Hydrophobic Modification of Chitosan via Reactive Solvent-Free Extrusion
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Structural Characteristics of the Obtained Derivatives
3.1.1. Elemental Analysis
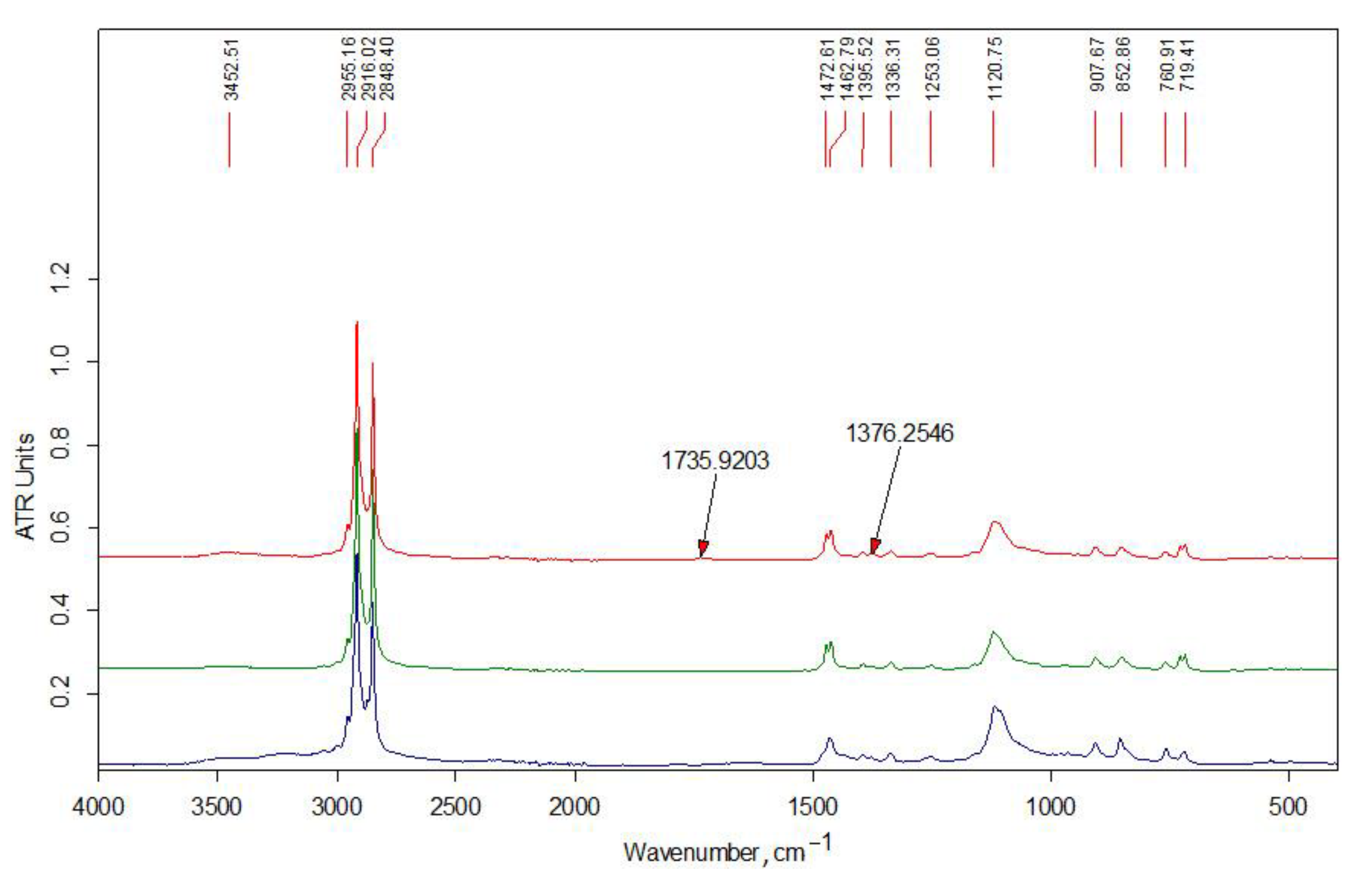
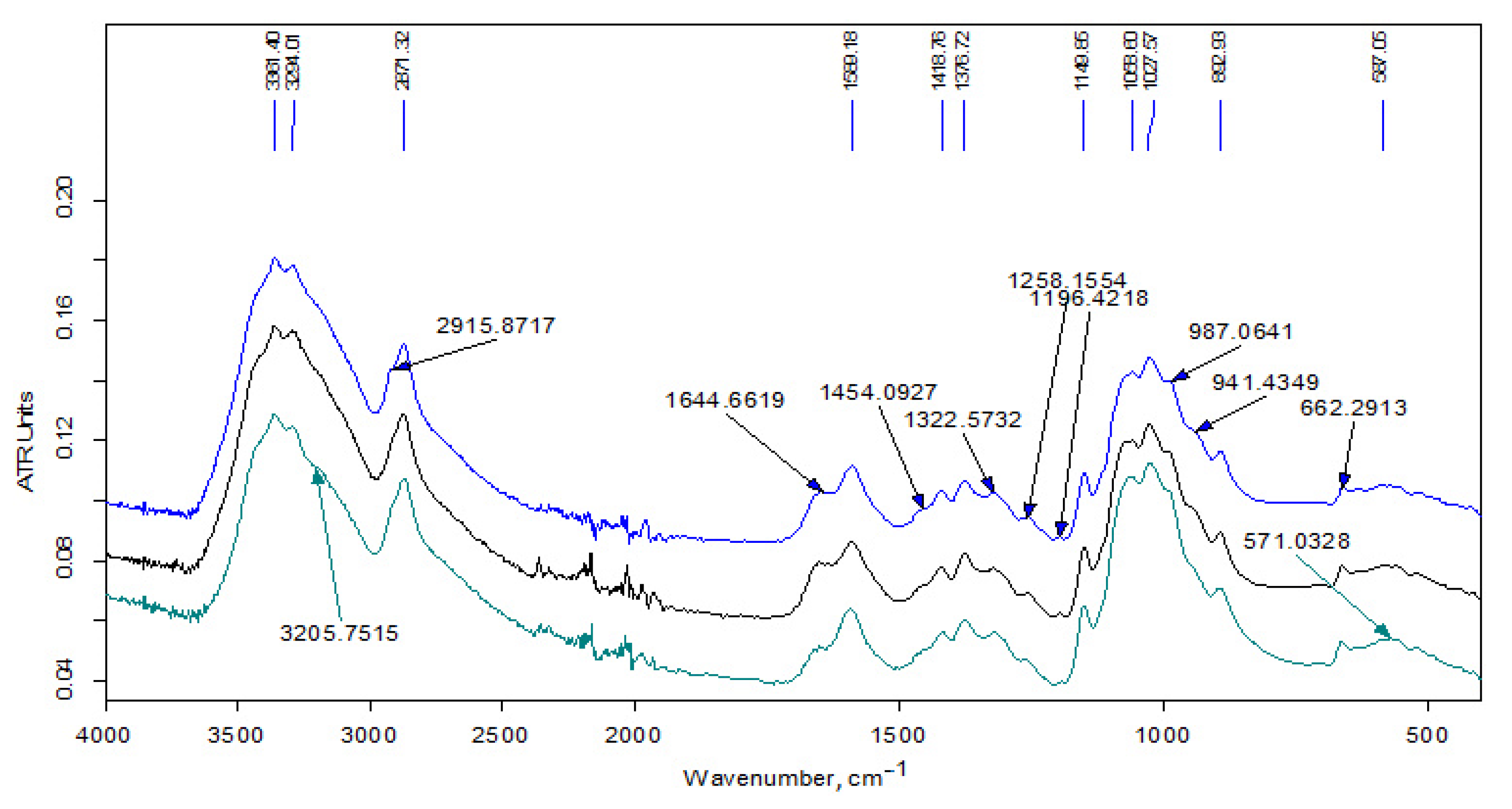
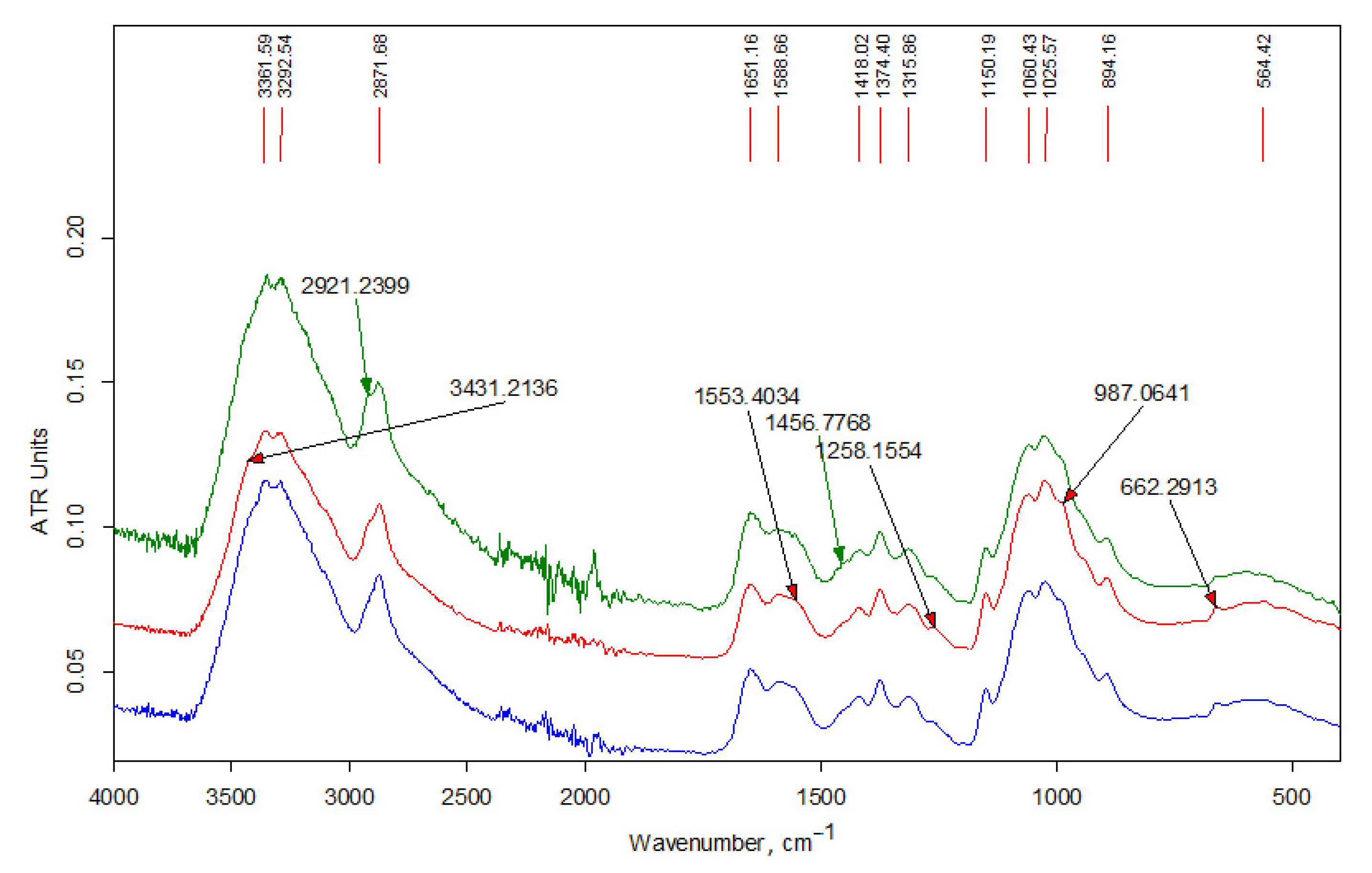
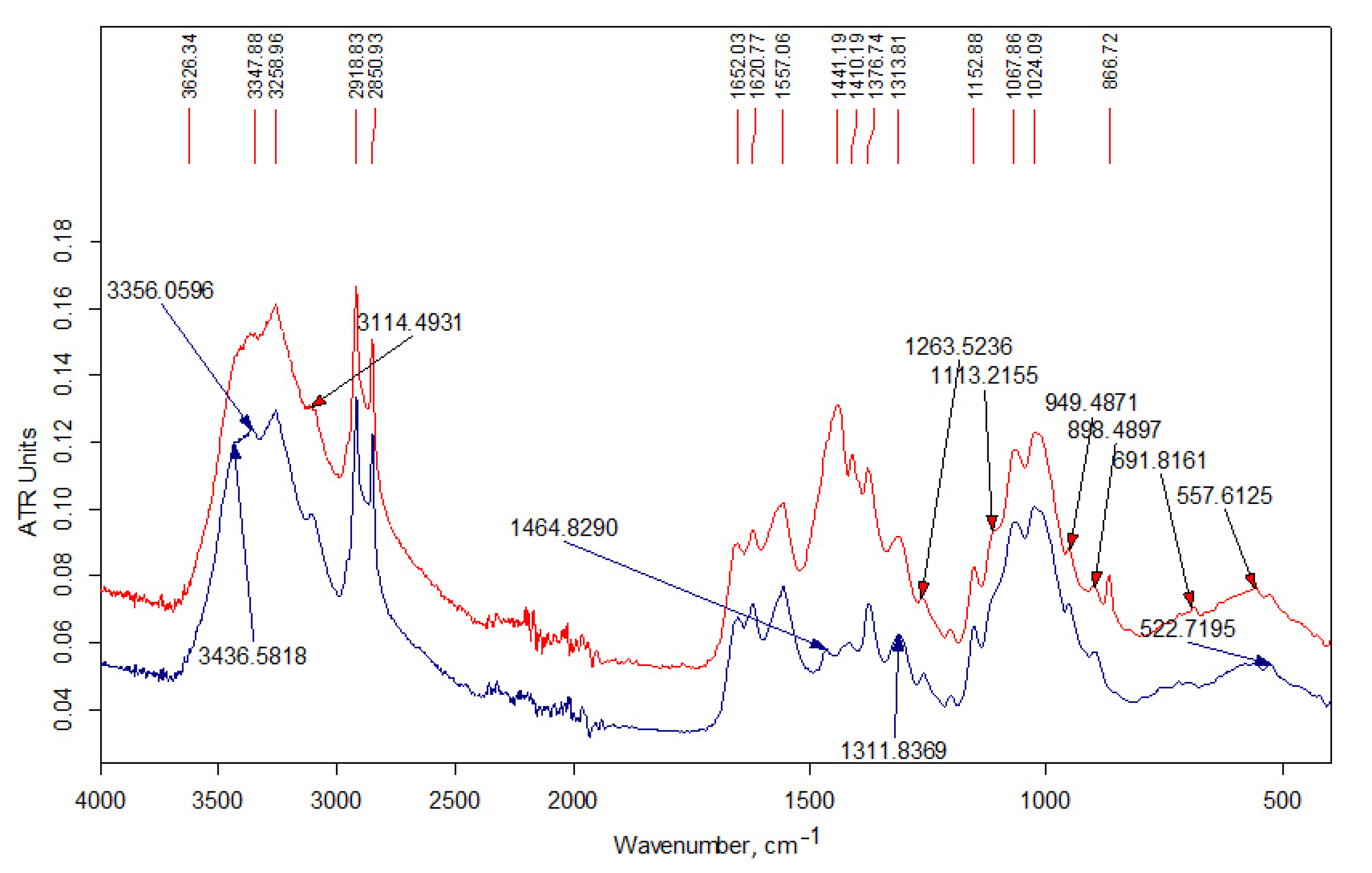
3.1.2. FT-IR Data
3.2. Behavior in Solution and Properties of the Films
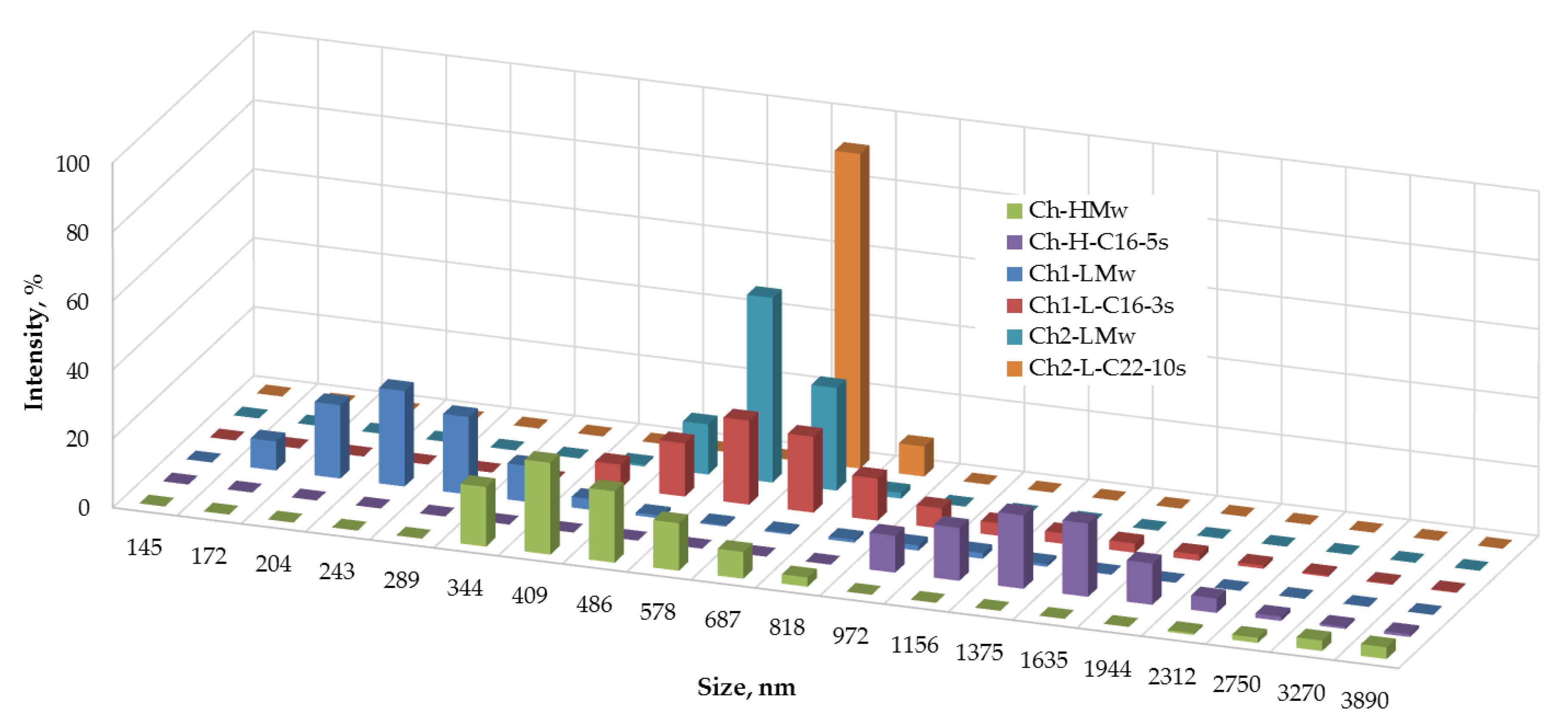
3.2.1. Dynamic Light Scattering
3.2.2. Mechanical Tests
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| C, 1 g dl−1 | t, sec | ηrel | ηsp | ηsp/C |
|---|---|---|---|---|
| 0.45 | 363 | 3.42 | 2.42 | 5.35 |
| 0.33 | 276 | 2.60 | 1.60 | 4.82 |
| 0.26 | 230 | 2.17 | 1.17 | 4.46 |
| 0.22 | 197 | 1.86 | 0.86 | 4.2 |
Appendix B

References
- Jothimani, B.; Sureshkumar, S.; Venkatachalapathy, B. Hydrophobic structural modification of chitosan and its impact on nanoparticle synthesis—A physicochemical study. Carbohydrate Polym. 2017, 173, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Korchagina, E.V.; Philippova, O.E. Effects of hydrophobic substituents and salt on core–shell aggregates of hydrophobically modified chitosan: Light scattering study. Langmuir 2012, 28, 7880–7888. [Google Scholar] [CrossRef]
- Desbrieres, J.; Martinez, C.; Rinaudo, M. Hydrophobic derivatives of chitosan: Characterization and rheological behavior. Int. J. Biol. Macromol. 1996, 19, 21–28. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, L.; Gan, W.; Zhou, J.; Zhang, L. Self-assembled micelles based on hydrophobically modified quaternized cellulose for drug delivery. Colloids Surf. B Biointerfaces 2011, 83, 313–320. [Google Scholar] [CrossRef]
