation of sonochemically grafted active materials based on polysaccharides. Colloids Surf. B Biointerfaces 2020, 190, 110931. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Singh, S.; Negi, Y.S. Ethylene scavengers for active packaging of fresh food produce. Environ. Chem. Lett. 2020, 18, 269. [Google Scholar] [CrossRef]
- Barden, L.; Decker, E.A. Lipid oxidation in low-moisture food: A review. Crit. Rev. Food Sci. Nutr. 2013, 56, 2467. [Google Scholar] [CrossRef]
- Xie, M.; Wang, J.; Zhao, H. A PVA film for detecting lipid oxidation intended for food application. Sens. Actuators B Chem. 2018, 273, 260. [Google Scholar] [CrossRef]
- Faas, N.; Röcker, B.; Smrke, S.; Yeretzian, C.; Yildirim, S. Prevention of lipid oxidation in linseed oil using a palladium-based oxygen scavenging film. Food Packag. Shelf Life 2020, 24, 100488. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Garrido, L.; Faba, S.; Guarda, A.; Galotto, M.J.; de Dicastillo, C.L. Cucumis metuliferus fruit extract loaded acetate cellulose coatings for antioxidant active packaging. Polymers 2020, 12, 1. [Google Scholar] [CrossRef]
- Shankar, S.; Wang, L.F.; Rhim, J.W. Effect of melanin nanoparticles on the mechanical, water vapor barrier, and antioxidant properties of gelatin-based films for food packaging application. Food Packag. Shelf Life 2019, 21, 100363. [Google Scholar] [CrossRef]
- Suppakul, P.; Miltz, J.; Sonneveld, K.; Bigger, S.W. Active Packaging Technologieswith an Emphasis on Antimicrobial Concise Reviews in Food Science. J. Food Sci. 2003, 68, 408. [Google Scholar] [CrossRef]
- Appendini, P.; Hotchkiss, J.H. Review of antimicrobial food packaging. Sci. Emerg. Technol. 2002, 3, 113. [Google Scholar] [CrossRef]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Preparation and physicochemical evaluation of chitosan/poly(vinyl alcohol)/pectin ternary film for food-packaging applications. Carbohydr. Polym. 2010, 79, 711. [Google Scholar] [CrossRef]
- Samantaray, P.K.; Little, A.; Haddleton, D.M.; McNally, T.; Tan, B.; Sun, Z.; Huang, W.; Ji, Y.; Wan, C. Poly(glycolic acid) (PGA): A versatile building block expanding high performance and sustainable bioplastic applications. Green Chem. 2020, 22, 4055. [Google Scholar] [CrossRef]
- Andrade-Del Olmo, J.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Vilas-Vilela, J.L. Antibacterial chitosan electrostatic/covalent coating onto biodegradable poly (L-lactic acid). Food Hydrocoll. 2020, 105, 105835. [Google Scholar] [CrossRef]
- Chen, H.; Li, L.; Ma, Y.; Mcdonald, T.P.; Wang, Y. Development of active packaging film containing bioactive components encapsulated in β-cyclodextrin and its application. Food Hydrocoll. 2019, 90, 360. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, X.; Wang, L.; Wu, S.; Li, D.; Liu, C.; Feng, Z.J. Fabrication and Characterization of Microemulsion Stabilized by Integrated Phosvitin and Gallic Acid. J. Agric. Food Chem. 2020, 68, 5437–5447. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Wang, L.; Zhu, M.; Wu, S.; Wang, X.; Li, D.; Liu, C.; Feng, Z.; Tian, B. Separation, structural characteristics and biological activity of lactic acid bacteria exopolysaccharides separated by aqueous two-phase system. LWT 2021, 147, 111617. [Google Scholar] [CrossRef]
- Kalayci, O.A.; Cömert, F.B.; Hazer, B.; Atalay, T.; Cavicchi, K.A.; Cakmak, M. Synthesis, characterization, and antibacterial activity of metal nanoparticles embedded into amphiphilic comb-type graft copolymers. Polym. Bull. 2010, 65, 215. [Google Scholar] [CrossRef]
