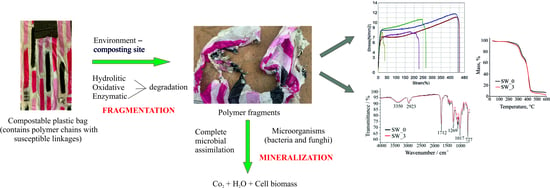
Influence of Home Composting on Tensile Properties of Commercial Biodegradable Plastic Films
Abstract
1. Introduction
2. Materials and Methods
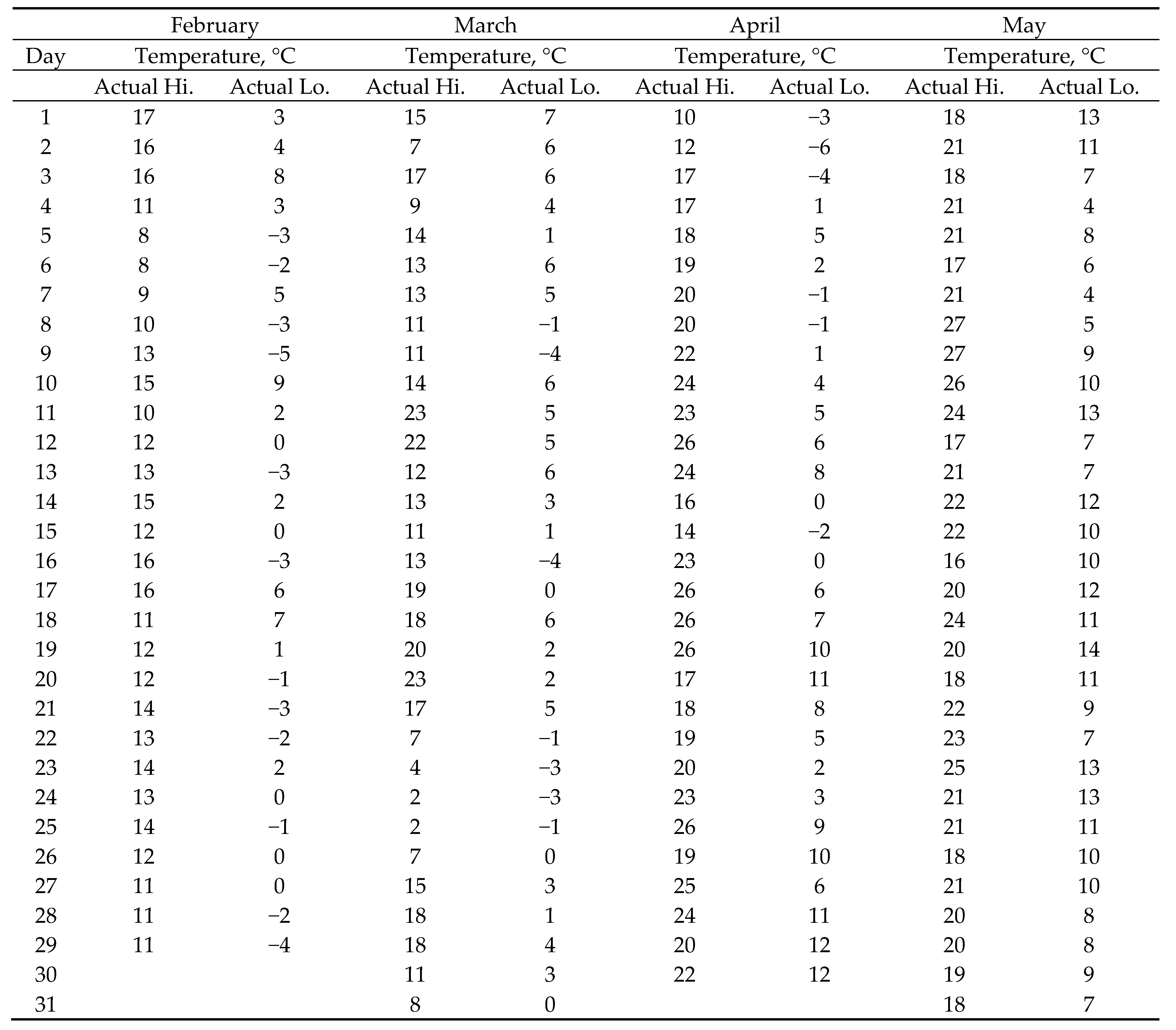
2.1. Atmospheric Conditions during Testing