- Li, Q.; Ye, L.; Cai, Y.; Huang, R. Study of Rheological Behavior of Hydrophobically Modified Hydroxyethyl Cellulose. J. App. Polym. Sci. 2006, 100, 3346–3352. [Google Scholar] [CrossRef]
- Sonia, T.A.; Rekha, M.R.; Sharma, C.P. Bioadhesive hydrophobic chitosan microparticles for oral delivery of insulin: In vitro characterization and in vivo uptake studies. J. Appl. Polym. Sci. 2011, 119, 2902–2910. [Google Scholar] [CrossRef]
- Venkataraman, P.; Tang, J.; Frenkel, E.; McPherson, G.L.; He, J.; Raghavan, S.R.; Kolesnichenko, V.; Bose, A.; John, V.T. Attachment of a Hydrophobically Modified Biopolymer at the Oil-Water Interface in the Treatment of Oil Spills. ACS Appl. Mater. Interfaces 2013, 5, 3572–3580. [Google Scholar] [CrossRef]
- Seo, T.; Ohtake, H.; Kanbara, T.; Yonetake, K.; Iijima, T. Preparation and permeability properties of chitosan membranes having hydrophobic groups. Macromol. Chem. Phisics 1991, 192, 2447–2461. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandía, M.L.; Caballero, A.H. Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demina, T.S.; Drozdova, M.G.; Sevrin, C.; Compère, P.; Akopova, T.A.; Markvicheva, E.; Grandfils, C. Biodegradable Cell Microcarriers Based on Chitosan/Polyester Graft-Copolymers. Molecules 2020, 25, 1949. [Google Scholar] [CrossRef]
- Demina, T.S.; Akopova, T.A.; Vladimirov, L.V.; Zelenetskii, A.N.; Markvicheva, E.A.; Grandfils, C. Polylactide-based microspheres prepared using solid-state copolymerized chitosan and D,L-lactide. Mater. Sci. Eng. C 2016, 59, 333–338. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Coelhoso, I.M.; Fernando, A.L. Chitosan Composites in Packaging Industry—Current Trends and Future Challenges. Polymers 2020, 12, 417. [Google Scholar] [CrossRef] [Green Version]
