Backbone Effects on the Thermoelectric Properties of Ultra-Small Bandgap Conjugated Polymers
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Monomers and Polymers
2.3. Preparation of Polymer Films
2.4. Instruments and Measurements
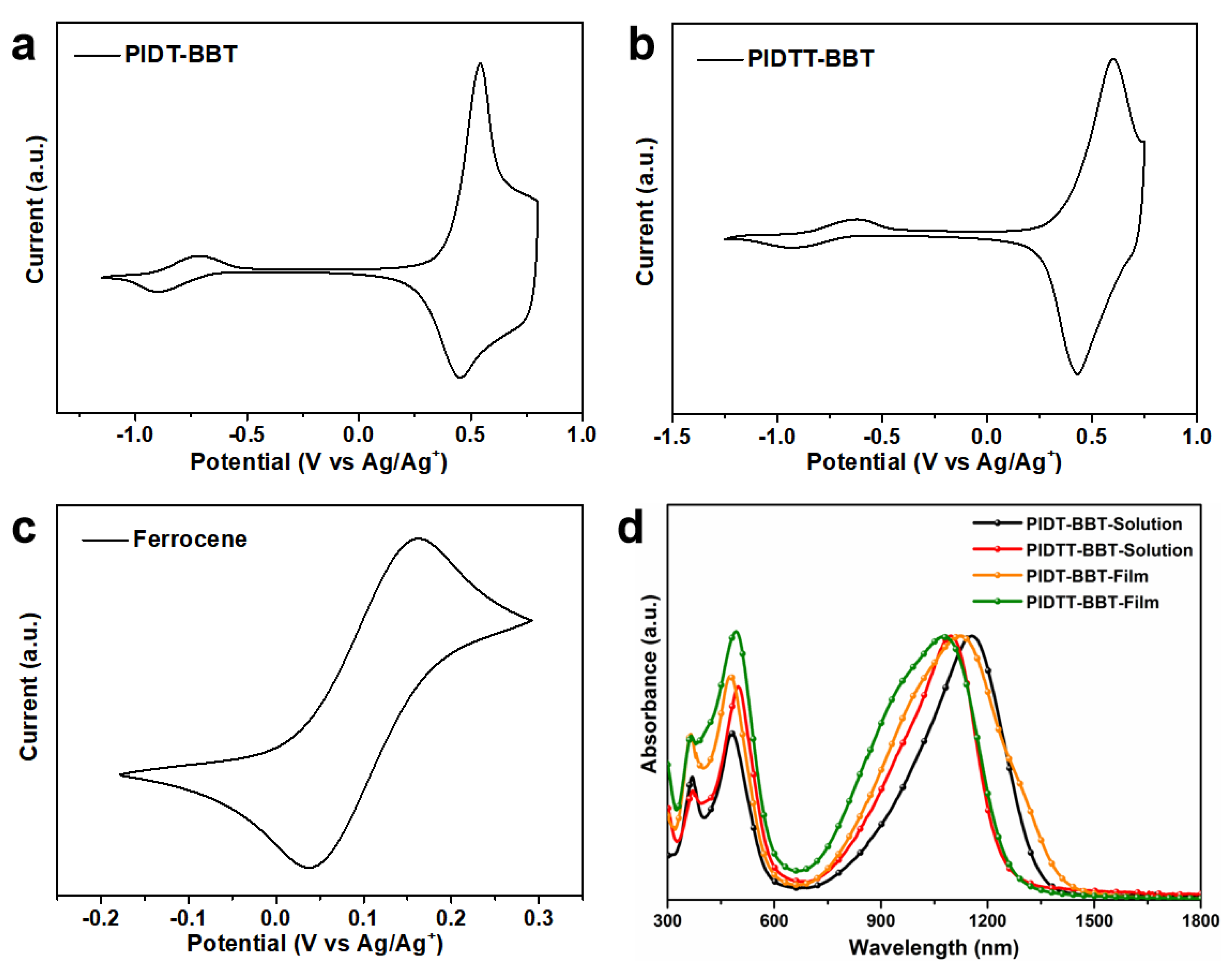
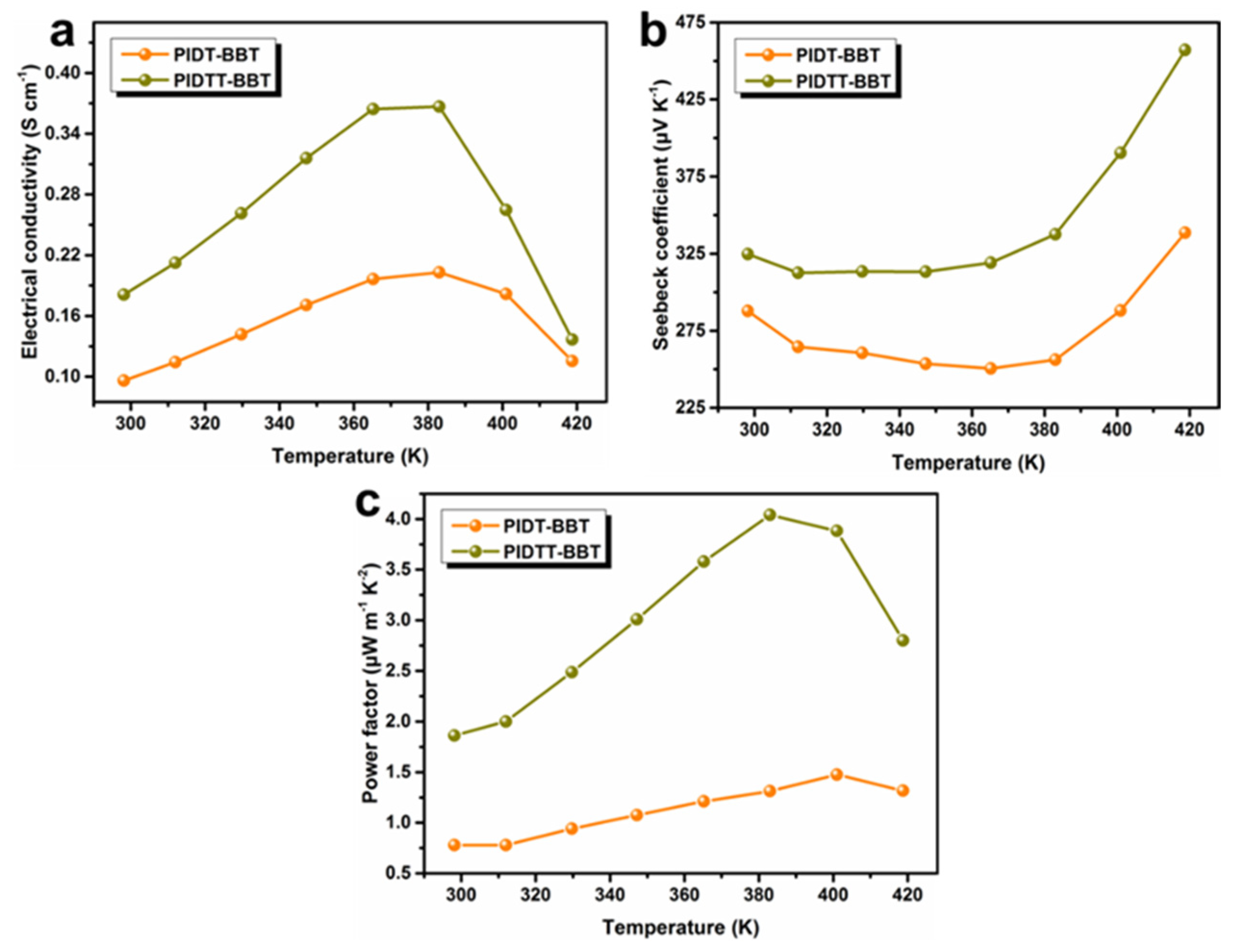
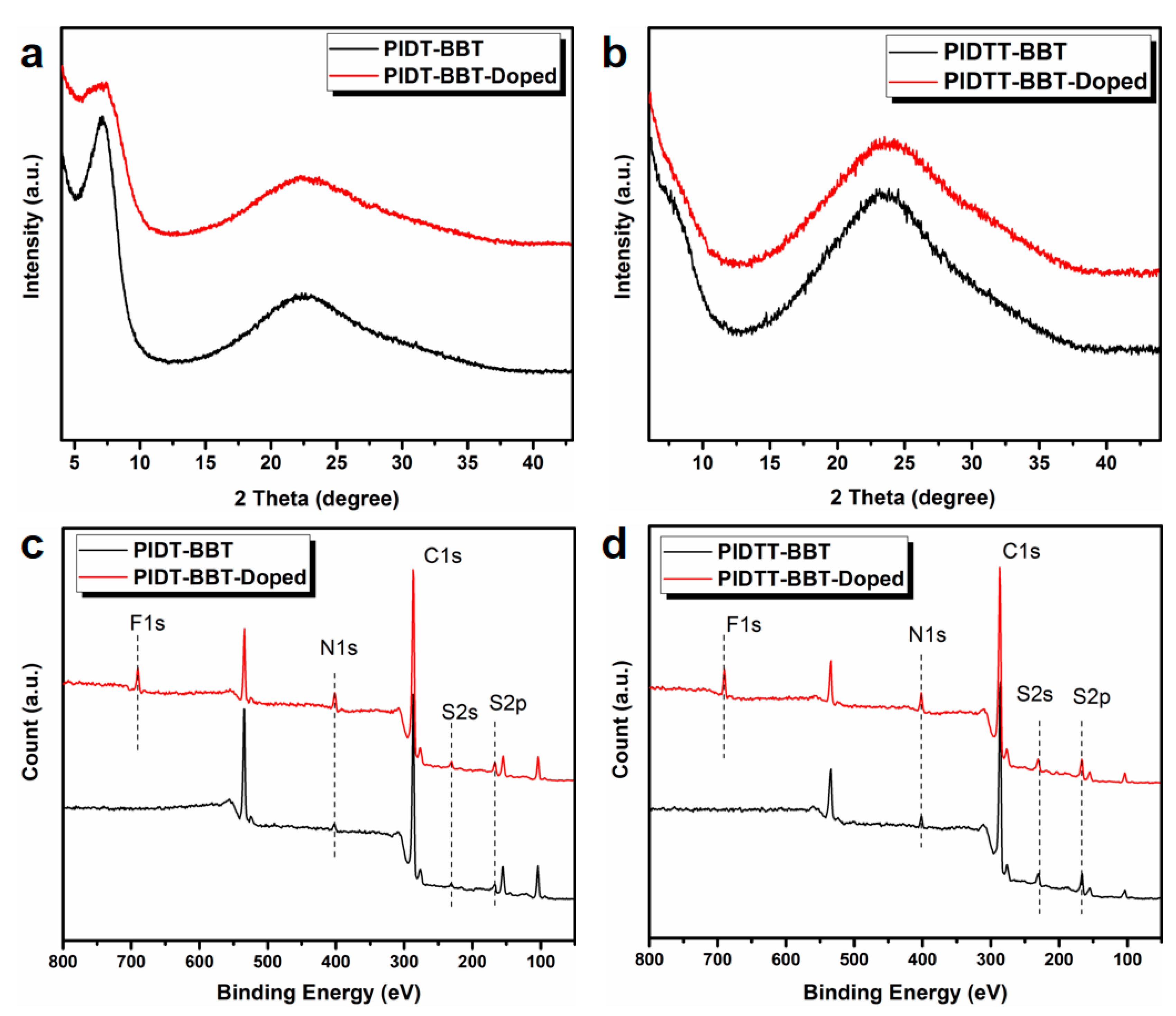
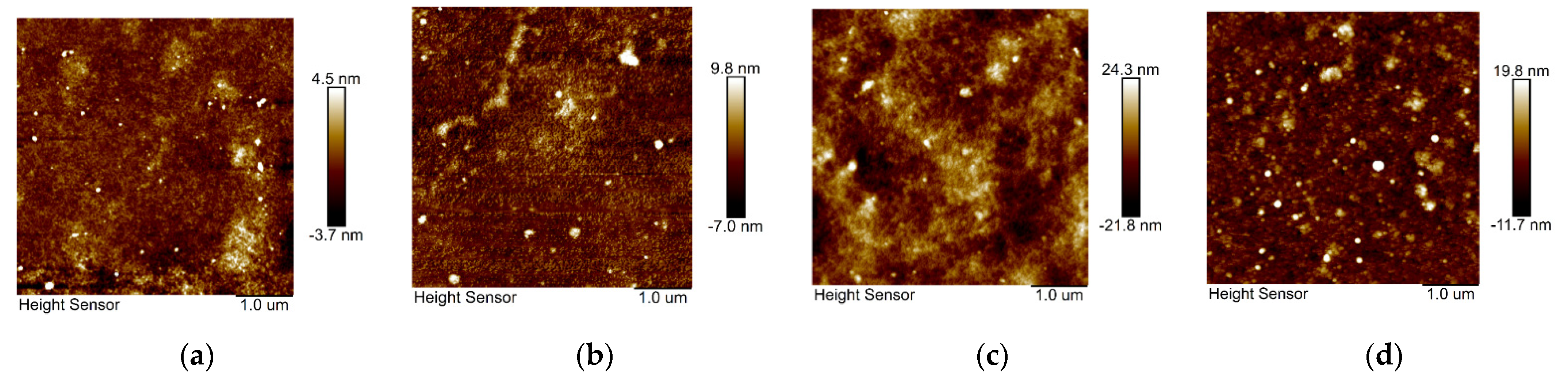
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, Z.; Boland, M.J.; Butrouna, K.; Strachan, D.R.; Graham, K.R. Increased Power Factors of organic–inorganic Nanocomposite Thermoelectric Materials and the Role of Energy Filtering. J. Mater. Chem. A 2017, 5, 15891–15900. [Google Scholar] [CrossRef]
- Liu, T.; Shinohara, A.; Tan, G.; Pan, C.; Wang, L. The Cross-Linking Effect on the Thermoelectric Properties of Conjugated Polymer/Carbon Nanotube Composite Films. Macromol. Mater. Eng. 2019, 304. [Google Scholar] [CrossRef]
- Gao, C.; Liu, Y.; Gao, Y.; Zhou, Y.; Zhou, X.; Yin, X.; Pan, C.; Yang, C.; Wang, H.; Chen, G.; et al. High-Performance N-Type Thermoelectric Composites of Acridones With Tethered Tertiary Amines and Carbon Nanotubes. J. Mater. Chem. A 2018, 6, 20161–20169. [Google Scholar] [CrossRef]
- Yue, R.; Xu, J. Poly(3,4-Ethylenedioxythiophene) As Promising Organic Thermoelectric Materials: A Mini-Review. Synth. Met. 2012, 162, 912–917. [Google Scholar] [CrossRef]
- Du, Y.; Shen, S.Z.; Cai, K.; Casey, P.S. Research Progress on polymer–inorganic Thermoelectric Nanocomposite Materials. Prog. Polym. Sci. 2012, 37, 820–841. [Google Scholar] [CrossRef]