2.2. Tested Films and Bags
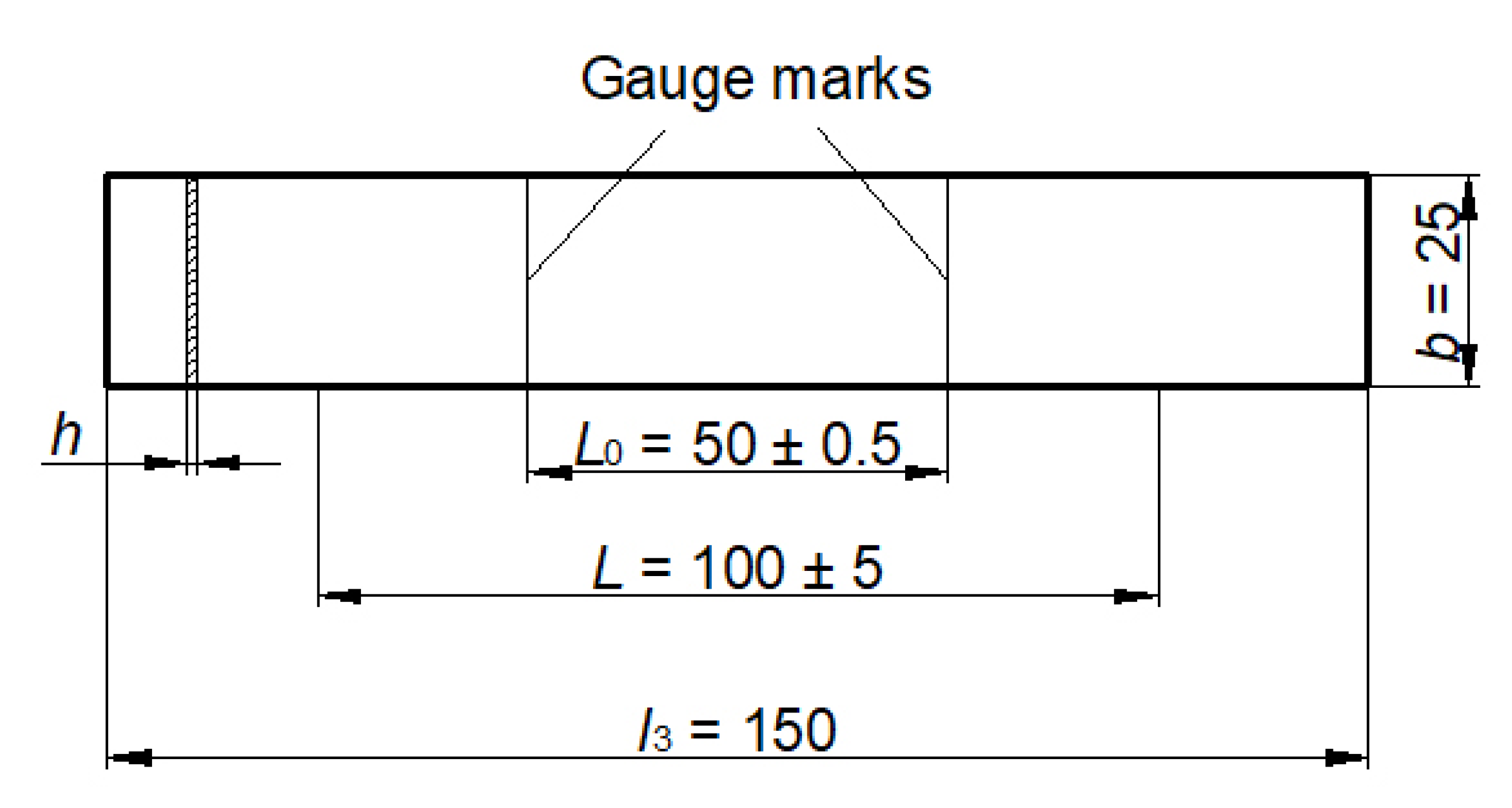
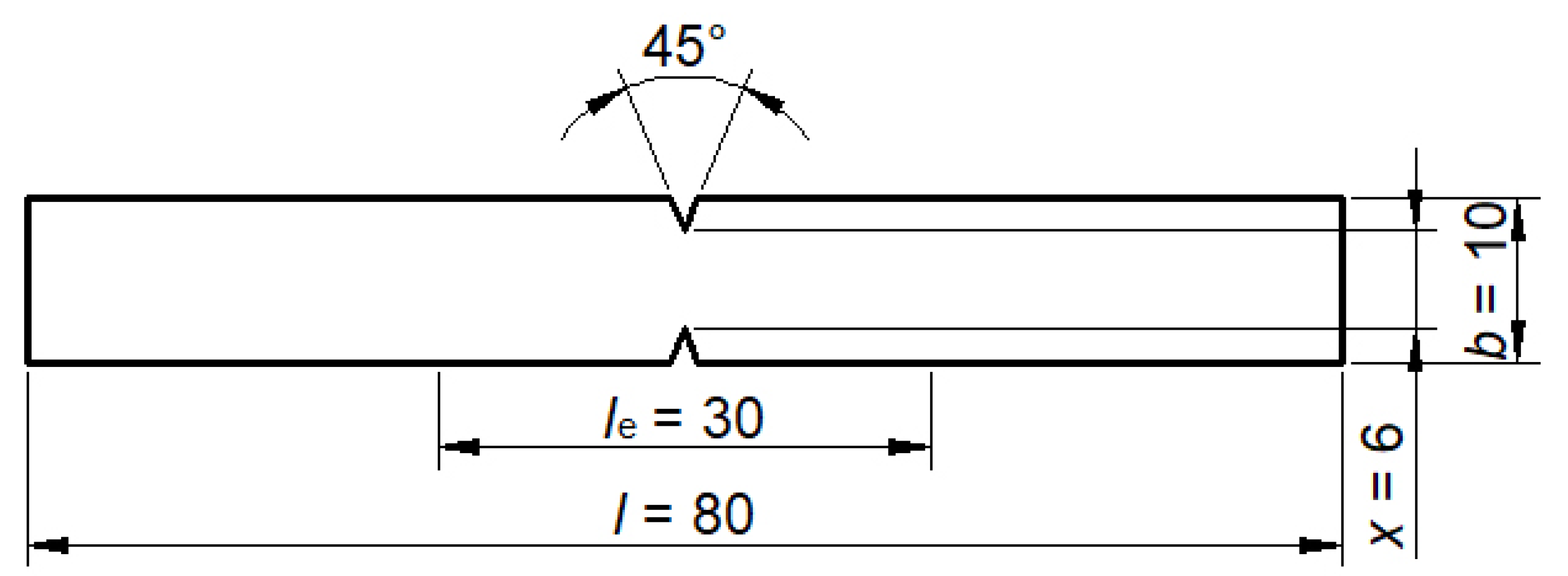
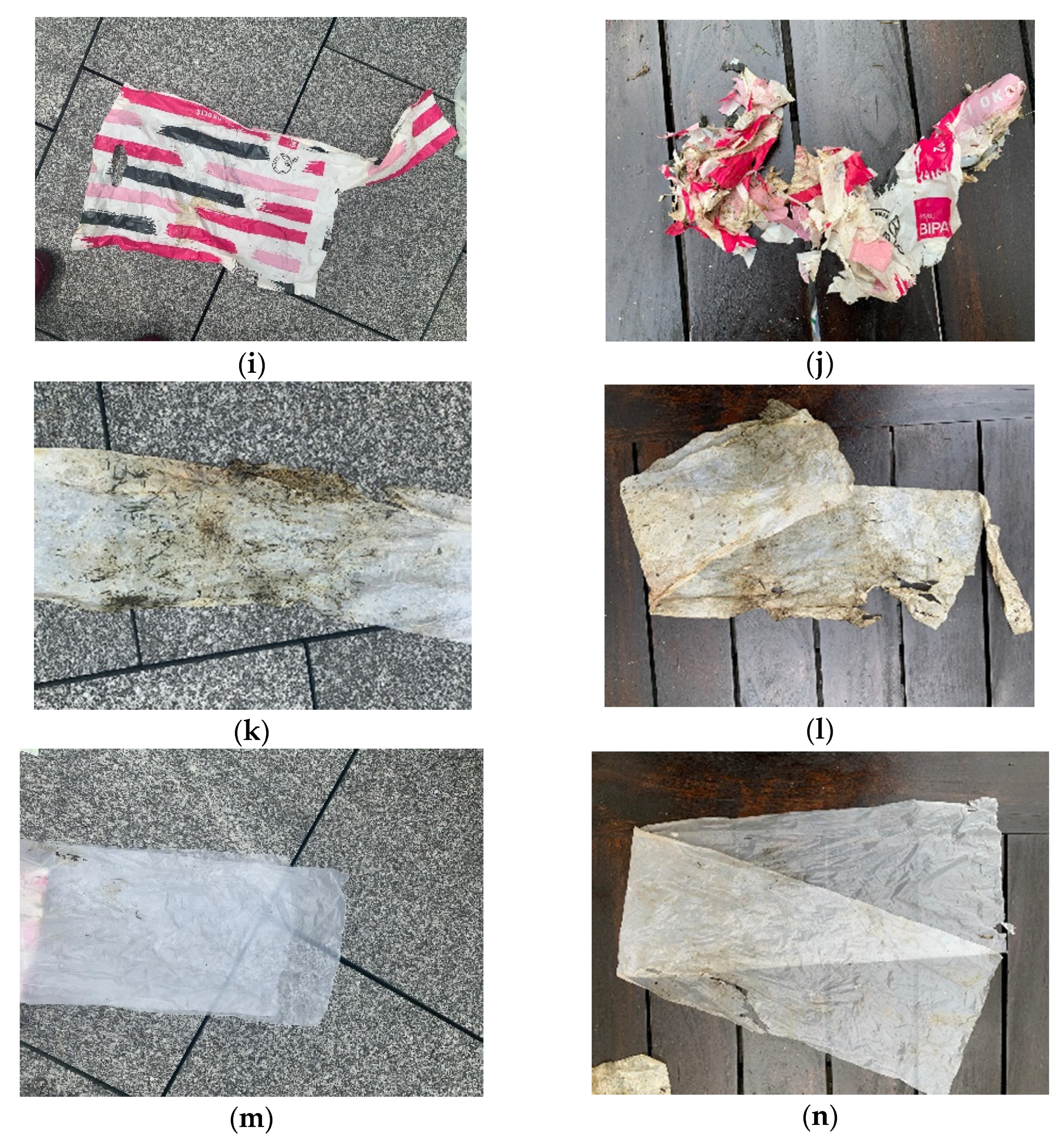
2.3. Conducting the Experiment
3. Results
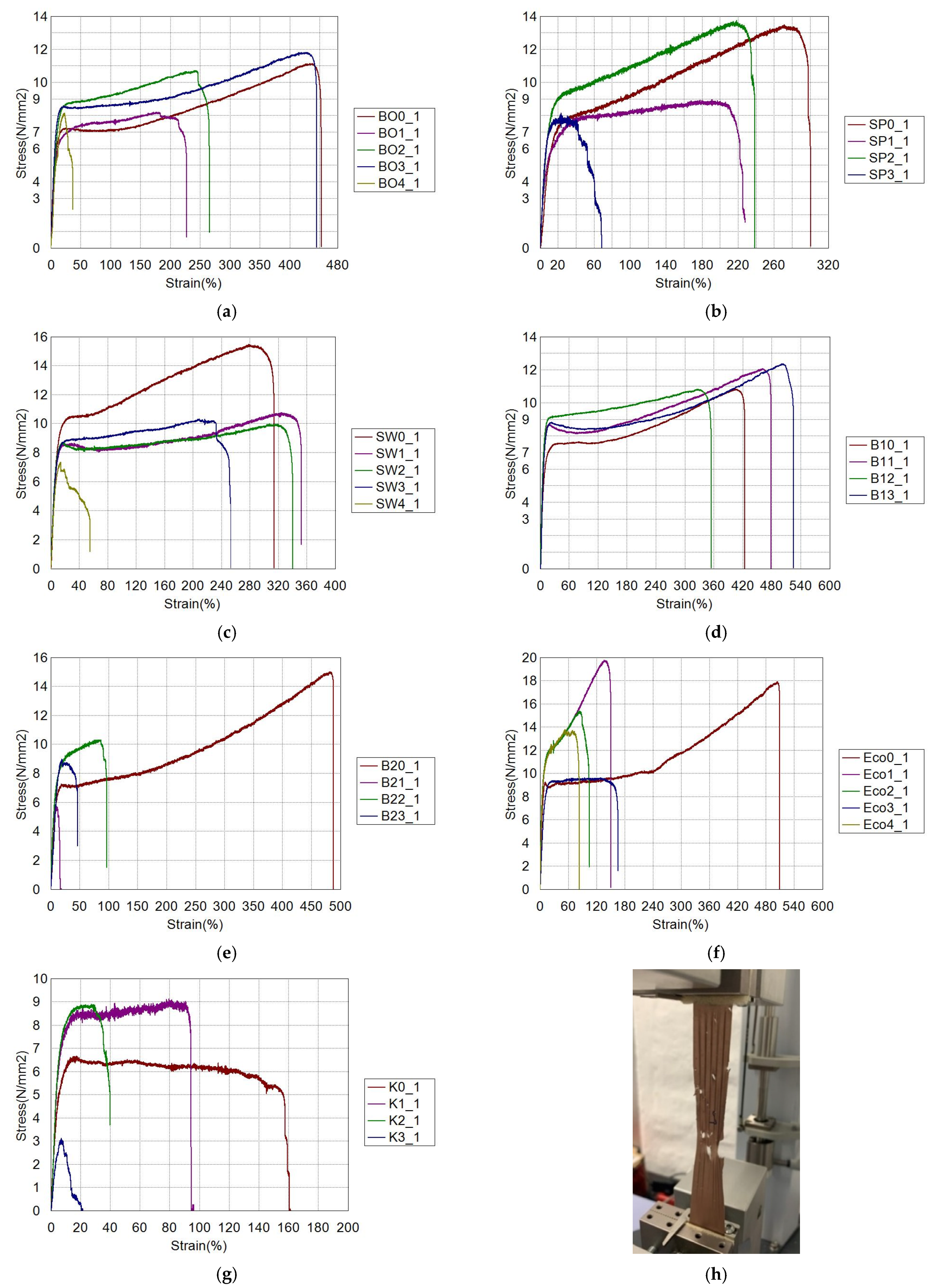
3.1. Tensile Properties
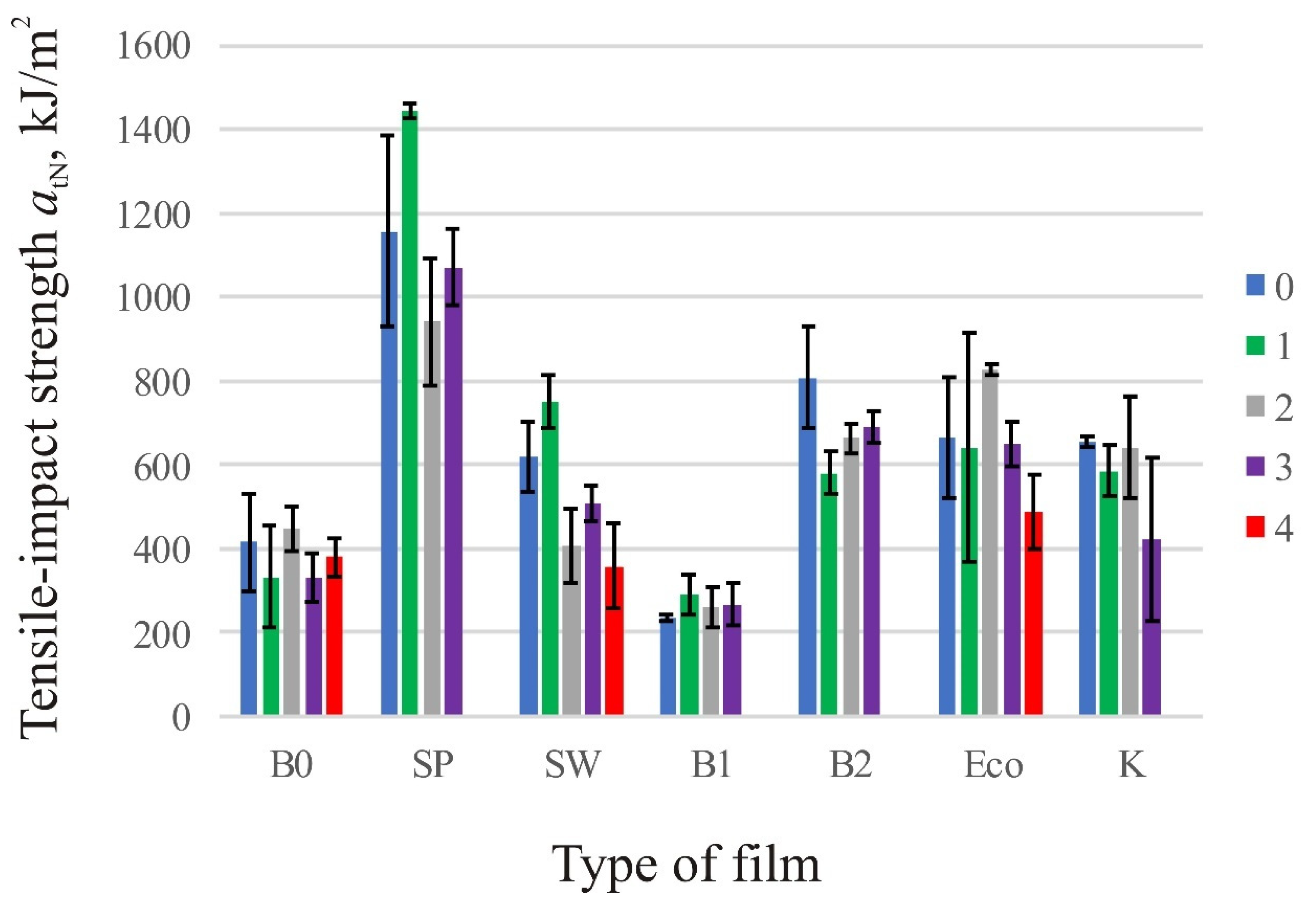