- Prashanth, K.V.H.; Tharanathan, R.N. Chitin/chitosan: Modifications and their unlimited application potential—an overview. Trends Food Sci. Technol. 2007, 18, 117–131. [Google Scholar] [CrossRef]
- Dowling, M.B.; Smith, W.; Balogh, P.; Duggan, M.J.; MacIntire, I.C.; Harris, E.; Mesar, T.; Raghavan, S.R.; King, D.R. Hydrophobically-modified chitosan foam: Description and hemostatic efficacy. J. Surg. Res. 2015, 193, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Schirmeister, C.G.; Hees, T.; Licht, E.H.; Mulhaupt, R. 3D printing of high density polyethylene by fused filament fabrication. Addit. Manuf. 2019, 28, 152–159. [Google Scholar] [CrossRef]
- Peng, F.; Jiang, H.; Woods, A.; Joo, P.; Amis, E.J.; Zacharia, N.S.; Vogt, B.D. 3D Printing with Core-Shell Filaments Containing High- or Low-Density Polyethylene Shells. ACS Appl. Polym. Mater. 2019, 1, 275–285. [Google Scholar] [CrossRef]
- Dubinenko, G.; Zinoviev, A.; Bolbasov, E.; Kozelskaya, A.; Shesterikov, E.; Novikov, V.; Tverdokhlebov, S. Highly filled poly(l-lactic acid)/hydroxyapatite composite for 3D printing of personalized bone tissue engineering scaffolds. J. Appl. Polym. Sci. 2021, 138, 49662. [Google Scholar] [CrossRef]
- Tarres, Q.; Melbo, J.K.; Delgado-Aguilar, M.; Espinach, F.X.; Mutje, P.; Chinga-Carrasco, G. Bio-polyethylene reinforced with thermomechanical pulp fibers: Mechanical and micromechanical characterization and its application in 3D-printing by fused deposition modelling. Compos. Part B Eng. 2018, 153, 70–77. [Google Scholar] [CrossRef]
