Synthesis and Characterization of Conducting PANDB/χ-Al2O3 Core-Shell Nanocomposites by In Situ Polymerization
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
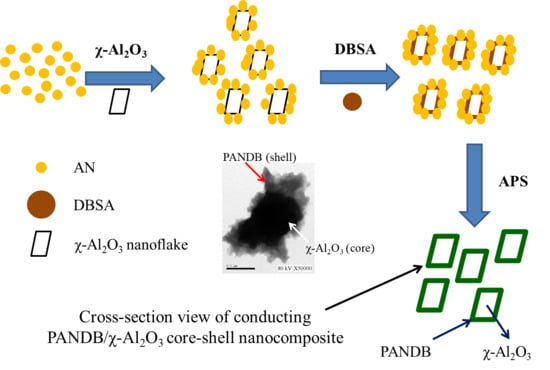
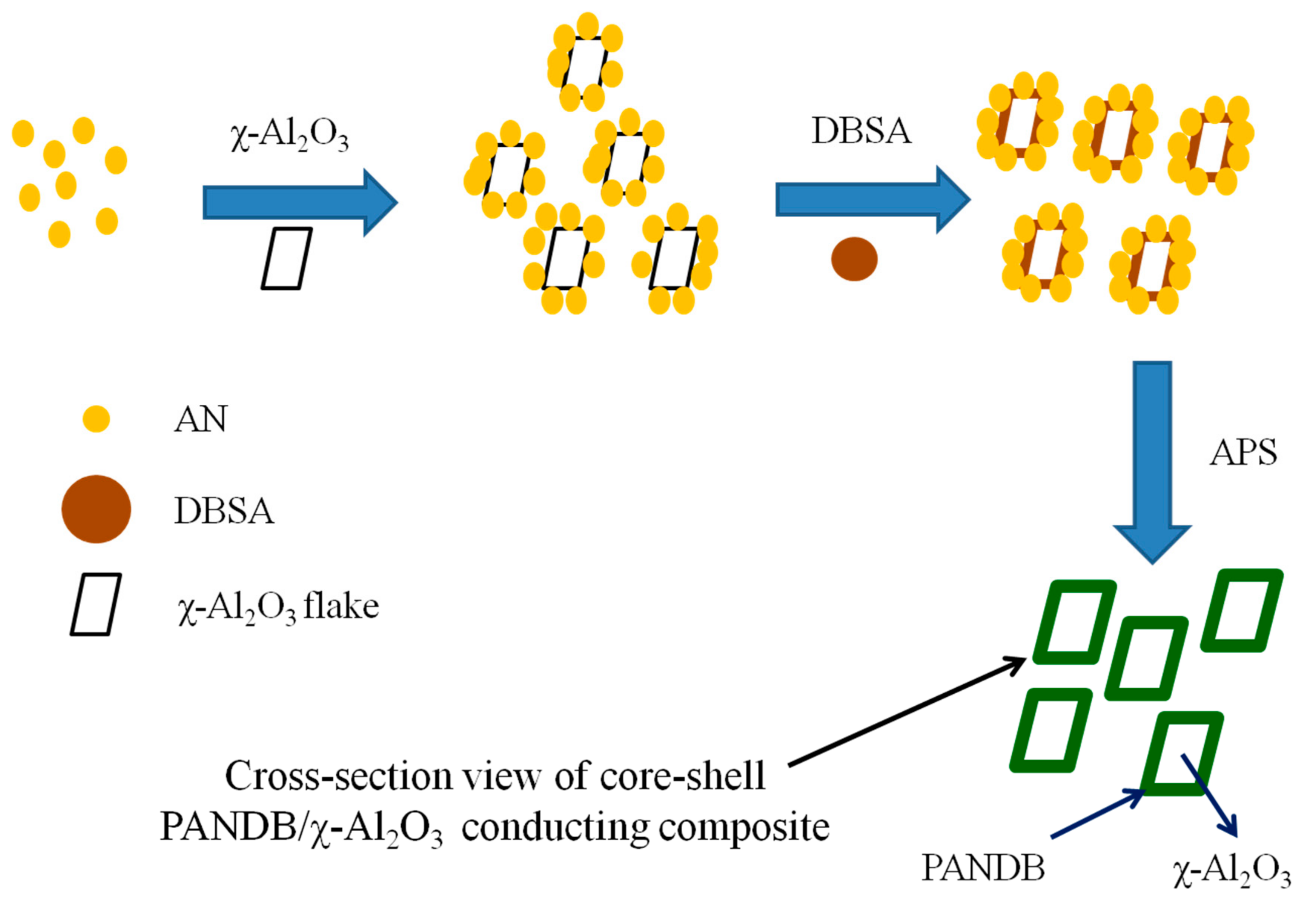
2.2. Synthesis of Pure PANDB and Conducting PANDB/χ-Al2O3 Core-Shell Nanocomposites
2.3. Fabrication of Conducting WPU/PANDB/χ-Al2O3 Blend Film
2.4. Characterization
2.4.1. Electrical Conductivity and Surface Resistance Analysis
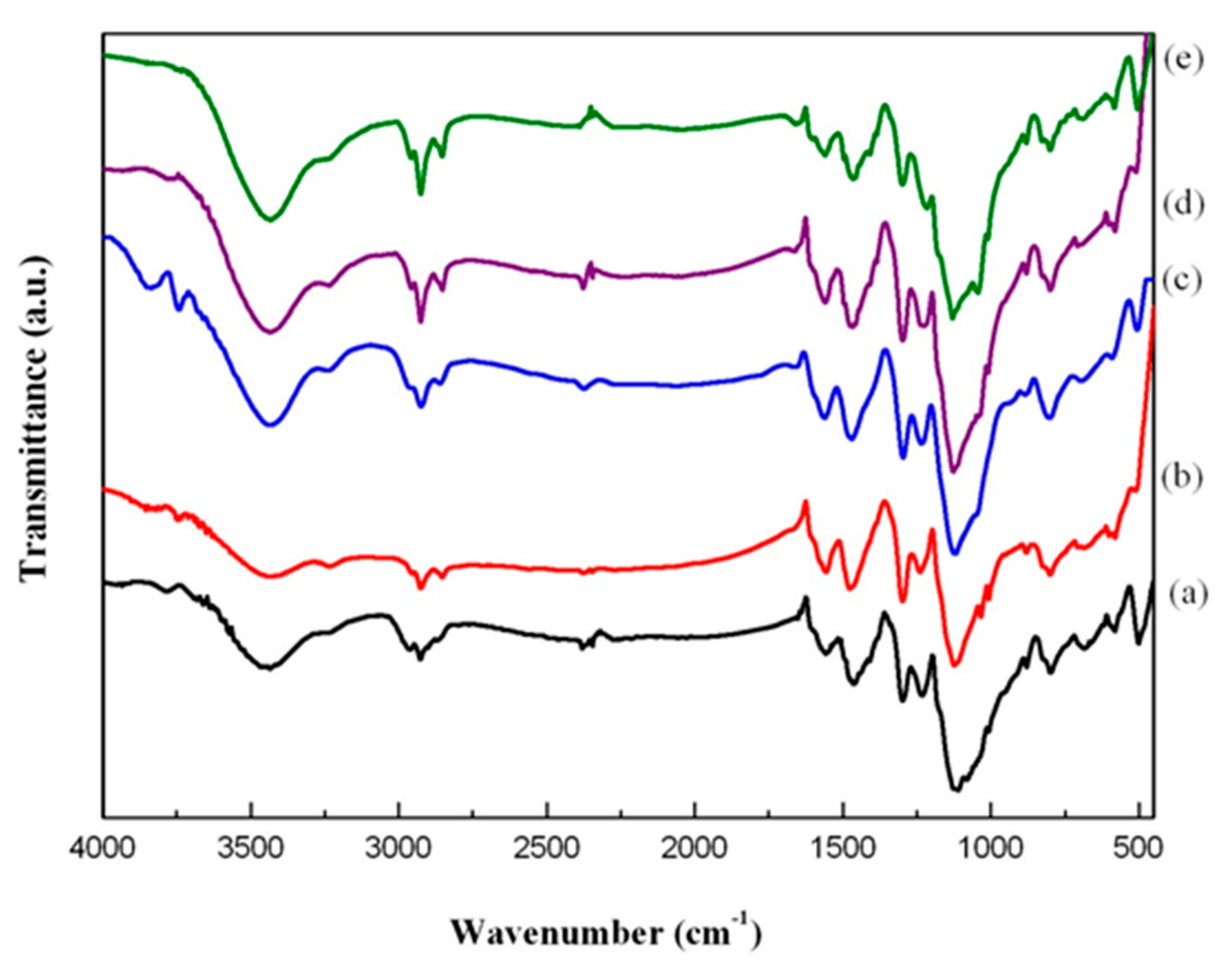
2.4.2. FTIR Analysis
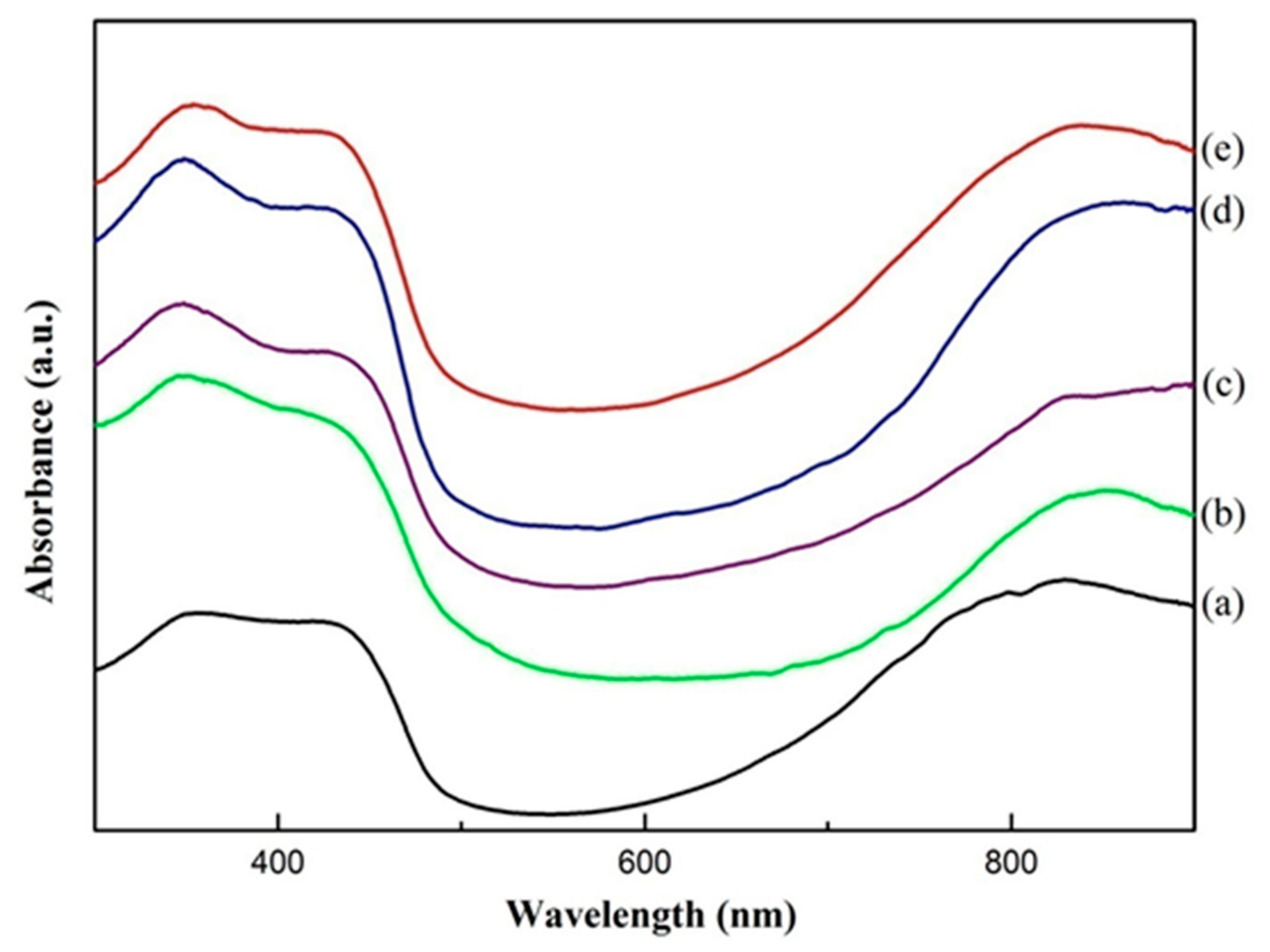
2.4.3. UV-Vis Analysis
2.4.4. XRD Analysis
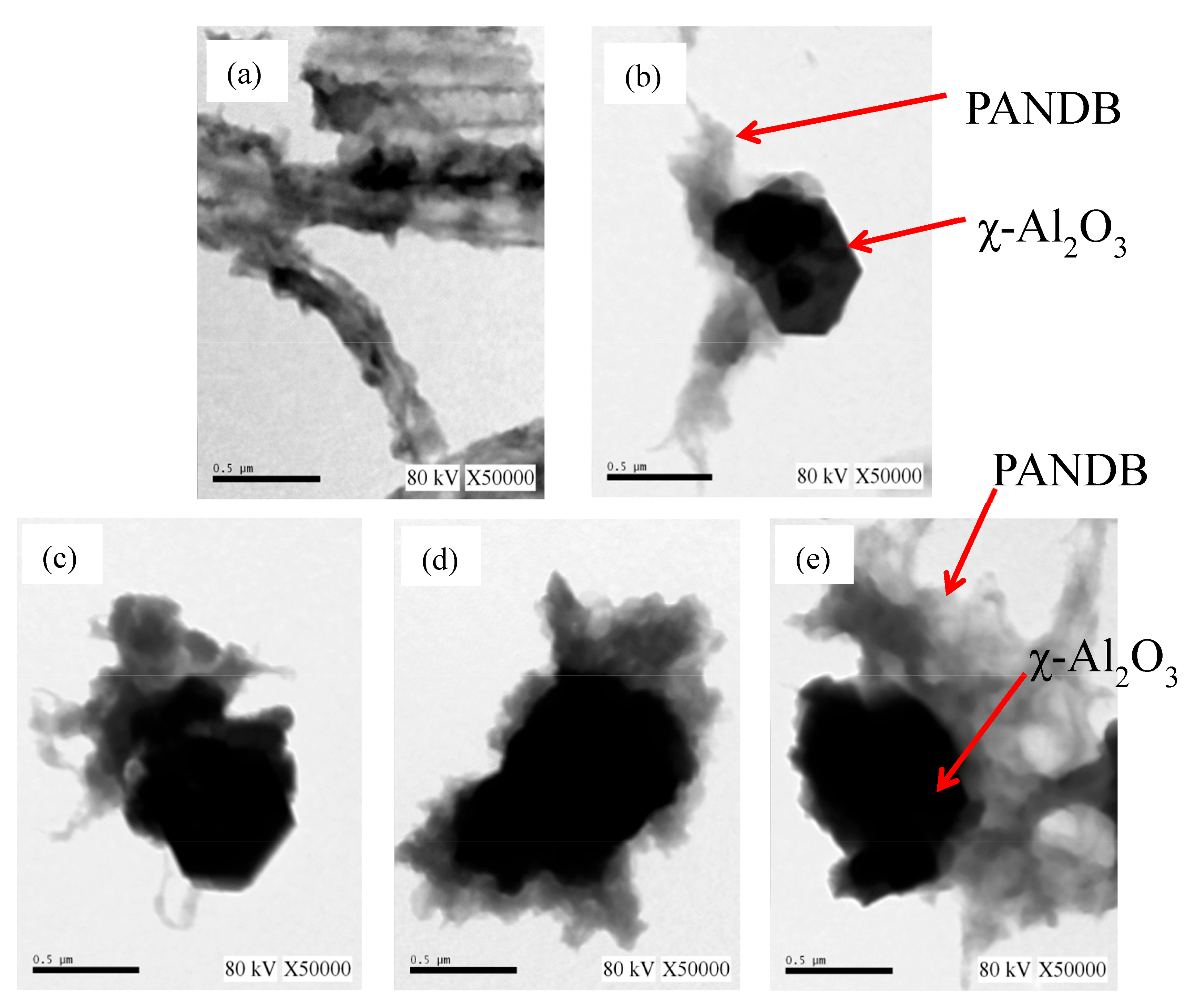
2.4.5. TEM Examination
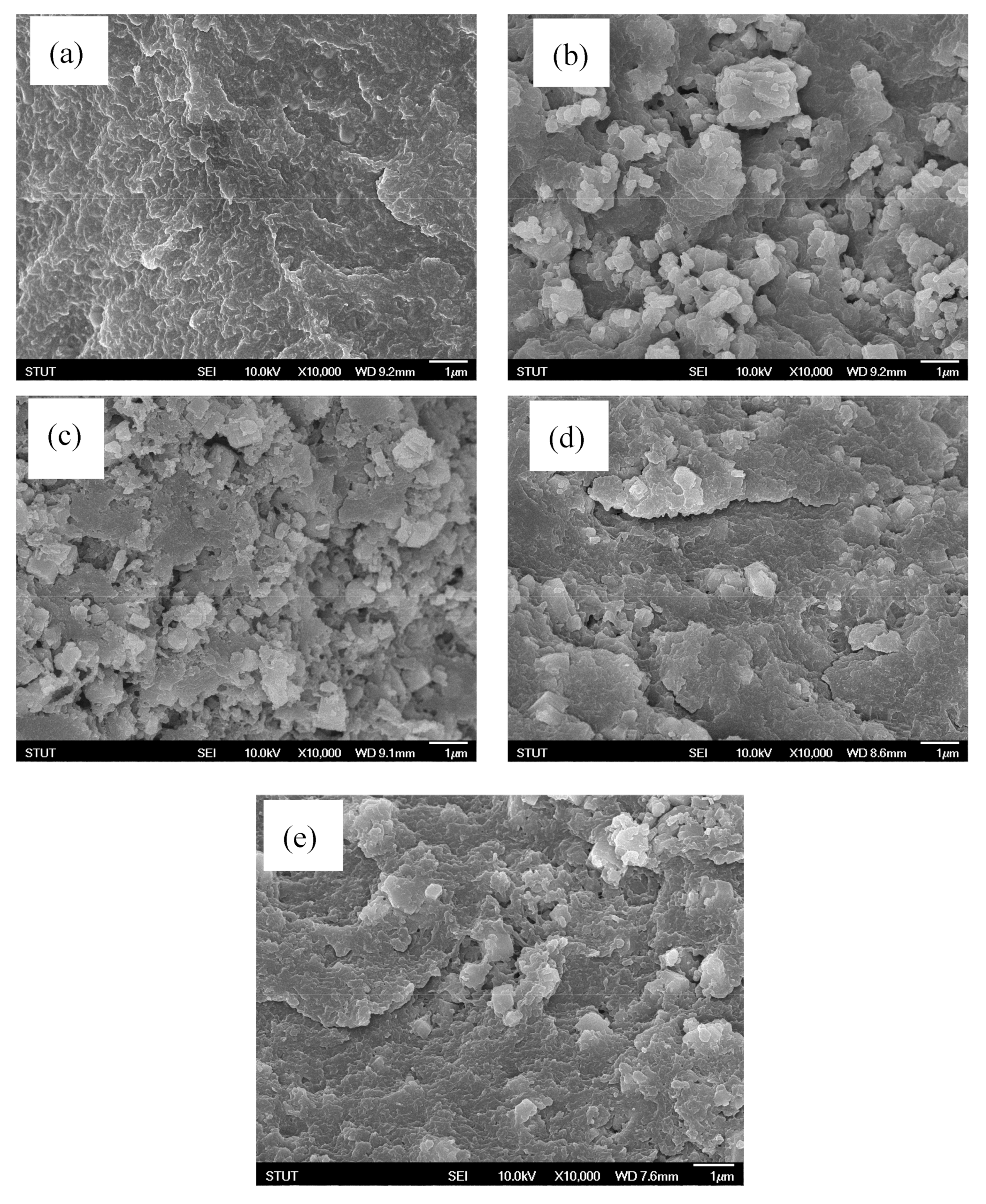
2.4.6. FE-SEM Examination
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, Y.; Jang, G.-W.; Hsueh, K.F.; Scherr, E.M.; MacDiarmid, A.G.; Epstein, A.J. Thermal transitions and mechanical properties of films of chemically prepared polyaniline. Polymers 1992, 33, 314–322. [Google Scholar] [CrossRef]
