Water-Preserving and Salt-Resistant Slow-Release Fertilizers of Polyacrylic Acid-Potassium Humate Coated Ammonium Dihydrogen Phosphate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Extraction of HA from Lignite by H2O2 Oxygenation
2.2.2. Preparation of PAA-KHA
2.2.3. Preparation of ADP@PAA-KHA
2.3. Characterization
2.3.1. Determination the Properties of HA
2.3.2. Determination of Water Absorbency of PAA-KHA in Deionized Water and Salty Water
2.3.3. Determination of Slow-Release Performance of the ADP@PAA-KHA Fertilizer
3. Results and Discussion
3.1. Extraction of HA from Lignite
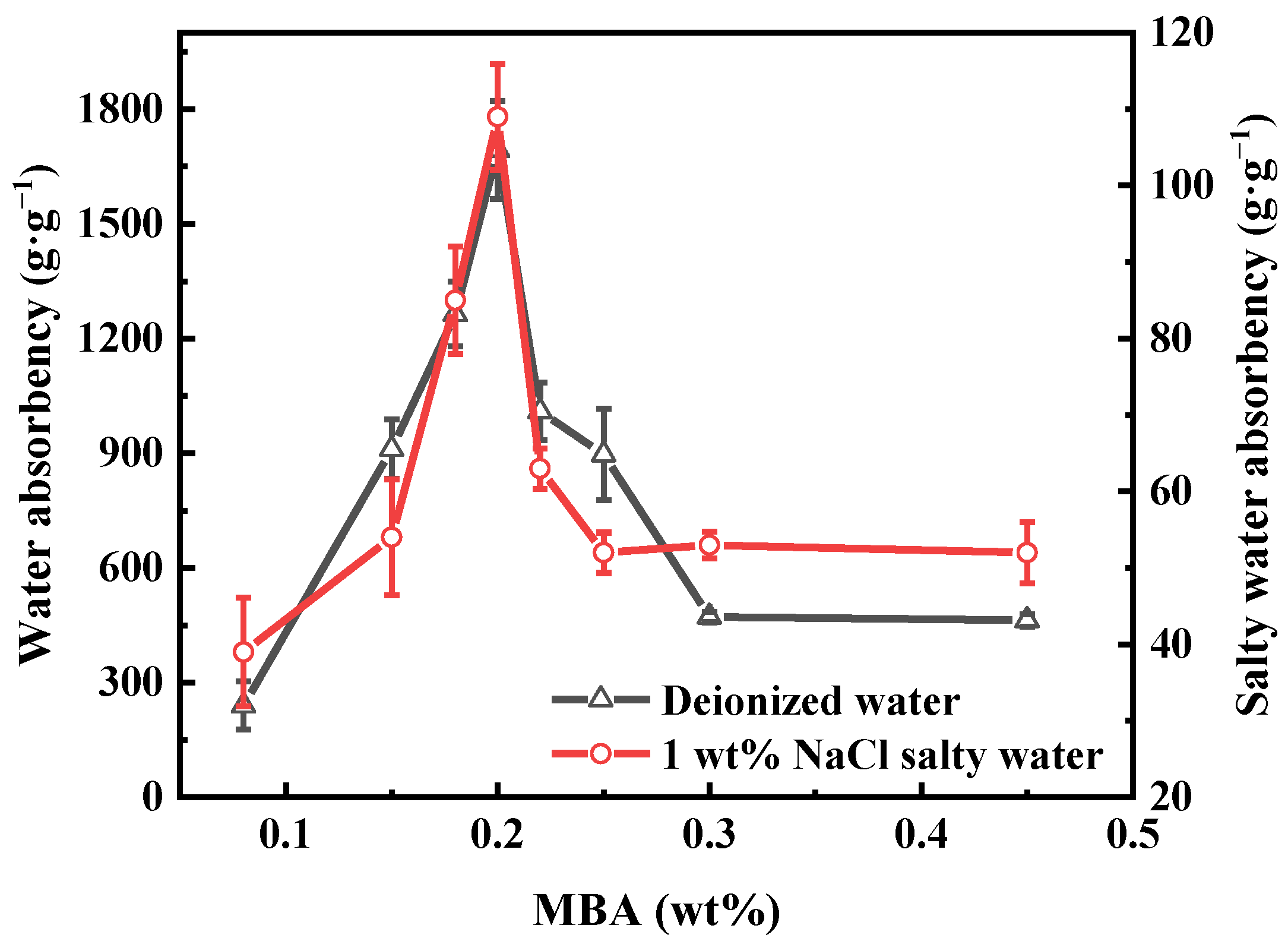
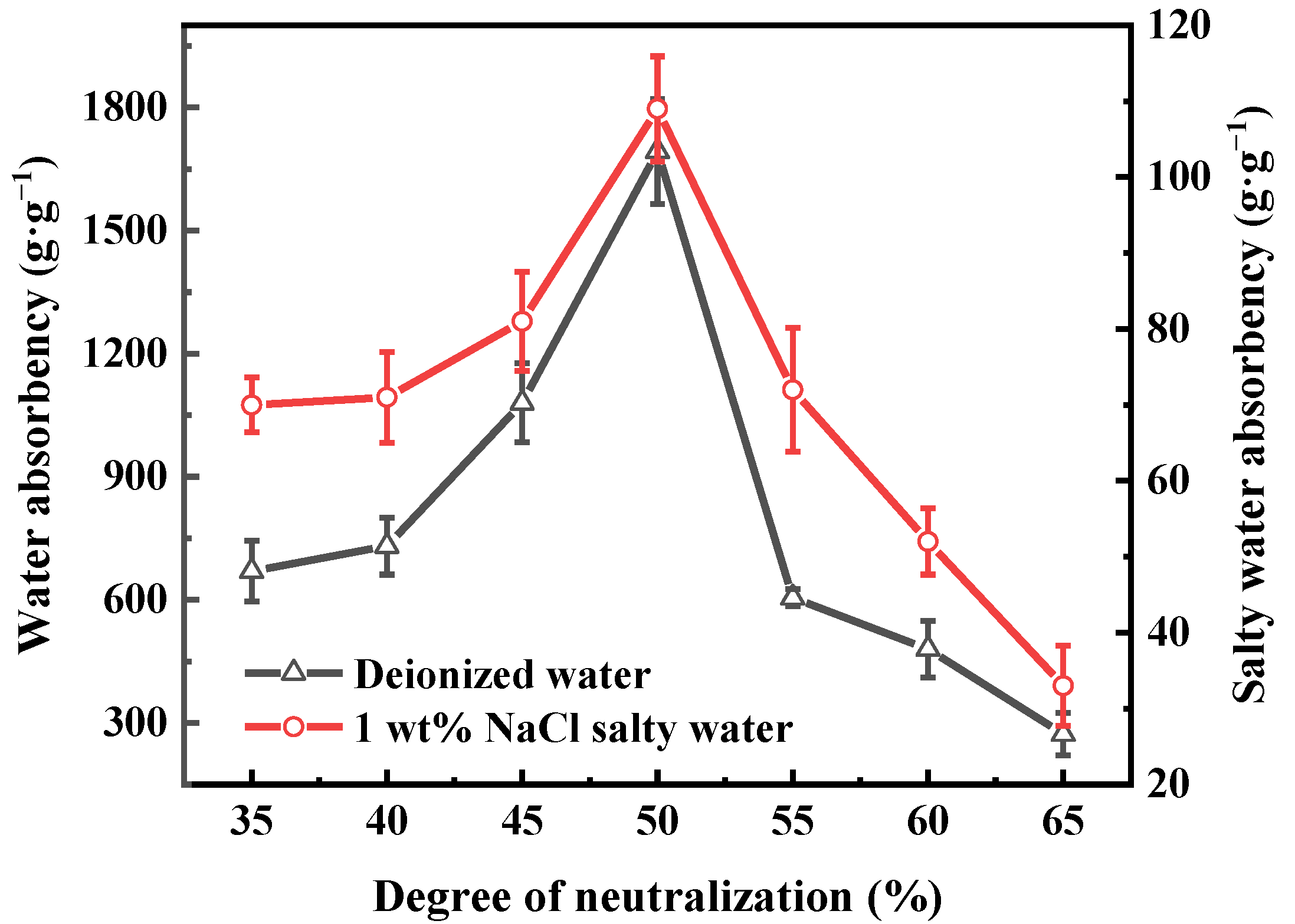
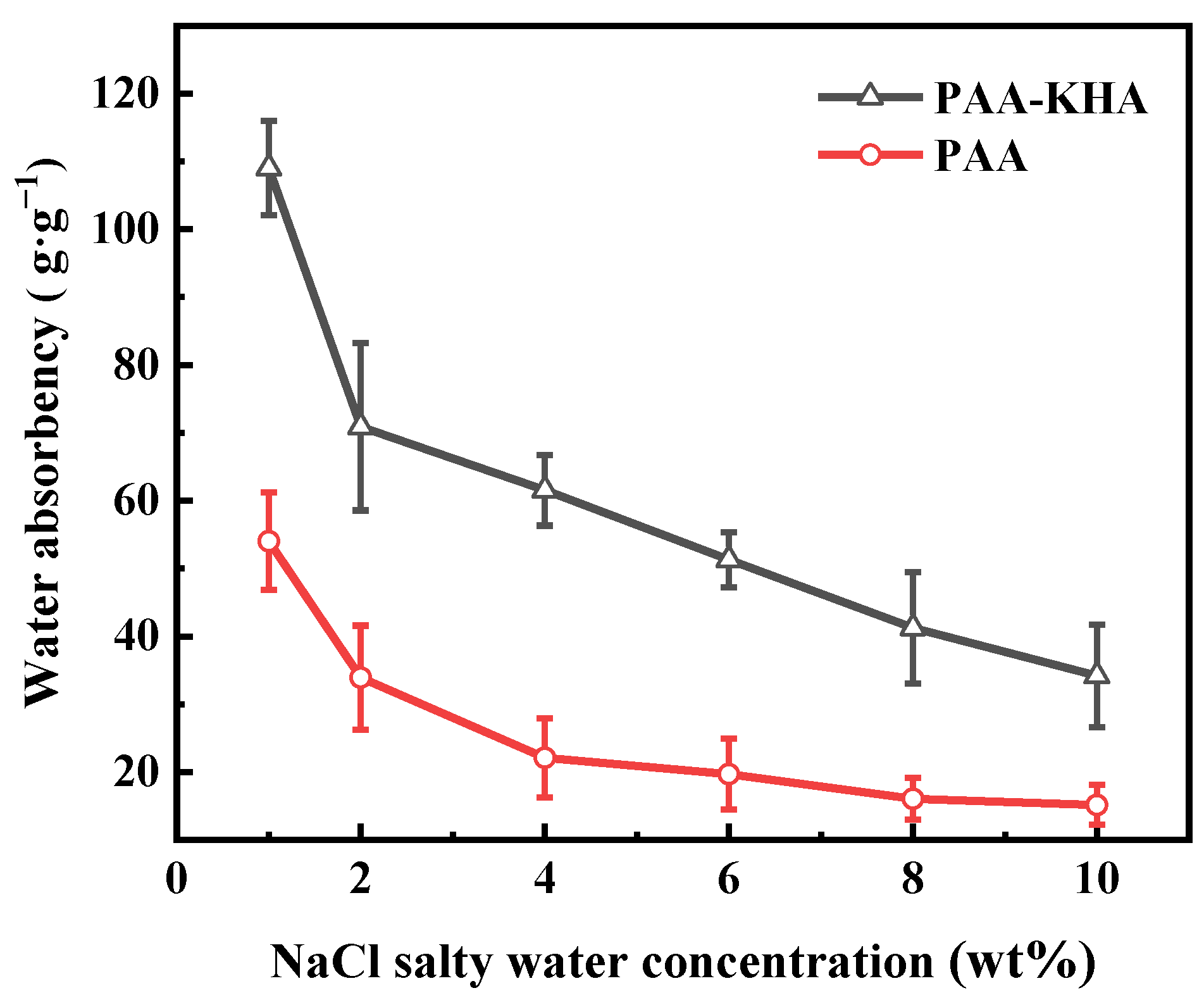
3.2. Effect of Synthesis Conditions on the Water Absorbency of PAA-KHA
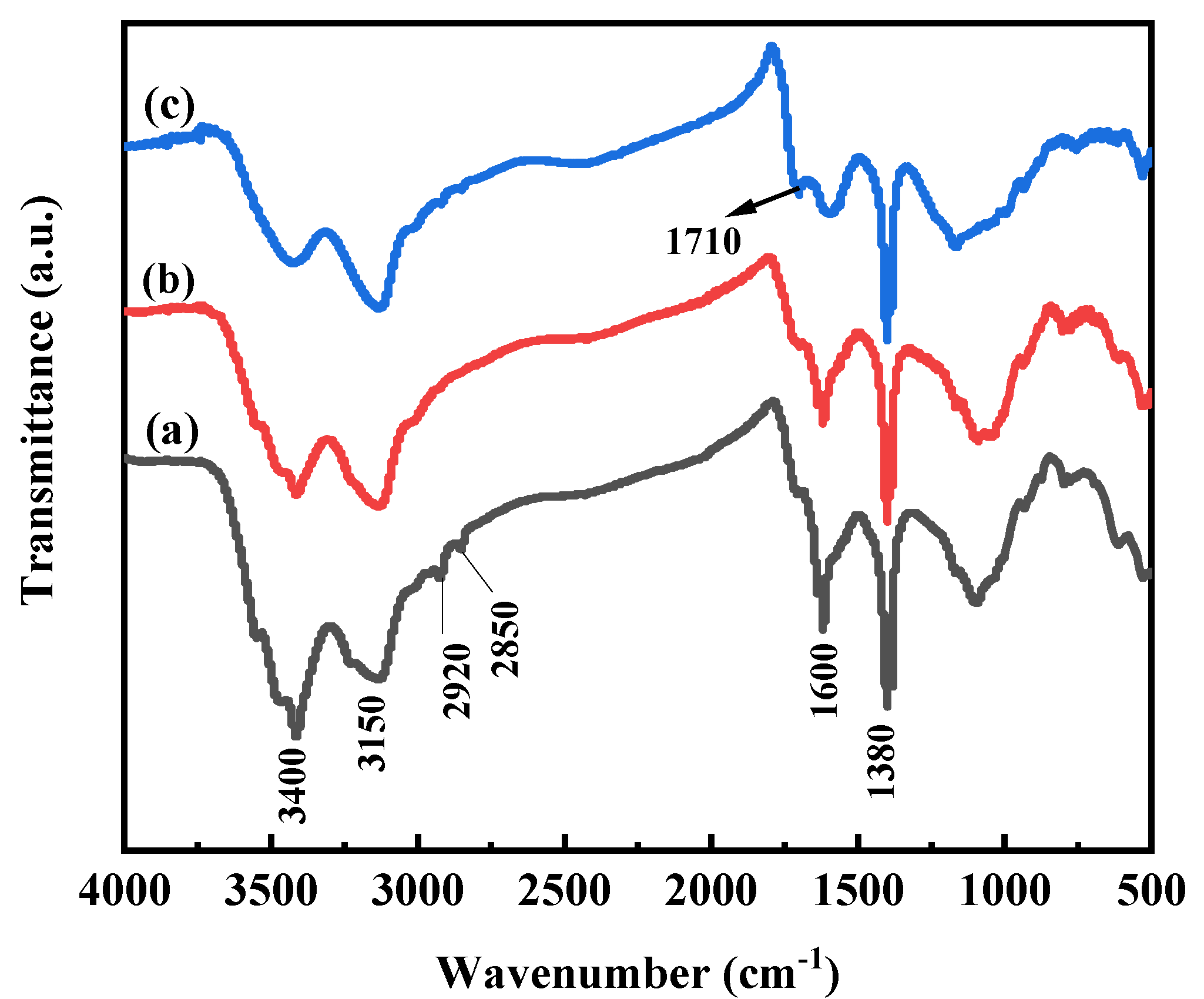
3.3. Properties of PAA-KHA
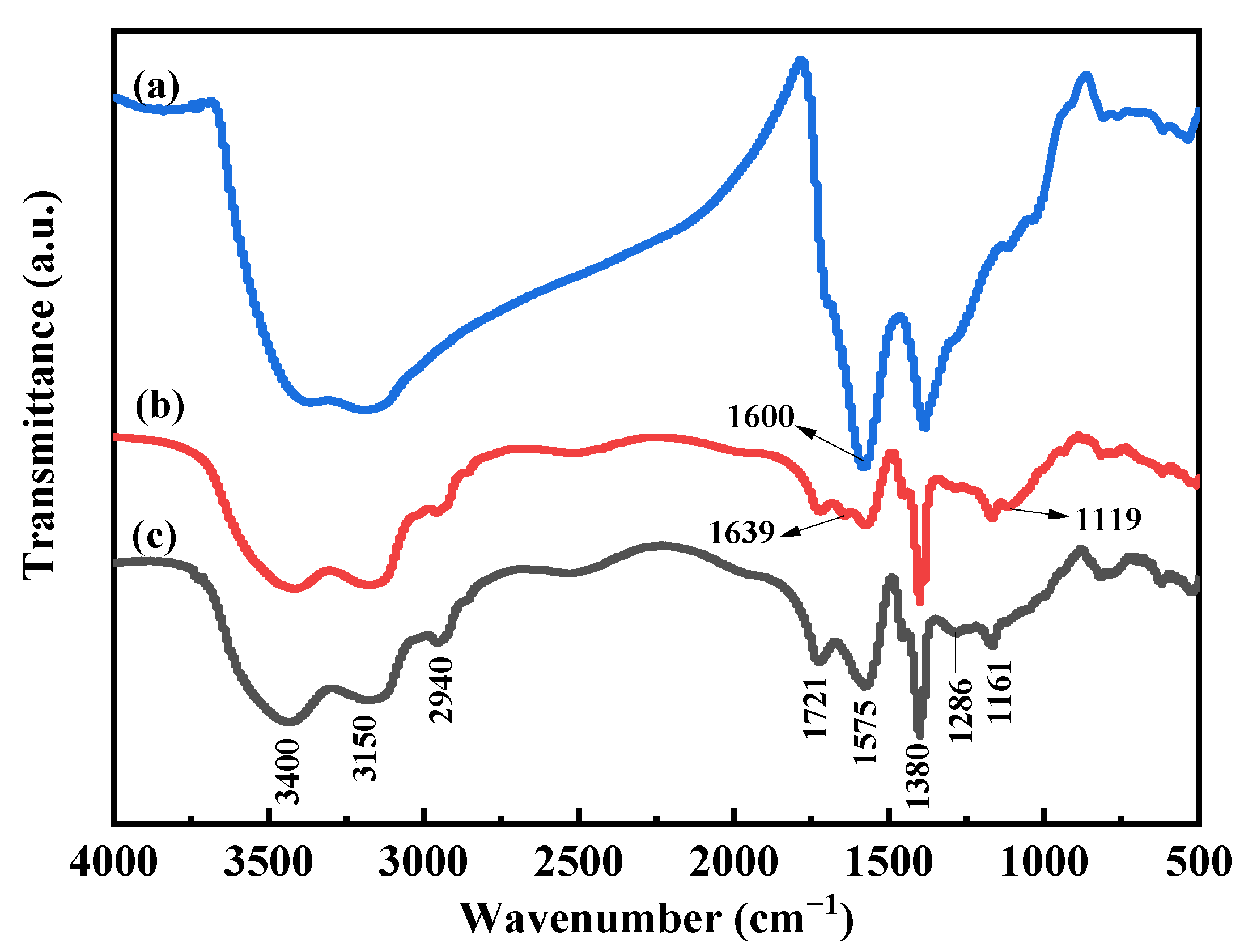
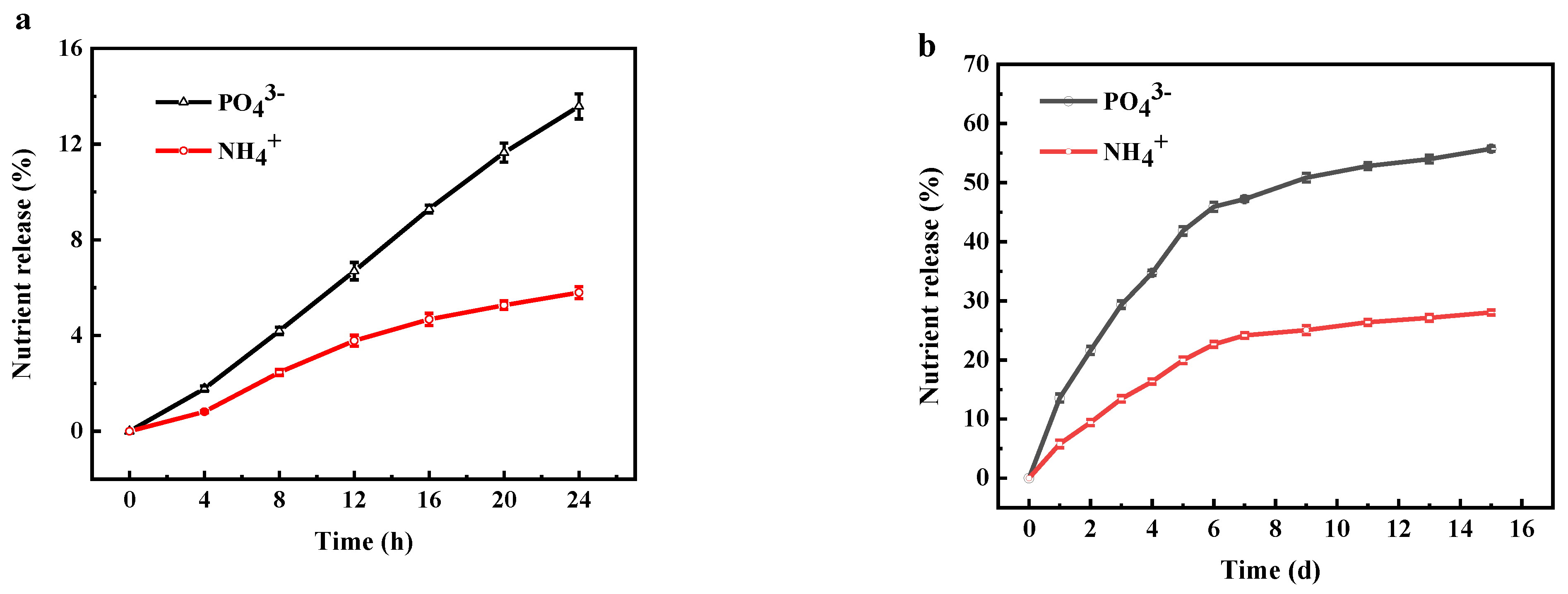
3.4. Properties of ADP@PAA-KHA Fertilizer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| PAA | Polyacrylic acid |
| HA | Humic acid |
| KHA | Potassium humate |
| PAA-KHA | Polyacrylic acid-potassium humate |
| ADP | Ammonium dihydrogen phosphate |
| ADP@PAA-KHA | Polyacrylic acid-potassium humate coated ammonium dihydrogen phosphate fertilizer |
| AA | Acrylic acid |
| APS | Ammonium persulfate |
| MBA | N, N′-methylene amide |
| w | coated rate (%) |
| m1 | the weight of fertilizer cores (g) |
