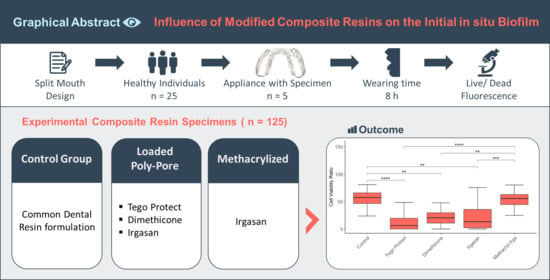
The Influence of Modified Experimental Dental Resin Composites on the Initial In Situ Biofilm—A Triple-Blinded, Randomized, Controlled Split-Mouth Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Its Modifications
2.2. Participants
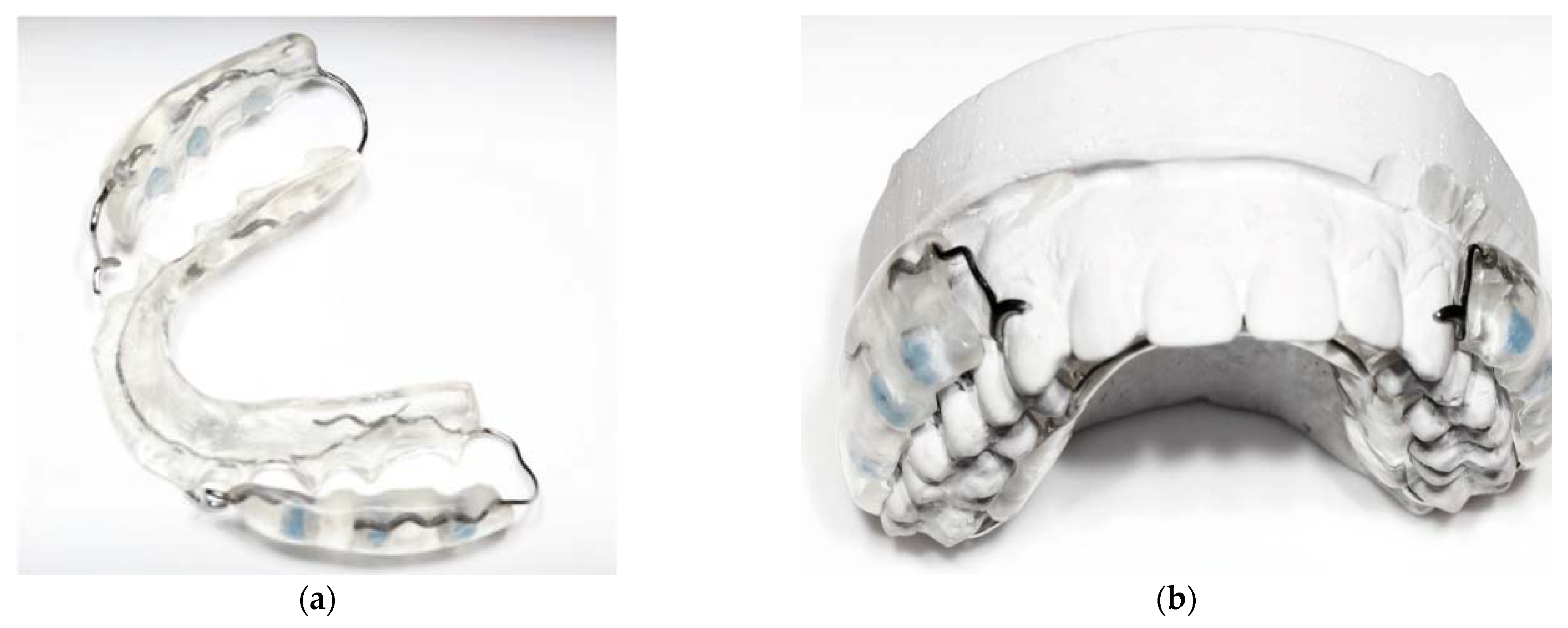
2.3. Intervention
2.4. Trial Design
2.5. Specimen Preparation
2.6. Cell Viability Determination
2.7. Statistical Analysis
3. Results
3.1. Participants
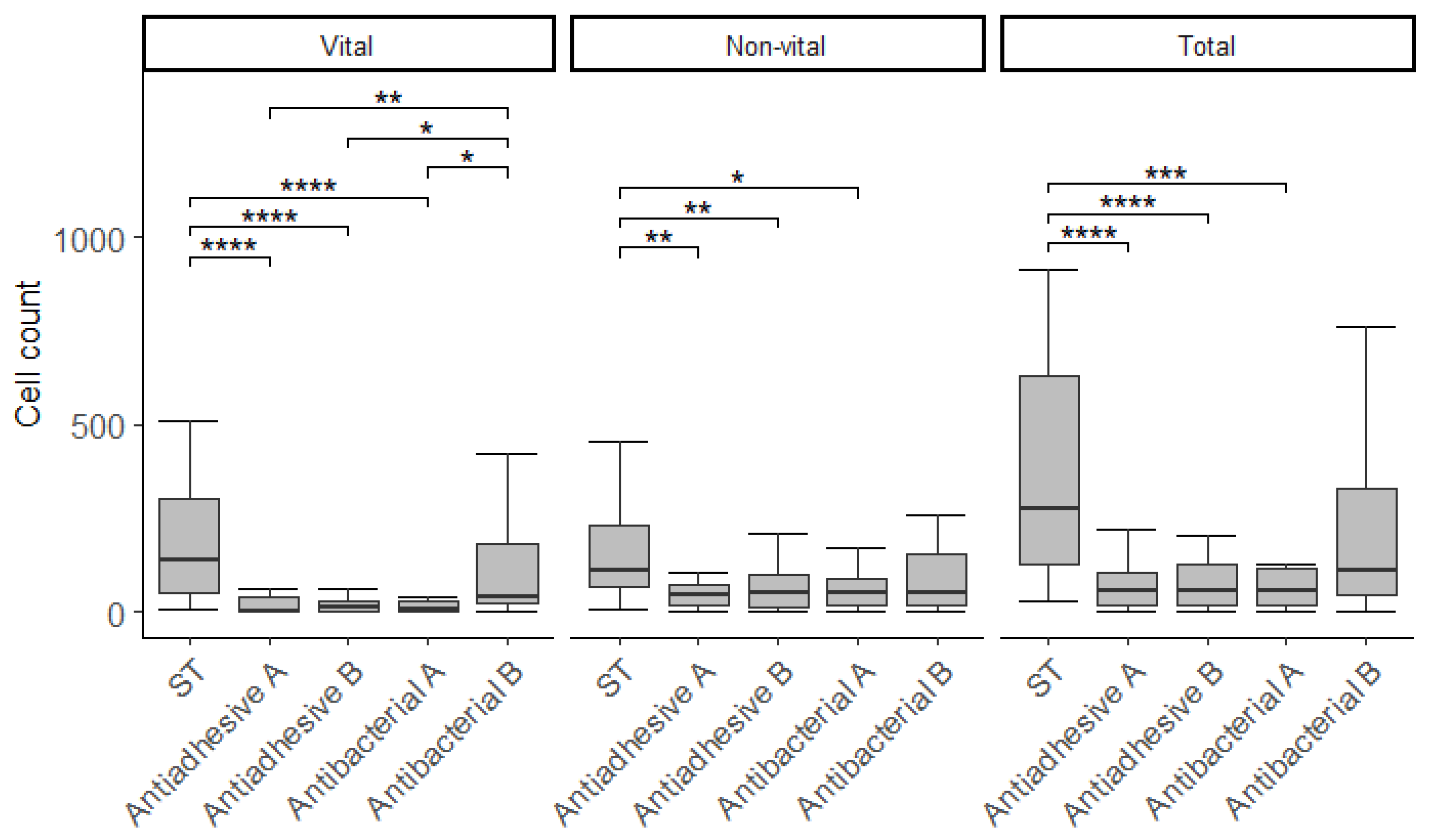
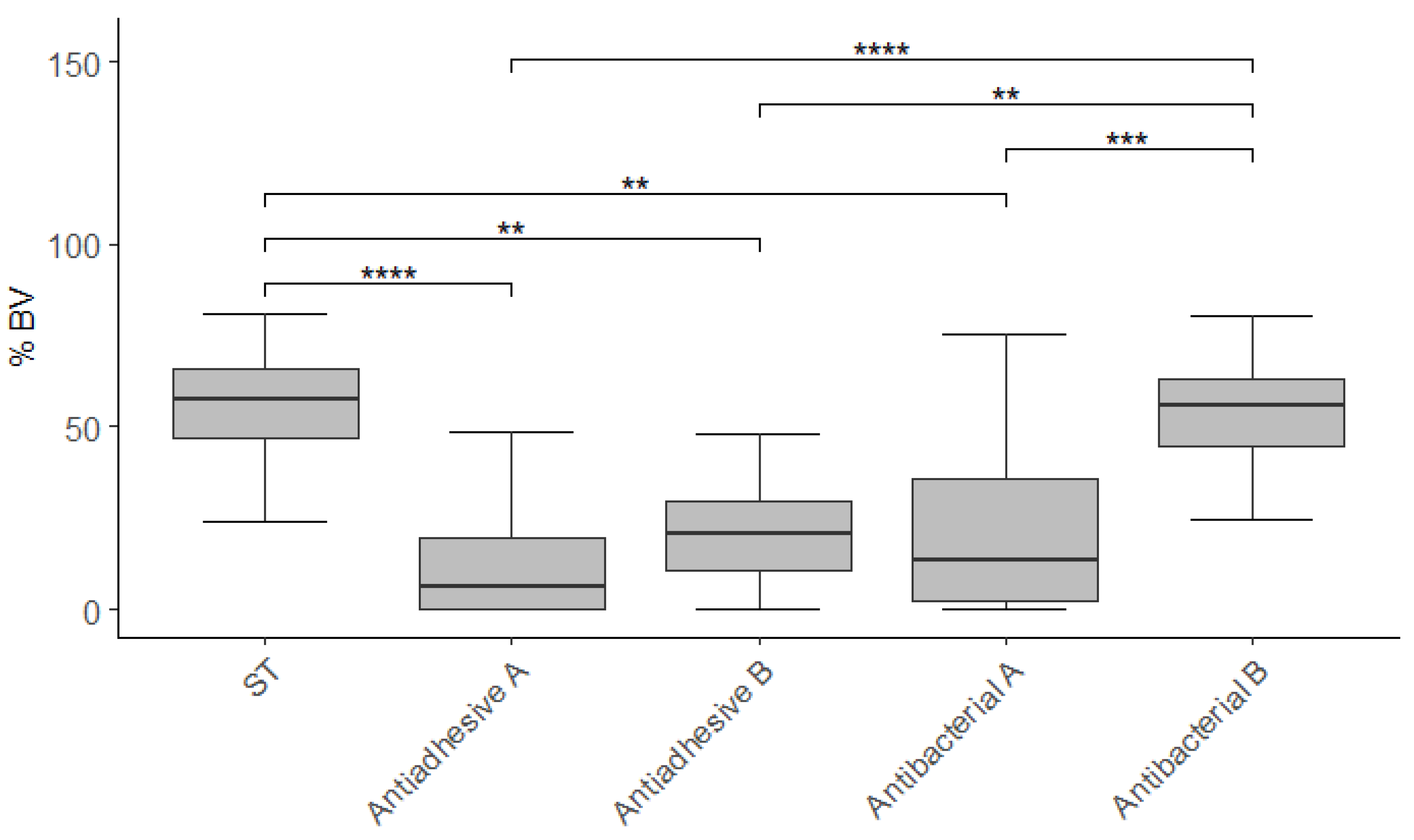
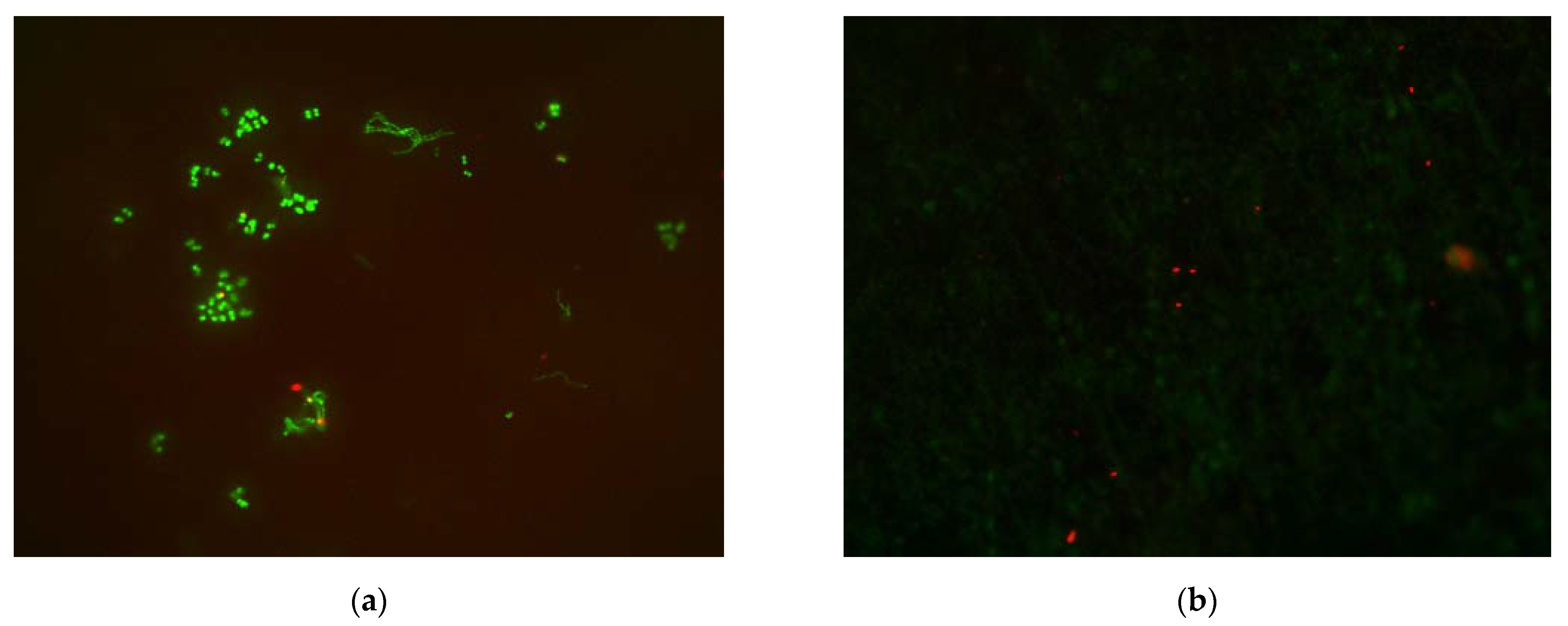
3.2. Cell Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mjor, I.A.; Toffenetti, F. Secondary caries: A literature review with case reports. Quintessence Int. 2000, 31, 165–179. [Google Scholar]
- Ruben, J.; Arends, J.; Christoffersen, J. The effect of window width on the demineralization of human dentine and enamel. Caries Res. 1999, 33, 214–219. [Google Scholar] [CrossRef]
- Hao, Y.; Huang, X.; Zhou, X.; Li, M.; Ren, B.; Peng, X.; Cheng, L. Influence of Dental Prosthesis and Restorative Materials Interface on Oral Biofilms. Int. J. Mol. Sci 2018, 19, 3157. [Google Scholar] [CrossRef] [Green Version]
- Quirynen, M.; Bollen, C.M. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J. Clin. Periodontol. 1995, 22, 1–14. [Google Scholar] [CrossRef]
- Quirynen, M.; Marechal, M.; Busscher, H.J.; Weerkamp, A.H.; Darius, P.L.; van Steenberghe, D. The influence of surface free energy and surface roughness on early plaque formation. An in vivo study in man. J. Clin. Periodontol. 1990, 17, 138–144. [Google Scholar] [CrossRef]
- Aykent, F.; Yondem, I.; Ozyesil, A.G.; Gunal, S.K.; Avunduk, M.C.; Ozkan, S. Effect of different finishing techniques for restorative materials on surface roughness and bacterial adhesion. J. Prosthet. Dent. 2010, 103, 221–227. [Google Scholar] [CrossRef]
- Beyth, N.; Bahir, R.; Matalon, S.; Domb, A.J.; Weiss, E.I. Streptococcus mutans biofilm changes surface-topography of resin composites. Dent. Mater. 2008, 24, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Bollen, C.M.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef]
- Quirynen, M.; Marechal, M.; Busscher, H.J.; Weerkamp, A.H.; Arends, J.; Darius, P.L.; van Steenberghe, D. The influence of surface free-energy on planimetric plaque growth in man. J. Dent. Res. 1989, 68, 796–799. [Google Scholar] [CrossRef] [Green Version]