3.2. Tensile-Impact Strength
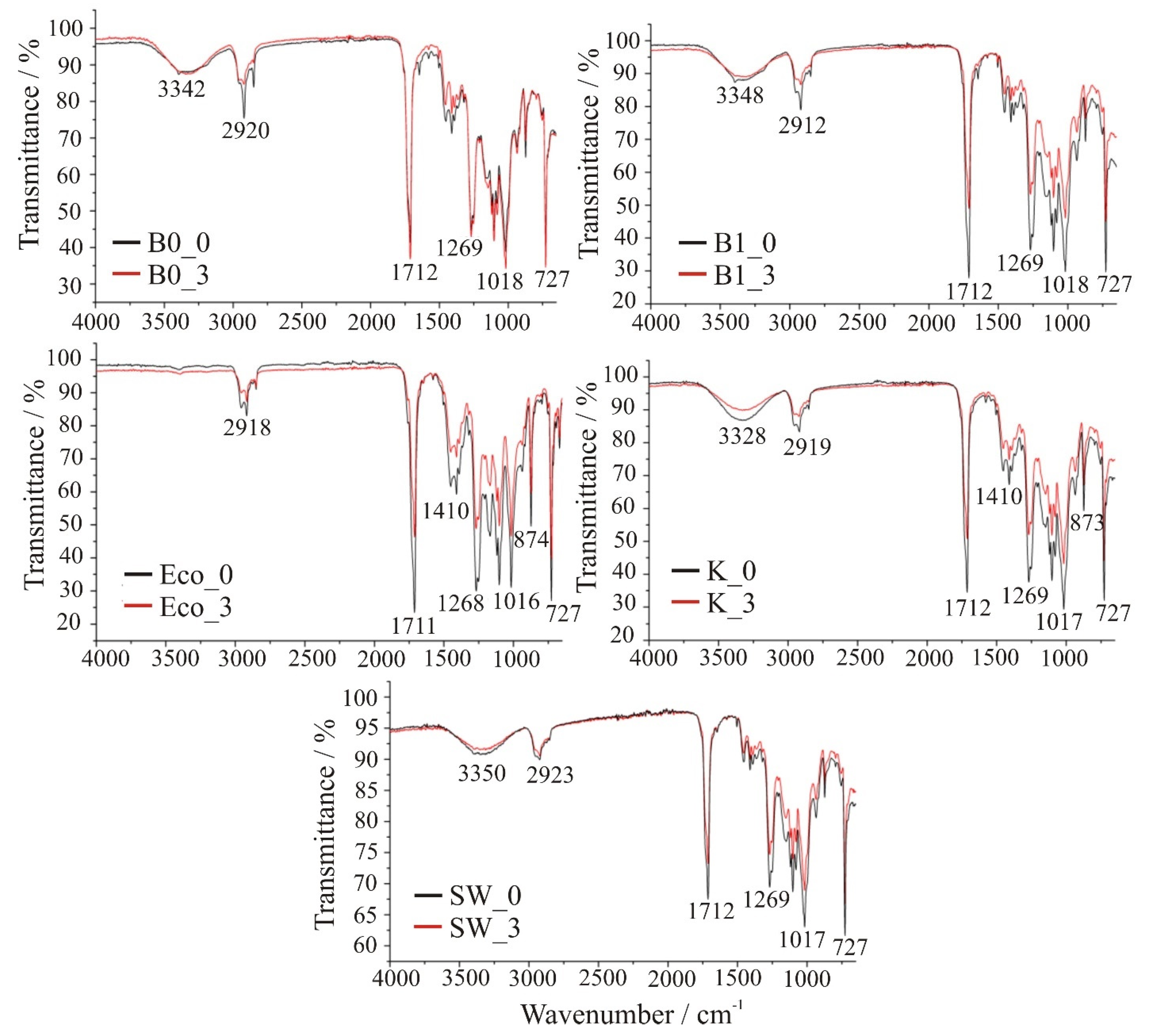
3.3. Fourier-Transform Infrared Spectroscopy
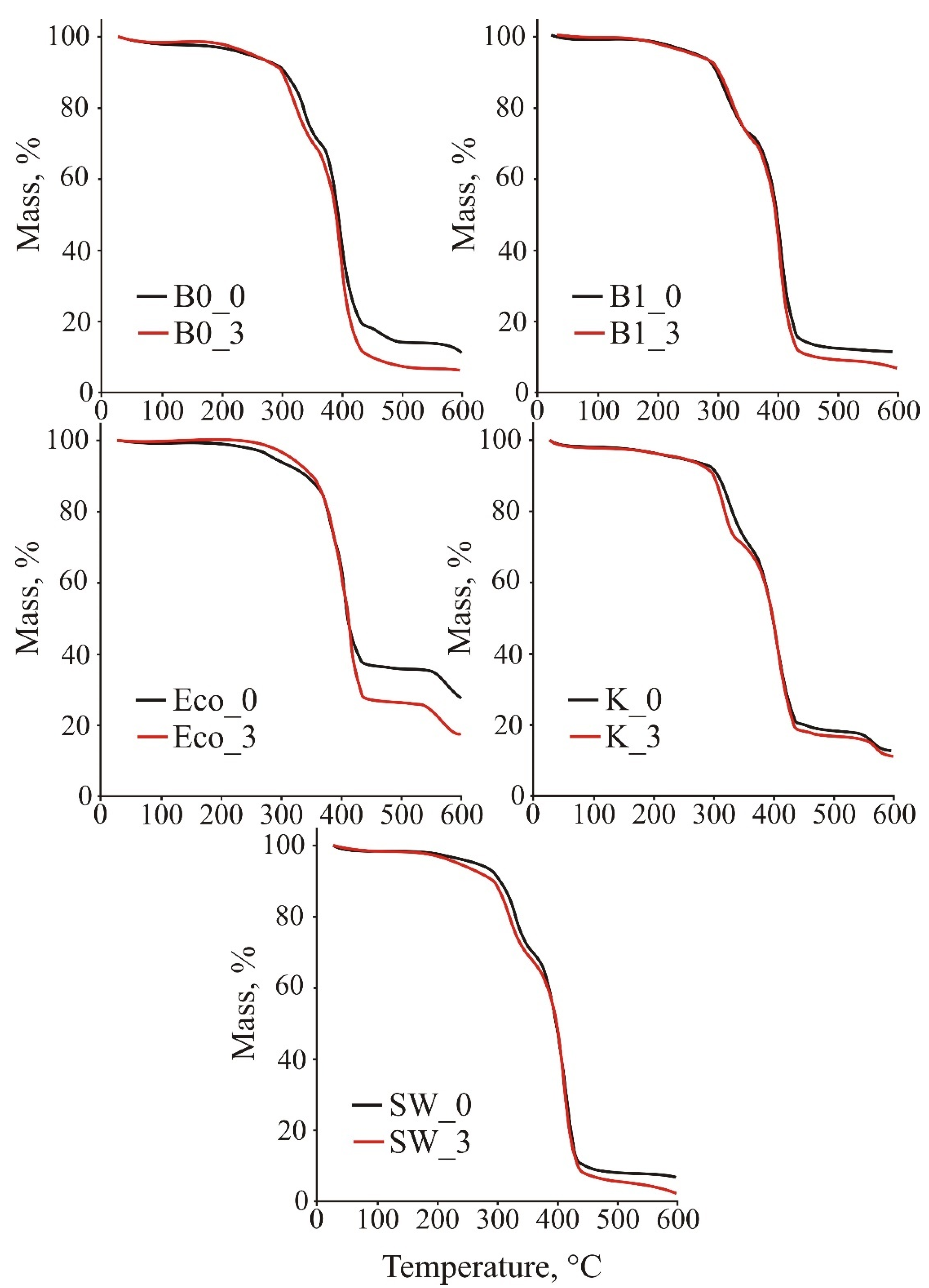
3.4. Thermogravimetric Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adamcová, D.; Vaverková, M.D.; Mašíček, T.; Břoušková, E. Analysis of biodegrability of degradable/biodegradable plastic material in controlled composting environment. J. Ecol. Eng. 2016, 17, 1–10. [Google Scholar] [CrossRef][Green Version]
- Letcher, T. Introduction to plastic waste and recycling. In Plastic Waste and Recycling: Environmental Impact, Societal Issues, Prevention, and Solutions; Letcher, T., Ed.; Academic Press: Cambridge, MA, USA, 2020; Available online: https://www.elsevier.com/books/plastic-waste-and-recycling/letcher/978-0-12-817880-5 (accessed on 1 May 2021).