| m2 | the weight of ADP@PAA-KHA (g) |
| H | water absorbency (g·g−1) |
| M1 | the mass of PAA-KHA (g) |
| M2 | the mass of PAA-KHA after fully swelling in deionized or salty water (g) |
| W | the cumulative release rate of each nutrient (%) |
| VE | the volume of sample solution (L) |
| V0 | the volume of solution in the container (L) |
| Ci | the concentration of nutrient in the sample solution (mg·L−1) |
| Cn | the concentration of the solution in the container (mg·L−1) |
| n and i | the number of samples |
| mx | the nutrient mass in the sample (mg) |
References
- Azeem, B.; KuShaari, K.; Man, Z.B.; Basit, A.; Thanh, T.H. Review on materials & methods to produce controlled release coated urea fertilizer. J. Control. Release 2014, 181, 11–21. [Google Scholar]
- Xu, Y.; Wang, J.; Gu, T.; Kong, J.; Moayedi, H. Geological Hazards in Loess Induced by Agricultural Irrigation in Arid and Semiarid Regions of China. Adv. Civil. Eng. 2020, 2020, 1–11. [Google Scholar]
- Fu, J.; Wang, C.; Chen, X.; Huang, Z.; Chen, D. Classification research and types of slow controlled release fertilizers (SRFs) used—A review. Commun. Soil Sci. Plant Anal. 2018, 49, 2219–2230. [Google Scholar] [CrossRef]
- Li, T.; Lü, S.; Zhang, S.; Gao, C.; Liu, M. Lignin-based multifunctional fertilizer for immobilization of Pb (II) in contaminated soil. J. Taiwan Inst. Chem. Eng. 2018, 91, 643–652. [Google Scholar] [CrossRef]