- Chen, S.A.; Lee, H.T. Polyaniline plasticized with 1-methyl-2-pyrrolidone: Structure and doping behavior. Macromolecules 1993, 26, 3254–3261. [Google Scholar] [CrossRef]
- Wei, Y.; Hsueh, K.F. Thermal analysis of chemically synthesized polyaniline and effects of thermal aging on conductivity. J. Polym. Sci. Part A Polym. Chem. 1989, 27, 4351–4363. [Google Scholar] [CrossRef]
- Zhang, F.; Cui, W.; Wang, B.; Xu, B.; Liu, X.; Liu, X.; Jia, Z.; Wu, G. Morphology-control synthesis of polyaniline decorative porous carbon with remarkable electromagnetic wave absorption capabilities. Compos. B Eng. 2021, 204, 108491. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Lu, H.; Wang, Y.; Xu, J.; Zhu, J.; Zhang, C.; Liu, T. Cryopolymerization enables anisotropic polyaniline hybrid hydrogels with superelasticity and highly deformation-tolerant electrochemical energy storage. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, H.; Shi, Y.; Yu, X.; Lan, G. Preparation and Gas Sensing Properties of PANI/SnO2 Hybrid Material. Polymers 2021, 13, 1360. [Google Scholar] [CrossRef] [PubMed]
