Optimisation of GBFS, Fly Ash, and Nano-Silica Contents in Alkali-Activated Mortars
Abstract
:1. Introduction
2. Materials and Optimisation Modelling
2.1. Materials
2.2. Mix Design
2.3. Microstructure and Morphology
2.4. Optimisation Using RSM Model
3. Results
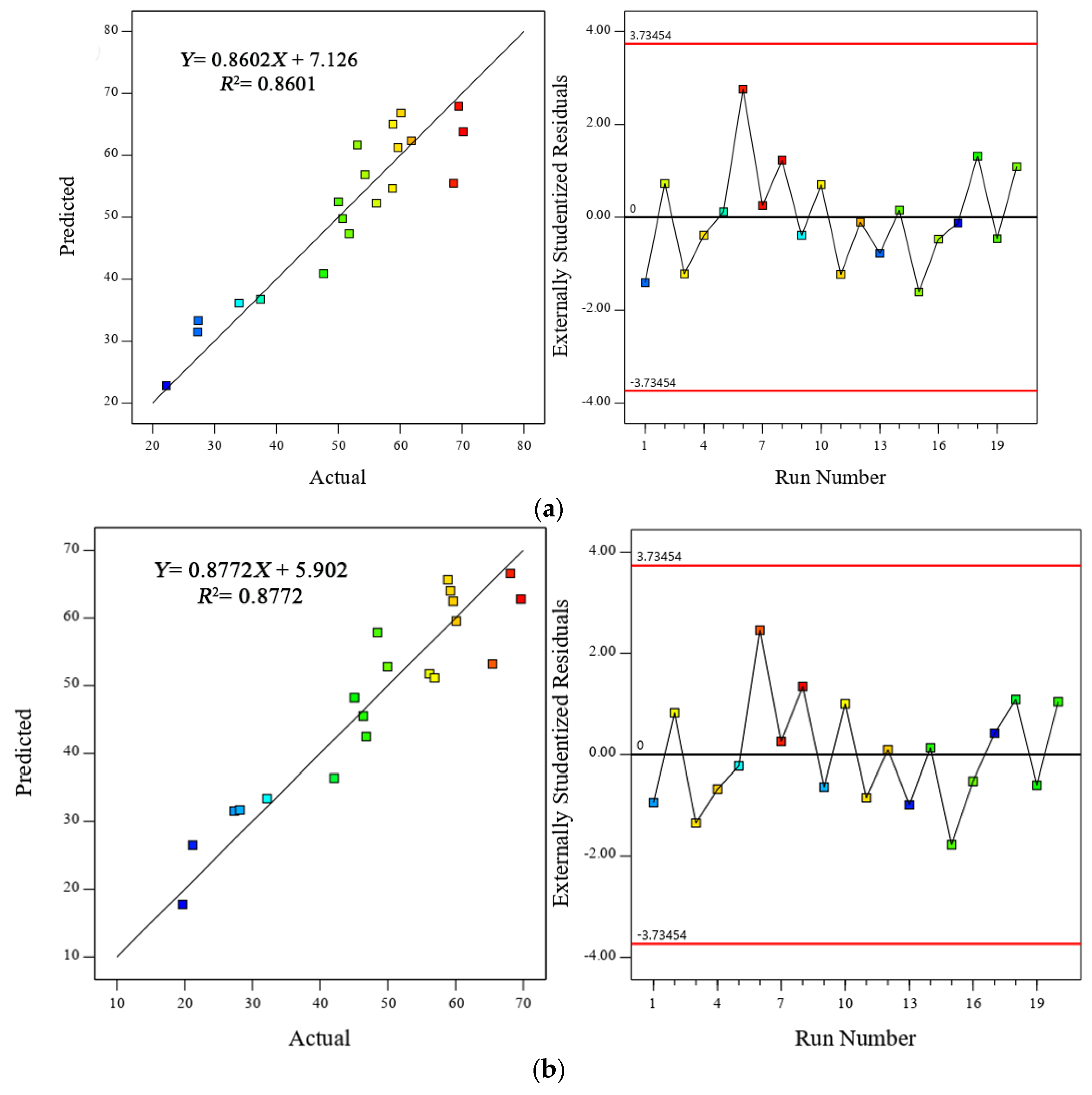
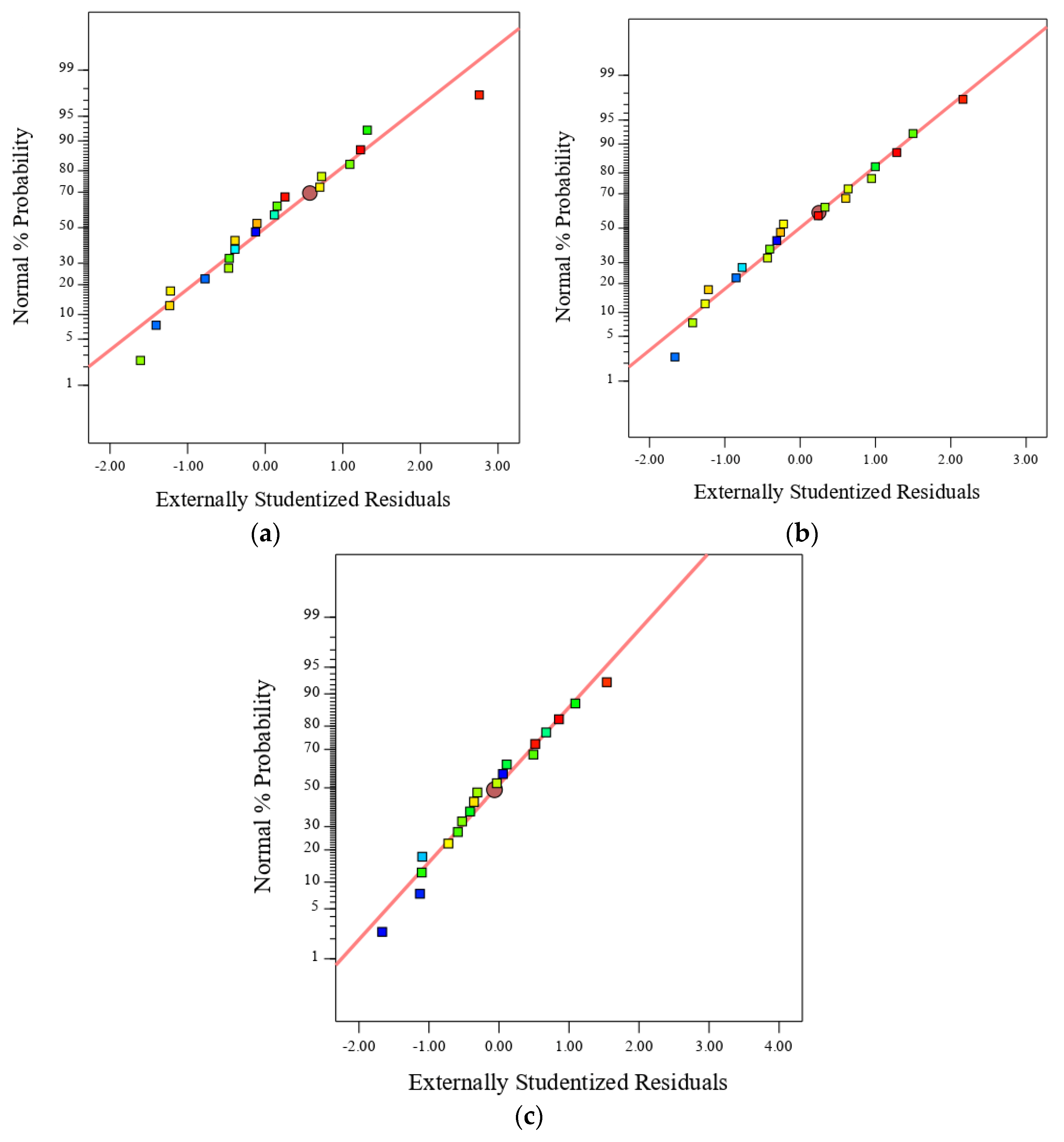
3.1. Predicted Equations and Their Validation
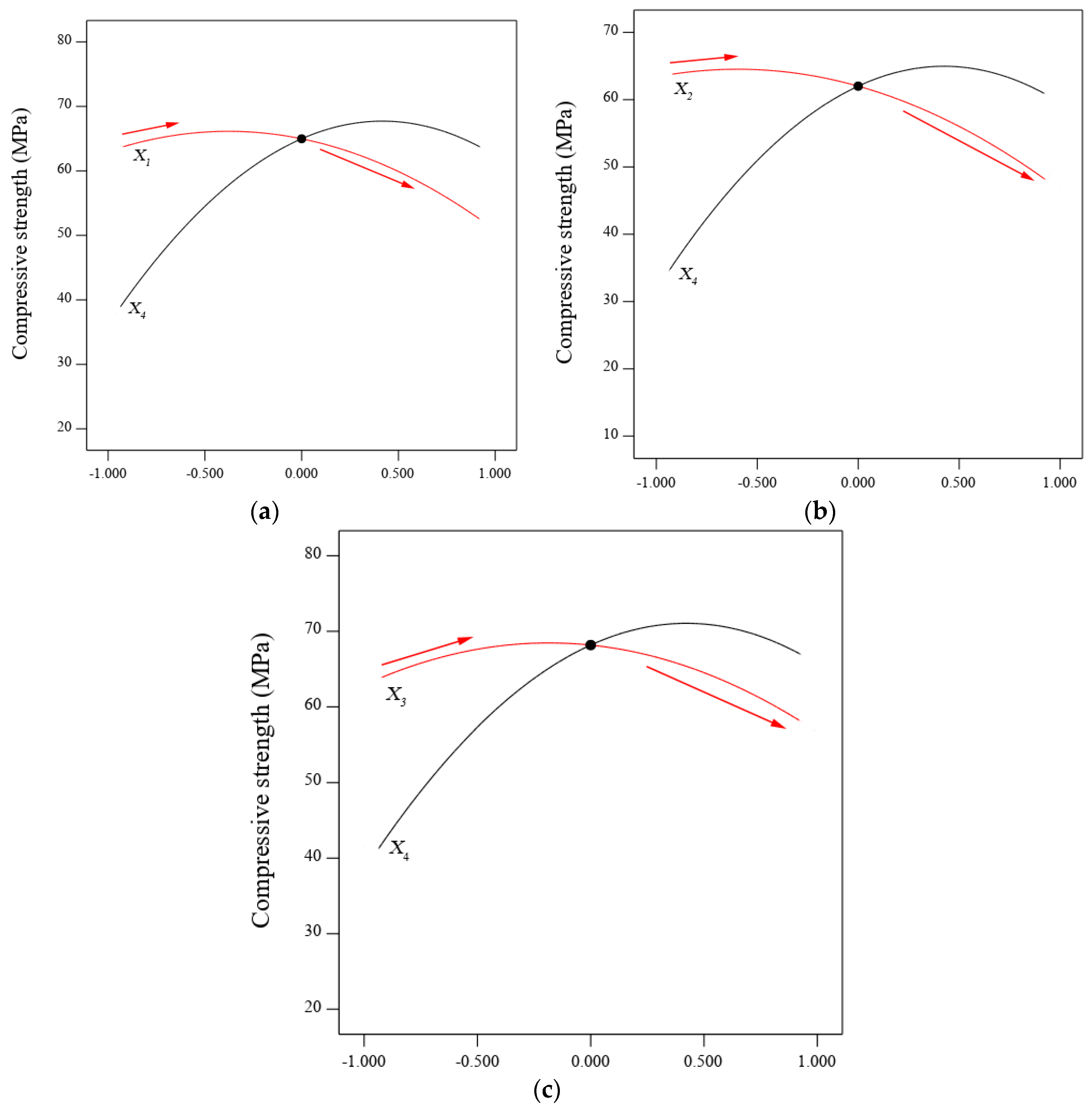
3.2. Interaction and Optimisation
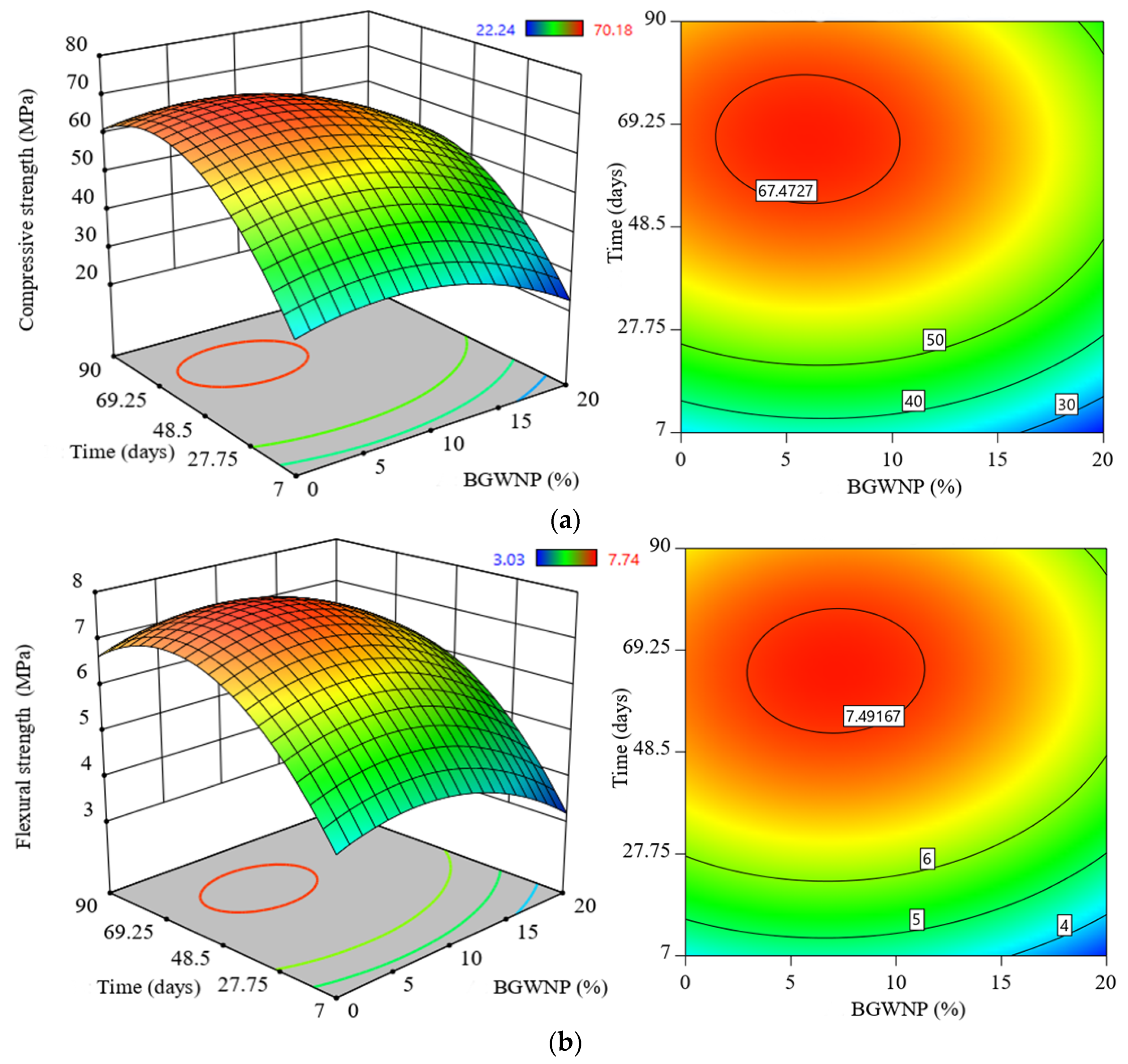