- Kim, G.-H.; Shao, L.; Zhang, K.; Pipe, K.P. Engineered Doping of Organic Semiconductors for Enhanced Thermoelectric Efficiency. Nat. Mater. 2013, 12, 719–723. [Google Scholar] [CrossRef]
- Zhou, X.; Liang, A.; Pan, C.; Wang, L. Effects of Oxidative Doping on the Thermoelectric Performance of Polyfluorene Deriva-tives/Carbon Nanotube Composite Films. Org. Electron. 2018, 52, 281–287. [Google Scholar] [CrossRef]
- Xuan, Y.; Liu, X.; Desbief, S.; Leclère, P.; Fahlman, M.; Lazzaroni, R.; Berggren, M.; Cornil, J.; Emin, D.; Crispin, X. Thermoelectric Properties of Conducting Polymers: The Case of poly(3-hexylthiophene). Phys. Rev. B 2010, 82, 115454. [Google Scholar] [CrossRef]
- Glaudell, A.M.; Cochran, J.E.; Patel, S.N.; Chabinyc, M.L. Impact of the Doping Method on Conductivity and Thermopower in Semiconducting Polythiophenes. Adv. Energy Mater. 2015, 5, 1401072. [Google Scholar] [CrossRef]
- Yuan, D.; Guo, Y.; Zeng, Y.; Fan, Q.; Wang, J.; Yi, Y.; Zhu, X. Air-Stable n-Type Thermoelectric Materials Enabled by Organic Diradicaloids. Angew. Chem. Int. Ed. 2019, 58, 4958–4962. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Chen, G.; Tang, M.Y.; Yang, R.G.; Lee, H.; Wang, D.Z.; Ren, Z.F.; Fleurial, J.-P.; Gogna, P. New Directions for Low-Dimensional Thermoelectric Materials. Adv. Mater. 2007, 19, 1043–1053. [Google Scholar] [CrossRef]