- Shim, G.; Tran, M.X.; Liu, G.; Byun, D.; Lee, J.K. Flexible, fiber-shaped, quasi-solid-state Zn-polyaniline batteries with methanesulfonic acid-doped aqueous gel electrolyte. Energy Storage Mater. 2021, 35, 739–749. [Google Scholar] [CrossRef]
- Ryu, K.S.; Kim, K.M.; Kang, S.-G.; Lee, G.J.; Joo, J.; Chang, S.H. Electrochemical and physical characterization of lithium ionic salt doped polyaniline as a polymer electrode of lithium secondary battery. Synth. Met. 2000, 110, 213–217. [Google Scholar] [CrossRef]
- Mello, H.J.N.P.D.; Mulato, M. Enzymatically functionalized polyaniline thin films produced with one-step electrochemical immobilization and its application in glucose and urea potentiometric biosensors. Biomed. Microdevices 2020, 22, 1–9. [Google Scholar] [CrossRef]
- Shoaie, N.; Daneshpour, M.; Azimzadeh, M.; Mahshid, S.; Khoshfetrat, S.M.; Jahanpeyma, F.; Gholaminejad, A.; Omidfar, K.; Foruzandeh, M. Electrochemical sensors and biosensors based on the use of polyaniline and its nanocomposites: A review on recent advances. Microchim. Acta 2019, 186, 465. [Google Scholar] [CrossRef]