- Ren, X. Biodegradable plastics: A solution or a challenge? J. Clean. Prod. 2003, 11, 27–40. [Google Scholar] [CrossRef]
- Rujnić Havstad, M. Biodegradable plastics. In Plastic Waste and Recycling: Environmental Impact, Societal Issues, Prevention, and Solutions; Letcher, T., Ed.; Academic Press: Cambridge, MA, USA, 2020; Available online: https://www.elsevier.com/books/plastic-waste-and-recycling/letcher/978-0-12-817880-5 (accessed on 12 May 2021).
- Lambert, S.; Wagner, M. Environmental performance of bio-based and biodegradable plastics: The road ahead. Chem. Soc. Rev. 2017, 46, 6855–6871. [Google Scholar] [CrossRef] [PubMed]
- Folino, A.; Karageorgiou, A.; Calabrò, P.S.; Komilis, D. Biodegradation of Wasted Bioplastics in Natural and Industrial Environments: A Review. Sustainability 2020, 12, 6030. [Google Scholar] [CrossRef]
- Engla, G.; Soni, L.K.; Dixit, V.K. Biodegradable polymers: A smart strategy for today’s crucial needs. Crit. Rev. Pharm. Sci. 2014, 3, 1–70, ISSN 2319-1082. [Google Scholar]
- Vaverková, M.; Adamcová, D.; Zloch, J. How do degradable/biodegradable plastic materials decompose in home composting environment? J. Ecol. Engineering. 2014, 15, 82–89. [Google Scholar] [CrossRef]
- Quecholac-Piña, X.; García-Rivera, M.A.; Espinosa-Valdemar, R.M.; Vázquez-Morillas, A.; Beltrán-Villavicencio, M.; Cisneros-Ramos, A.L. Biodegradation of compostable and oxodegradable plastic films by backyard composting and bioaugmentation. Env. Sci. Pollut. Res. Int. 2017, 24, 25725–25730. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Hernandez, J.C.; Capowiez, Y.; Ro, K.S. Potential Use of Earthworms to Enhance Decaying of Biodegradable Plastics. ACS Sustain. Chem. Eng. 2020, 8, 4292–4316. [Google Scholar] [CrossRef]
- Narancic, T.; Verstichel, S.; Chaganti, S.R.; Morales-Gamez, L.; Kenny, S.T.; De Wilde, B.; Padamati, R.P.; O’Connor, K.E. Biodegradable Plastic Blends Create New Possibilities for End-of-Life Management of Plastics but They Are Not a Panacea for Plastic Pollution. Environ. Sci. Technol. 2018, 52, 10441–10452. [Google Scholar] [CrossRef] [PubMed]
- Juroš, L. Comparison of Mechanical Properties of Biodegradable Household Bags. Diploma Thesis, FSB, Zagreb, Croatia, 2020. [Google Scholar]
- Hazrati, H.; Shayegan, J.; Seyedi, S.M. Biodegradation kinetics and interactions of styrene and ethylbenzene as single and dual substrates for a mixed bacterial culture. J. Environ. Health Sci. Eng. 2015, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Tosin, M.; Pischedda, A.; Degli-Innocenti, F. Biodegradation kinetics in soil of a multi-constituent biodegradable plastic. Polym. Degrad. Stab. 2019, 166, 213–218. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H. Biodegradable Polymers “Biodegradation-Life of Science”. Chamy, R., Rosenkranz, F., Eds.; IntechOpen, Open Access Peer-Reviewed Chapter 2013. pp. 141–185. Available online: https://www.intechopen.com/chapters/45095 (accessed on 2 June 2020). [CrossRef]
- Accuweather. Available online: https://www.accuweather.com/hr/hr/zagreb/117910/may-weather/117910?year=2020 (accessed on 2 June 2020).
- HRN EN ISO 527-3:2019 Plastics—Determination of Tensile Properties—Part 3: Test Conditions for Films and Sheets (ISO 527-3:2019); International Organization for Standardization, Croatian Standards Institute: Zagreb, Croatia, 2019.
- HRN EN ISO 8256:2004 Plastics—Determination of Tensile-Impact Strength (ISO 8256:2004); International Organization for Standardization, Croatian Standards Institute: Zagreb, Croatia, 2014.