- Morrier, J.J.; Suchett-Kaye, G.; Nguyen, D.; Rocca, J.P.; Blanc-Benon, J.; Barsotti, O. Antimicrobial activity of amalgams, alloys and their elements and phases. Dent. Mater. 1998, 14, 150–157. [Google Scholar] [CrossRef]
- Shahal, Y.; Steinberg, D.; Hirschfeld, Z.; Bronshteyn, M.; Kopolovic, K. In vitro bacterial adherence onto pellicle-coated aesthetic restorative materials. J. Oral Rehabil. 1998, 25, 52–58. [Google Scholar] [CrossRef]
- van de Sande, F.H.; Opdam, N.J.M.; Truin, G.J.; Bronkhorst, E.M.; de Soet, J.J.; Cenci, M.S.; Huysmans, M.-C. The influence of different restorative materials on secondary caries development in situ. J. Dent. 2014, 42, 1171–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opdam, N.J.M.; Bronkhorst, E.M.; Loomans, B.A.C.; Huysmans, M.-C.D.N.J.M. 12-year Survival of Composite vs. Amalgam Restorations. J. Dent. Res. 2010, 89, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.; Luis, H.; Martin, M.D.; Leroux, B.G.; Rue, T.; Leitão, J.; DeRouen, T.A. Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trial. J. Am. Dent. Assoc. 2007, 138, 775–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Ahmad, A.; Follo, M.; Selzer, A.C.; Hellwig, E.; Hannig, M.; Hannig, C. Bacterial colonization of enamel in situ investigated using fluorescence in situ hybridization. J. Med. Microbiol. 2009, 58, 1359–1366. [Google Scholar] [CrossRef] [Green Version]
- Jung, D.J.; Al-Ahmad, A.; Follo, M.; Spitzmuller, B.; Hoth-Hannig, W.; Hannig, M.; Hannig, C. Visualization of initial bacterial colonization on dentine and enamel in situ. J. Microbiol. Methods 2010, 81, 166–174. [Google Scholar] [CrossRef]
- Rüttermann, S.; Trellenkamp, T.; Bergmann, N.; Beikler, T.; Ritter, H.; Janda, R. Bacterial viability and physical properties of antibacterially modified experimental dental resin composites. PLoS ONE 2013, 8, e79119. [Google Scholar] [CrossRef] [Green Version]
- Burgers, R.; Hahnel, S.; Reichert, T.E.; Rosentritt, M.; Behr, M.; Gerlach, T.; Handel, G.; Gosau, M. Adhesion of Candida albicans to various dental implant surfaces and the influence of salivary pellicle proteins. Acta Biomater. 2010, 6, 2307–2313. [Google Scholar] [CrossRef]
- Knorr, S.D.; Combe, E.C.; Wolff, L.F.; Hodges, J.S. The surface free energy of dental gold-based materials. Dent. Mater. 2005, 21, 272–277. [Google Scholar] [CrossRef]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implant. Res. 2006, 17 (Suppl. 2), 68–81. [Google Scholar] [CrossRef]
