Effect of Process Conditions on the Properties of Resorcinol-Formaldehyde Aerogel Microparticles Produced via Emulsion-Gelation Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
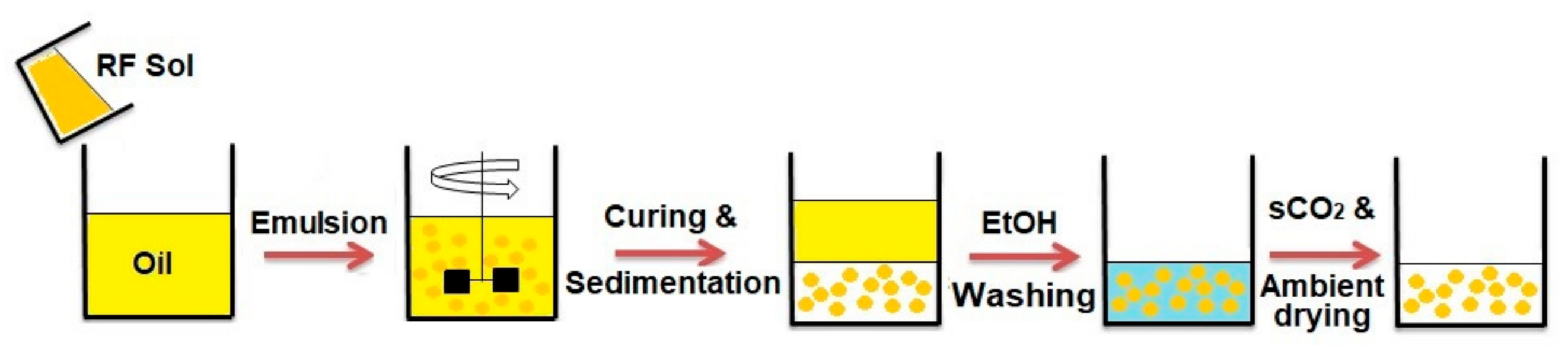
2.2. Preparation of RF Aerogel Microparticles
2.3. Characterization
3. Results and Discussion
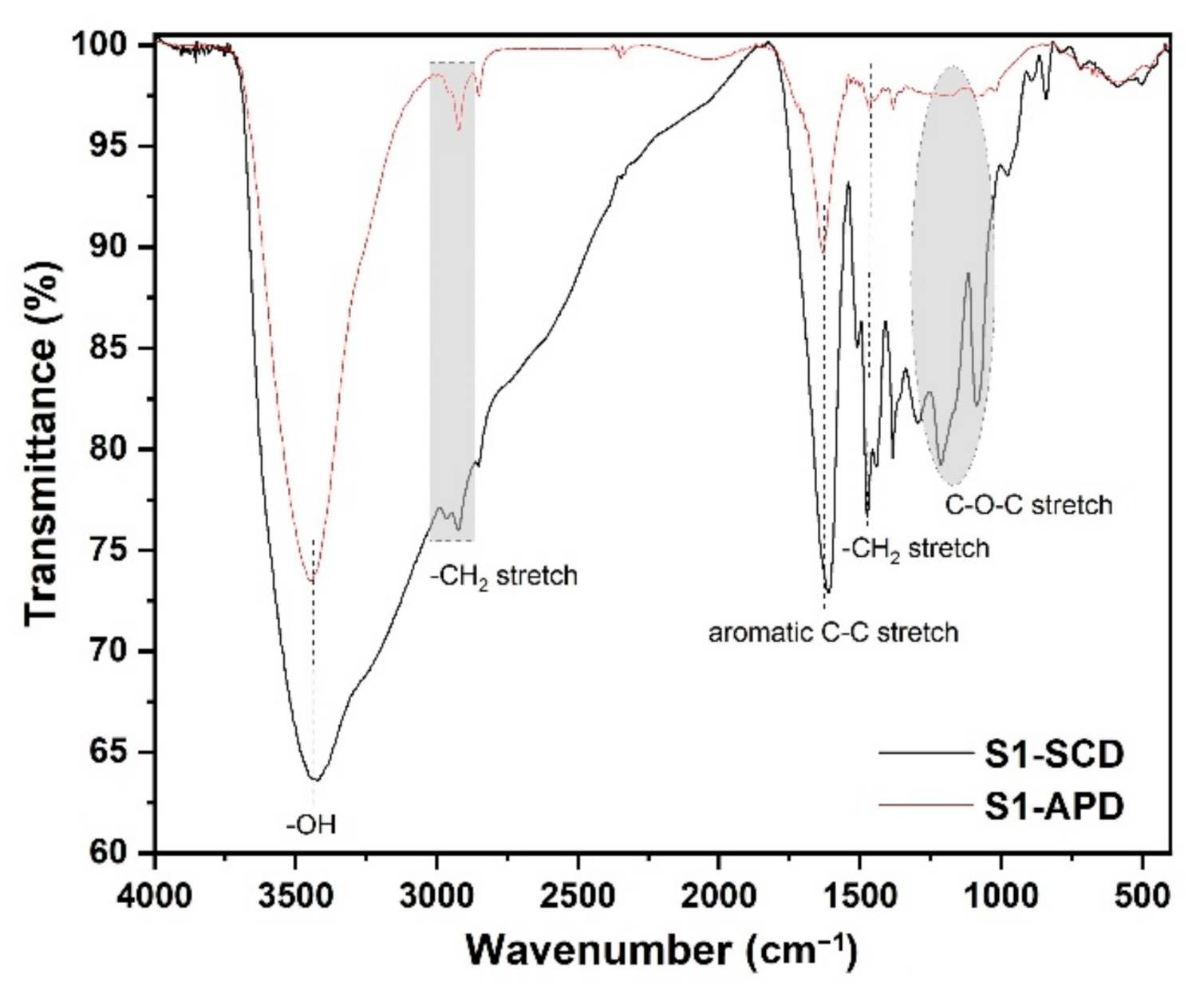
3.1. FT-IR Analysis
3.2. WAXS Analysis
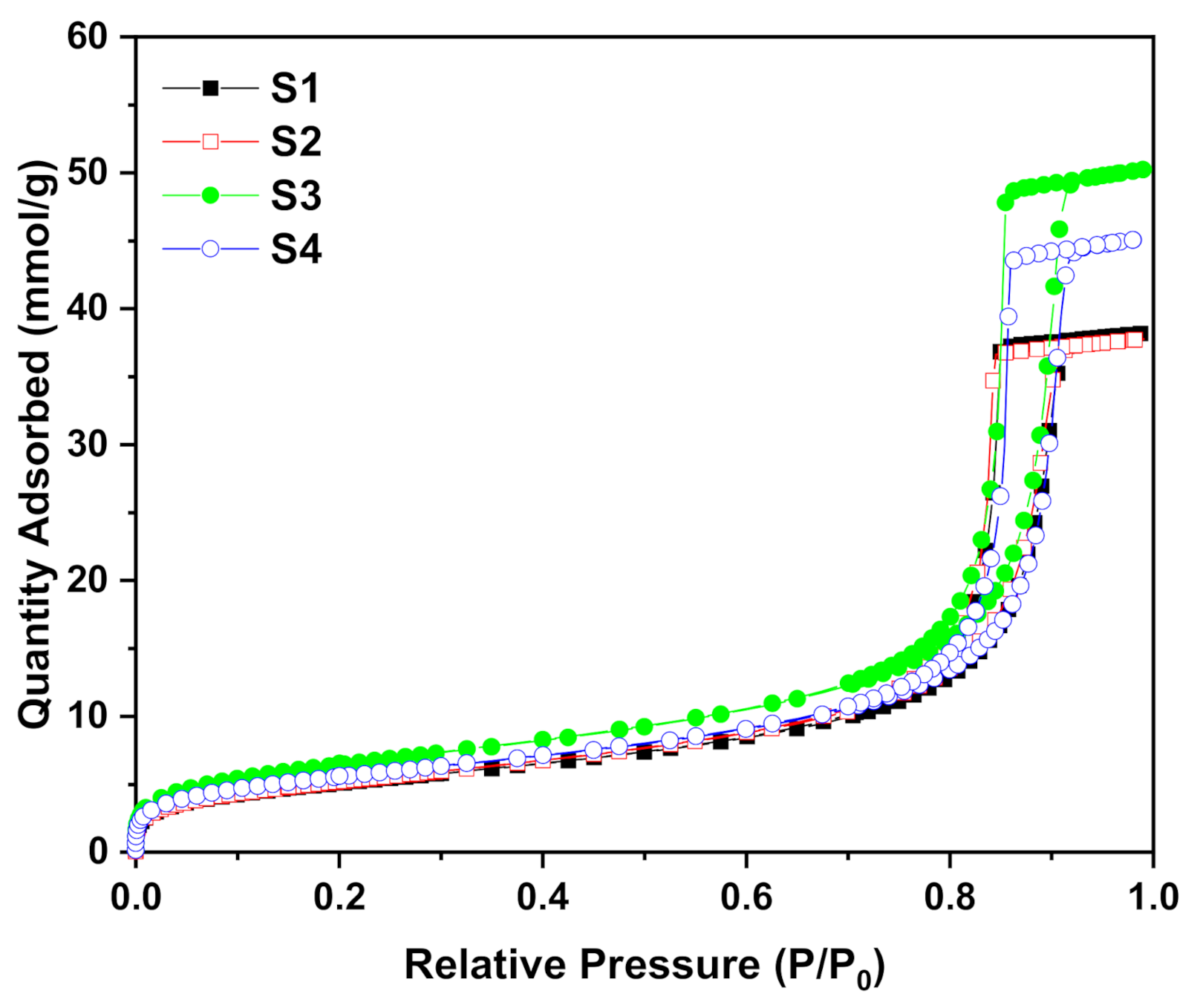
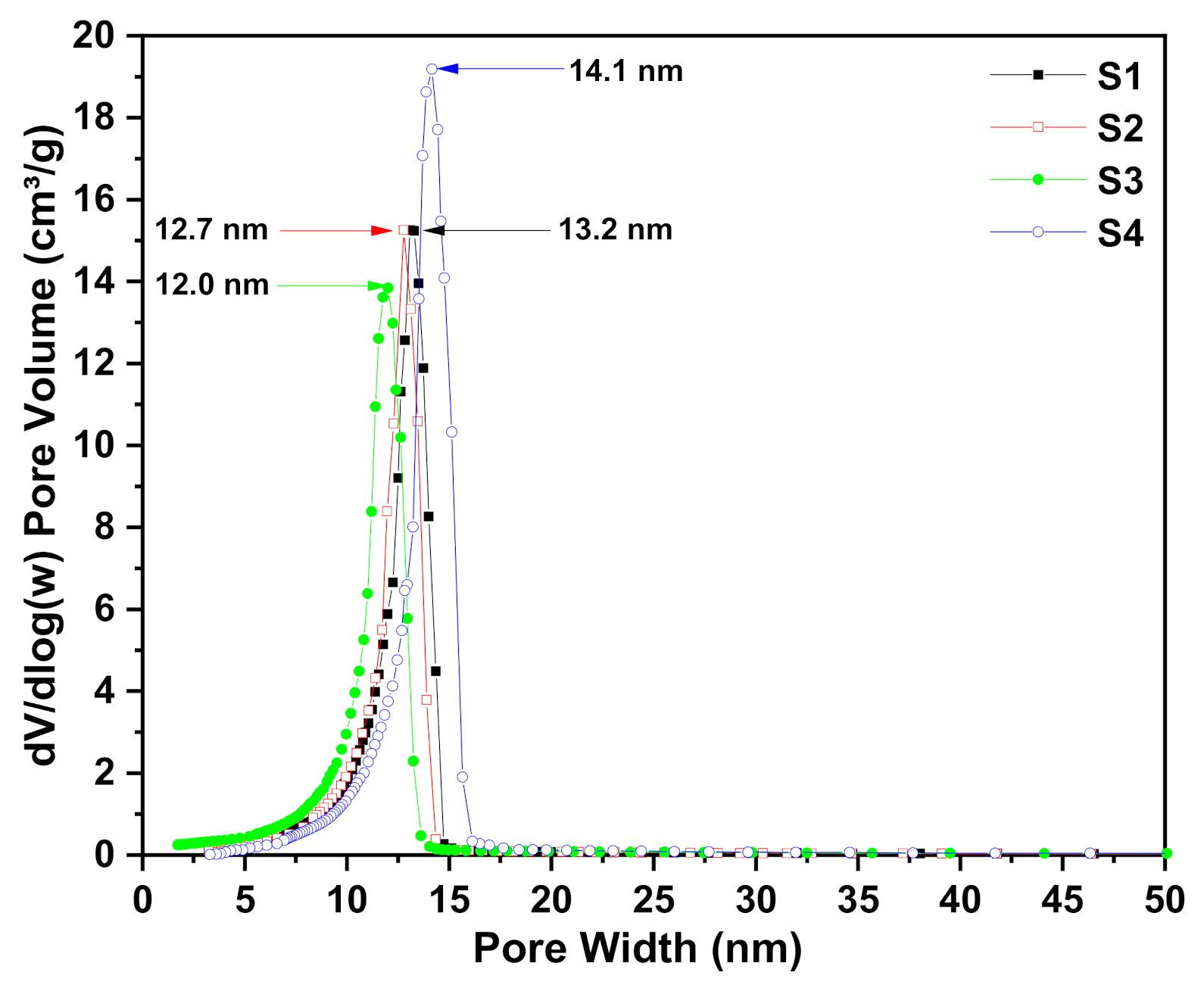
3.3. N2 Adsorption/Desorption Isotherm
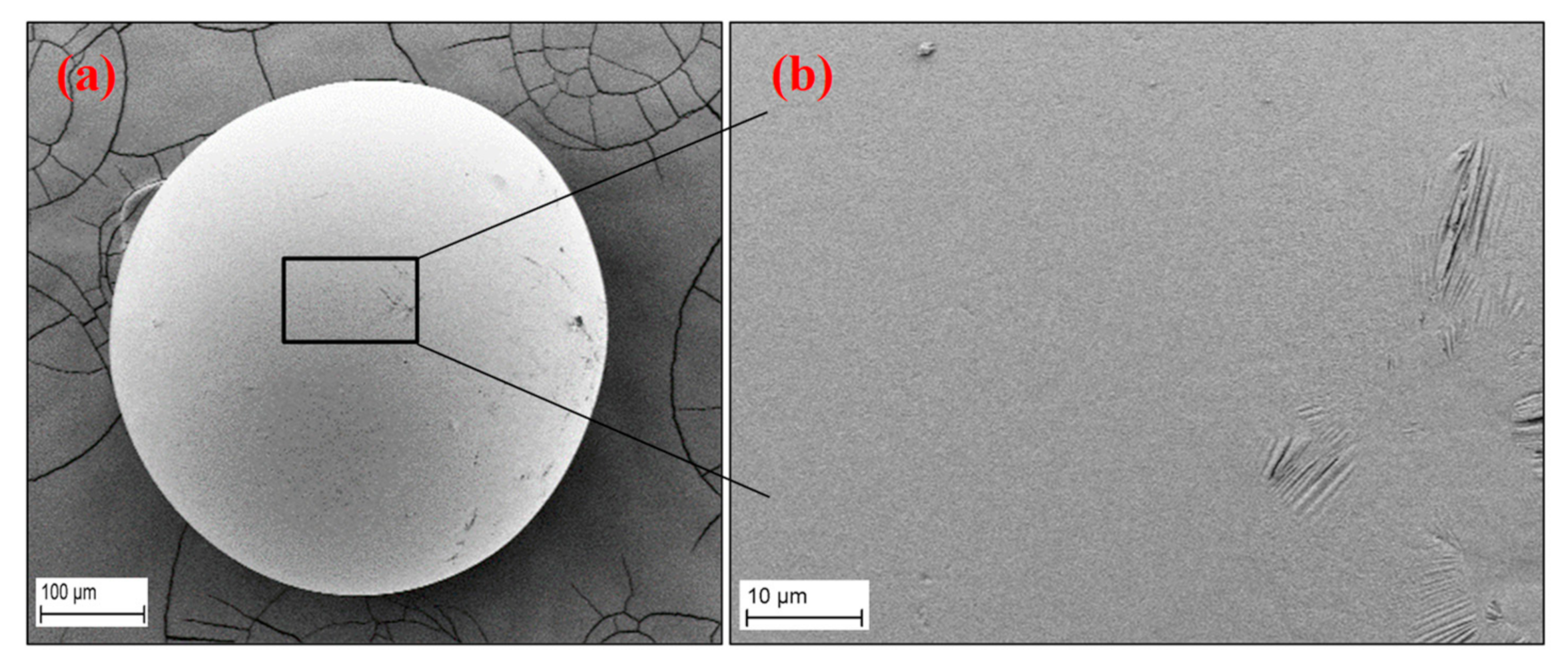
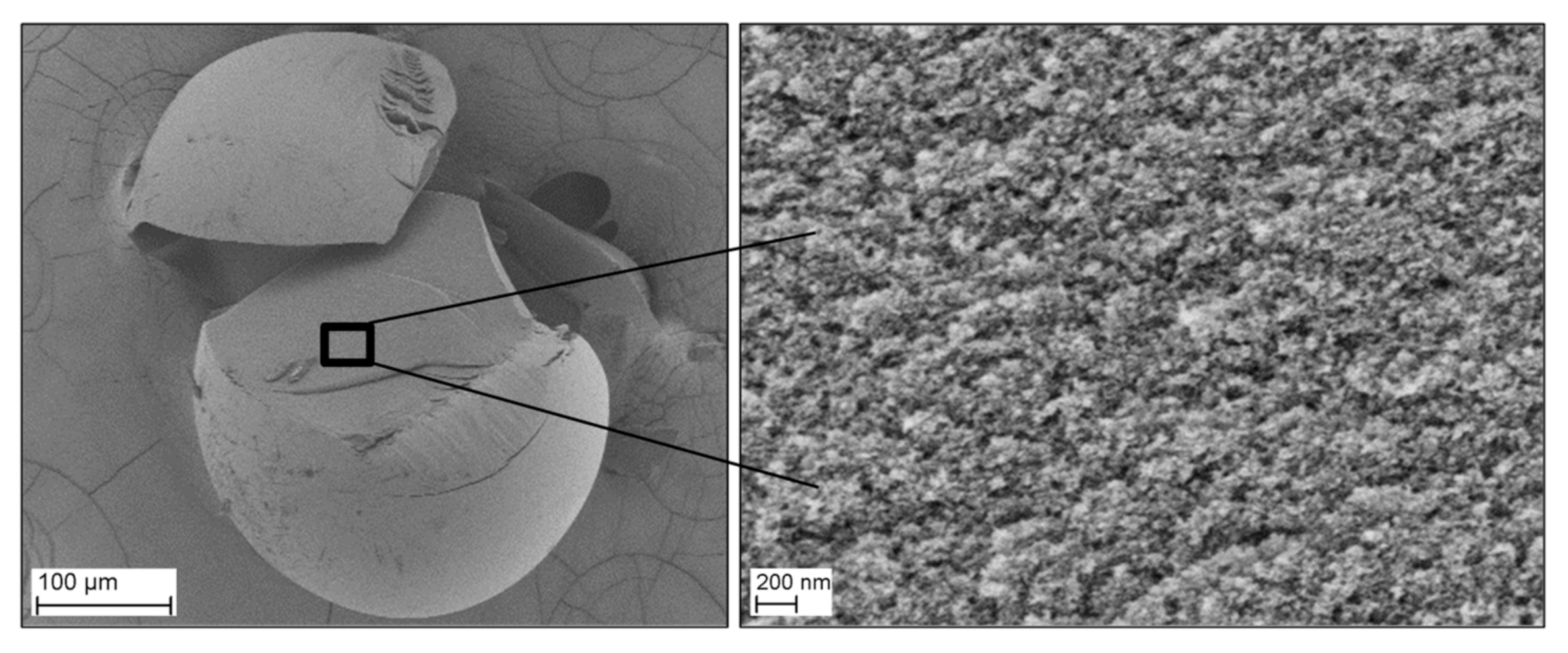
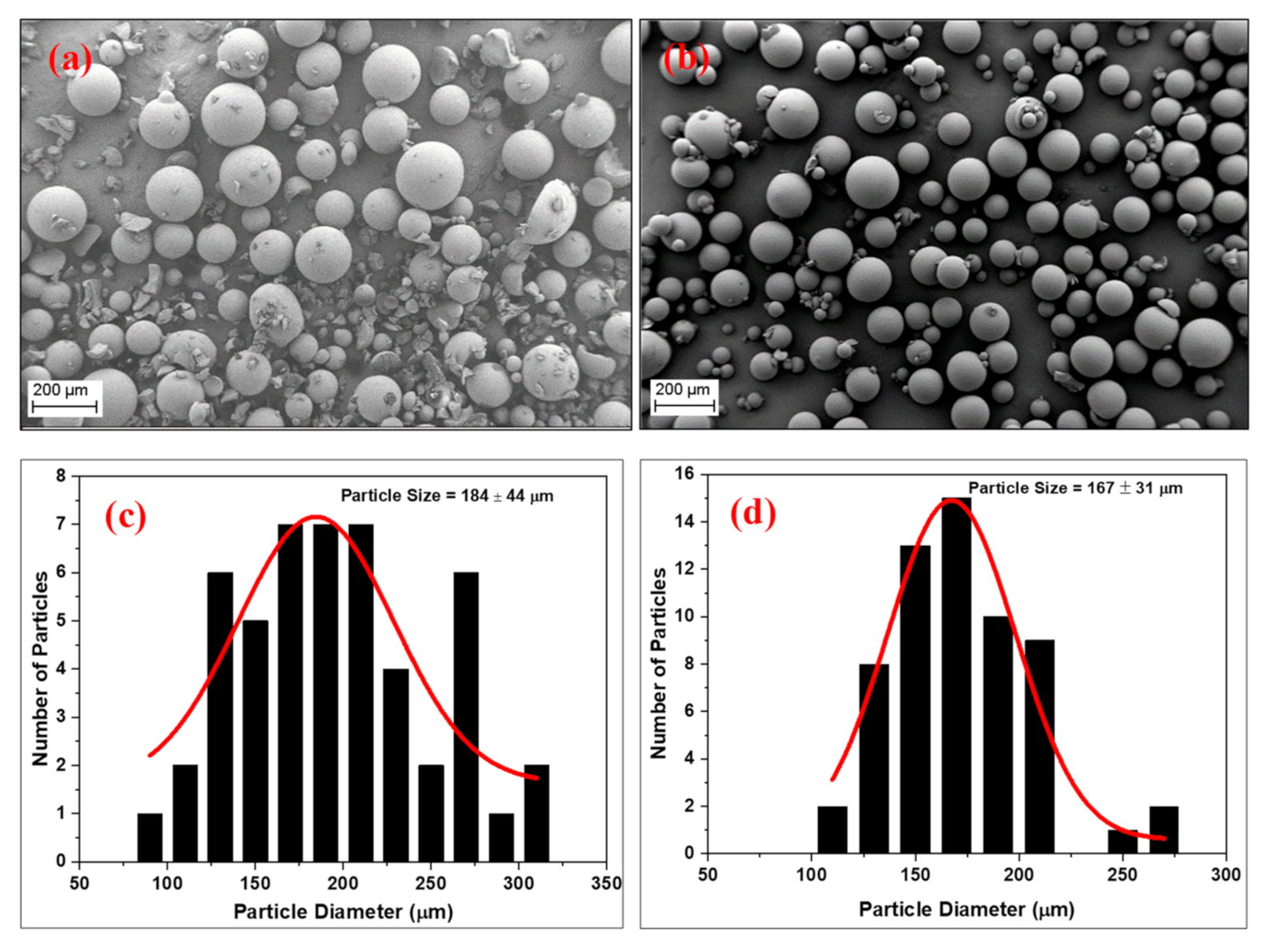
3.4. Morphology of the RF Aerogel Microparticles
3.5. Effect of Process Conditions
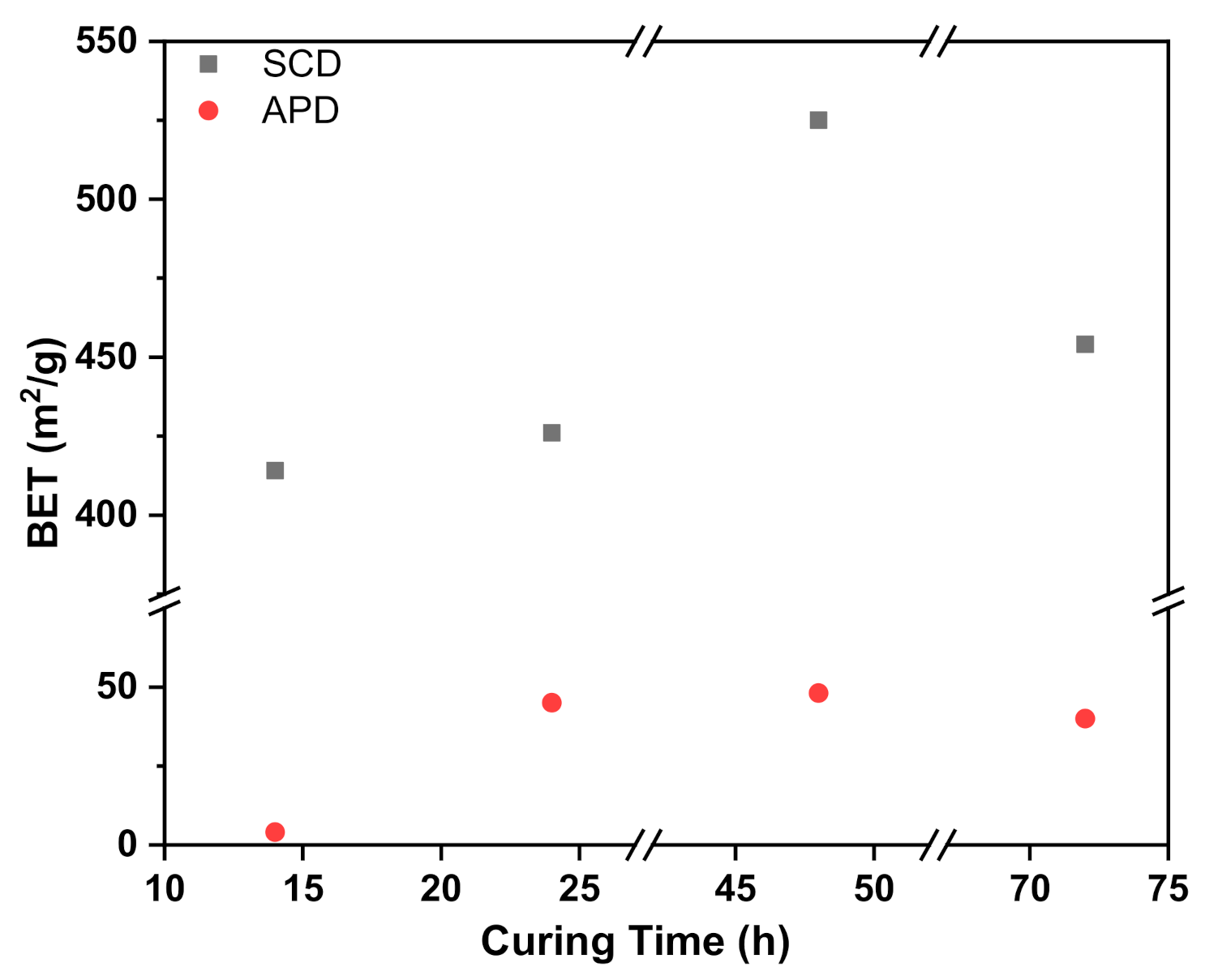
3.5.1. Stirring Rate
3.5.2. RF Sol:Oil Ratio
3.5.3. Initial pH of the RF Sol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paraskevopoulou, P.; Chriti, D.; Raptopoulos, G.; Anyfantis, G.C. Synthetic polymer aerogels in particulate form. Materials 2019, 12, 1543. [Google Scholar] [CrossRef] [Green Version]
- Mulik, S.; Sotiriou-Leventis, C. Resorcinol–formaldehyde aerogels. In Aerogels Handbook; Aegerter, M.A., Leventis, N., Koebel, M.M., Eds.; Springer: New York, NY, USA, 2011; pp. 215–234. [Google Scholar] [CrossRef]