3.3. Effect of BGWNP Addition on the Mechanical Properties of FA-GBFS-Based AAM
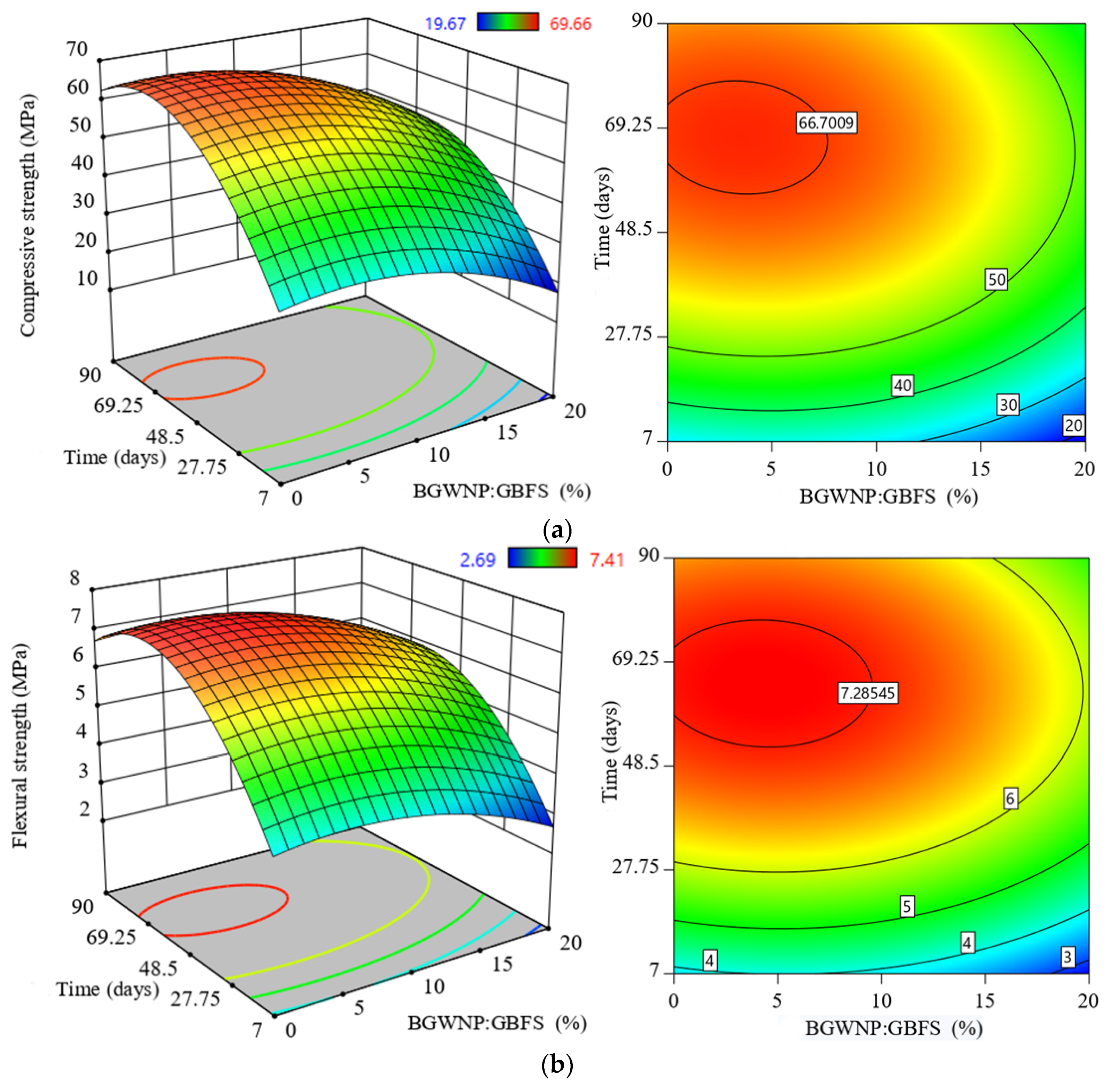
3.4. Effect of BGWNP Addition as GBFS Replacement on the Mechanical Properties of FA-GBFS-Based AAM
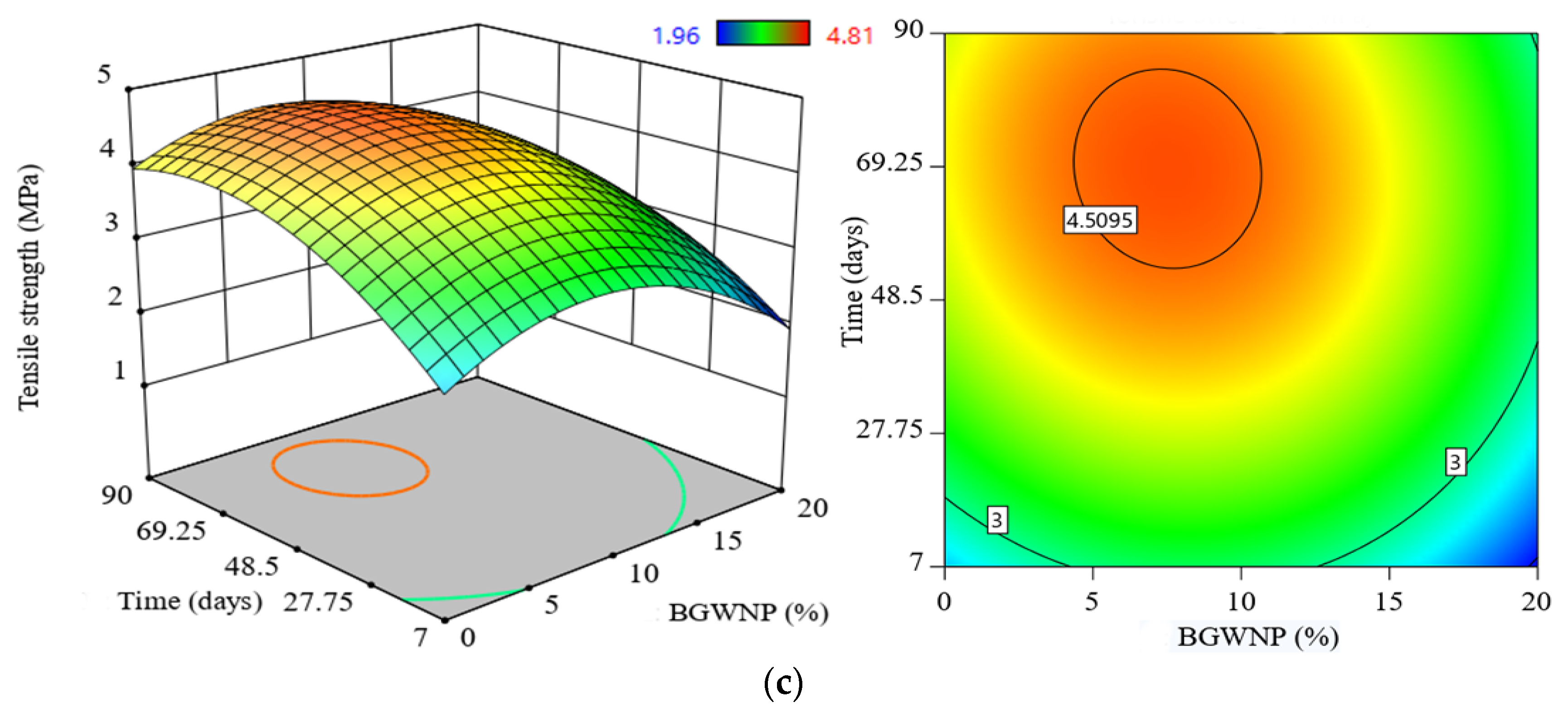
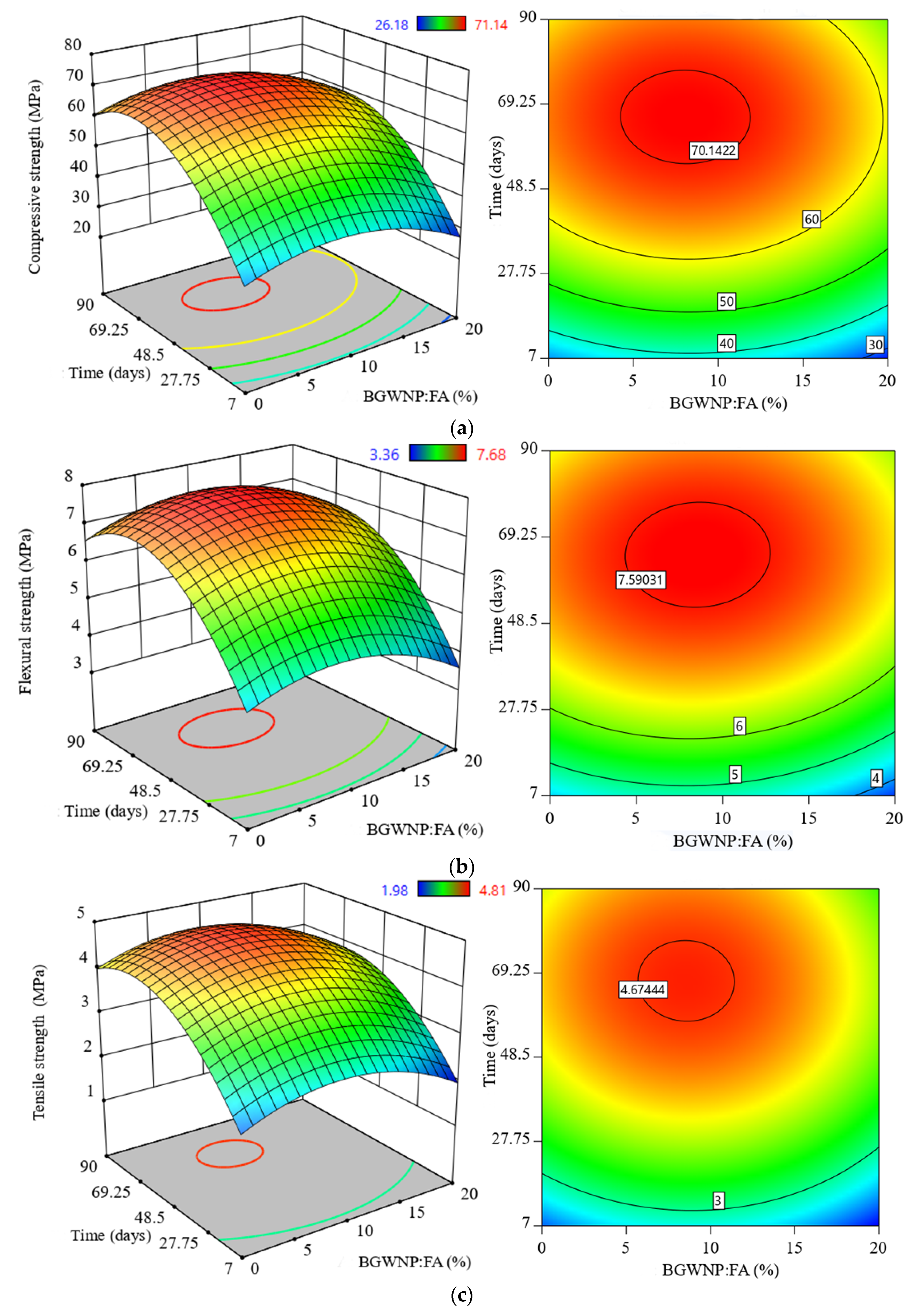
3.5. Effect of BGWNP Addition as FA Replacement on the Mechanical Properties of FA-GBFS-Based AAM
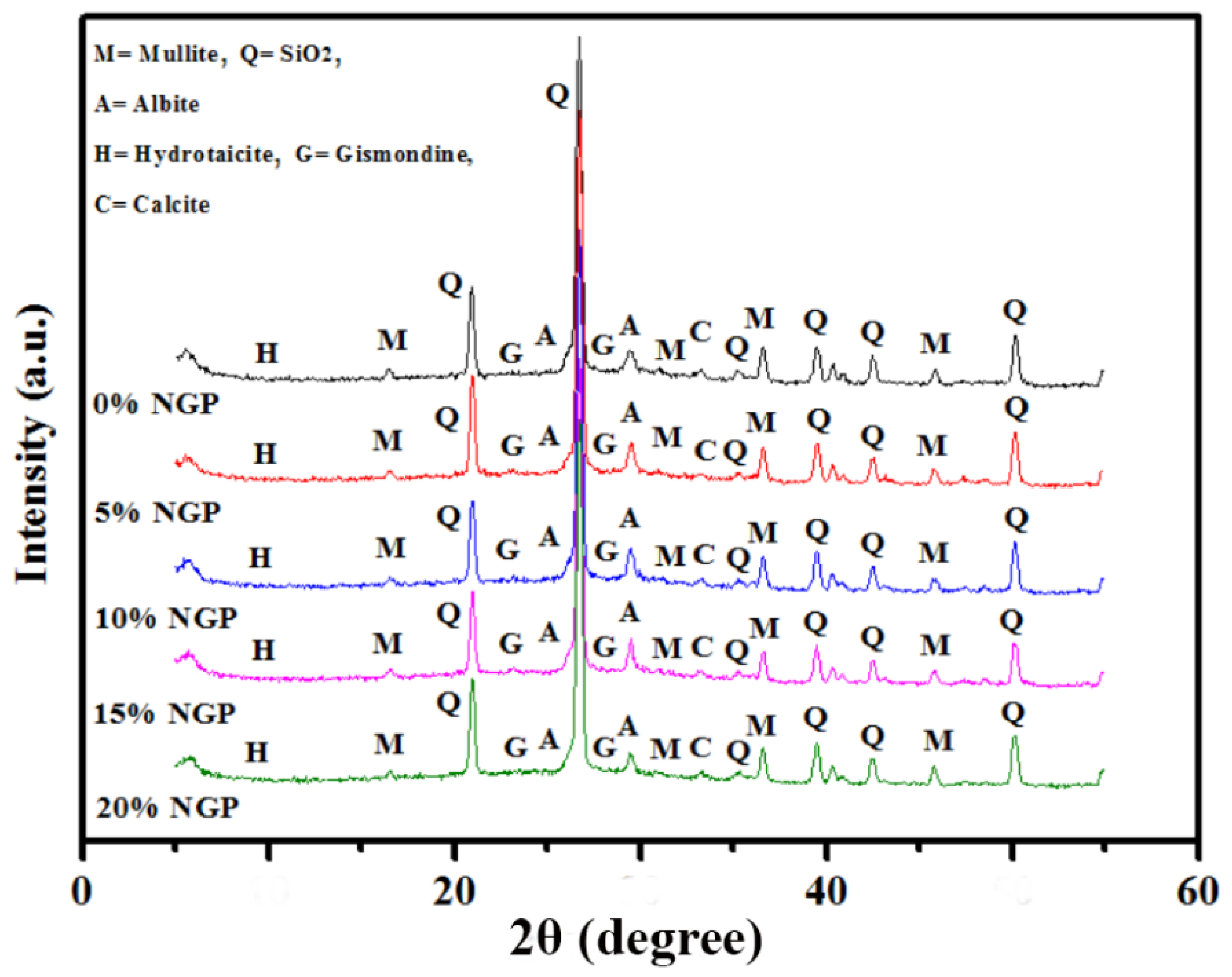
3.6. Microstructure
4. Conclusions
- BGWNP demonstrated its ability to improve the mechanical properties of AAM, however, the incorporation of BGWNP as the replacement of FA was found to be the most significant, while the replacement of GBFS by BGWNP was less significant.
- The compressive, tensile and flexural strength increased with an increase of BGWNP content as the replacement of FA by up to 10%. Nevertheless, the recorded optimum percentage was 8.4%, in which the values of compressive, tensile and flexural strength were 70.1, 4.67 and 7.59 MPa, respectively, which were higher than that of the control mixture (59.6, 3.8 and 6.5 MPa).
- The addition of BGWNP as the replacement of GBFS was limited up to 5% and the maximum compressive, tensile and flexural strength were 66.7, 4.2 and 7.2 MPa, respectively, at BGWNP of 3.4%. Beyond the said value, a significant strength reduction was observed.
- The insulation of BGWNP in AAM made with FA and GBFS (30:70) had a positive effect in which the compressive, tensile and flexural strength were 76.4, 4.5 and 7.4 MPa at an optimal BGWNP content of 6.9%.
