Tin Complexes of 4-(Benzylideneamino)benzenesulfonamide: Synthesis, Structure Elucidation and Their Efficiency as PVC Photostabilizers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments and Chemicals
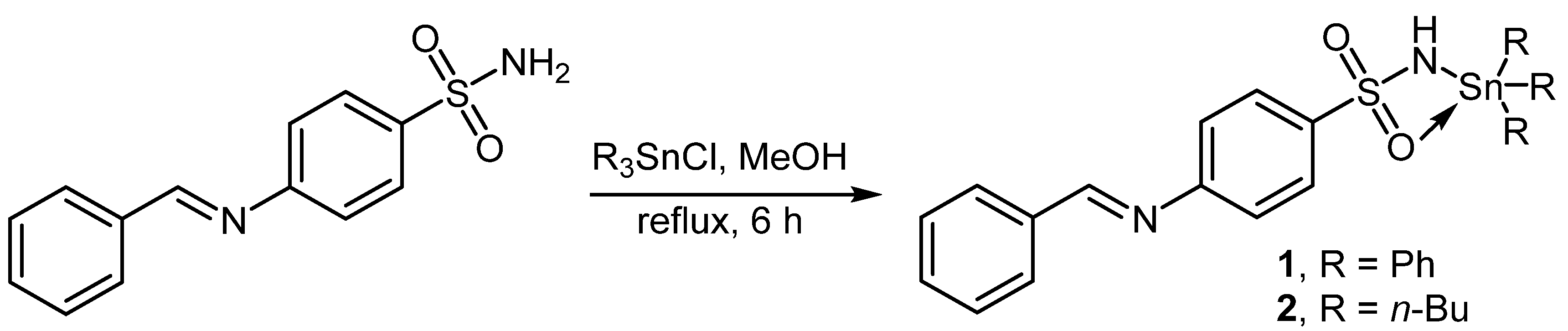
2.2. Synthesis of Complexes 1 and 2
2.3. Synthesis of Complex 3
2.4. Preparation of PVC Films
2.5. Assessment of PVC by FTIR Spectroscopy
2.6. Assessment of PVC Photodegradation by Weight Loss (%)
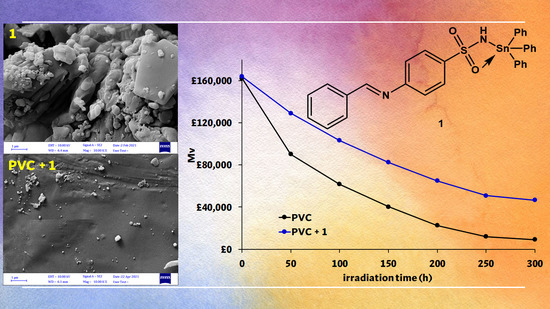
2.7. Assessment of PVC Photodegradation by Average Molecular Weight ()
3. Results and Discussion
3.1. Synthesis of Complexes 1–3
3.2. Characterization of Complexes 1–3
3.3. FESEM of Complexes 1–3
3.4. Assessment of PVC Photodegradation by EDX Mapping
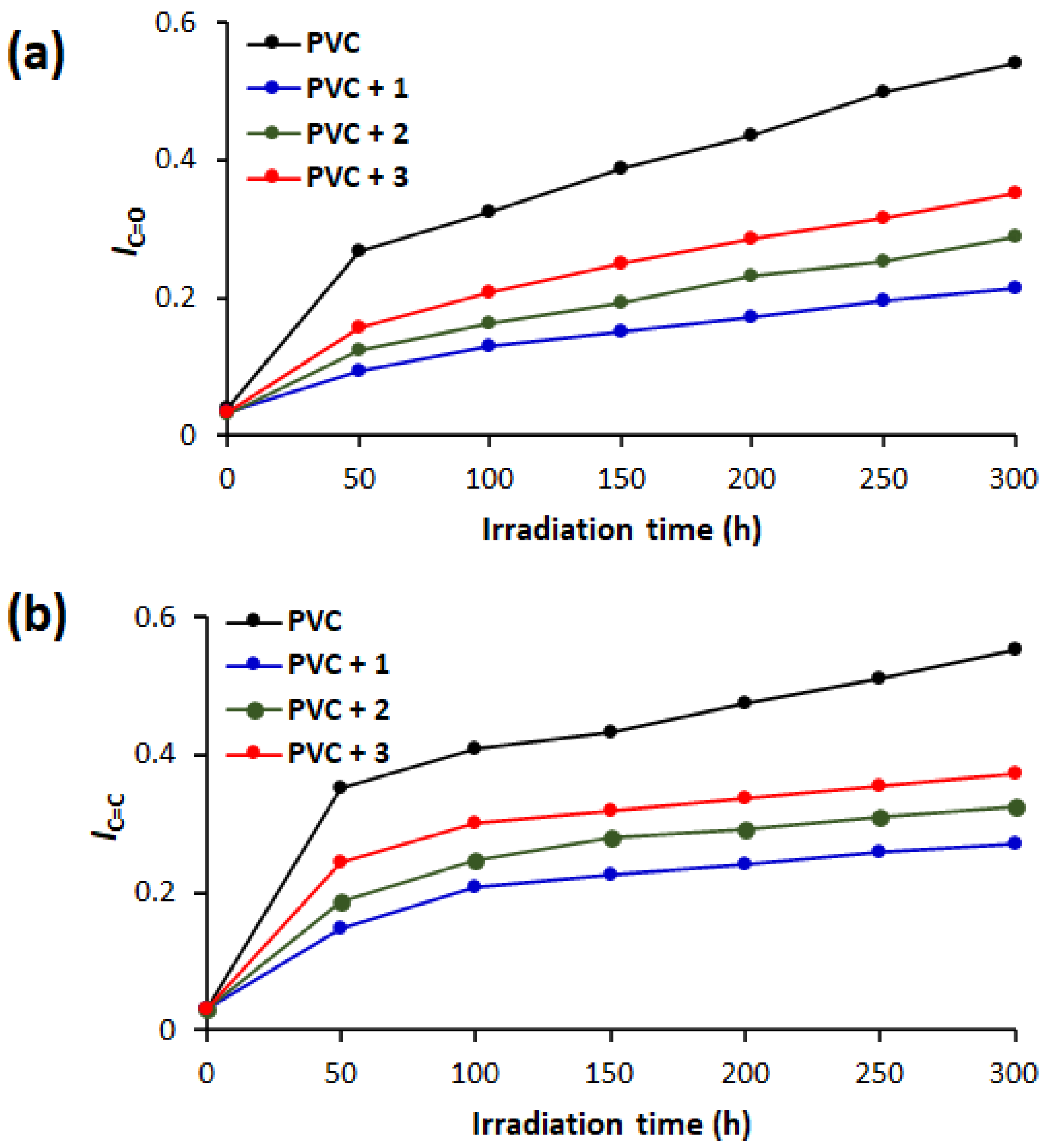
3.5. Assessment of PVC Photodegradation by FTIR Spectroscopy
3.6. Assessment of PVC Photodegradation by Weight Loss
3.7. Assessment of PVC Photodegradation by the
3.8. Assessment of PVC Photodegradation by Optical Microscopy
3.9. Assessment of PVC Photodegradation by the FESEM
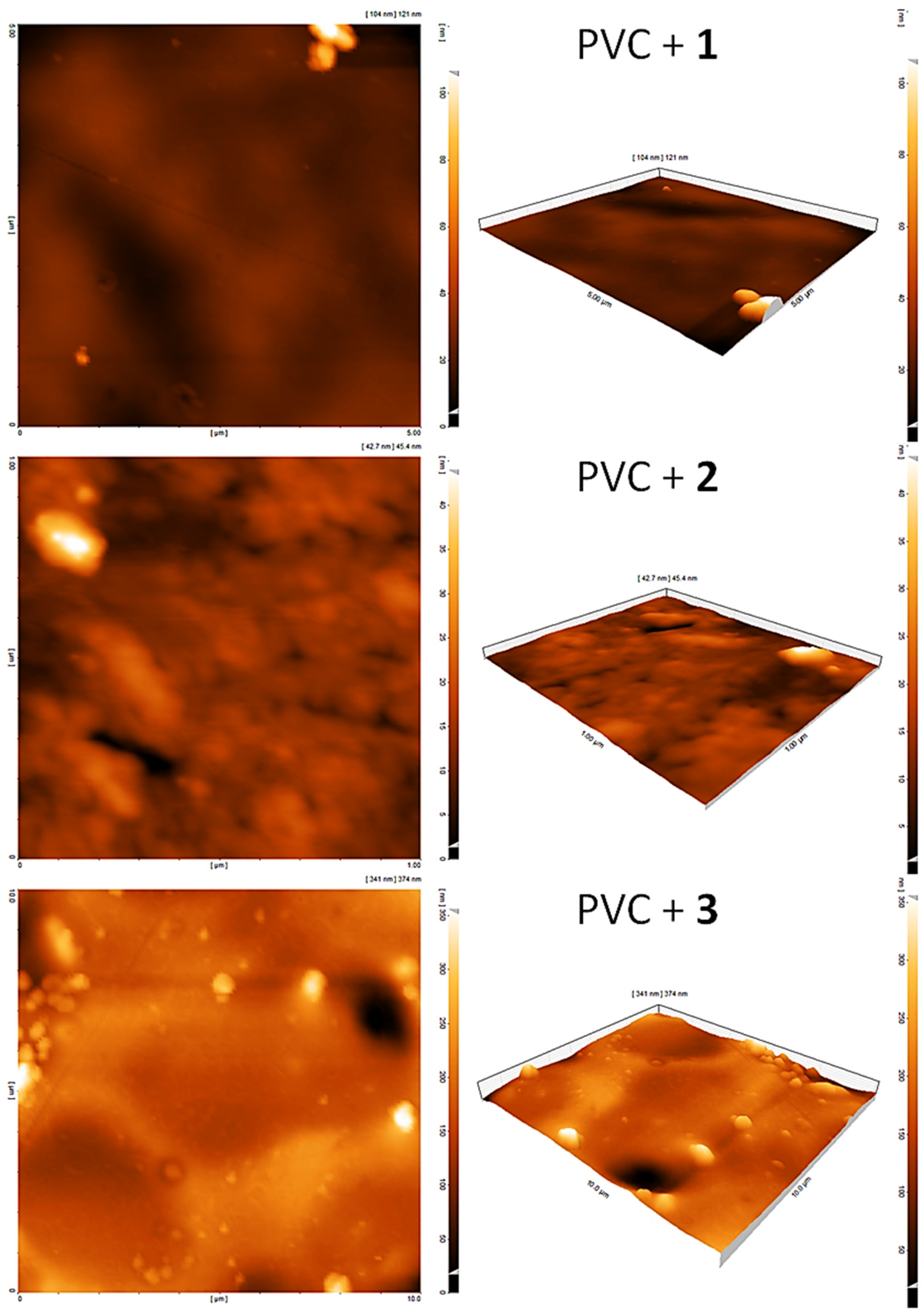
3.10. Assessment of PVC Photodegradation by the AFM
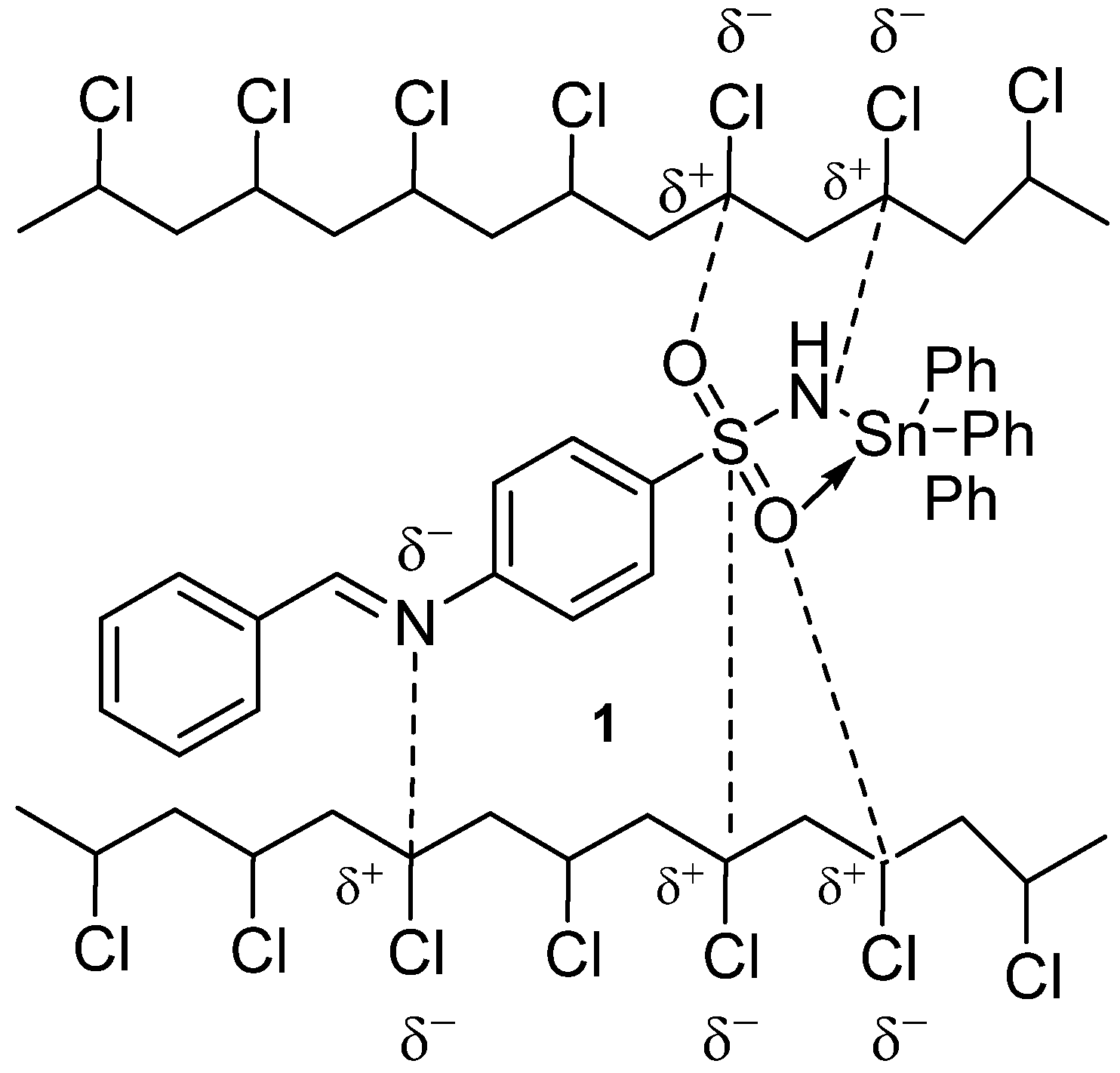
3.11. Suggested Mechanisms of the Role Played by Organotin Complexes as PVC Photostabilizers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millet, H.; Vangheluwe, P.; Block, C.; Sevenster, A.; Garcia, L.; Antonopoulos, R. The nature of plastics and their societal usage. In Plastics and the Environment; Royal Society of Chemistry: London, UK, 2018; pp. 1–20. [Google Scholar] [CrossRef]
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Cadogan, D.F.; Howick, C.J. Plasticizers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Patrick, S.G. Practical Guide to Polyvinyl Chloride; Rapra Technology Limited: Shrewsbury, UK, 2005. [Google Scholar]
- Shi, W.; Zhang, J.; Shi, X.-M.; Jiang, G.-D. Different photodegradation processes of PVC with different average degrees of polymerization. J. Appl. Polym. Sci. 2008, 107, 528–540. [Google Scholar] [CrossRef]
- Al-Hashem, H.A.S.; Al-Naeem, M.A.H. Effect of temperature on the stiffness of polyvinyl chloride and chlorinated polyvinyl chloride joints under bending. J. Appl. Sci. 2007, 7, 3442–3450. [Google Scholar] [CrossRef]