- Shen, Y.; Du, A.; Cheng, F.; Li, X.; Liu, C.; Liu, Y.; Zhao, M.; Shen, J.; Zhou, B. Preparation and characterization of inhomogeneous RF aerogels with continuously varying densities. J. Sol-Gel Sci. Technol. 2019, 90, 478–486. [Google Scholar] [CrossRef]
- Schwan, M.; Ratke, L. Flexibilisation of resorcinol–formaldehyde aerogels. J. Mater. Chem. A 2013, 1, 13462–13468. [Google Scholar] [CrossRef]
- Guo, H.; Meador, M.A.B.; McCorkle, L.; Quade, D.J.; Guo, J.; Hamilton, B.; Cakmak, M.; Sprowl, G. Polyimide aerogels cross-linked through amine functionalized polyoligomeric silsesquioxane. ACS Appl. Mater. Interfaces 2011, 3, 546–552. [Google Scholar] [CrossRef]
- Al-Muhtaseb, S.A.; Ritter, J.A. Preparation and properties of resorcinol–formaldehyde organic and carbon gels. Adv. Mater. 2003, 15, 101–114. [Google Scholar] [CrossRef]
- ElKhatat, A.M.; Al-Muhtaseb, S.A. Advances in tailoring resorcinol-formaldehyde organic and carbon gels. Adv. Mater. 2011, 23, 2887–2903. [Google Scholar] [CrossRef]
- Liu, N.; Shen, J.; Liu, D. Activated high specific surface area carbon aerogels for EDLCs. Microporous Mesoporous Mater. 2013, 167, 176–181. [Google Scholar] [CrossRef]