- Pallavicini, P.; Dacarro, G.; Taglietti, A.S. Self-Assembled Monolayers of Silver Nanoparticles: From Intrinsic to Switchable Inorganic Antibacterial Surfaces. Eur. J. Inorg. Chem. 2018, 45, 4846. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Galus, S.; Gniewosz, M. Biopolymers-based materials containing silver nanoparticles as active packaging for food applications—A review. Int. J. Mol. Sci. 2020, 21, 698. [Google Scholar] [CrossRef]
- Goñi-Ciaurriz, L.; González-Gaitano, G.; Vélaz, I. Cyclodextrin-grafted nanoparticles as food preservative carriers. Int. J. Pharm. 2020, 588, 119664. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Azimi, F. Using Sonochemistry for the Production of Poly(Vinyl Alcohol)/MWCNT-Vitamin B1 Nanocomposites: Exploration of Morphology, Thermal and Mechanical Properties. New J. Chem. 2019, 43, 7502. [Google Scholar] [CrossRef]
- Yu, Z.; Lu, L.; Lu, L.; Pan, L. Multilayers assembly of bio-polyelectrolytes onto surface modified polypropylene films: Characterization, chelating and antioxidant activity. Carbohydr. Polym. 2020, 245, 116456. [Google Scholar] [CrossRef] [PubMed]
- Khung, Y.L.; Narducci, D. Surface modification strategies on mesoporous silica nanoparticles for anti-biofouling zwitterionic film grafting. Adv. Colloid Interface Sci. 2015, 226, 166. [Google Scholar] [CrossRef]
- Tang, H.; Liu, P.; Lu, M.; Ding, Y.; Wang, F.; Gao, C.; Zhang, S.; Yang, M. Thermal-oxidative effect of a co-condensed nanosilica-based antioxidant in polypropylene. Polymer 2017, 112, 369. [Google Scholar] [CrossRef]
- Hu, Y.; Shi, L.; Ren, Z.; Hao, G.; Chen, J.; Weng, W.J. Characterization of emulsion films prepared from soy protein isolate at different preheating temperatures. Food Eng. 2021, 309, 110697. [Google Scholar] [CrossRef]
- González, A.; Gastelú, G.; Barrera, G.N.; Ribotta, P.D.; Igarzabal, C.I.Á. Preparation and characterization of soy protein films reinforced with cellulose nanofibers obtained from soybean by-products. Food Hydrocoll. 2019, 89, 758. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, W.; Tian, B.; Li, D.; Liu, C.; Jiang, B.; Feng, Z. Preparation and Characterization of Coating Based on Protein Nanofibers and Polyphenol and Application for Salted Duck Egg Yolks. Foods 2020, 9, 449. [Google Scholar] [CrossRef]
- Quilez-Molina, A.I.; Heredia-Guerrero, J.A.; Armirotti, A.; Paul, U.C.; Athanassiou, A.; Bayer, I.S. Comparison of physicochemical, mechanical and antioxidant properties of polyvinyl alcohol films containing green tealeaves waste extracts and discarded balsamic vinegar. Food Packag. Shelf Life 2020, 23, 100445. [Google Scholar] [CrossRef]
- Haghighi, H.; Leugoue, S.K.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Fava, P.; Pulvirenti, A. Development of antimicrobial films based on chitosan-polyvinyl alcohol blend enriched with ethyl lauroyl arginate (LAE) for food packaging applications. Food Hydrocoll. 2020, 100, 105419. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, M.H.; Ko, J.A.; Kang, D.H.; Bae, H.; Park, H.J. Influence of Food with High Moisture Content on Oxygen Barrier Property of Polyvinyl Alcohol (PVA)/Vermiculite Nanocomposite Coated Multilayer Packaging Film. J. Food Sci. 2018, 83, 349. [Google Scholar] [CrossRef] [PubMed]
- Perrone, D.; Ardito, F.; Giannatempo, G.; Dioguardi, M.; Troiano, G.; Lo Russo, L.; De Lillo, A.; Laino, L.; Lo Muzio, L. Biological and therapeutic activities, and anticancer properties of curcumin. Exp Ther. Med. 2015, 10, 1615. [Google Scholar] [CrossRef]