- Liu, J.; Lv, Y.; Luo, Z.; Wang, H.; Wei, Z. Molecular chain model construction, thermo-stability, and thermo-oxidative degradation mechanism of poly(vinyl chloride). RSC Adv. 2016, 6, 31898–31905. [Google Scholar] [CrossRef]
- Rabek, J.F. Polymer Photodegradation—Mechanisms and Experimental Methods; Chapman & Hall: London, UK, 1995. [Google Scholar]
- Veronelli, M.; Mauro, M.; Bresadpla, S. Influence of thermal dehydrochlorination on the photooxidation kinetics of PVC samples. Polym. Degrad. Stab. 1999, 66, 349–357. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Świątek, M.; Kamińska, A. Modification of polystyrene and poly(vinyl chloride) for the purpose of obtaining packaging materials degradable in the natural environment. Polym. Degrad. Stab. 2004, 83, 35–40. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Kowalonek, J.; Ołdak, D. The influence of UV-irradiation on poly(vinyl chloride) modified by iron and cobalt chlorides. Polym. Degrad. Stab. 2003, 79, 231–240. [Google Scholar] [CrossRef]
- Chaochanchaikul, K.; Rosarpitak, V.; Sombatsompop, N. Photodegradation profiles of PVC compound and wood/PVC composites under UV weathering. Express Polym. Lett. 2013, 7, 146–160. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Ding, A.; Guo, H.; Lu, G.; Huang, X. Construction of nontoxic polymeric UV-absorber with great resistance to UV-photoaging. Sci. Rep. 2016, 6, 25508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafari, A.J.; Donaldson, J.D. Determination of HCl and VOC emission from thermal degradation of PVC in the absence and presence of copper, copper(II) oxide and copper(II) chloride. J. Chem. 2009, 6, 685–692. [Google Scholar] [CrossRef] [Green Version]
- Real, L.P.; Ferraria, A.M.; Rego, A.B. Comparison of different photo-oxidation conditions of poly(vinyl chloride) for outdoor applications. Polym. Test. 2008, 27, 743–751. [Google Scholar] [CrossRef]
- Babinsky, R. PVC additives: A global review. Plast. Addit. Compd. 2006, 8, 38–40. [Google Scholar] [CrossRef]
- Lucarini, M.; Pedulli, G.F. Free radical intermediates in the inhibition of the autoxidation reaction. Chem. Soc. Rev. 2010, 39, 2106–2119. [Google Scholar] [CrossRef]
- Gryn’ova, G.; Hodgson, J.L.; Coote, M.L. Revising the mechanism of polymer autooxidation. Org. Biomol. Chem. 2011, 9, 480–490. [Google Scholar] [CrossRef]
- Grossman, R.F. Mixed metal vinyl stabilizer synergism. II: Reactions with zinc replacing cadmium. J. Vinyl Addit. Technol. 1990, 12, 142–145. [Google Scholar] [CrossRef]