- Wiegand, A.; Buchalla, W.; Attin, T. Review on fluoride-releasing restorative materials--fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent. Mater. 2007, 23, 343–362. [Google Scholar] [CrossRef]
- Rüttermann, S.; Trellenkamp, T.; Bergmann, N.; Raab, W.H.; Ritter, H.; Janda, R. A new approach to influence contact angle and surface free energy of resin-based dental restorative materials. Acta Biomater. 2011, 7, 1160–1165. [Google Scholar] [CrossRef]
- Rüttermann, S.; Beikler, T.; Janda, R. Contact angle and surface free energy of experimental resin-based dental restorative materials after chewing simulation. Dent. Mater. 2014, 30, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Rüttermann, S.; Bergmann, N.; Beikler, T.; Raab, W.H.; Janda, R. Bacterial viability on surface-modified resin-based dental restorative materials. Arch. Oral Biol. 2012, 57, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Henrich, B.; Hermann, I.; Di Giulio, M.; Köhrer, K.; Deenen, R.; Sivalingam, S.; Peters, U.; Beikler, T.; Janda, R.; Rüttermann, S. Reexamination In Vitro and In Situ of an Antibacterially Modified Experimental Dental Resin Composite with Molecular Methods: A Pilot Study. Adv. Mater. Sci. Eng. 2016, 2016, 6367234. [Google Scholar] [CrossRef] [Green Version]
- Kolenbrander, P.E.; Palmer, R.J., Jr.; Periasamy, S.; Jakubovics, N.S. Oral multispecies biofilm development and the key role of cell-cell distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef] [PubMed]
- American Dental Association. Periodontal screening & recording an early detection system. J. N. J. Dent. Assoc. 1993, 64, 7–9, 11. [Google Scholar]
- Silness, J.; Loe, H. Periodontal disease in pregnancy. Ii. Correlation between oral hygiene and periodontal condtion. Acta Odontol. Scand. 1964, 22, 121–135. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- International Organization for Standardization. ISO 4049: Dentistry-Polymer-Based Filling, Restortive and Luting Materials; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Sachs, L. Angewandte Statistik, 8th ed.; Springer: Berlin/Heidelberg, Germany, 1997; p. 252. [Google Scholar]
- Tomczak, M.; Tomczak, E. The need to report effect size estimates revisited. An overview of some recommended measures of effect size. Trends Sport Sci. 2014, 1, 19–25. [Google Scholar]