- Pud, A.; Shapoval, G.; Kamarchik, P.; Ogurtsov, N.; Gromovaya, V.; Myronyuk, I.; Kontsur, Y. Electrochemical behavior of mild steel coated by polyaniline doped with organic sulfonic acids. Synth. Met. 1999, 107, 111–115. [Google Scholar] [CrossRef]
- Yang, N.; Yang, T.; Wang, W.; Chen, H.; Li, W. Polydopamine modified polyaniline-graphene oxide composite for enhancement of corrosion resistance. J. Hazard. Mater. 2019, 377, 142–151. [Google Scholar] [CrossRef]
- Wong, P.-Y.; Phang, S.-W.; Baharum, A. Effects of synthesised polyaniline (PAni) contents on the anti-static properties of PAni-based polylactic acid (PLA) films. RSC Adv. 2020, 10, 39693–39699. [Google Scholar] [CrossRef]
- Ahn, S.; Park, M.; Jeong, S.; Kim, Y.; Park, J.; Kim, S.; Kim, H.; Cho, H.; Wolf, C.; Pei, M.; et al. Fine Control of Perovskite Crystallization and Reducing Luminescence Quenching Using Self-Doped Polyaniline Hole Injection Layer for Efficient Perovskite Light-Emitting Diodes. Adv. Funct. Mater. 2019, 29, 1807535. [Google Scholar] [CrossRef]
- Kowsari, E.; Faraghi, G. Ultrasound and ionic-liquid-assisted synthesis and characterization of polyaniline/Y2O3 nanocomposite with controlled conductivity. Ultrason. Sonochem. 2010, 17, 718–725. [Google Scholar] [CrossRef]