- Gregory, S.A.; Menon, A.K.; Ye, S.; Seferos, D.S.; Reynolds, J.R.; Yee, S.K. Effect of Heteroatom and Doping on the Thermoelectric Properties of Poly(3-alkylchalcogenophenes). Adv. Energy Mater. 2018, 8, 1802419. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Pan, C.; Chen, Z.; Zhou, X.; Gao, C.; Wang, L. A Study of the Thermoelectric Properties of benzo[1,2-b:4,5-b′]dithiophene–based donor–acceptor Conjugated Polymers. Polym. Chem. 2018, 9, 4440–4447. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Jiao, F.; Zhang, J.; Xu, W.; Zhu, D. Effects of Structural Order in the Pristine State on the Thermoelectric Pow-Er-Factor of Doped PBTTT Films. Synth. Met. 2012, 162, 788–793. [Google Scholar] [CrossRef]
- Chen, K.-S.; Zhang, Y.; Yip, H.-L.; Sun, Y.; Davies, J.A.; Ting, C.; Chen, C.; Jen, A.K.-Y. Highly Efficient Indacenodithiophene-Based Polymeric Solar Cells in Conventional and Inverted Device Configurations. Org. Electron. 2011, 12, 794–801. [Google Scholar] [CrossRef]
- Patel, S.N.; Glaudell, A.M.; Peterson, K.A.; Thomas, E.M.; O’Hara, K.A.; Lim, E.; Chabinyc, M.L. Morphology Controls the Ther-Moelectric Power Factor of a Doped Semiconducting Polymer. Sci. Adv. 2017, 3, e1700434. [Google Scholar] [CrossRef] [Green Version]
- Vijayakumar, V.; Zaborova, E.; Biniek, L.; Zeng, H.; Herrmann, L.; Carvalho, A.; Boyron, O.; Leclerc, N.; Brinkmann, M. Effect of Alkyl Side Chain Length on Doping Kinetics, Thermopower, and Charge Transport Properties in Highly Oriented F4TCNQ-Doped PBTTT Films. ACS Appl. Mater. Interfaces 2019, 11, 4942–4953. [Google Scholar] [CrossRef]
- Fan, J.; Yuen, J.D.; Wang, M.; Seifter, J.; Seo, J.H.; Mohebbi, A.R.; Zakhidov, D.; Heeger, A.; Wudl, F. High-Performance Am-Bipolar Transistors and Inverters from an Ultralow Bandgap Polymer. Adv. Mater. 2012, 24, 2186–2190. [Google Scholar] [CrossRef]