- Czaja-Jagielska, N.; Melski, K. Biodegradation of Starch-Based Films in Conditions of Nonindustrial Composting. Pol. J. Environ. Stud. 2013, 22, 1039–1044. Available online: http://www.pjoes.com/Biodegradation-of-Starch-Based-Films-r-nin-Conditions-of-Nonindustrial-Composting,89060,0,2.html (accessed on 12 May 2021).
- Mostafa, H.M.; Sourell, H.; Bockisch, F.J. Mechanical properties of some bioplastics under different soil types used as biodegradable drip tubes. Agric. Eng. Int. CIGR J. 2010, 12, 12–21. Available online: https://www.researchgate.net/publication/277994731_Mechanical_properties_of_some_bioplastics_under_different_soil_types_used_as_biodegradable_drip_tubes (accessed on 20 May 2021).
- Scarascia-Mugnozza, G.; Schettini, E.; Vox, G.; Malinconico, M.; Immirzi, B.; Pagliara, S. Mechanical properties decay and morphological behaviour of biodegradable films for agricultural mulching in real scale experiment. Polym. Degrad. Stab. 2006, 91, 2801–2808. [Google Scholar] [CrossRef]
- Irska, I.; Paszkiewicz, S.; Gorący, K.; Linares, A.; Ezquerra, T.A.; Jędrzejewski, R.; Rosłaniec, Z.; Piesowicz, E. Poly(butylene terephthalate)/polylactic acid based copolyesters and blends: Miscibility-structure-property relationship. Express Polym. Lett. 2020, 14, 26–47. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, W.; Wu, Q.; Ren, J. Preparation, Mechanical, and Thermal Properties of Biodegradable Polyesters/Poly(Lactic Acid) Blends. J. Nanomater. 2010, 287082, 1–8. [Google Scholar] [CrossRef]
- Weng, Y.-X.; Jin, Y.-J.; Meng, Q.-Y.; Wang, L.; Zhang, M.; Wang, Y.-Z. Biodegradation behavior of poly(butylene adipate-co-terephthalate) (PBAT), poly(lactic acid) (PLA), and their blend under soil conditions. Polym. Test. 2013, 32, 918–926. [Google Scholar] [CrossRef]
- Lomelí-Ramírez, M.G.; Barrios-Guzmán, A.J.; García-Enriquez, S.; Rivera-Prado, J.J.; Manríquez-González, R. Chemical and Mechanical Evaluation of Bio-composites Based on Thermoplastic Starch and Wood Particles Prepared by Thermal Compression. Bioresources 2014, 9, 2960–2974. Available online: https://bioresources.cnr.ncsu.edu/resources/chemical-and-mechanical-evaluation-of-bio-composites-based-on-thermoplastic-starch-and-wood-particles-prepared-by-thermal-compression/ (accessed on 24 May 2021). [CrossRef]
- Pigłowska, M.; Kurc, B.; Rymaniak, Ł.; Lijewski, P.; Fuć, P. Kinetics and Thermodynamics of Thermal Degradation of Different Starches and Estimation the OH Group and H2O Content on the Surface by TG/DTG-DTA. Polymers 2020, 12, 357. [Google Scholar] [CrossRef] [PubMed]

| Label | Certificates | Average Thickness, mm | Photo |
|---|---|---|---|
| B0 | DIN CERTCO 7P0324 TÜV AUSTRIA S0426 Industrial composting | 0.028 |  |
| SP | DIN CERTCO 7P03240 TÜV AUSTRIA S0426 Home composting | 0.011 |  |
| SW | DIN CERTCO 9G0087 TÜV AUSTRIA 7P0002 Industrial composting | 0.022 |  |
| B1, B2 | DIN CERTCO 7P0324 TÜV AUSTRIA S0426 B1 − Industrial composting B2 − Industrial and home composting | B1−0.04 B2−0.018 |  |
| Eco | DIN CERTCO 7W0188 Industrial composting | 0.017 |  |
| K | - | 0.019 |  |
| Tensile Strength, MPa | |||||
|---|---|---|---|---|---|
| Sample Name/ Months | 0 | 1 | 2 | 3 | 4 |
| B0 | 11.2 ± 0.5 | 8.7 ± 0.9 | 10.9 ± 0.9 | 12.1 ± 1.9 | 8.3 ± 0.1 |
| SP | 13.7 ± 2.2 | 9.9 ± 0.8 | 14.4 ± 4.3 | 8.4 ± 0.6 | - |
| SW | 15.9 ± 2.0 | 11.5 ± 1.7 | 10.2 ± 0.9 | 10.7 ± 0.8 | 7.6 ± 1.7 |
| B1 | 10.9 ± 1.7 | 12.2 ± 1.6 | 10.9 ± 0.8 | 12.5 ± 1.7 | - |
| B2 | 15.3 ± 0.9 | 6.2 ± 2.4 | 10.5 ± 0.9 | 9.3 ± 1.4 | - |
| Eco | 18.2 ± 3.6 | 20.2 ± 4.4 | 16.1 ± 6.2 | 10.5 ± 1.2 | 14.7 ± 3.5 |
| K | 7.3 ± 0.3 | 9.4 ± 0.8 | 9.0 ± 0.4 | 3.8 ± 1.3 | - |
| Tensile Strength at Break, MPa | |||||
|---|---|---|---|---|---|