- In general, the optimal replacement percentages of FA, GBFS, and BGWNP that maximise the compressive, tensile and flexural strength of AAM were 61.6%, 30%, and 8.4%, respectively.
- The non-linear equations proposed here proved their ability to predict the compressive, tensile and flexural strength with minimum error (RRMSE < 0.107, RRSE < 0.116 and RAE < 0.068) and high correlation between the actual and predicted data (R2 > 0.8601, R > 0.927), thus confirming both the robustness and reliability of the models.
- The differences between the adjusted R2 and predicted R2 were less than 0.2 for all equations, indicating that the models could be used for further observation in the future.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Prakash, R.; Thenmozhi, R.; Raman, S.N.; Subramanian, C. Characterization of eco-friendly steel fiber-reinforced concrete containing waste coconut shell as coarse aggregates and fly ash as partial cement replacement. Struct. Concr. 2020, 21, 437–447. [Google Scholar] [CrossRef]
- Ibrahim, M.H.B.W.; Shahidan, S.; Algaifi, H.A.; Hamzah, A.F.B.; Jaya, R.P. CBA Self-compacting Concrete Exposed to Seawater by Wetting and Drying Cycles. In Properties of Self-Compacting Concrete with Coal Bottom Ash Under Aggressive Environments; Springer: Singapore, 2021; pp. 59–75. [Google Scholar]
- Ibrahim, M.H.B.W.; Shahidan, S.; Algaifi, H.A.; Hamzah, A.F.B.; Jaya, R.P. CBA Self-compacting Concrete Exposed to Chloride and Sulphate. In Properties of Self-Compacting Concrete with Coal Bottom Ash Under Aggressive Environments; Springer: Singapore, 2021; pp. 33–57. [Google Scholar]
- Amin, M.N.; Iqtidar, A.; Khan, K.; Javed, M.F.; Shalabi, F.I.; Qadir, M.G. Comparison of Machine Learning Approaches with Traditional Methods for Predicting the Compressive Strength of Rice Husk Ash Concrete. Crystals 2021, 11, 779. [Google Scholar] [CrossRef]
- Raju, S.; Dharmar, B. Studies on flexural behavior of reinforced concrete beams with copper slag and fly ash. Struct. Concr. 2020, 21, 107–116. [Google Scholar] [CrossRef]
- Roy, K.; Akhtar, A.; Sachdev, S.; Hsu, M.; Lim, J.; Sarmah, A. Development and characterization of novel biochar-mortar composite utilizing waste derived pyrolysis biochar. Int. J. Eng. Res. 2017, 8, 1912–1919. [Google Scholar]
- Mohammadhosseini, H.; Ngian, S.P.; Alyousef, R.; Tahir, M.M. Synergistic effects of waste plastic food tray as low-cost fibrous materials and palm oil fuel ash on transport properties and drying shrinkage of concrete. J. Build. Eng. 2021, 42, 102826. [Google Scholar] [CrossRef]
- Rajamony Laila, L.; Gurupatham, B.G.A.; Roy, K.; Lim, J.B. Influence of super absorbent polymer on mechanical, rheological, durability, and microstructural properties of self-compacting concrete using non-biodegradable granite pulver. Struct. Concr. 2021, 22, E1093–E1116. [Google Scholar]
- Rajamony Laila, L.; Gurupatham, B.G.A.; Roy, K.; BP Lim, J. Effect of super absorbent polymer on microstructural and mechanical properties of concrete blends using granite pulver. Struct. Concr. 2021, 22, E898–E915. [Google Scholar] [CrossRef]
- Alaskar, A.; Alabduljabbar, H.; Mohamed, A.M.; Alrshoudi, F.; Alyousef, R. Abrasion and skid resistance of concrete containing waste polypropylene fibers and palm oil fuel ash as pavement material. Constr. Build. Mater. 2021, 282, 122681. [Google Scholar] [CrossRef]
- Zhang, P.; Zheng, Y.; Wang, K.; Zhang, K. Combined influence of nano-CaCO3 and polyvinyl alcohol fibers on fresh and mechanical performance of concrete incorporating fly ash. Struct. Concr. 2020, 21, 724–734. [Google Scholar] [CrossRef]
- Rajaei, S.; Shoaei, P.; Shariati, M.; Ameri, F.; Musaeei, H.R.; Behforouz, B.; de Brito, J. Rubberized alkali-activated slag mortar reinforced with polypropylene fibres for application in lightweight thermal insulating materials. Constr. Build. Mater. 2021, 270, 121430. [Google Scholar] [CrossRef]
- Kubba, Z.; Hewayde, E.; Huseien, G.F.; Sam, A.R.M.; Asaad, M. Effect of sodium silicate content on setting time and mechanical properties of multi blend geopolymer mortars. J. Eng. Appl. Sci. 2019, 14, 2262–2267. [Google Scholar] [CrossRef] [Green Version]
- Kubba, Z.; Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Asaad, M.A.; Ismail, M.; Tahir, M.M.; Mirza, J. Impact of curing temperatures and alkaline activators on compressive strength and porosity of ternary blended geopolymer mortars. Case Stud. Constr. Mater. 2018, 9, e00205. [Google Scholar] [CrossRef]
- Faridmehr, I.; Fahim Huseien, G.; Hajmohammadian Baghban, M. Evaluation of Mechanical and Environmental Properties of Engineered Alkali-Activated Green Mortar. Materials 2020, 13, 4098. [Google Scholar] [CrossRef]
- Huseien, G.F.; Ismail, M.; Tahir, M.M.; Mirza, J.; Khalid, N.H.A.; Asaad, M.A.; Husein, A.A.; Sarbini, N.N. Synergism between palm oil fuel ash and slag: Production of environmental-friendly alkali activated mortars with enhanced properties. Constr. Build. Mater. 2018, 170, 235–244. [Google Scholar] [CrossRef]
- Al-Majidi, M.H.; Lampropoulos, A.; Cundy, A.; Meikle, S. Development of geopolymer mortar under ambient temperature for in situ applications. Constr. Build. Mater. 2016, 120, 198–211. [Google Scholar] [CrossRef] [Green Version]
- Huseien, G.F.; Sam, A.R.M.; Alyousef, R. Texture, morphology and strength performance of self-compacting alkali-activated concrete: Role of fly ash as GBFS replacement. Constr. Build. Mater. 2021, 270, 121368. [Google Scholar] [CrossRef]
- Nuaklong, P.; Jongvivatsakul, P.; Pothisiri, T.; Sata, V.; Chindaprasirt, P. Influence of rice husk ash on mechanical properties and fire resistance of recycled aggregate high-calcium fly ash geopolymer concrete. J. Clean. Prod. 2020, 252, 119797. [Google Scholar] [CrossRef]
- Ibrahim, M.; Johari, M.A.M.; Rahman, M.K.; Maslehuddin, M. Effect of alkaline activators and binder content on the properties of natural pozzolan-based alkali activated concrete. Constr. Build. Mater. 2017, 147, 648–660. [Google Scholar] [CrossRef]
- Ibrahim, M.; Johari, M.A.M.; Maslehuddin, M.; Rahman, M.K. Influence of nano-SiO2 on the strength and microstructure of natural pozzolan based alkali activated concrete. Constr. Build. Mater. 2018, 173, 573–585. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.A.; Aguirre-Guerrero, A.M.; de Gutiérrez, R.M. Carbonation-induced corrosion of alkali-activated binary concrete based on natural volcanic pozzolan. Constr. Build. Mater. 2020, 232, 117189. [Google Scholar] [CrossRef]
- Faridmehr, I.; Nehdi, M.L.; Huseien, G.F.; Baghban, M.H.; Sam, A.R.M.; Algaifi, H.A. Experimental and Informational Modeling Study of Sustainable Self-Compacting Geopolymer Concrete. Sustainability 2021, 13, 7444. [Google Scholar] [CrossRef]