- Porta, M.; Zumeta, E. Implementing the Stockholm treaty on persistent organic pollutants. Occup. Environ. Med. 2002, 59, 651–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Xie, L.; Fu, M.; Zhang, J.; Indrawirawan, S.; Zhang, Y.; Tang, S. Synergistic effects of lanthanum-pentaerythritol alkoxide with zinc stearates and with beta-diketone on the thermal stability of poly(vinyl chloride). Polym. Degd. Stab. 2015, 114, 52–59. [Google Scholar] [CrossRef]
- Fu, M.; Li, D.; Liu, H.; Ai, H.; Zhang, Y.; Zhang, L. Synergistic effects of zinc-mannitol alkoxide with calcium/zinc stearates and with β-diketone on thermal stability of rigid poly (vinyl chloride). J. Polym. Res. 2016, 23, 13. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Ahmed, D.S.; El-Hiti, G.A.; Alotaibi, M.H.; Hashim, H.; Yousif, E. SEM morphological analysis of irradiated polystyrene film doped by a Schiff base containing a 1,2,4-triazole ring system. Appl. Petrochem. Res. 2019, 9, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Hashim, H.; El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, D.S.; Yousif, E. Fabrication of ordered honeycomb porous poly(vinyl chloride) thin film doped with a Schiff base and nickel(II) chloride. Heliyon 2018, 4, e00743. [Google Scholar] [CrossRef] [Green Version]
- Shaalan, N.; Laftah, N.; El-Hiti, G.A.; Alotaibi, M.H.; Muslih, R.; Ahmed, D.S.; Yousif, E. Poly(vinyl chloride) photostabilization in the presence of Schiff bases containing a thiadiazole moiety. Molecules 2018, 23, 913. [Google Scholar] [CrossRef] [Green Version]
- Ali, G.Q.; El-Hiti, G.A.; Tomi, I.H.R.; Haddad, R.; Al-Qaisi, A.J.; Yousif, E. Photostability and performance of polystyrene films containing 1,2,4-triazole-3-thiol ring system Schiff bases. Molecules 2016, 21, 1699. [Google Scholar] [CrossRef] [Green Version]
- Balakit, A.A.; Ahmed, A.; El-Hiti, G.A.; Smith, K.; Yousif, E. Synthesis of new thiophene derivatives and their use as photostabilizers for rigid poly(vinyl chloride). Int. J. Polym. Sci. 2015, 2015, 510390. [Google Scholar] [CrossRef]
- Jin, D.; Khanal, S.; Zhang, C.; Xu, S. Photodegradation of polybenzimidazole/polyvinyl chloride composites and polybenzimidazole: Density functional theory and experimental study. J. Appl. Polym. Sci. 2021, 138, 49693. [Google Scholar] [CrossRef]