- Brück, S.; Ratke, L. RF-aerogels: A new binding material for foundry application. J. Sol-Gel Sci. Technol. 2003, 26, 663–666. [Google Scholar] [CrossRef]
- Cai, X.; Tan, G.; Deng, Z.; Liu, J.; Gui, D. Preparation of hierarchical porous carbon aerogels by microwave assisted sol-gel process for supercapacitors. Polymers 2019, 11, 429. [Google Scholar] [CrossRef] [Green Version]
- Reuß, M.; Ratke, L. Subcritically dried RF-aerogels catalysed by hydrochloric acid. J. Sol-Gel Sci. Technol. 2008, 47, 74–80. [Google Scholar] [CrossRef]
- Ratke, L.; Milow, B. Aerogels for foundry applications. In Aerogels Handbook; Aegerter, M.A., Leventis, N., Koebel, M.M., Eds.; Springer: New York, NY, USA, 2011; pp. 763–788. [Google Scholar] [CrossRef] [Green Version]
- Meyer, E.; Milow, B.; Ratke, L. Development of aerogel additives for the foundry industry. J. Supercrit. Fluids 2015, 106, 62–68. [Google Scholar] [CrossRef]
- Ratke, L.; Brück, S. Mechanical properties of aerogel composites for casting purposes. J. Mater. Sci. 2006, 41, 1019–1024. [Google Scholar] [CrossRef]
- Reuß, M.; Ratke, L. Characterization of carbon-aerosands. Int. J. Foundry Res. 2009, 61, 24–33. [Google Scholar]