- Zahid, M.; Shafiq, N.; Isa, M.H.; Gil, L. Statistical modeling and mix design optimization of fly ash based engineered geopolymer composite using response surface methodology. J. Clean. Prod. 2018, 194, 483–498. [Google Scholar] [CrossRef]
- Alabduljabbar, H.; Huseien, G.F.; Sam, A.R.M.; Alyouef, R.; Algaifi, H.A.; Alaskar, A. Engineering Properties of Waste Sawdust-Based Lightweight Alkali-Activated Concrete: Experimental Assessment and Numerical Prediction. Materials 2020, 13, 5490. [Google Scholar] [CrossRef] [PubMed]
- Altowayti, W.A.H.; Algaifi, H.A.; Bakar, S.A.; Shahir, S. The adsorptive removal of As (III) using biomass of arsenic resistant Bacillus thuringiensis strain WS3: Characteristics and modelling studies. Ecotoxicol. Environ. Saf. 2019, 172, 176–185. [Google Scholar] [CrossRef]
- Su, M.; Zhong, Q.; Peng, H.; Li, S. Selected machine learning approaches for predicting the interfacial bond strength between FRPs and concrete. Constr. Build. Mater. 2021, 270, 121456. [Google Scholar] [CrossRef]
- Zhao, Y.; Moayedi, H.; Bahiraei, M.; Foong, L.K. Employing TLBO and SCE for optimal prediction of the compressive strength of concrete. Smart Struct. Syst. 2020, 26, 753–763. [Google Scholar]
- Zhao, Y.; Zhong, X.; Foong, L.K. Predicting the splitting tensile strength of concrete using an equilibrium optimization model. Steel Compos. Struct. 2021, 39, 81–93. [Google Scholar]
- Algaifi, H.A.; Alqarni, A.S.; Alyousef, R.; Bakar, S.A.; Ibrahim, M.W.; Shahidan, S.; Ibrahim, M.; Salami, B.A. Mathematical prediction of the compressive strength of bacterial concrete using gene expression programming. Ain Shams Eng. J. 2021, 42, 102826. [Google Scholar]
- Chu, H.-H.; Khan, M.A.; Javed, M.F.; Zafar, A.; Khan, M.I.; Alabduljabbar, H.; Qayyum, S. Sustainable use of fly-ash: Use of gene-expression programming (GEP) and multi-expression programming (MEP) for forecasting the compressive strength geopolymer concrete. Ain Shams Eng. J. 2021, in press. [Google Scholar] [CrossRef]
- Ali Khan, M.; Zafar, A.; Akbar, A.; Javed, M.F.; Mosavi, A. Application of Gene Expression Programming (GEP) for the prediction of compressive strength of geopolymer concrete. Materials 2021, 14, 1106. [Google Scholar] [CrossRef]
- Yeddula, B.S.R.; Karthiyaini, S. Experimental investigations and GEP modelling of compressive strength of ferrosialate based geopolymer mortars. Constr. Build. Mater. 2020, 236, 117602. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Algaifi, H.A.; Alyouef, R. Development of a sustainable concrete incorporated with effective microorganism and fly Ash: Characteristics and modeling studies. Constr. Build. Mater. 2021, 285, 122899. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, K.; Wang, J.; Guo, J.; Hu, S.; Ling, Y. Mechanical properties and prediction of fracture parameters of geopolymer/alkali-activated mortar modified with PVA fiber and nano-SiO2. Ceram. Int. 2020, 46, 20027–20037. [Google Scholar] [CrossRef]
- Jalal, M.; Grasley, Z.; Gurganus, C.; Bullard, J.W. Experimental investigation and comparative machine-learning prediction of strength behavior of optimized recycled rubber concrete. Constr. Build. Mater. 2020, 256, 119478. [Google Scholar] [CrossRef]
- Ting, M.Z.Y.; Wong, K.S.; Rahman, M.E.; Selowarajoo, M. Prediction model for hardened state properties of silica fume and fly ash based seawater concrete incorporating silicomanganese slag. J. Build. Eng. 2021, 41, 102356. [Google Scholar] [CrossRef]
- Ray, S.; Haque, M.; Ahmed, T.; Nahin, T.T. Comparison of artificial neural network (ANN) and response surface methodology (RSM) in predicting the compressive and splitting tensile strength of concrete prepared with glass waste and tin (Sn) can fiber. J. King Saud Univ. Eng. Sci. 2021, in press. [Google Scholar]
- Pinheiro, C.; Rios, S.; da Fonseca, A.V.; Fernández-Jiménez, A.; Cristelo, N. Application of the response surface method to optimize alkali activated cements based on low-reactivity ladle furnace slag. Constr. Build. Mater. 2020, 264, 120271. [Google Scholar] [CrossRef]
- Mokhtar, N.; Megat Johari, M.A.; Tajarudin, H.A.; Al-Gheethi, A.A.; Algaifi, H.A. A sustainable enhancement of bio-cement using immobilised Bacillus sphaericus: Optimization, microstructural properties, and techno-economic analysis for a cleaner production of bio-cementitious mortars. J. Clean. Prod. 2021, 318, 128470. [Google Scholar] [CrossRef]
- Khan, A.; Do, J.; Kim, D. Experimental optimization of high-strength self-compacting concrete based on D-optimal design. J. Constr. Eng. Manag. 2017, 143, 04016108. [Google Scholar] [CrossRef]
- Hassan, W.N.F.W.; Ismail, M.A.; Lee, H.-S.; Meddah, M.S.; Singh, J.K.; Hussin, M.W.; Ismail, M. Mixture optimization of high-strength blended concrete using central composite design. Constr. Build. Mater. 2020, 243, 118251. [Google Scholar] [CrossRef]
- Basri, M.S.M.; Mustapha, F.; Mazlan, N.; Ishak, M.R. Optimization of rice husk ash-based geopolymers coating composite for enhancement in flexural properties and microstructure using response surface methodology. Coatings 2020, 10, 165. [Google Scholar] [CrossRef] [Green Version]
- Shahmansouri, A.A.; Nematzadeh, M.; Behnood, A. Mechanical properties of GGBFS-based geopolymer concrete incorporating natural zeolite and silica fume with an optimum design using response surface method. J. Build. Eng. 2021, 36, 102138. [Google Scholar] [CrossRef]
- Venkatesan, M.; Zaib, Q.; Shah, I.H.; Park, H.S. Optimum utilization of waste foundry sand and fly ash for geopolymer concrete synthesis using D-optimal mixture design of experiments. Resour. Conserv. Recycl. 2019, 148, 114–123. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, J.; Luo, X.; Zhu, J.; Nie, L. Experiment research on mix design and early mechanical performance of alkali-activated slag using response surface methodology (RSM). Ceram. Int. 2016, 42, 11666–11673. [Google Scholar] [CrossRef]
- Chong, B.W.; Othman, R.; Putra Jaya, R.; Mohd Hasan, M.R.; Sandu, A.V.; Nabiałek, M.; Jeż, B.; Pietrusiewicz, P.; Kwiatkowski, D.; Postawa, P. Design of Experiment on Concrete Mechanical Properties Prediction: A Critical Review. Materials 2021, 14, 1866. [Google Scholar] [CrossRef]
- Mirrashid, M.; Naderpour, H. Recent trends in prediction of concrete elements behavior using soft computing (2010–2020). Arch. Comput. Methods Eng. 2021, 28, 3307–3327. [Google Scholar] [CrossRef]