- Al-Ahmad, A.; Wunder, A.; Auschill, T.M.; Follo, M.; Braun, G.; Hellwig, E.; Arweiler, N.B. The in vivo dynamics of Streptococcus spp., Actinomyces naeslundii, Fusobacterium nucleatum and Veillonella spp. in dental plaque biofilm as analysed by five-colour multiplex fluorescence in situ hybridization. J. Med. Microbiol. 2007, 56, 681–687. [Google Scholar] [CrossRef]
- Hannig, C.; Follo, M.; Hellwig, E.; Al-Ahmad, A. Visualization of adherent micro-organisms using different techniques. J. Med. Microbiol. 2010, 59, 1–7. [Google Scholar] [CrossRef]
- Hannig, C.; Hannig, M.; Rehmer, O.; Braun, G.; Hellwig, E.; Al-Ahmad, A. Fluorescence microscopic visualization and quantification of initial bacterial colonization on enamel in situ. Arch. Oral Biol. 2007, 52, 1048–1056. [Google Scholar] [CrossRef]
- ten Cate, J.M. Biofilms, a new approach to the microbiology of dental plaque. Odontology 2006, 94, 1–9. [Google Scholar] [CrossRef]
- Landenberger, P.; Baumann, L.; Gerhardt-Szép, S.; Rüttermann, S. The effect of new anti-adhesive and antibacterial dental resin filling materials on gingival fibroblasts. Dent. Mater. 2021, 37, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Sojka, M.F. Precipitation Polymerization Process for Producing an Oil Adsorbent Polymer Capable of Entrapping Solid Particles and Liquids and the Product Thereof. U.S. Patent 5830960, 1998. [Google Scholar]
- Oh, S.T.; Han, S.H.; Ha, C.S.; Cho, W.J. Synthesis and biocidal activities of polymer. IV. Antibacterial activity and hydrolysis of polymers containing diphenyl ether. J. Appl. Polym. Sci. 1996, 59, 1871–1878. [Google Scholar] [CrossRef]
- Oh, S.T.; Ha, C.S.; Cho, W.J. Synthesis and biocidal activities of polymer. III. Bactericical activity of homopolymer of AcDP and copolymer of acdp with St. J. Appl. Polym. Sci. 1994, 54, 859–866. [Google Scholar] [CrossRef]
- Choi, S.-b.; Jepperson, J.; Jarabek, L.; Thomas, J.; Chisholm, B.; Boudjouk, P. Novel Approach to Anti-Fouling and Fouling-Release Marine Coatings Based on Dual-Functional Siloxanes. Macromol. Symp. 2007, 249–250, 660–667. [Google Scholar] [CrossRef]
- Rüttermann, S.; Krüger, S.; Raab, W.H.; Janda, R. Polymerization shrinkage and hygroscopic expansion of contemporary posterior resin-based filling materials—a comparative study. J. Dent. 2007, 35, 806–813. [Google Scholar] [CrossRef]
- Janda, R.; Roulet, J.F.; Latta, M.; Rüttermann, S. Water sorption and solubility of contemporary resin-based filling materials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 82, 545–551. [Google Scholar] [CrossRef]