- Shang, Q.; Feng, H.; Liu, J.; Lian, Q.; Feng, Z.; Chen, N.; Qiu, J.; Wu, H. Constructing and optimizing hollow ZnxFe3-xO4@polyaniline composites as high-performance microwave absorbers. J. Colloid Interface Sci. 2021, 584, 80–91. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Z.; Lu, W.; Huang, T.; Hu, S. Preparation of core–shell structured T-ZnOw/polyaniline composites via graft polymerization. Mater. Chem. Phys. 2009, 115, 258–262. [Google Scholar] [CrossRef]
- Jangid, N.K.; Jadoun, S.; Yadav, A.; Srivastava, M.; Kaur, N. Polyaniline-TiO2-based photocatalysts for dyes degradation. Polym. Bull. 2021, 78, 4743–4777. [Google Scholar] [CrossRef]
- Pan, L.; Pu, L.; Shi, Y.; Song, S.; Xu, Z.; Zhang, R.; Zheng, Y. Synthesis of polyaniline nanotubes with a reactive template of manganese oxide. Adv. Mater. 2007, 19, 461–464. [Google Scholar] [CrossRef]
- Kumar, N.; Bahl, T.; Kumar, R. Study of the methylene blue adsorption mechanism using ZrO2/Polyaniline nanocomposite. Nano Express 2020, 1, 030025. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Rajakarthihan, S. Antimicrobial study on gamma-irradiated polyaniline–aluminum oxide (PANI–Al2O3) nanoparticles. Int. Nano Lett. 2020, 10, 97–110. [Google Scholar] [CrossRef]
- Resan, S.A.; Essa, A.F. Preparation and study of the optical properties for polyaniline-Al2O3 nanocomposite. Mater. Today Proc. 2021, 45, 5819–5822. [Google Scholar] [CrossRef]
- Qi, Y.-N.; Xu, F.; Sun, L.-X.; Zeng, J.-L.; Liu, Y.-Y. Thermal stability and glass transition behavior of PANI/α-Al2O3 composites. J. Therm. Anal. Calorim. 2008, 94, 553–557. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, P.; Su, Z. Core-shell attapulgite@polyaniline composite particles via in situ oxidative polymerization. Synth. Met. 2007, 157, 585–591. [Google Scholar] [CrossRef]
- Kalotra, S.; Mehta, R. Synthesis of polyaniline/clay nanocomposites by in situ polymerization and its application for the removal of Acid Green 25 dye from wastewater. Polym. Bull. 2021, 78, 2439–2463. [Google Scholar] [CrossRef]