- American Society for Testing and Materials International. C618-19, A. Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- American Society for Testing and Materials International. C117-17, A. Standard Test Method for Materials Finer than 75-μm (No. 200) Sieve in Mineral Aggregates by Washing; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- American Society for Testing and Materials International. C33M-18, A.C. Standard Specification for Concrete Aggregates; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- American Society for Testing and Materials International. C109M-20b, A.C. Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50 mm] Cube Specimens); ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- American Society for Testing and Materials International. C579-18, A. Standard Test Methods for Compressive Strength of Chemical-Resistant Mortars, Grouts, Monolithic Surfacings, and Polymer Concretes; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- American Society for Testing and Materials International. C78M-21, A.C. Standard Test Method for Flexural Strength of Concrete (Using Simple Beam with Third-Point Loading; ASTM International: West Conshohocken, PA, USA, 2021. [Google Scholar]
- American Society for Testing and Materials International. C496M-17, A.C. Standard Test Method for Splitting Tensile Strength of Cylindrical Concrete Specimens; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Abdulkadir, I.; Mohammed, B.S.; Liew, M.; Wahab, M. Modelling and multi-objective optimization of the fresh and mechanical properties of self-compacting high volume fly ash ECC (HVFA-ECC) using response surface methodology (RSM). Case Stud. Constr. Mater. 2021, 14, e00525. [Google Scholar]
- Nematzadeh, M.; Maghferat, A.; Herozi, M.R.Z. Mechanical Properties and Durability of Compressed Nylon Aggregate Concrete Reinforced with Forta-Ferro Fiber: Experiments and Optimization. J. Build. Eng. 2021, 41, 102771. [Google Scholar] [CrossRef]
- Habibi, A.; Ramezanianpour, A.M.; Mahdikhani, M. RSM-based optimized mix design of recycled aggregate concrete containing supplementary cementitious materials based on waste generation and global warming potential. Resour. Conserv. Recycl. 2021, 167, 105420. [Google Scholar] [CrossRef]
- Revilla-Cuesta, V.; Skaf, M.; Espinosa, A.B.; Santamaría, A.; Ortega-López, V. Statistical Approach for the Design of Structural Self-Compacting Concrete with Fine Recycled Concrete Aggregate. Mathematics 2020, 8, 2190. [Google Scholar] [CrossRef]
- Oskouei, A.V.; Nazari, R.; Khaneghahi, M.H. Laboratory and in situ investigation of the compressive strength of CFRD concrete. Constr. Build. Mater. 2020, 242, 118166. [Google Scholar] [CrossRef]
- Jalal, M.; Arabali, P.; Grasley, Z.; Bullard, J.W.; Jalal, H. Behavior assessment, regression analysis and support vector machine (SVM) modeling of waste tire rubberized concrete. J. Clean. Prod. 2020, 273, 122960. [Google Scholar] [CrossRef]
- Elemam, W.E.; Abdelraheem, A.H.; Mahdy, M.G.; Tahwia, A.M. Optimizing fresh properties and compressive strength of self-consolidating concrete. Constr. Build. Mater. 2020, 249, 118781. [Google Scholar] [CrossRef]
- Algaifi, H.A.; Bakar, S.A.; Sam, A.R.M.; Abidin, A.R.Z.; Shahir, S.; AL-Towayti, W.A.H. Numerical modeling for crack self-healing concrete by microbial calcium carbonate. Constr. Build. Mater. 2018, 189, 816–824. [Google Scholar] [CrossRef]
- Algaifi, H.A.; Bakar, S.A.; Sam, A.R.M.; Ismail, M.; Abidin, A.R.Z.; Shahir, S.; Altowayti, W.A.H. Insight into the role of microbial calcium carbonate and the factors involved in self-healing concrete. Constr. Build. Mater. 2020, 254, 119258. [Google Scholar] [CrossRef]
- Mohammed, B.S.; Khed, V.C.; Nuruddin, M.F. Rubbercrete mixture optimization using response surface methodology. J. Clean. Prod. 2018, 171, 1605–1621. [Google Scholar] [CrossRef]
- Mohammed, M.K.; Al-Hadithi, A.I.; Mohammed, M.H. Production and optimization of eco-efficient self compacting concrete SCC with limestone and PET. Constr. Build. Mater. 2019, 197, 734–746. [Google Scholar] [CrossRef]
- Carrillo, J.; Ramirez, J.; Lizarazo-Marriaga, J. Modulus of elasticity and Poisson’s ratio of fiber-reinforced concrete in Colombia from ultrasonic pulse velocities. J. Build. Eng. 2019, 23, 18–26. [Google Scholar] [CrossRef]
- Khan, M.A.; Memon, S.A.; Farooq, F.; Javed, M.F.; Aslam, F.; Alyousef, R. Compressive strength of fly-ash-based geopolymer concrete by gene expression programming and random forest. Adv. Civ. Eng. 2021, 2021, 6618407. [Google Scholar]
- Ashrafian, A.; Gandomi, A.H.; Rezaie-Balf, M.; Emadi, M. An evolutionary approach to formulate the compressive strength of roller compacted concrete pavement. Measurement 2020, 152, 107309. [Google Scholar] [CrossRef]
- Farooq, F.; Nasir Amin, M.; Khan, K.; Rehan Sadiq, M.; Faisal Javed, M.; Aslam, F.; Alyousef, R. A Comparative Study of Random Forest and Genetic Engineering Programming for the Prediction of Compressive Strength of High Strength Concrete (HSC). Appl. Sci. 2020, 10, 7330. [Google Scholar] [CrossRef]
- Tinoco, J.; Alberto, A.; da Venda, P.; Correia, A.G.; Lemos, L. A novel approach based on soft computing techniques for unconfined compression strength prediction of soil cement mixtures. Neural. Comput. Appl. 2020, 32, 8985–8991. [Google Scholar] [CrossRef]
- Mohammed, A.; Rafiq, S.; Sihag, P.; Kurda, R.; Mahmood, W. Soft computing techniques: Systematic multiscale models to predict the compressive strength of HVFA concrete based on mix proportions and curing times. J. Build. Eng. 2020, 101851. [Google Scholar] [CrossRef]
- Shah, M.I.; Amin, M.N.; Khan, K.; Niazi, M.S.K.; Aslam, F.; Alyousef, R.; Javed, M.F.; Mosavi, A. Performance Evaluation of Soft Computing for Modeling the Strength Properties of Waste Substitute Green Concrete. Sustainability 2021, 13, 2867. [Google Scholar] [CrossRef]
- Shah, M.I.; Memon, S.A.; Khan Niazi, M.S.; Amin, M.N.; Aslam, F.; Javed, M.F. Machine Learning-Based Modeling with Optimization Algorithm for Predicting Mechanical Properties of Sustainable Concrete. Adv. Civ. Eng. 2021, 2021, 6682283. [Google Scholar]
- Ren, Z.; Liu, Y.; Yuan, L.; Luan, C.; Wang, J.; Cheng, X.; Zhou, Z. Optimizing the content of nano-SiO2, nano-TiO2 and nano-CaCO3 in Portland cement paste by response surface methodology. J. Build. Eng. 2021, 35, 102073. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, Z.; Zhang, K.; Tang, X.; Huang, S. Statistical modeling and multi-objective optimization of road geopolymer grouting material via RSM and MOPSO. Constr. Build. Mater. 2021, 271, 121534. [Google Scholar] [CrossRef]
- Yu, R.; Spiesz, P.; Brouwers, H. Effect of nano-silica on the hydration and microstructure development of Ultra-High Performance Concrete (UHPC) with a low binder amount. Constr. Build. Mater. 2014, 65, 140–150. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.J.; Jennings, H.M.; Chen, J.J. Influence of nucleation seeding on the hydration mechanisms of tricalcium silicate and cement. J. Phys. Chem. C 2009, 113, 4327–4334. [Google Scholar] [CrossRef] [Green Version]