- Janda, R.; Roulet, J.F.; Latta, M.; Rüttermann, S. The effects of thermocycling on the flexural strength and flexural modulus of modern resin-based filling materials. Dent. Mater. 2006, 22, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.M.; Almeida, G.S.; Poskus, L.T.; Guimarães, J.G. Relationship between the degree of conversion, solubility and salivary sorption of a hybrid and a nanofilled resin composite. J. Appl. Oral Sci. 2008, 16, 161–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, L.; Filho, J.D.; Guimarães, J.G.; Poskus, L.T.; Silva, E.M. Solubility, salivary sorption and degree of conversion of dimethacrylate-based polymeric matrixes. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 85, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Sadr, A.; Inai, N.; Tagami, J. Evaluation of resin composite polymerization by three dimensional micro-CT imaging and nanoindentation. Dent. Mater. 2011, 27, 1070–1078. [Google Scholar] [CrossRef]
- Feng, L.; Suh, B.I. The effect of curing modes on polymerization contraction stress of a dual cured composite. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 76, 196–202. [Google Scholar] [CrossRef]
- Sharifi, S.; Mirzadeh, H.; Imani, M.; Atai, M.; Ziaee, F. Photopolymerization and shrinkage kinetics of in situ crosslinkable N-vinyl-pyrrolidone/poly(ε-caprolactone fumarate) networks. J. Biomed. Mater. Res. Part A 2008, 84A, 545–556. [Google Scholar] [CrossRef]
- Sideridou, I.D.; Karabela, M.M.; Micheliou, C.N.; Karagiannidis, P.G.; Logothetidis, S. Physical properties of a hybrid and a nanohybrid dental light-cured resin composite. J. Biomater. Sci. Polym. Ed. 2009, 20, 1831–1844. [Google Scholar] [CrossRef]
- Silikas, N.; Eliades, G.; Watts, D.C. Light intensity effects on resin-composite degree of conversion and shrinkage strain. Dent. Mater. 2000, 16, 292–296. [Google Scholar] [CrossRef]
- Quirynen, M.; Bollen, C.M.; Papaioannou, W.; Van Eldere, J.; van Steenberghe, D. The influence of titanium abutment surface roughness on plaque accumulation and gingivitis: Short-term observations. Int. J. Oral Maxillofac. Implant. 1996, 11, 169–178. [Google Scholar]
- Bollen, C.M.; Papaioanno, W.; Van Eldere, J.; Schepers, E.; Quirynen, M.; van Steenberghe, D. The influence of abutment surface roughness on plaque accumulation and peri-implant mucositis. Clin. Oral Implant. Res. 1996, 7, 201–211. [Google Scholar] [CrossRef]