- Beerthuis, R.; Rothenberg, G.; Shiju, N.R. Catalytic routes towards acrylic acid, adipic acid and ε-caprolactam starting from biorenewables. Green Chem. 2015, 17, 1341–1361. [Google Scholar] [CrossRef] [Green Version]
- Du, W.; Slany, M.; Wang, X.; Chen, G.; Zhang, J. The Inhibition Property and Mechanism of a Novel Low Molecular Weight Zwitterionic Copolymer for Improving Wellbore Stability. Polymers 2020, 12, 708. [Google Scholar] [CrossRef] [Green Version]
- Zohuriaan-Mehr, M.J.; Kabiri, K. Superabsorbent Polymer Materials: A Review. Iran. Polym. J. 2008, 17, 451–477. [Google Scholar]
- Chang, L.; Xu, L.; Liu, Y.; Qiu, D. Superabsorbent polymers used for agricultural water retention. Polym. Test. 2021, 94, 107021. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, R.; Li, A.; Wang, A. Preparation, Swelling Behaviors, and Slow-Release Properties of a Poly(acrylic acid-co-acrylamide)/Sodium Humate Superabsorbent Composite. Ind. Eng. Chem. Res. 2006, 45, 48–53. [Google Scholar] [CrossRef]
- Zaini, M.A.; Amano, Y.; Machida, M. Adsorption of heavy metals onto activated carbons derived from polyacrylonitrile fiber. J. Hazard. Mater. 2010, 180, 552–560. [Google Scholar] [CrossRef]
- Chai, W.; Huang, Y.; Han, G.; Liu, J.; Yang, S.; Cao, Y. An Enhanced Study on Adsorption of Al(iii) onto Bentonite and Kaolin: Kinetics, Isotherms, and Mechanisms. J. Min. Proc. Ext. Met. Rev. 2016, 38, 106–115. [Google Scholar] [CrossRef]