- Chen, C.-H. Thermal Studies of Polyaniline Doped with Dodecyl Benzene Sulfonic Acid Directly Prepared via Aqueous Dispersions. J. Polym. Res. 2002, 9, 195–200. [Google Scholar] [CrossRef]
- Chen, C.-H.; Kan, Y.-T.; Mao, C.-F.; Liao, W.-T.; Hsieh, C.-D. Fabrication and characterization of water-based polyurethane/polyaniline conducting blend films. Surf. Coat. Technol. 2013, 231, 71–76. [Google Scholar] [CrossRef]
- Zhang, Z.; Wan, M. Composite films of nanostructured polyaniline with poly(vinyl alcohol). Synth. Met. 2002, 128, 83–89. [Google Scholar] [CrossRef]
- Zhou, S.; Wu, T.; Kan, J. Effect of methanol on morphology of polyaniline. Eur. Polym. J. 2007, 43, 395–402. [Google Scholar] [CrossRef]
- Abdiryim, T.; Xiao-Gang, Z.; Jamal, R. Comparative studies of solid-state synthesized polyaniline doped with inorganic acids. Mater. Chem. Phys. 2005, 90, 367–372. [Google Scholar] [CrossRef]
- Gao, Y.; Shan, D.; Cao, F.; Gong, J.; Li, X.; Ma, H.-Y.; Su, Z.-M.; Qu, L.-Y. Silver/Polyaniline Composite Nanotubes: One-Step Synthesis and Electrocatalytic Activity for Neurotransmitter Dopamine. J. Phys. Chem. C 2009, 113, 15175–15181. [Google Scholar] [CrossRef]