- Uyen, M.; Busscher, H.J.; Weerkamp, A.H.; Arends, J. Surface free energies of oral streptococci and their adhesion to solids. FEMS Microbiol. Lett. 1985, 30, 103–106. [Google Scholar] [CrossRef]
- Mei, L.; Busscher, H.J.; van der Mei, H.C.; Chen, Y.; de Vries, J.; Ren, Y. Oral bacterial adhesion forces to biomaterial surfaces constituting the bracket-adhesive-enamel junction in orthodontic treatment. Eur. J. Oral Sci. 2009, 117, 419–426. [Google Scholar] [CrossRef]
- Gyo, M.; Nikaido, T.; Okada, K.; Yamauchi, J.; Tagami, J.; Matin, K. Surface response of fluorine polymer-incorporated resin composites to cariogenic biofilm adherence. Appl. Environ. Microbiol. 2008, 74, 1428–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buergers, R.; Schneider-Brachert, W.; Hahnel, S.; Rosentritt, M.; Handel, G. Streptococcal adhesion to novel low-shrink silorane-based restorative. Dent. Mater. 2009, 25, 269–275. [Google Scholar] [CrossRef]
- Eick, J.D.; Kotha, S.P.; Chappelow, C.C.; Kilway, K.V.; Giese, G.J.; Glaros, A.G.; Pinzino, C.S. Properties of silorane-based dental resins and composites containing a stress-reducing monomer. Dent. Mater. 2007, 23, 1011–1017. [Google Scholar] [CrossRef]
- Weinmann, W.; Thalacker, C.; Guggenberger, R. Siloranes in dental composites. Dent. Mater. 2005, 21, 68–74. [Google Scholar] [CrossRef]
- Vogel, B.S.; Wildung, M.R.; Vogel, G.; Croteau, R. Abietadiene synthase from grand fir (Abies grandis). cDNA isolation, characterization, and bacterial expression of a bifunctional diterpene cyclase involved in resin acid biosynthesis. J. Biol. Chem. 1996, 271, 23262–23268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.P.; Lee, S.J.; Lim, B.S.; Ahn, S.J. Surface characteristics of orthodontic materials and their effects on adhesion of mutans streptococci. Angle Orthod. 2009, 79, 353–360. [Google Scholar] [CrossRef]
| Raw Material | ST | Antiadhesive A | Antiadhesive B | Antibacterial A | Antibacterial B |
|---|---|---|---|---|---|
| Glass | 73.0 | 68.0 | 68.2 | 68.0 | 73.0 |
| Poly-Tego | - | 5.0 | - | - | - |
| Poly-Dimeth | - | - | 5.0 | - | - |
| Poly-Irga | - | - | - | 5.0 | - |
| Methacryl-Irga | - | - | - | - | 8.0 |
| Matrix | 27.0 | 27.0 | 26.8 | 27.0 | 19.0 |
| Total | 100 | 100 | 100 | 100 | 100 |
| Active agent | 0 | 4.0 | 4.0 | 4.0 | 8.0 |
| Code | Product/Properties | Batch | Company |
|---|---|---|---|