- El-Hiti, G.A.; Ahmed, D.S.; Yousif, E.; Alotaibi, M.H.; Star, H.A.; Ahmed, A.A. Influence of polyphosphates on the physicochemical properties of poly(vinyl chloride) after irradiation with ultraviolet light. Polymers 2020, 12, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiller, M. PVC Additives: Performance, Chemistry, Developments, and Sustainability; Carl Hanser Verlag: Munich, Germany, 2015. [Google Scholar]
- Chakrabarti, S.; Chaudhuri, B.; Bhattacharjee, S.; Das, P.; Dutta, B.K. Degradation mechanism and kinetic model for photocatalytic oxidation of PVC-ZnO composite film in presence of a sensitizing dye and UV radiation. J. Hazard. Mater. 2008, 154, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.C.; Noguchi, T.; Isshiki, M.; Wu, J.H. Effect of titanium dioxide particles on the surface morphology and the mechanical properties of PVC composites during QUV accelerated weathering. Polym. Compos. 2016, 37, 3391–3397. [Google Scholar] [CrossRef]
- Yang, T.C.; Noguchi, T.; Isshiki, M.; Wu, J.H. Effect of titanium dioxide on chemical and molecular changes in PVC sidings during QUV accelerated weathering. Polym. Degrad. Stab. 2014, 104, 33–39. [Google Scholar] [CrossRef]
- Devi, J.; Yadav, J. Recent advancements in organotin(IV) complexes as potential anticancer agents. Anticancer Agents Med. Chem. 2018, 18, 335–353. [Google Scholar] [CrossRef]
- Romero-Chávez, M.M.; Pineda-Urbina, K.; Pérez, D.J.; Obledo-Benicio, F.; Flores-Parra, A.; Gómez-Sandoval, Z.; Ramos-Organillo, Á. Organotin(IV) compounds derived from ibuprofen and cinnamic acids, an alternative into design of anti-inflammatory by the cyclooxygenases (COX-1 and COX-2) pathway. J. Organomet. Chem. 2018, 862, 58–70. [Google Scholar] [CrossRef]
- Sirajuddin, M.; Ali, S. Organotin(IV) carboxylates as promising potential drug candidates in the field of cancer chemotherapy. Curr. Pharm. Des. 2016, 22, 6665–6681. [Google Scholar] [CrossRef]
- Da Silva, M.A.; dos Santos, A.S.S.; dos Santos, T.V.; Meneghetti, M.R.; Meneghetti, S.M.P. Organotin(IV) compounds with high catalytic activities and selectivities in the glycerolysis of triacylglycerides. Catal. Sci. Technol. 2017, 7, 5750–5757. [Google Scholar] [CrossRef]
- Ghazi, D.; Rasheed, Z.; Yousif, E. A review of organotin compounds: Chemistry and applications. Arch. Org. Inorg. Chem. Sci. 2018, 3, 344–352. [Google Scholar] [CrossRef]