- An, C.; Li, M.; Marszalek, T.; Li, D.; Berger, R.; Pisula, W.; Baumgarten, M. Thiadizoloquinoxaline-Based Low-Bandgap Conjugated Polymers As Ambipolar Semiconductors for Organic Field Effect Transistors. Chem. Mater. 2014, 26, 5923–5929. [Google Scholar] [CrossRef] [Green Version]
- Kawabata, K.; Saito, M.; Osaka, I.; Takimiya, K. Very Small Bandgap Pi-Conjugated Polymers with Extended Thienoquinoids. J. Am. Chem. Soc. 2016, 138, 7725–7732. [Google Scholar] [CrossRef]
- Song, X.; Fan, M.; Zhang, K.; Ding, D.; Chen, W.; Li, Y.; Yu, L.; Sun, M.; Yang, R. Fusing Benzo[c][1,2,5]oxadiazole Unit With Thi-Ophene for Constructing Wide-Bandgap High-Performance IDT-Based Polymer Solar Cell Donor Material. Macromol. Rapid. Commun. 2018, 39, e1700782. [Google Scholar] [CrossRef]
- Suh, E.H.; Jeong, Y.J.; Oh, J.G.; Lee, K.; Jung, J.; Kang, Y.S.; Jang, J. Doping of Donor-Acceptor Polymers With Long Side Chains via Solution Mixing for Advancing Thermoelectric Properties. Nano Energy 2019, 58, 585–595. [Google Scholar] [CrossRef]
- Tang, L.-M.; Xiao, J.; Bai, W.-Y.; Li, Q.-Y.; Wang, H.-C.; Miao, M.-S.; Yip, H.-L.; Xu, Y.-X. End-Chain Effects of Non-Fullerene Acceptors on Polymer Solar Cells. Org. Electron. 2019, 64, 1–6. [Google Scholar] [CrossRef]
- Patel, S.N.; Glaudell, A.M.; Kiefer, D.; Chabinyc, M.L. Increasing the Thermoelectric Power Factor of a Semiconducting Polymer by Doping from the Vapor Phase. ACS Macro Lett. 2016, 5, 268–272. [Google Scholar] [CrossRef] [Green Version]