| Photoinitiator | α.α-dimethoxy-α-phenylacetophenone | 0066162S | Ciba Specialty Chemicals, Basel, Switzerland |
| Stabilizer | Pentaerythrityl-tetrakis[3-(3,5-di-tert-butyl-4-hydroxyphenyl)-propionate | 26099IC3 | Ciba Speciality Chemicals |
| TTEGDMA | Tetraethyleneglycole dimethacrylate, standard monomer, functionality = 2, MW = 330 g·mol−1, good chemical and physical properties, very low viscosity (14 Pa s, 25 °C), diluting | J1620 | Cray Valley, Paris, France |
| UV stabilizer | 2-Hydroxy-4-methoxy-bezophenone | 411351/ 143302 | Fluka, Buchs, Switzerland |
| UDMA | 7,7,9-Trimethyl-4,13-dioxo-3,14-dioxa-5,12-diaza-hexadecan-1,16-diol-dimethacrylate, standard monomer, functionality = 2, MW = 471 g·mol−1, flexible, tough, very good chemical resistance, medium viscosity(10,000 m·Pas,25 °C) | 330503057 | Rahn A.G, Zürich, Switzerland |
| Bis-GMA | Bis-GMA, standard monomer, functionality = 2, MW = 513 g·mol−1, rigid, very good chemical resistance, very high viscosity (4500 m·Pas, 60 °C) | 2008218303 | Rahn A.G |
| CQ | D,L-Camphorquinone | 0148990002 | Rahn A.G |
| Amine | Ethyl-4-(dimethylamino)-benzoate | 310170 | Rahn A.G |
| Glass | Strontium borosilicate glass (GO 18–093, d50 = 0.7 µm). silaned (3-methacryloyloxypropyl trimethoxy silane), D = 2.6 g·cm−3, | Lab14701 | Schott Electronic Packaging, GmbH, Landshut, Germany |
| Poly- | Poly-Pore, cross-linked polyallyl methacrylate, adsorber, hollow beads, diameter 20–40 µm | L07070303AB | AMCOL Health & Beauty Solutions, Arlington Heights, IL, USA |
| Tego | Tego Protect 5000, hydroxyfunctional polydimethylsiloxane, hydro- and oleophobic, D = 1.05 g·cm−3 | ES57608918 | Evonik Tego Chemie, Essen, Germany |
| Dimeth | Dimethicone 200/350 cst, polydimethylsiloxane, D = 0.965 g·cm−3 | 4962250 | Dow Corning Corp., Midland, MI, USA |
| Irga | Irgasan, 5-chloro-2-(2,4-dichlorophenoxy)phenole | 1124816 | Sigma Aldrich GmbH, Steinheim, Germany |
| Poly-Dimeth | Poly-Pore loaded with 80% dimethicone, D = 1.0 g·cm−3 | Experimental product | University laboratory |
| Poly-Tego | Poly-Pore loaded with 80% Tego Protect 5000, D = 1.0 g·cm−3 | Experimental product | University laboratory |
| Poly-Irga | loaded with 80% Irgasan, D = 1.0 cm−3 | Experimental product | University laboratory |
| Methacryl-Irga | 5-chloro-2-(2,4-dichlorophenoxy)phenyl methacrylate | Experimental product | University laboratory |
| n | % | |
|---|---|---|
| Participants | 25 | 100 |
| Female/Male | 19/6 | 76/24 |