- Sullivan, D.J.; Azlin-Hasim, S.; Cruz-Romero, M.; Cummins, E.; Kerry, J.P.; Morris, M.A. Antimicrobial effect of benzoic and sorbic acid salts and nano-solubilisates against Staphylococcus aureus, Pseudomonas fluorescens and chicken microbiota biofilms. Food Control. 2020, 107, 106786. [Google Scholar] [CrossRef]
- Vinokur, Y.; Rodov, V.; Reznick, N.; Goldman, G.; Horev, B.; Umiel, N.; Friedman, H. Rose petal tea as an antioxidant-rich beverage: Cultivar effects. J. Food Sci. 2006, 71, 1365. [Google Scholar] [CrossRef]
- Kong, Y.; Hay, J.N. The measurement of the crystallinity of polymers by DSC. Polymer 2002, 43, 3873–3878. [Google Scholar] [CrossRef]
- Kietzig, A.M.; Hatzikiriakos, S.G.; Englezos, P. Ice friction: The effects of surface roughness, structure, and hydrophobicity. J. Appl. Phys. 2009, 106, 535. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Bhushan, B. Roughness optimization for biomimetic superhydrophobic surfaces. Microsyst. Technol. 2005, 11, 535. [Google Scholar] [CrossRef]
- Gao, Q.; Zhu, Q.; Guo, Y.; Yang, C.Q. Formation of highly hydrophobic surfaces on cotton and polyester fabrics using silica sol nanoparticles and nonfluorinated alkylsilane. Ind. Eng. Chem. Res. 2009, 48, 9797. [Google Scholar] [CrossRef]
- Mastronicolis, S.K.; Berberi, A.; Diakogiannis, I.; Petrova, E.; Kiaki, I.; Baltzi, T.; Xenikakis, P. Alteration of the phospho- or neutral lipid content and fatty acid composition in Listeria monocytogenes due to acid adaptation mechanisms for hydrochloric, acetic and lactic acids at pH 5.5 or benzoic acid at neutral pH. Antonie Van Leeuwenhoek 2010, 98, 307–316. [Google Scholar] [CrossRef][Green Version]
- Vinokur, Y.; Rodov, V. Method for determining total (hydrophilic and lipophilic) radical-scavenging activity in the same sample of fresh produce. Acta Hortic. 2006, 709, 53. [Google Scholar] [CrossRef]

| Thickness (mm) | Contact Angle (degree) | WVP (×10−10 g s−1 m–1 Pa−1) | |
|---|---|---|---|
| PVA | 0.12 ± 0.02 | 31.71 ± 0.05 | 0.81 ± 0.07 |
| PVA-SNP | 0.21 ± 0.05 | 86.73 ± 1.31 | 0.75 ± 0.01 |
| PVA-SNP-cur | 0.22 ± 0.05 | 52.41 ± 0.08 | 0.46 ± 0.09 |
| PVA-SNP-ba | 0.17 ± 0.01 | 102.16 ± 0.33 | 0.24 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, M.; Molad Filossof, A.; Ashur, I.; Vernick, S.; Natan-Warhaftig, M.; Rodov, V.; Banin, E.; Poverenov, E. In Situ Grafting of Silica Nanoparticle Precursors with Covalently Attached Bioactive Agents to Form PVA-Based Materials for Sustainable Active Packaging. Polymers 2021, 13, 2889. https://doi.org/10.3390/polym13172889
Klein M, Molad Filossof A, Ashur I, Vernick S, Natan-Warhaftig M, Rodov V, Banin E, Poverenov E. In Situ Grafting of Silica Nanoparticle Precursors with Covalently Attached Bioactive Agents to Form PVA-Based Materials for Sustainable Active Packaging. Polymers. 2021; 13(17):2889. https://doi.org/10.3390/polym13172889
Chicago/Turabian StyleKlein, Miri, Anat Molad Filossof, Idan Ashur, Sefi Vernick, Michal Natan-Warhaftig, Victor Rodov, Ehud Banin, and Elena Poverenov. 2021. "In Situ Grafting of Silica Nanoparticle Precursors with Covalently Attached Bioactive Agents to Form PVA-Based Materials for Sustainable Active Packaging" Polymers 13, no. 17: 2889. https://doi.org/10.3390/polym13172889
APA StyleKlein, M., Molad Filossof, A., Ashur, I., Vernick, S., Natan-Warhaftig, M., Rodov, V., Banin, E., & Poverenov, E. (2021). In Situ Grafting of Silica Nanoparticle Precursors with Covalently Attached Bioactive Agents to Form PVA-Based Materials for Sustainable Active Packaging. Polymers, 13(17), 2889. https://doi.org/10.3390/polym13172889