- Alkemper, J.; Diefenbach, S.; Ratke, L. Chill casting into aerogels. Scr. Metall. Mater. 1993, 29, 1495–1500. [Google Scholar] [CrossRef]
- Dwivedi, C.; Pathak, S.K.; Kumar, M.; Tripathi, S.C.; Bajaj, P.N. Removal of cesium by spherical resorcinol–formaldehyde resin beads: Sorption and kinetic studies. J. Radioanal. Nucl. Chem. 2013, 297, 1–8. [Google Scholar] [CrossRef]
- Eskenazi, D.; Kreit, P.; Pirard, J.-P.; Job, N.; Compère, P. Toward a continuous synthesis of porous carbon xerogel beads. AIChE J. 2018, 64, 1049–1058. [Google Scholar] [CrossRef]
- Ganesan, K.; Budtova, T.; Ratke, L.; Gurikov, P.; Baudron, V.; Preibisch, I.; Niemeyer, P.; Smirnova, I.; Milow, B. Review on the production of polysaccharide aerogel particles. Materials 2018, 11, 2144. [Google Scholar] [CrossRef] [Green Version]
- Alnaief, M.; Smirnova, I. In Situ production of spherical aerogel microparticles. J. Supercrit. Fluids 2011, 55, 1118–1123. [Google Scholar] [CrossRef]
- Baudron, V.; Taboada, M.; Gurikov, P.; Smirnova, I.; Whitehouse, S. Production of starch aerogel in form of monoliths and microparticles. Colloid Polym. Sci. 2020, 298, 477–494. [Google Scholar] [CrossRef] [Green Version]