- Zong, Z.; Kimura, Y.; Takahashi, M.; Yamane, H. Characterization of chemical and solid state structures of acylated chitosans. Polymer 2000, 41, 899–906. [Google Scholar] [CrossRef]
- Dowling, M.B.; Kumar, R.; Keibler, M.A.; Hess, J.R.; Bochicchio, G.V.; Raghavan, S.R. A self-assembling hydrophobically modified chitosan capable of reversible hemostatic action. Biomaterials 2011, 32, 3351–3357. [Google Scholar] [CrossRef]
- Kurita, K.; Mori, S.; Nishiyama, Y.; Harata, M. N-Alkylation of chitin and some characteristics of the novel derivatives. Polym. Bull. 2002, 48, 159–166. [Google Scholar] [CrossRef]
- Salmon, S.; Hudson, S.M. Crystal morphology, biosynthesis, and physical assembly of cellulose, chitin, and chitosan. J. Macromol. Sci. Rev. Macromol. Chem. Phys. 1997, 37, 199–276. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Heinze, T.; El Seoud, O.A.; Koschella, A. Principles of Cellulose Derivatization. In Cellulose Derivatives; Springer Series on Polymer and Composite Materials; Springer: Cham, Switzerland, 2018; Volume 4, pp. 259–292. [Google Scholar]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friscic, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaz, P.; Achimovicova, M.; Balaz, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutkova, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [Green Version]
- Torres, E.; Gaona, A.; García-Bosch, N.; Muñoz, M.; Fombuena, V.; Moriana, R.; Vallés-Lluch, A. Improved Mechanical, Thermal, and Hydrophobic Properties of PLA Modified with Alkoxysilanes by Reactive Extrusion Process. Polymers 2021, 13, 2475. [Google Scholar] [CrossRef] [PubMed]