- Ahmed, A.; El-Hiti, G.A.; Hadi, A.G.; Ahmed, D.S.; Baashen, M.A.; Hashim, H.; Yousif, E. Photostabilization of poly(vinyl chloride) films blended with organotin complexes of mefenamic acid for outdoor applications. Appl. Sci. 2021, 11, 2853. [Google Scholar] [CrossRef]
- Mousa, O.G.; El-Hiti, G.A.; Baashen, M.A.; Bufaroosha, M.; Ahmed, A.; Ahmed, A.A.; Ahmed, D.S.; Yousif, E. Synthesis of carvedilol-organotin complexes and their effects on reducing photodegradation of poly(vinyl chloride). Polymers 2021, 13, 500. [Google Scholar] [CrossRef]
- Mahmood, Z.N.; Yousif, E.; Alias, M.; El-Hiti, G.A.; Ahmed, D.S. Synthesis, characterization, properties, and use of new fusidate organotin complexes as additives to inhibit poly(vinyl chloride) photodegradation. J. Polym. Res. 2020, 27, 267. [Google Scholar] [CrossRef]
- Jasem, H.; Hadi, A.G.; El-Hiti, G.A.; Baashen, M.A.; Hashim, H.; Ahmed, A.A.; Ahmed, D.S.; Yousif, E. Tin-naphthalene sulfonic acid complexes as photostabilizers for poly(vinyl chloride). Molecules 2021, 26, 3629. [Google Scholar] [CrossRef]
- Gaumet, S.; Gardette, J.-L. Photo-oxidation of poly(vinyl chloride): Part 2—A comparative study of the carbonylated products in photo-chemical and thermal oxidations. Polym. Degrad. Stab. 1991, 33, 17–34. [Google Scholar] [CrossRef]
- Pospíšil, J.; Nešpurek, S. Photostabilization of coatings. Mechanisms and performance. Prog. Polym. Sci. 2000, 25, 1261–1335. [Google Scholar] [CrossRef]
- Pepperl, G. Molecular weight distribution of commercial PVC. J. Vinyl Addit. Technol. 2000, 6, 88–92. [Google Scholar] [CrossRef]
- Morozov, O.S.; Vyshinskii, N.N.; Rudnevskii, N.K. Investigation of some organotin compounds and their complexes by IR spectroscopy. J. Appl. Spectrosc. 1981, 35, 1019–1023. [Google Scholar] [CrossRef]
- Shahid, K.; Ali, S.; Shahzadi, S.; Badshah, A.; Khan, K.M.; Maharvi, G.M. Organotin(IV) complexes of aniline derivatives. I. Synthesis, spectral and antibacterial studies of di- and triorganotin(IV) derivatives of 4-bromomaleanilic acid. Synth. React. Inorg. Met-Org. Chem. 2003, 33, 1221–1235. [Google Scholar] [CrossRef]
- Masood, H.; Ali, S.; Mazhar, M.; Shahzadi, S.; Shahid, K. 1H, 13C, 119Sn NMR, Mass, Mössbauer and biological studies of tri-, di- and chlorodiorganotin(IV) carboxylates. Turk. J. Chem. 2004, 28, 75–86. [Google Scholar]