- Baudron, V.; Gurikov, P.; Smirnova, I. A continuous approach to the emulsion gelation method for the production of aerogel micro-particle. Colloids Surf. A Physicochem. Eng. Asp. 2019, 566, 58–69. [Google Scholar] [CrossRef]
- Mayer, S.T.; Kong, F.M.; Pekala, R.W.; Kaschmitter, J.L. Organic Aerogel Microspheres and Fabrication Method Therefor. U.S. Patent Application No. 5,508,341, 16 April 1996. [Google Scholar]
- Chaichanawong, J.; Kongcharoen, K.; Areerat, S. Preparation of carbon aerogel microspheres by a simple-injection emulsification method. Adv. Powder Technol. 2013, 24, 891–896. [Google Scholar] [CrossRef]
- Sharma, C.S.; Kulkarni, M.M.; Sharma, A.; Madou, M. Synthesis of carbon xerogel particles and fractal-like structures. Chem. Eng. Sci. 2009, 64, 1536–1543. [Google Scholar] [CrossRef]
- Zapata-Benabithe, Z.; Carrasco-Marín, F.; de Vicente, J.; Moreno-Castilla, C. Carbon xerogel microspheres and monoliths from resorcinol–formaldehyde mixtures with varying dilution ratios: Preparation, surface characteristics, and electrochemical double-layer capacitances. Langmuir 2013, 29, 6166–6173. [Google Scholar] [CrossRef]