- Kaupp, G. Organic solid-state reactions with 100% yield. In Organic Solid State Reactions, Topics in Current Chemistry; Toda, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 254, pp. 95–183. [Google Scholar]
- Crawford, D.E.; Miskimmin, C.K.G.; Albadarin, A.B.; Walker, G.; James, S.L. Organic synthesis by Twin Screw Extrusion (TSE): Continuous, scalable and solvent-free. Green Chem. 2017, 6, 1507–1518. [Google Scholar] [CrossRef] [Green Version]
- Andersen, J.; Mack, J. Mechanochemistry and organic synthesis: From mystical to practical. Green Chem. 2018, 20, 1435–1443. [Google Scholar] [CrossRef]
- Shakhtshneider, T.; Boldyrev, V. Mechanochemical synthesis and mechanical activation of drugs. In Reactivity of Molecular Solids; Boldyreva, E., Boldyrev, V., Eds.; John Wiley & Sons, LTD: Chichester, UK, 1999; pp. 271–312. [Google Scholar]
- Xu, W.; Wen, M.; Yu, J.; Zhang, Q.; Polyakov, N.E.; Dushkin, A.V.; Su, W. Mechanochemical preparation of kaempferol intermolecular complexes for enhancing the solubility and bioavailability. Drug Dev. Ind. Pharm. 2018, 44, 1924–1932. [Google Scholar] [CrossRef]
- Akopova, T.A.; Zelenetskii, A.N.; Ozerin, A.N. Solid State Synthesis and Modification of Chitosan. In Focus on Chitosan Research; Ferguson, A.N., O’Neill, A.G., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2011; Chapter 8; pp. 223–254. [Google Scholar]