- Karlsson, R.P.; Larsson, P.T.; Hansson, P.; Wagberg, L. Thermodynamics of the Water-Retaining Properties of Cellulose-Based Networks. Biomacromolecules 2019, 20, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Xiang, Y.; Xu, Y.; Wei, J.; Zhang, Z.; Li, L.; Li, J. Poly-acrylic acid grafted natural rubber for multi-coated slow release compound fertilizer: Preparation, properties and slow-release characteristics. Int. J. Biol. Macromol. 2020, 146, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, M.; Liu, Z.; Guo, Y.; Zhang, Q. Salt-Tolerant Superabsorbent Polymer with High Capacity of Water-Nutrient Retention Derived from Sulfamic Acid-Modified Starch. ACS Omega 2019, 4, 5923–5930. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Liu, M.; Zhan, F.; Wu, L. Preparation and properties of a slow-release membrane-encapsulated urea fertilizer with superabsorbent and moisture preservation. Ind. Eng. Chem. Res. 2005, 44, 4206–4211. [Google Scholar] [CrossRef]
- Fang, S.; Wang, G.; Xing, R.; Chen, X.; Liu, S.; Qin, Y.; Li, K.; Wang, X.; Li, R.; Li, P. Synthesis of superabsorbent polymers based on chitosan derivative graft acrylic acid-co-acrylamide and its property testing. Int. J. Biol. Macromol. 2019, 132, 575–584. [Google Scholar] [CrossRef]
- Chen, J.; Lu, S.; Zhang, Z.; Zhao, X.; Li, X.; Ning, P.; Liu, M. Environmentally friendly fertilizers: A review of materials used and their effects on the environment. Sci. Total Environ. 2018, 613–614, 829–839. [Google Scholar] [CrossRef]