- Rehman, W.; Baloch, M.K.; Badshah, A.; Ali, S. Synthesis and characterization of biologically potent di-organotin(IV) complexes of mono-methyl glutarate. J. Chin. Chem. Soc. 2005, 52, 231–236. [Google Scholar] [CrossRef]
- Nief, O.A. Photostabilization of polyvinyl chloride by some new thiadiazole derivatives. Eur. J. Chem. 2015, 6, 242–247. [Google Scholar] [CrossRef] [Green Version]
- Gardette, J.L.; Gaumet, S.; Lemaire, J. Photooxidation of poly(vinyl chloride). 1. A reexamination of the mechanism. Macromolecules 1989, 22, 2576–2581. [Google Scholar] [CrossRef]
- Mark, J.E. (Ed.) Physical Properties of Polymers Handbook; Springer: New York, NY, USA, 2007. [Google Scholar]
- Yousif, E.; El-Hiti, G.A.; Hussain, Z.; Altaie, A. Viscoelastic, spectroscopic and microscopic study of the photo irradiation effect on the stability of PVC in the presence of sulfamethoxazole Schiff’s bases. Polymers 2015, 7, 2190–2204. [Google Scholar] [CrossRef] [Green Version]
- Valko, L.; Klein, E.; Kovařik, P.; Bleha, T.; Šimon, P. Kinetic study of thermal dehydrochlorination of poly(vinyl chloride) in the presence of oxygen: III. Statistical thermodynamic interpretation of the oxygen catalytic activity. Eur. Polym. J. 2001, 37, 1123–1132. [Google Scholar] [CrossRef]
- Yousif, E.; Hasan, A.; El-Hiti, G.A. Spectroscopic, physical and topography of photochemical process of PVC films in the presence of Schiff base metal complexes. Polymers 2016, 8, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, D.S.; El-Hiti, G.A.; Hameed, A.S.; Yousif, E.; Ahmed, A. New tetra-Schiff bases as efficient photostabilizers for poly(vinyl chloride). Molecules 2017, 22, 1506. [Google Scholar] [CrossRef] [Green Version]
- El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, A.A.; Hamad, B.A.; Ahmed, D.S.; Ahmed, A.; Hashim, H.; Yousif, E. The morphology and performance of poly(vinyl chloride) containing melamine Schiff bases against ultraviolet light. Molecules 2019, 24, 803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadi, A.G.; Yousif, E.; El-Hiti, G.A.; Ahmed, D.S.; Jawad, K.; Alotaibi, M.H.; Hashim, H. Long-term effect of ultraviolet irradiation on poly(vinyl chloride) films containing naproxen diorganotin(IV) complexes. Molecules 2019, 24, 2396. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, R.; El-Hiti, G.A.; Ahmed, A.; Yousif, E. Poly(vinyl chloride) doped by 2-(4-isobutylphenyl)propanoate metal complexes: Enhanced resistance to UV irradiation. Arab. J. Sci. Eng. 2017, 42, 4307–4315. [Google Scholar] [CrossRef]