| Sample Name/ Months | 0 | 1 | 2 | 3 | 4 |
| B0 | 11.1 ± 0.6 | 5.4 ± 3 | 7.9 ± 4.3 | 9.5 ± 3.1 | 4.8 ± 1.4 |
| SP | 10.0 ± 2.9 | 4.6 ± 1.3 | 10.9 ± 5.9 | 2.4 ± 1.0 | - |
| SW | 12.8 ± 5.1 | 8.3 ± 3 | 7.3 ± 1.8 | 6.7 ± 3.2 | 3.2 ± 3.1 |
| B1 | 9.9 ± 1.7 | 10.7 ± 1.2 | 7.7 ± 1.5 | 9.5 ± 3.5 | - |
| B2 | 14.3 ± 1.4 | 3.4 ± 2.4 | 8.2 ± 3.2 | 5.5 ± 2.3 | - |
| Eco | 17 ± 3.9 | 16.4 ± 6.2 | 10.9 ± 7.5 | 6.8 ± 2.1 | 10 ± 1.3 |
| K | 4.1 ± 0.6 | 7.8 ± 1.0 | 4.7 ± 1.0 | 0.4 ± 0.05 | - |
| Tensile Strain at Break, % | |||||
|---|---|---|---|---|---|
| Sample Name/ Months | 0 | 1 | 2 | 3 | 4 |
| B0 | 441 ± 10.1 | 227.3 ± 142.5 | 265.5 ± 56.9 | 444.9 ± 83.4 | 36.8 ± 7.7 |
| SP | 299 ± 65.6 | 225 ± 126.6 | 237.4 ± 80.2 | 66.7 ± 27.9 | - |
| SW | 313.6 ± 73.1 | 352.4 ± 162.2 | 339.2 ± 27.0 | 252 ± 111.7 | 55.5 ± 33.2 |
| B1 | 423.3 ± 156.2 | 477.8 ± 75.7 | 355 ± 53.7 | 525.2 ± 100.6 | - |
| B2 | 487.7 ± 45.0 | 15.8 ± 6.7 | 96.1 ± 40.8 | 47 ± 14.9 | - |
| Eco | 508.8 ± 24.0 | 150.6 ± 28.6 | 101.7 ± 46.5 | 165.5 ± 124.6 | 83.1 ± 43.0 |
| K | 159.5 ± 90.9 | 104 ± 21.6 | 40.1 ± 13.9 | 18.1 ± 13.5 | - |
| Tensile Modulus, MPa | |||||
|---|---|---|---|---|---|
| Sample Name/ Months | 0 | 1 | 2 | 3 | 4 |
| B0 | 98.1 ± 24.5 | 103.7 ± 17.3 | 91.8 ± 16.2 | 119.4 ± 20.8 | 103.4 ± 5.8 |
| SP | 73.9 ± 11.8 | 102.3 ± 2.3 | 105.5 ± 14.3 | 113.1 ± 27.9 | - |
| SW | 151.3 ± 28.8 | 123.7 ± 26.5 | 133.6 ± 27.9 | 144.5 ± 10.1 | 116.3 ± 17.1 |
| B1 | 105.2 ± 14.5 | 128.3 ± 19.2 | 122.4 ± 19.9 | 130.6 ± 10.0 | - |
| B2 | 100.5 ± 11.1 | 119.2 ± 21.5 | 118.1 ± 25.4 | 121.1 ± 48.7 | - |
| Eco | 182.3 ± 41.0 | 199.2 ± 55.3 | 243.7 ± 36.2 | 153 ± 40.7 | 230 ± 56.0 |
| K | 153.7 ± 0.6 | 151.8 ± 8.7 | 176.2 ± 23.8 | 106.6 ± 15.8 | - |
| Tensile-Impact Strength, kJ/m2 | |||||
|---|---|---|---|---|---|
| Sample Name/ Months | 0 | 1 | 2 | 3 | 4 |
| B0 | 414.4 ± 117.6 | 331.7 ± 121.2 | 448.6 ± 53.4 | 329.5 ± 57.2 | 379.5 ± 46.5 |
| SP | 1156.8 ± 226.1 | 1444.2 ± 16.5 | 940.5 ± 149.7 | 1070.8 ± 89.4 | - |
| SW | 619.1 ± 83.0 | 749.9 ± 63.2 | 406.6 ± 89.6 | 506.4 ± 43.9 | 358 ± 102.1 |
| B1 | 234.1 ± 8.1 | 292.1 ± 48.6 | 257.8 ± 48.2 | 266 ± 50.3 | - |
| B2 | 807 ± 121.1 | 578.9 ± 50.7 | 662.9 ± 33.8 | 690.2 ± 38.1 | - |
| Eco | 664 ± 143.6 | 640.9 ± 273.6 | 826 ± 14.1 | 651.5 ± 53.0 | 487 ± 88.9 |
| K | 654.8 ± 12.9 | 584.7 ± 61.5 | 641.7 ± 120.6 | 421.8 ± 194.2 | - |
| T95 (°C) | Tmax (°C) | r600 (%) | |
|---|---|---|---|
| B0_0 | 238.8 | 390.3 | 11.4 |
| B0_3 | 252.7 | 395.2 | 6.2 |
| B1_0 | 261.4 | 411.0 | 11.0 |
| B1_3 | 253.2 | 411.3 | 6.9 |
| Eco_0 | 293.2 | 401.6 | 28.0 |
| Eco_3 | 318.9 | 405.2 | 17.9 |
| K_0 | 260.5 | 410.3 | 12.8 |
| K_3 | 259.1 | 410.4 | 11.3 |
| SW_0 | 260.3 | 408.6 | 7.0 |
| SW_3 | 234.3 | 413.9 | 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rujnić Havstad, M.; Juroš, L.; Katančić, Z.; Pilipović, A. Influence of Home Composting on Tensile Properties of Commercial Biodegradable Plastic Films. Polymers 2021, 13, 2785. https://doi.org/10.3390/polym13162785
Rujnić Havstad M, Juroš L, Katančić Z, Pilipović A. Influence of Home Composting on Tensile Properties of Commercial Biodegradable Plastic Films. Polymers. 2021; 13(16):2785. https://doi.org/10.3390/polym13162785
Chicago/Turabian StyleRujnić Havstad, Maja, Ljerka Juroš, Zvonimir Katančić, and Ana Pilipović. 2021. "Influence of Home Composting on Tensile Properties of Commercial Biodegradable Plastic Films" Polymers 13, no. 16: 2785. https://doi.org/10.3390/polym13162785
APA StyleRujnić Havstad, M., Juroš, L., Katančić, Z., & Pilipović, A. (2021). Influence of Home Composting on Tensile Properties of Commercial Biodegradable Plastic Films. Polymers, 13(16), 2785. https://doi.org/10.3390/polym13162785