- Björnström, J.; Martinelli, A.; Matic, A.; Börjesson, L.; Panas, I. Accelerating effects of colloidal nano-silica for beneficial calcium–silicate–hydrate formation in cement. Chem. Phys. Lett. 2004, 392, 242–248. [Google Scholar] [CrossRef]
- Chithra, S.; Kumar, S.S.; Chinnaraju, K. The effect of colloidal nano-silica on workability, mechanical and durability properties of high performance concrete with copper slag as partial fine aggregate. Constr. Build. Mater. 2016, 113, 794–804. [Google Scholar] [CrossRef]
- Adak, D.; Sarkar, M.; Mandal, S. Effect of nano-silica on strength and durability of fly ash based geopolymer mortar. Constr. Build. Mater. 2014, 70, 453–459. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, J.; Kaur, M. Microstructure and strength development of fly ash-based geopolymer mortar: Role of nano-metakaolin. Constr. Build. Mater. 2018, 190, 672–679. [Google Scholar] [CrossRef]
- Samadi, M.; Shah, K.W.; Huseien, G.F.; Lim, N.H.A.S. Influence of glass silica waste nano powder on the mechanical and microstructure properties of alkali-activated mortars. Nanomaterials 2020, 10, 324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindgreen, H.; Geiker, M.; Krøyer, H.; Springer, N.; Skibsted, J. Microstructure engineering of Portland cement pastes and mortars through addition of ultrafine layer silicates. Cem. Concr. Compos. 2008, 30, 686–699. [Google Scholar] [CrossRef]
- Aydın, A.C.; Nasl, V.J.; Kotan, T. The synergic influence of nano-silica and carbon nano tube on self-compacting concrete. J. Build. Eng. 2018, 20, 467–475. [Google Scholar] [CrossRef]
- Lim, N.H.A.S.; Mohammadhosseini, H.; Tahir, M.M.; Samadi, M.; Sam, A.R.M. Microstructure and Strength Properties of Mortar Containing Waste Ceramic Nanoparticles. Arab. J. Sci. Eng. 2018, 43, 5305–5313. [Google Scholar] [CrossRef]
- Fu, J.; Kamali-Bernard, S.; Bernard, F.; Cornen, M. Comparison of mechanical properties of CSH and portlandite between nano-indentation experiments and a modelling approach using various simulation techniques. Compos. B Eng. 2018, 151, 127–138. [Google Scholar] [CrossRef]
- Indumathi, P.; Shabhudeen, S.; Saraswathy, C. Synthesis and characterization of nano silica from the Pods of Delonix Regia ash. Int. J. Adv. Eng. Technol. 2011, 2, 421–426. [Google Scholar]
- Qing, Y.; Zenan, Z.; Deyu, K.; Rongshen, C. Influence of nano-SiO2 addition on properties of hardened cement paste as compared with silica fume. Constr. Build. Mater. 2007, 21, 539–545. [Google Scholar] [CrossRef]
- Bai, P.; Sharratt, P.; Yeo, T.Y.; Bu, J. A facile route to preparation of high purity nanoporous silica from acid-leached residue of serpentine. J. Nanosci. Nanotechnol. 2014, 14, 6915–6922. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.G.; Lee, H.-K. Effect of fly ash characteristics on delayed high-strength development of geopolymers. Constr. Build. Mater. 2016, 102, 260–269. [Google Scholar] [CrossRef]








| Composition | BGWNP | GBFS | FA |
|---|---|---|---|
| SiO2 | 69.14 | 30.7 | 57.25 |
| Al2O3 | 13.86 | 11.1 | 28.75 |
| Fe2O3 | 0.24 | 0.54 | 3.57 |
| CaO | 3.16 | 51.9 | 5.10 |
| MgO | 0.68 | 4.47 | 1.38 |
| K2O | 0.01 | 0.36 | 0.94 |
| Na2O | 0.01 | 0.45 | 0.09 |
| SO3 | 4.08 | 0.06 | 0.10 |
| LOI | 0.16 | 0.22 | 0.11 |
| Independent Variables | Code | Units | Level (−1) | Level (+1) |
|---|---|---|---|---|
| BGWNP | X1 | % | 0 | 20 |
| BGWNP:GBFS | X2 | % | 0 | 20 |
| BGWNP:FA | X3 | % | 0 | 20 |
| Time | X4 | days | 7 | 90 |
| Model | Response | Predicted Equation |
|---|---|---|
| Model 1 | Compressive strength (CS) | |
| Flexural strength (FS) | ||
| Tensile strength (TS) | ||
| Model 2 | Compressive strength (CS) | |
| Flexural strength (FS) | ||
| Tensile strength (TS) | ||
| Model 3 | Compressive strength (CS) | |
| Flexural strength (FS) | ||
| Tensile strength (TS) |
| Item | Compressive Strength | Flexural Strength | Tensile Strength | ||||||
|---|---|---|---|---|---|---|---|---|---|
| p-Value | F-value | Sig. | p-Value | F-Value | Sig. | p-Value | F-Value | Sig. | |
| Model 1 | <0.0001 | 17.22 | Y | <0.0001 | 13.4 | Y | 0.0036 | 6.02 | Y |
| X1 | 0.0081 | 9.5 | 0.0375 | 5.28 | 0.0486 | 4.67 | |||
| X4 | <0.0001 | 49.69 | <0.0001 | 38.81 | 0.0031 | 12.67 | |||
| X1X4 | 0.7526 | 0.103 | 0.8195 | 0.0541 | 0.6657 | 0.1948 | |||
| 0.0295 | 5.88 | 0.0402 | 5.11 | 0.0109 | 8.6 | ||||
| 0.0002 | 24.24 | 0.0005 | 20.17 | 0.0453 | 4.83 | ||||
| Model 2 | <0.0001 | 20.01 | Y | <0.0001 | 18.13 | Y | <0.0001 | 15.08 | Y |
| X2 | 0.0008 | 17.85 | 0.0056 | 10.68 | 0.0015 | 15.49 | |||
| X4 | <0.0001 | 55.24 | <0.0001 | 50.71 | <0.0001 | 39.87 | |||
| X2X4 | 0.5722 | 0.3345 | 0.7524 | 0.1035 | 0.3985 | 0.7585 | |||
| 0.0538 | 4.43 | 0.0902 | 3.31 | 0.0630 | 4.08 | ||||
| 0.0002 | 25.98 | <0.0001 | 29.49 | 0.0008 | 18.22 | ||||
| Model 3 | <0.0001 | 18.65 | Y | <0.0001 | 13.91 | Y | <0.0001 | 19.39 | Y |
| X3 | 0.1232 | 2.69 | 0.216 | 1.68 | 0.1505 | 2.31 | |||
| X4 | <0.0001 | 59.18 | <0.0001 | 42.55 | <0.0001 | 61.14 | |||
| X3X4 | 0.8966 | 0.0175 | 0.7803 | 0.0808 | 0.8319 | 0.0468 | |||
| 0.0193 | 6.99 | 0.0329 | 5.6 | 0.0043 | 11.59 | ||||
| 0.0001 | 28.16 | 0.0003 | 22.4 | 0.0002 | 25.53 | ||||
| Item | R | RRMSE | RMSE | RRSE | RSE | RAE | MAE |
|---|---|---|---|---|---|---|---|
| Model 1 | 0.860 | 0.102 | 5.218 | 0.114 | 0.012 | 0.063 | 4.165 |
| Model 2 | 0.877 | 0.107 | 5.253 | 0.116 | 0.013 | 0.068 | 4.411 |
| Model 3 | 0.870 | 0.0925 | 4.973 | 0.106 | 0.011 | 0.061 | 4.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algaifi, H.A.; Mustafa Mohamed, A.; Alsuhaibani, E.; Shahidan, S.; Alrshoudi, F.; Huseien, G.F.; Bakar, S.A. Optimisation of GBFS, Fly Ash, and Nano-Silica Contents in Alkali-Activated Mortars. Polymers 2021, 13, 2750. https://doi.org/10.3390/polym13162750
Algaifi HA, Mustafa Mohamed A, Alsuhaibani E, Shahidan S, Alrshoudi F, Huseien GF, Bakar SA. Optimisation of GBFS, Fly Ash, and Nano-Silica Contents in Alkali-Activated Mortars. Polymers. 2021; 13(16):2750. https://doi.org/10.3390/polym13162750
Chicago/Turabian StyleAlgaifi, Hassan Amer, Abdeliazim Mustafa Mohamed, Eyad Alsuhaibani, Shahiron Shahidan, Fahed Alrshoudi, Ghasan Fahim Huseien, and Suhaimi Abu Bakar. 2021. "Optimisation of GBFS, Fly Ash, and Nano-Silica Contents in Alkali-Activated Mortars" Polymers 13, no. 16: 2750. https://doi.org/10.3390/polym13162750
APA StyleAlgaifi, H. A., Mustafa Mohamed, A., Alsuhaibani, E., Shahidan, S., Alrshoudi, F., Huseien, G. F., & Bakar, S. A. (2021). Optimisation of GBFS, Fly Ash, and Nano-Silica Contents in Alkali-Activated Mortars. Polymers, 13(16), 2750. https://doi.org/10.3390/polym13162750