- Ali, M.M.; El-Hiti, G.A.; Yousif, E. Photostabilizing efficiency of poly(vinyl chloride) in the presence of organotin(IV) complexes as photostabilizers. Molecules 2016, 21, 1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadi, A.G.; Jawad, K.; El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, A.A.; Ahmed, D.S.; Yousif, E. Photostabilization of poly(vinyl chloride) by organotin(IV) compounds against photodegradation. Molecules 2019, 24, 3557. [Google Scholar] [CrossRef] [Green Version]
- Ghazi, D.; El-Hiti, G.A.; Yousif, E.; Ahmed, D.S.; Alotaibi, M.H. The effect of ultraviolet irradiation on the physicochemical properties of poly(vinyl chloride) films containing organotin(IV) complexes as photostabilizers. Molecules 2018, 23, 254. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, D.S.; El-Hiti, G.A.; Yousif, E.; Hameed, A.S. Polyphosphates as inhibitors for poly(vinyl chloride) photodegradation. Molecules 2017, 22, 1849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabaa, M.W.; Oraby, E.H.; Abdel Naby, A.S.; Mohamed, R.R. Anthraquinone derivatives as organic stabilizers for rigid poly(vinyl chloride) against photo-degradation. Eur. Polym. J. 2005, 41, 2530–2543. [Google Scholar] [CrossRef]
- Zheng, X.-G.; Tang, L.-H.; Zhang, N.; Gao, Q.-H.; Zhang, C.-F.; Zhu, Z.-B. Dehydrochlorination of PVC materials at high temperature. Energy Fuels 2003, 17, 896–900. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Oraby, E.H.; Abdel-Naby, A.S.; Mohamed, R.R. Organic thermal stabilizers for rigid poly (vinyl chloride). Part XII: N-phenyl-3-substituted-5-pyrazolone derivatives. Polym. Degrad. Stab. 2006, 91, 911–923. [Google Scholar] [CrossRef]
- Mohammed, A.; El-Hiti, G.A.; Yousif, E.; Ahmed, A.A.; Ahmed, D.S.; Alotaibi, M.H. Protection of poly(vinyl chloride) films against photodegradation using various valsartan tin complexes. Polymers 2020, 12, 969. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Complex. | Color | MP (°C) | Yield (%) | Calculated (Found; %) | |||
|---|---|---|---|---|---|---|---|
| C | H | N | Sn | ||||
| 1 | Off-White | 112–114 | 86 | 61.11 (61.16) | 4.30 (4.32) | 4.60 (4.71) | 19.48 (19.55) |
| 2 | Off-White | 132–134 | 63 | 54.66 (54.70) | 6.97 (7.00) | 5.10 (5.12) | 21.61 (21.68) |