- Thomas, E.M.; Davidson, E.; Katsumata, R.; Segalman, R.A.; Chabinyc, M.L. Branched Side Chains Govern Counterion Position and Doping Mechanism in Conjugated Polythiophenes. ACS Macro Lett. 2018, 7, 1492–1497. [Google Scholar] [CrossRef]
- Scholes, D.T.; Yee, P.Y.; Lindemuth, J.R.; Kang, H.; Onorato, J.; Ghosh, R.; Luscombe, C.K.; Spano, F.C.; Tolbert, S.H.; Schwartz, B.J. The Effects of Crystallinity on Charge Transport and the Structure of Sequentially Processed F 4 TCNQ-Doped Conjugated Polymer Films. Adv. Funct. Mater. 2017, 27, 1702654. [Google Scholar] [CrossRef]
- Mazaheripour, A.; Thomas, E.M.; Segalman, R.A.; Chabinyc, M.L. Nonaggregating Doped Polymers Based on Poly(3,4-Propylenedioxythiophene). Macromolecules 2019, 52, 2203–2213. [Google Scholar] [CrossRef]
- Gueye, M.N.; Carella, A.; Massonnet, N.; Yvenou, E.; Brenet, S.; Faure-Vincent, J.; Pouget, S.; Rieutord, F.; Okuno, H.; Benayad, A.; et al. Structure and Dopant Engineering in PEDOT Thin Films: Practical Tools for a Dramatic Conduc-Tivity Enhancement. Chem. Mater. 2016, 28, 3462–3468. [Google Scholar] [CrossRef]
- Thomas, E.M.; Popere, B.C.; Fang, H.; Chabinyc, M.L.; Segalman, R.A. Role of Disorder Induced by Doping on the Thermoelectric Properties of Semiconducting Polymers. Chem. Mater. 2018, 30, 2965–2972. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, Y.; Souri, M.; Luo, X.; Boehm, A.M.; Li, R.; Zhang, Y.; Wang, T.; Kim, D.Y.; Mei, J.; et al. Influence of Dopant Size and Electron Affinity on the Electrical Conductivity and Thermoelectric Properties of a Series of Con-Jugated Polymers. J. Mater. Chem. A 2018, 6, 6495–16505. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Lee, Y.-H.; Lee, Y.-P.; Chiang, C.-J.; Shen, C.; Wu, C.-C.; Ohta, Y.; Yokozawa, T.; Dai, C.-A. Synthesis and Characterization of poly(3-Hexylthiophene)-poly(3-Hexyloxythiophene) Random Copolymers With Tunable Band Gap via Grignard Metathesis Polymerization. Polym. Int. 2014, 63, 2068–2075. [Google Scholar] [CrossRef]
- Kumar, N.S.S.; Gujrati, M.D.; Wilson, J.N. Evidence of Preferential π-Stacking: A Study of Intermolecular and Intramolecular Charge Transfer Complexes. Chem. Commun. 2010, 46, 5464–5466. [Google Scholar] [CrossRef]
- Wang, S.; Ha, M.; Manno, M.; Frisbie, C.D.; Leighton, C. Hopping Transport and the Hall Effect Near the insulator–metal Transition in Electrochemically Gated poly(3-Hexylthiophene) Transistors. Nat. Commun. 2012, 3, 1210. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Pan, C.; Gao, C.; Shinohara, A.; Yin, X.; Wang, L.; Li, Y.; Jiang, Q.; Yang, C.; Wang, L. Thermoelectrics of Two-Dimensional Conjugated Benzodithiophene-Based Polymers: Density-of-States Enhancement and Semi-Metallic Behavior. J. Mater. Chem. A 2019, 7, 10422–10430. [Google Scholar] [CrossRef]
- Chen, X.; Wu, H.; Cui, J.; Xiao, Y.; Zhang, Y.; He, J.; Chen, Y.; Cao, J.; Cai, W.; Pennycook, S.J.; et al. Extraordinary Thermoelectric Performance in N-Type Manganese Doped Mg3Sb2 Zintl: High Band Degeneracy, Tuned Carrier Scattering Mechanism and Hierarchical Microstructure. Nano Energy 2018, 52, 246–255. [Google Scholar] [CrossRef]