| Weight Ratio of AN/χ-Al2O3 | Conductivity (S/cm) |
|---|---|
| Pure PANDB | 6.3 × 10−1 |
| 0.5 | 0.6 × 10−1 |
| 1.0 | 1.1 × 10−1 |
| 1.5 | 1.7 × 10−1 |
| 2.0 | 1.7 × 10−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-H.; Lin, Y.-C.; Yen, F.-S. Synthesis and Characterization of Conducting PANDB/χ-Al2O3 Core-Shell Nanocomposites by In Situ Polymerization. Polymers 2021, 13, 2787. https://doi.org/10.3390/polym13162787
Chen C-H, Lin Y-C, Yen F-S. Synthesis and Characterization of Conducting PANDB/χ-Al2O3 Core-Shell Nanocomposites by In Situ Polymerization. Polymers. 2021; 13(16):2787. https://doi.org/10.3390/polym13162787
Chicago/Turabian StyleChen, Cheng-Ho, Ying-Chen Lin, and Fu-Su Yen. 2021. "Synthesis and Characterization of Conducting PANDB/χ-Al2O3 Core-Shell Nanocomposites by In Situ Polymerization" Polymers 13, no. 16: 2787. https://doi.org/10.3390/polym13162787
APA StyleChen, C.-H., Lin, Y.-C., & Yen, F.-S. (2021). Synthesis and Characterization of Conducting PANDB/χ-Al2O3 Core-Shell Nanocomposites by In Situ Polymerization. Polymers, 13(16), 2787. https://doi.org/10.3390/polym13162787