- Gamzazade, A.I.; Shlimak, V.M.; Sklar, A.M.; Shtykova, E.V.; Pavlova, S.A.; Rogozhin, S.V. Study of the hydrodynamic properties of chitosan solutions. Acta Polym. 1985, 36, 420–424. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Pearson, F.G.; Marchessault, R.H.; Liang, C.Y. Infrared Spectra of Crystalline Polysaccharides. V. Chitin. J. Polym. Sci. 1960, 43, 101–116. [Google Scholar] [CrossRef]
- Duarte, M.L.; Ferreira, M.C.; Marvao, M.R.; Rocha, J. An optimised method to determine the degree of acetylation of chitin and chitosan by FTIR spectroscopy. Int. J. Biol. Macromol. 2002, 31, 1–8. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infra-Red Spectra of Complex Molecules, 2nd ed.; Methuen: London, UK; Wiley: New York, NY, USA, 1964. [Google Scholar]
- Picart, C. Natural-based multilayer films for biomedical applications. In Natural-Based Polymers for Biomedical Applications; Reis, R.L., Ed.; Woodhead Publishing Limited: Cambridge, UK; CRC Press LLC: Boca Raton, FL, USA; Boston, MA, USA; New York, NY, USA; Washington, DC, USA, 2008; pp. 231–259. [Google Scholar]
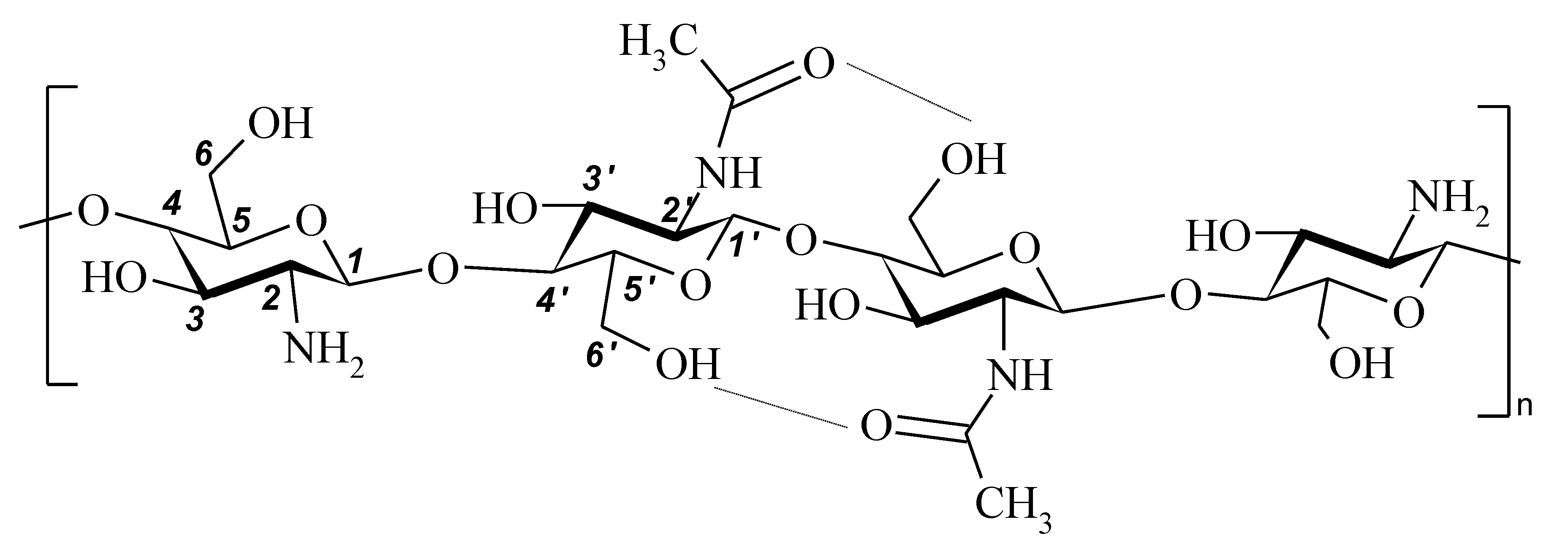

| Sample Code | Modifier Content, wt-% | Temperature of Treatment 1, °C | Solubility in 2% Aqueous CH3COOH | Mw, 2 kDa | DD, 3 % |
|---|---|---|---|---|---|
| Ch-HMw | - | - | 95 | 350 | 80 |
| Ch1-LMw | - | - | 94 | 80 | 87 |
| Ch2-LMw | - | - | 92 | 140 | 93 |
| Ch2-L-C22-3 | 3 | 50–60 | 75 | – | – |
| Ch2-L-C22-10 | 10 | 50–70 | 57 | – | – |
| Ch1-L-C16-3 | 3 | 40–60 | 76 | – | – |
| Ch-H-C16-5 | 5 | 40–60 | 72 | – | – |
| Wave Number, cm−1 | Interpretation [35,36,37,38] |
|---|---|
| 3480 | νОН in hydrogen bonds of (C6) H2OH groups |
| 3450 | νОН in hydrogen bonds between O3 and O2 atoms |
| 3370 | νNН asymmetrical |
| 3295 | νNН symmetrical |