- Xie, D.-X.; Liu, T.-C.; Xiao, J.; Fang, J.-K.; Pan, C.-J.; Shao, G. Effect of Side Chain Substituent Volume on Thermoelectric Properties of IDT-Based Conjugated Polymers. Molecules 2021, 26, 963. [Google Scholar] [CrossRef] [PubMed]

| Polymer | EHOMO (eV) | ELUMO (eV) | Egec (eV) | Egopt (eV) | λonset (nm) | EgDFT (eV) | Carrier Mobility (10−4 cm2/V s) |
|---|---|---|---|---|---|---|---|
| PIDT-BBT | −4.90 | −4.11 | 0.80 | 0.87 | 1423 | 0.60 | 4.52 |
| PIDTT-BBT | −4.92 | −4.06 | 0.86 | 0.95 | 1300 | 0.60 | 274 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, D.; Xiao, J.; Li, Q.; Liu, T.; Xu, J.; Shao, G. Backbone Effects on the Thermoelectric Properties of Ultra-Small Bandgap Conjugated Polymers. Polymers 2021, 13, 2486. https://doi.org/10.3390/polym13152486
Xie D, Xiao J, Li Q, Liu T, Xu J, Shao G. Backbone Effects on the Thermoelectric Properties of Ultra-Small Bandgap Conjugated Polymers. Polymers. 2021; 13(15):2486. https://doi.org/10.3390/polym13152486
Chicago/Turabian StyleXie, Dexun, Jing Xiao, Quanwei Li, Tongchao Liu, Jinjia Xu, and Guang Shao. 2021. "Backbone Effects on the Thermoelectric Properties of Ultra-Small Bandgap Conjugated Polymers" Polymers 13, no. 15: 2486. https://doi.org/10.3390/polym13152486
APA StyleXie, D., Xiao, J., Li, Q., Liu, T., Xu, J., & Shao, G. (2021). Backbone Effects on the Thermoelectric Properties of Ultra-Small Bandgap Conjugated Polymers. Polymers, 13(15), 2486. https://doi.org/10.3390/polym13152486