- Kakunuri, M.; Vennamalla, S.; Sharma, C.S. Synthesis of carbon xerogel nanoparticles by inverse emulsion polymerization of resorcinol–formaldehyde and their use as anode materials for lithium-ion battery. RSC Adv. 2015, 5, 4747–4753. [Google Scholar] [CrossRef]
- Lee, H.-J.; Song, J.-H.; Kim, J.-H. Synthesis of resorcinol/formaldehyde gel particles by the sol-emulsion–gel technique. Mater. Lett. 1998, 37, 197–200. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, S.; Fu, R.; Dresselhaus, M.S.; Dresselhaus, G. Carbon aerogel spheres prepared via alcohol supercritical drying. Carbon 2006, 44, 2430–2436. [Google Scholar] [CrossRef]
- Mulik, S.; Sotiriou-Leventis, C.; Leventis, N. Time-efficient acid-catalyzed synthesis of resorcinol−formaldehyde aerogels. Chem. Mater. 2007, 19, 6138–6144. [Google Scholar] [CrossRef]
- Pekala, R.W. Organic aerogels from the polycondensation of resorcinol with formaldehyde. J. Mater. Sci. 1989, 24, 3221–3227. [Google Scholar] [CrossRef]
- Hebalkar, N.; Arabale, G.; Sainkar, S.R.; Pradhan, S.D.; Mulla, I.S.; Vijayamohanan, K.; Ayyub, P.; Kulkarni, S.K. Study of correlation of structural and surface properties with electrochemical behaviour in carbon aerogels. J. Mater. Sci. 2005, 40, 3777–3782. [Google Scholar] [CrossRef]