- Meng, Y.; Ye, L. Synthesis and swelling property of superabsorbent starch grafted with acrylic acid/2-acrylamido-2-methyl-1-propanesulfonic acid. J. Sci. Food Agric. 2017, 97, 3831–3840. [Google Scholar] [CrossRef]
- Olad, A.; Doustdar, F.; Gharekhani, H. Starch-based semi-IPN hydrogel nanocomposite integrated with clinoptilolite: Preparation and swelling kinetic study. Carbohydr. Polym. 2018, 200, 516–528. [Google Scholar] [CrossRef]
- Motamedi, E.; Motesharezedeh, B.; Shirinfekr, A.; Samar, S.M. Synthesis and swelling behavior of environmentally friendly starch-based superabsorbent hydrogels reinforced with natural char nano/micro particles. J. Environ. Chem. Eng. 2020, 8, 103583. [Google Scholar] [CrossRef]
- Xu, S.; Yin, Y.; Wang, Y.; Li, X.; Hu, Z.; Wang, R. Amphoteric superabsorbent polymer based on waste collagen as loading media and safer release systems for herbicide 2, 4-D. J. Appl. Polym. Sci. 2019, 137, 48480. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, L.; Li, W.; Lin, Z.; Li, Y.; Hu, S.; Zhao, B. Effects of urea enhanced with different weathered coal-derived humic acid components on maize yield and fate of fertilizer nitrogen. J. Integr. Agric. 2019, 18, 656–666. [Google Scholar] [CrossRef]
- Li, Y. Research Progress of Humic Acid Fertilizer on the Soil. J. Phys. Conf. Ser. 2020, 1549, 022004. [Google Scholar] [CrossRef]