| Age (mean ± SD) (years) | 29.5 ± 3.3 | - |
| Tooth hard tissue | ||
| Decay | 0 | - |
| Oral hygiene (PLI) | ||
| Excellent (0) | 16 | 64.0 |
| Good (0.1–0.9) | 9 | 36.0 |
| Fair (1.0–1.9) | 0 | - |
| Poor (2.0–3.0) | 0 | - |
| Periodontal Screening and Recording Index (PSR) | ||
| Grade 0 | 104 | 69.3 |
| Grade 1 | 27 | 18 |
| Grade 2 | 19 | 12.7 |
| Grade 3 | 0 | - |
| Grade 4 | 0 | - |
| Material | n | Vital | Non-Vital | Total | % BV |
|---|---|---|---|---|---|
| ST | 25 | 137.7 (251.4)1 | 108.5 (163.4)1 | 276.2 (506.4)1 | 57.6 (19.4)1 |
| [463.7 ± 913.2] | [274.4 ± 540.0] | [738.1 ± 1434.2] | [55.2 ± 18.7] | ||
| Antiadhesive A | 25 | 2.7 (37.0)2 | 44.0 (53.9)2 | 57.7 (84.9)2 | 6.4 (19.7)2 |
| [66.6 ± 235.3] | [135.4 ± 286.1] | [202.0 ± 507.6] | [15.3 ± 20.8] | ||
| Antiadhesive B | 25 | 10.8 (27.0)2 | 48.1 (89.2)2 | 54.1 (113.0)2 | 20.6 (18.9)2 |
| [141.9 ± 604.2] | [132.8 ± 345.6] | [274.7 ± 946.9] | [23.6 ± 22.2] | ||
| Antibacterial A | 25 | 5.0 (26.9)2 | 50.9 (70.6)2 | 53.3 (96.3)2 | 13.2 (33.6)2 |
| [105.0 ± 321.7] | [182.0 ± 429.0] | [287.0 ± 712.2] | [22.2 ± 23.0] | ||
| Antibacterial B | 25 | 41.6 (160.4)1 | 52.5 (138.3)1,2 | 111.6 (286.9)1,2 | 55.6 (18.4)1 |
| [298.7 ± 926.5] | [206.6 ± 620.0] | [505.3 ± 1545.3] | [51.9 ± 17.7] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgard, N.; Kienitz, M.; Jourdan, C.; Rüttermann, S. The Influence of Modified Experimental Dental Resin Composites on the Initial In Situ Biofilm—A Triple-Blinded, Randomized, Controlled Split-Mouth Trial. Polymers 2021, 13, 2814. https://doi.org/10.3390/polym13162814
Burgard N, Kienitz M, Jourdan C, Rüttermann S. The Influence of Modified Experimental Dental Resin Composites on the Initial In Situ Biofilm—A Triple-Blinded, Randomized, Controlled Split-Mouth Trial. Polymers. 2021; 13(16):2814. https://doi.org/10.3390/polym13162814
Chicago/Turabian StyleBurgard, Niklas, Melanie Kienitz, Claudia Jourdan, and Stefan Rüttermann. 2021. "The Influence of Modified Experimental Dental Resin Composites on the Initial In Situ Biofilm—A Triple-Blinded, Randomized, Controlled Split-Mouth Trial" Polymers 13, no. 16: 2814. https://doi.org/10.3390/polym13162814
APA StyleBurgard, N., Kienitz, M., Jourdan, C., & Rüttermann, S. (2021). The Influence of Modified Experimental Dental Resin Composites on the Initial In Situ Biofilm—A Triple-Blinded, Randomized, Controlled Split-Mouth Trial. Polymers, 13(16), 2814. https://doi.org/10.3390/polym13162814