| 3265 | νNН (Amid II band) |
| 3100 | combined band νNН |
| 2950 | νCН3 asymmetrical |
| 2930 | νCН2 asymmetrical |
| 2890–2880 | νCН3, νCН2 symmetrical, νCН in the ring |
| 3000–2840 | νCН in alkyl substituents |
| 1660 | νC=О (пoлoса амид I) in hydrogen bonds C=О---НN |
| 1630 | νC=О (пoлoса амид I) in hydrogen bonds with О3 |
| 1600 | δNН2 |
| 1560 | δNН (Amid II band), νCN |
| 1475–1450 | δCН2 in alkyl substituents |
| 1470–1430 | δCН3 in alkyl substituents |
| 1430–1420 | δCН, δCН2, δCН3 in chitin and chitosan |
| 1395–1365 | δCН3 in alkyl substituents |
| 1390 | νCН, δCН symmetrical |
| 1380 | δCН3 symmetrical |
| 1375 | νCN, δCН2 |
| 1320 | δNН, δCН2 symmetrical |
| 1310 | δNН (Amid III band), δCН2 symmetrical |
| 1260–1200 | in (C3)НОН groups |
| 1155 | νCО asymmetrical of acetal bond |
| 1110 | νCО asymmetrical in the ring |
| 1070–1030 | νCО in the ring, in (C3)НОН and (C6)Н2ОН groups |
| 975 | C-CН3 |
| 895 | νCО asymmetrical, δCН |
| 770–720 | δCН2 in alkyl substituents |
| Sample | %C | %Н | %N | C/N | DS |
|---|---|---|---|---|---|
| Ch-HMw | 43.74 ± 0.14 | 6.79 ± 0.11 | 7.82 ± 0.02 | 6.53 | – |
| Ch1-LMw | 43.62 ± 0.12 | 6.67 ± 0.12 | 7.93 ± 0.02 | 6.42 | – |
| Ch2-LMw | 43.03 ± 0.21 | 6.82 ± 0.04 | 8.17 ± 0.03 | 6.23 | – |
| Ch1-L-C16-3s | 40.85 ± 0.10 | 7.85 ± 0.08 | 7.21 ± 0.01 | 6.61 | 0.012 |
| Ch1-L-C16-3ins | 43.56 ± 0.01 | 7.05 ± 0.01 | 5.33 ± 0.01 | 9.53 | 0.19 |
| Ch-H-C16-5s | 40.85 ± 0.10 | 7.70 ± 0.10 | 7.20 ± 0.01 | 6.62 | 0.006 |
| Ch-H-C16-5ins | 43.56 ± 0.01 | 7.05 ± 0.01 | 5.32 ± 0.01 | 9.55 | 0.19 |
| Ch2-L-C22-3s | 40.15 ± 0.08 | 6.65 ± 0.12 | 7.84 ± 0.02 | 5.98 | – |