- Radwan, E.K.; Ibrahim, M.B.M.; Moursy, A.S.; Ghafar, H.H.A. Characterization of Humic Acids Extracted From Egyptian Sediment by Elemental Composition, NMR and FTIR. J. Environ. Sci. Technol. 2019, 12, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.-L.; Huang, H.-H.; Wei, Y.J. Reduction of toxic Cr(VI)-humic acid in an ionic liquid. Spectrochim. Acta Part B At. Spectrosc. 2017, 133, 9–13. [Google Scholar] [CrossRef]
- Yong, S.L.; Bartlett, R.J. Stimulation of Plant Growth by Humic Substances. Soil Sci. Soc. Am. J. 1976, 40, 876–879. [Google Scholar]
- Shen, Y.; Jiao, S.; Ma, Z.; Lin, H.; Gao, W.; Chen, J. Humic acid-modified bentonite composite material enhances urea-nitrogen use efficiency. Chemosphere 2020, 255, 126976. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X. Preparation and Properties of Chitosan-g-Poly(acrylic acid)/humic Acid and Water-Retaining Slow-Release Fertilizer. Master’s Thesis, Guizhou University, Guizhou, China, 2016. [Google Scholar]
- Zhou, L.; Slany, M.; Bai, B.; Du, W.; Qu, C.; Zhang, J.; Tang, Y. Enhanced Removal of Sulfonated Lignite from Oil Wastewater with Multidimensional MgAl-LDH Nanoparticles. Nanomaterials 2021, 11, 861. [Google Scholar] [CrossRef]
- Cui, X.; Yang, M.; Zhang, X.; Gao, H.; Wang, G. Introduction to the Analysis Methods of Oxygen Functional Groups in Coal. J. Chem. 2012, 75, 808–814. [Google Scholar]
- Wang, W.; Yang, Z.; Zhang, A.; Yang, S. Water retention and fertilizer slow release integrated superabsorbent synthesized from millet straw and applied in agriculture. Ind. Crop. Prod. 2021, 160, 113126. [Google Scholar] [CrossRef]