| 3 | White | 168–170 | 76 | 50.39 (50.41) | 4.23 (4.16) | 8.40 (8.45) | 17.79 (17.86) |
| Complex | Wave Number (ν, cm–1) | ||||||
|---|---|---|---|---|---|---|---|
| NH | CH=N | C=C | SO2 asym | SO2 sym | Sn–O | Sn–N | |
| 1 | 3459 | 1678 | 1624 | 1307 | 1188 | 552 | 440 |
| 2 | 3460 | 1684 | 1630 | 1304 | 1179 | 533 | 439 |
| 3 | 3461 | 1667 | 1623 | 1307 | 1185 | 550 | 454 |
| Complex | 1H NMR (500 MHz: δ, ppm, J in Hz) |
|---|---|
| 1 | 8.11–8.00 (m, 8H, Ar), 7.55–7.42 (m, 14H, Ar), 7.01 (s, 1H, CH), 6.68 (d, J = 7.8 Hz, 2H, Ar), 6.01 (s, exch., 1H, NH) |
| 2 | 7.58–7.52 (m, 5H, Ar), 7.05 (s, 1H, CH), 6.74–6.70 (m, 4H, Ar), 5.94 (s, exch., 1H, NH), 1.79 (t, J = 7.4 Hz, 6H, 3CH2), 168 (m, 6H, 3CH2), 1.46 (m, 6H, 3CH2), 1.00 (t, J = 7.4 Hz, 9H, 3Me) |
| 3 | 8.08–7.66 (m, 12H, Ar), 7.12 (s, 2H, 2CH), 6.68 (d, J = 7.9 Hz, 6H, Ar), 5.95 (s, 2H, exch., 2NH), 1.15 (s, 6H, 2Me). |
| Complex | 13C NMR | 119Sn NMR |
|---|---|---|
| 1 | 162.8, 151.9, 143.7, 136.2, 135.7, 130.0, 129.5, 129.2, 129.0, 128.9, 128.7, 121.2, 112.4 | −227.4 |
| 2 | 161.9, 151.9, 140.3, 135.0, 130.0, 128.6, 127.4, 122.4, 112.4, 27.9, 26.2, 21.0, 13.6 | −241.3 |
| 3 | 162.8, 151.9, 141.1, 134.6, 132.0, 128.9, 126.9, 121.2, 112.4, 22.8 | −143.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghani, H.; Yousif, E.; Ahmed, D.S.; Kariuki, B.M.; El-Hiti, G.A. Tin Complexes of 4-(Benzylideneamino)benzenesulfonamide: Synthesis, Structure Elucidation and Their Efficiency as PVC Photostabilizers. Polymers 2021, 13, 2434. https://doi.org/10.3390/polym13152434
Ghani H, Yousif E, Ahmed DS, Kariuki BM, El-Hiti GA. Tin Complexes of 4-(Benzylideneamino)benzenesulfonamide: Synthesis, Structure Elucidation and Their Efficiency as PVC Photostabilizers. Polymers. 2021; 13(15):2434. https://doi.org/10.3390/polym13152434
Chicago/Turabian StyleGhani, Hassan, Emad Yousif, Dina S. Ahmed, Benson M. Kariuki, and Gamal A. El-Hiti. 2021. "Tin Complexes of 4-(Benzylideneamino)benzenesulfonamide: Synthesis, Structure Elucidation and Their Efficiency as PVC Photostabilizers" Polymers 13, no. 15: 2434. https://doi.org/10.3390/polym13152434
APA StyleGhani, H., Yousif, E., Ahmed, D. S., Kariuki, B. M., & El-Hiti, G. A. (2021). Tin Complexes of 4-(Benzylideneamino)benzenesulfonamide: Synthesis, Structure Elucidation and Their Efficiency as PVC Photostabilizers. Polymers, 13(15), 2434. https://doi.org/10.3390/polym13152434