| Ch2-L-C22-3ins | 43.58 ± 0.01 | 6.79 ± 0.11 | 6.28 ± 0.02 | 8.10 | 0.085 |
| Ch2-L-C22-10s | 40.72 ± 0.09 | 7.30 ± 0.10 | 7.21 ± 0.02 | 6.59 | 0.016 |
| Ch2-L-C22-10ins | 45.90 ± 0.10 | 8.31 ± 0.08 | 5.6 ± 0.01 | 9.56 | 0.15 |
| Sample | Tensile Strength, MPa | Elongation at Break, % | Elastic Modulus, MPa |
|---|---|---|---|
| Ch-HMw | 71 ± 2.3 | 19 ± 2.1 | 1650 ± 35 |
| Ch-H-C16-5s | 65 ± 1.5 | 23 ± 1.4 | 750 ± 18 |
| Ch1-LMw | 37 ± 1.3 | 4 ± 3.3 | 1623 ± 32 |
| Ch1-L-C16-3s | 30 ± 1.1 | 8 ± 2.1 | 877 ± 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akopova, T.A.; Demina, T.S.; Khavpachev, M.A.; Popyrina, T.N.; Grachev, A.V.; Ivanov, P.L.; Zelenetskii, A.N. Hydrophobic Modification of Chitosan via Reactive Solvent-Free Extrusion. Polymers 2021, 13, 2807. https://doi.org/10.3390/polym13162807
Akopova TA, Demina TS, Khavpachev MA, Popyrina TN, Grachev AV, Ivanov PL, Zelenetskii AN. Hydrophobic Modification of Chitosan via Reactive Solvent-Free Extrusion. Polymers. 2021; 13(16):2807. https://doi.org/10.3390/polym13162807
Chicago/Turabian StyleAkopova, Tatiana A., Tatiana S. Demina, Mukhamed A. Khavpachev, Tatiana N. Popyrina, Andrey V. Grachev, Pavel L. Ivanov, and Alexander N. Zelenetskii. 2021. "Hydrophobic Modification of Chitosan via Reactive Solvent-Free Extrusion" Polymers 13, no. 16: 2807. https://doi.org/10.3390/polym13162807
APA StyleAkopova, T. A., Demina, T. S., Khavpachev, M. A., Popyrina, T. N., Grachev, A. V., Ivanov, P. L., & Zelenetskii, A. N. (2021). Hydrophobic Modification of Chitosan via Reactive Solvent-Free Extrusion. Polymers, 13(16), 2807. https://doi.org/10.3390/polym13162807