- Slaný, M.; Jankovič, Ľ.; Madejová, J. Structural characterization of organo-montmorillonites prepared from a series of primary alkylamines salts: Mid-IR and near-IR study. Appl. Clay Sci. 2019, 176, 11–20. [Google Scholar] [CrossRef]
- Wu, J.; Wei, Y.; Lin, J.; Lin, S. Study on starch-graft-acrylamide/mineral powder superabsorbent composite. Polymer 2003, 44, 6513–6520. [Google Scholar] [CrossRef]
- Wu, M.; Song, M.; Liu, M.; Jiang, C.; Li, Z. Fungicidal activities of soil humic/fulvic acids as related to their chemical structures in greenhouse vegetable fields with cultivation chronosequence. J. Sci. Rep. 2016, 6, 32858. [Google Scholar] [CrossRef]
- Wang, W.; Yang, S.; Zhang, A.; Yang, Z. Synthesis of a slow-release fertilizer composite derived from waste straw that improves water retention and agricultural yield. Sci. Total Environ. 2021, 768, 144978. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.S.; Rempel, G.L. Preparation of superabsorbent polymers by crosslinking acrylic acid and acrylamide copolymers. J. Appl. Polym. Sci. 2015, 64, 1345–1353. [Google Scholar] [CrossRef]







| Content | Lignite | Oxygenated Lignite |
|---|---|---|
| Total acid groups (mmol·g−1) | 4.63 | 10.63 |
| Carboxyl (mmol·g−1) | 2.35 | 3.20 |
| Phenolic hydroxyl (mmol·g−1) | 2.28 | 7.60 |
| HA ad (%) | 18.78 | 35.13 |
| Regions | Element Mass Percent (wt%) | Atom Percent (at%) | ||||
|---|---|---|---|---|---|---|
| C | O | K | C | O | K | |
| The first layer | 31.91 | 40.73 | 27.35 | 45.01 | 43.13 | 11.85 |
| The second layer | 39.57 | 39.60 | 20.83 | 52.28 | 39.27 | 8.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Yang, L.; Cao, J.; Nie, C.; Liu, H.; Tian, J.; Chen, W.; Geng, P.; Xie, G. Water-Preserving and Salt-Resistant Slow-Release Fertilizers of Polyacrylic Acid-Potassium Humate Coated Ammonium Dihydrogen Phosphate. Polymers 2021, 13, 2844. https://doi.org/10.3390/polym13172844
Li H, Yang L, Cao J, Nie C, Liu H, Tian J, Chen W, Geng P, Xie G. Water-Preserving and Salt-Resistant Slow-Release Fertilizers of Polyacrylic Acid-Potassium Humate Coated Ammonium Dihydrogen Phosphate. Polymers. 2021; 13(17):2844. https://doi.org/10.3390/polym13172844
Chicago/Turabian StyleLi, Hongping, Lanwen Yang, Jianxin Cao, Chenchen Nie, Hao Liu, Juan Tian, Wenxing Chen, Pinglan Geng, and Guiming Xie. 2021. "Water-Preserving and Salt-Resistant Slow-Release Fertilizers of Polyacrylic Acid-Potassium Humate Coated Ammonium Dihydrogen Phosphate" Polymers 13, no. 17: 2844. https://doi.org/10.3390/polym13172844
APA StyleLi, H., Yang, L., Cao, J., Nie, C., Liu, H., Tian, J., Chen, W., Geng, P., & Xie, G. (2021). Water-Preserving and Salt-Resistant Slow-Release Fertilizers of Polyacrylic Acid-Potassium Humate Coated Ammonium Dihydrogen Phosphate. Polymers, 13(17), 2844. https://doi.org/10.3390/polym13172844