- Kumar, V.; Kandasubramanian, B. Ionic-liquid-assisted three-dimensional caged silica ablative nanocomposites. J. Appl. Polym. Sci. 2017, 134, 45328. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Thommes, M. Progress in the physisorption characterization of nanoporous gas storage materials. Engineering 2018, 4, 559–566. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ohmori, T.; Kim, Y.H. Preparation and characterization of monodisperse carbon cryogel microspheres. Microporous Mesoporous Mater. 2008, 112, 211–218. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental methods in chemical engineering: Specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Zhang, G.; Ni, C.; Liu, L.; Zhao, G.; Fina, F.; Irvine, J.T.S. Macro-mesoporous resorcinol–formaldehyde polymer resins as amorphous metal-free visible light photocatalysts. J. Mater. Chem. A 2015, 3, 15413–15419. [Google Scholar] [CrossRef] [Green Version]
- Ratke, L.; Hajduk, A. On the size effect of gelation kinetics in RF aerogels. Gels 2015, 1, 276–290. [Google Scholar] [CrossRef]
- Tannert, R.; Schwan, M.; Ratke, L. Reduction of shrinkage and brittleness for resorcinol-formaldehyde aerogels by means of a pH-controlled sol–gel process. J. Supercrit. Fluids 2015, 106, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Laskowski, J.; Milow, B.; Ratke, L. Subcritically dried resorcinol–formaldehyde aerogels from a base–acid catalyzed synthesis route. Microporous Mesoporous Mater. 2014, 197, 308–315. [Google Scholar] [CrossRef]


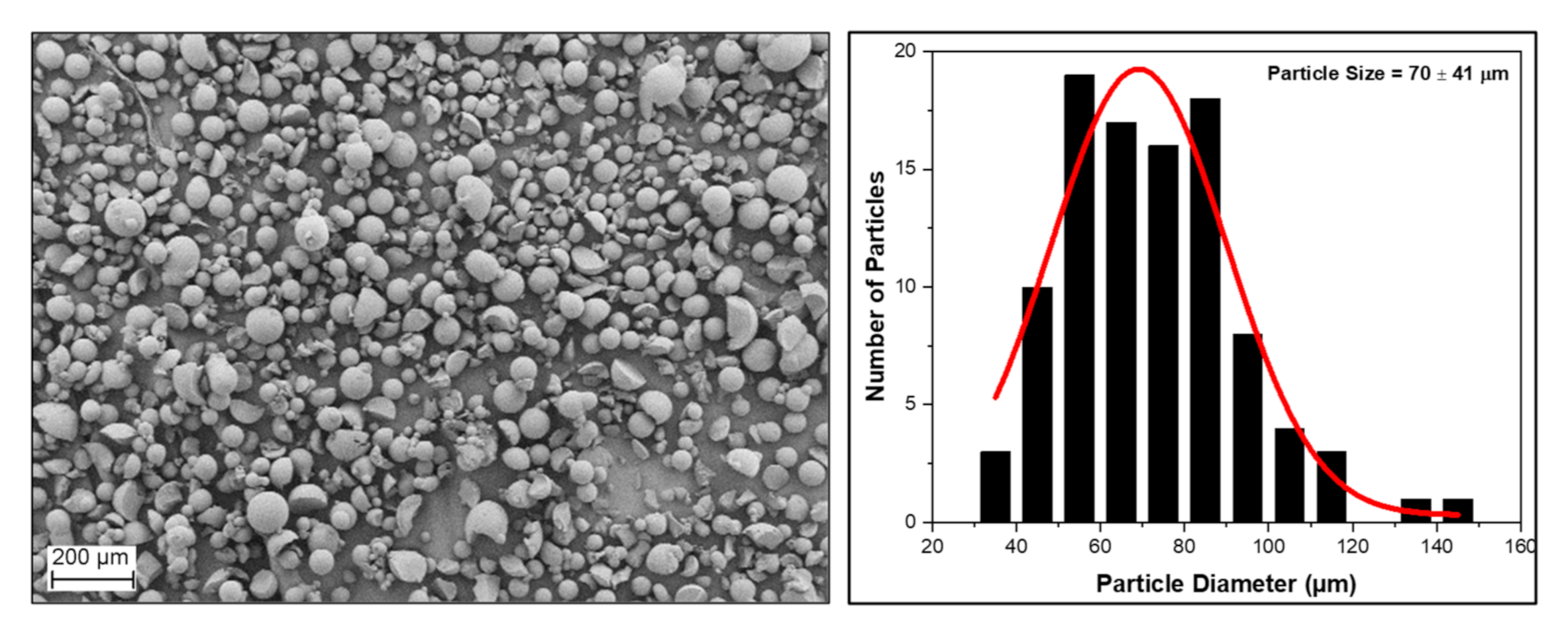
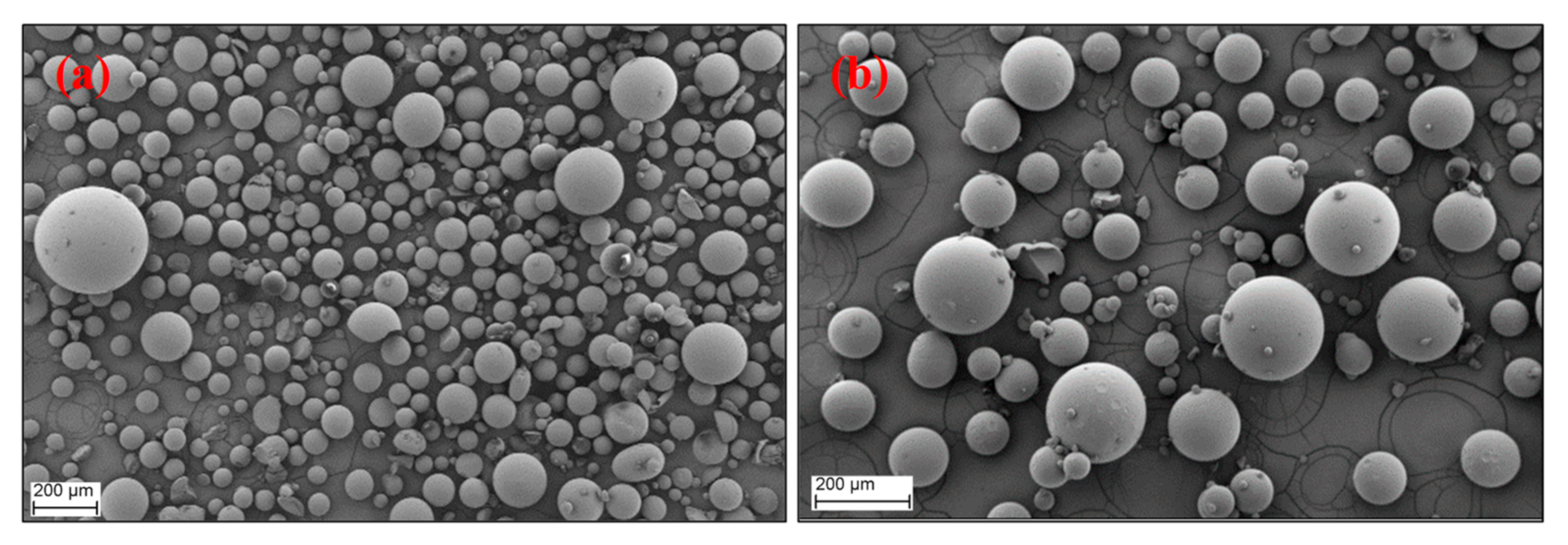
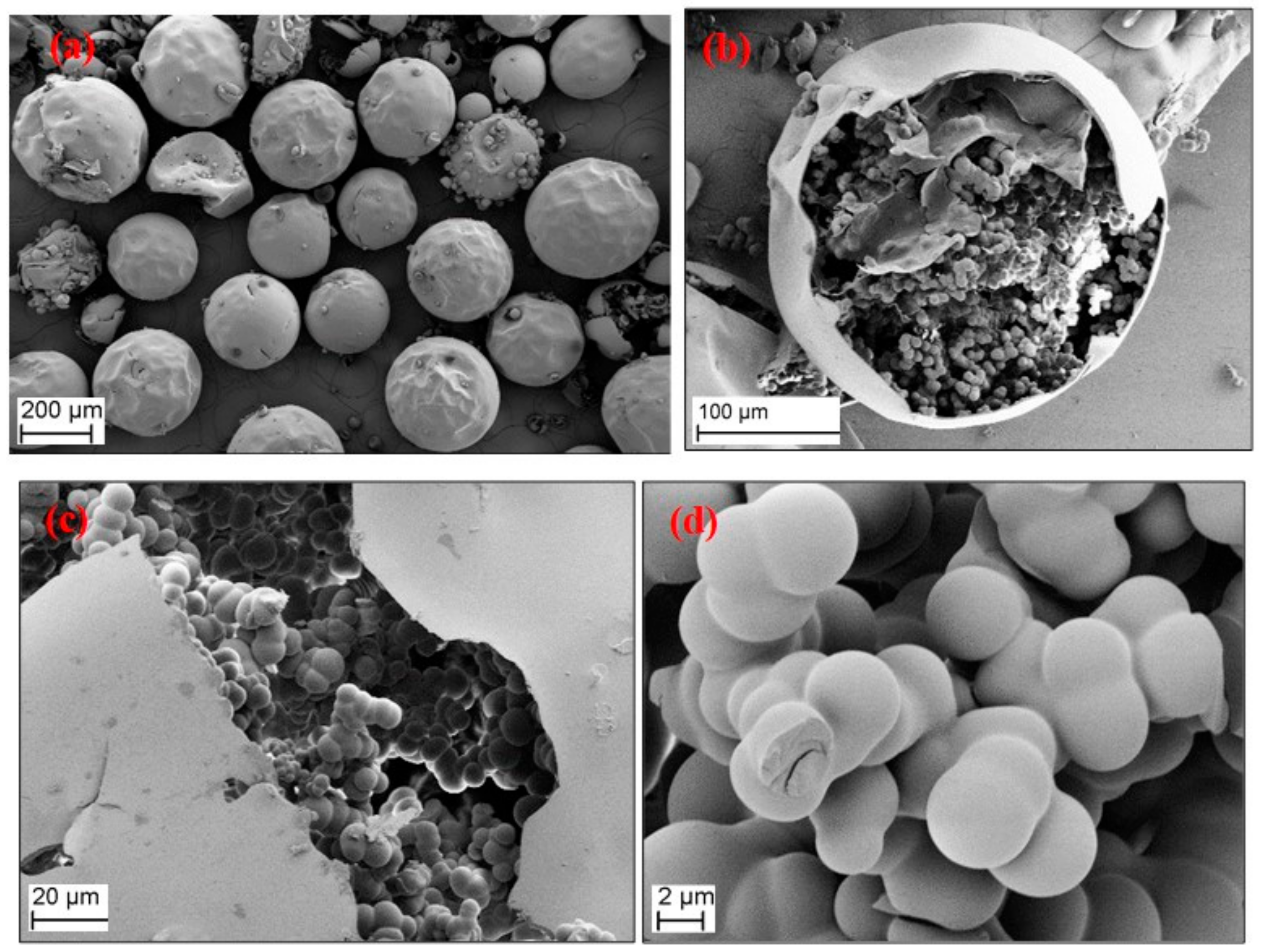
| Sample Codes | R/C | R/F | R/W | Sol:Oil Ratio | Stirring Rate (rpm) | Curing Time (h) | Drying Method * |
|---|---|---|---|---|---|---|---|
| S1 | 200 | 0.74 | 0.044 | 1:3 | 200 | 14 | SCD |
| APD | |||||||
| S2 | 24 | SCD | |||||
| APD | |||||||
| S3 | 48 | SCD | |||||
| APD | |||||||
| S4 | 72 | SCD | |||||
| APD | |||||||
| S5 | 1:3 | 500 | 24 | SCD | |||
| APD | |||||||
| S6 | 1:2 | 200 | 24 | SCD | |||
| APD | |||||||
| S7 | 1:1 | SCD | |||||
| APD | |||||||
| S8a * | 1:3 | SCD | |||||
| APD |
| Sample Codes | Drying method | SBET (m2/g) | Vp (cm3/g) | Dp (nm) |
|---|---|---|---|---|
| S1 | SCD | 414 | 1.32 | 13.2 |
| APD | 4 | 0.01 | - | |
| S2 | SCD | 426 | 1.31 | 12.7 |
| APD | 45 | 0.05 | - | |
| S3 | SCD | 525 | 1.74 | 12.0 |
| APD | 48 | 0.14 | - | |
| S4 | SCD | 454 | 1.56 | 14.1 |
| APD | 40 | 0.05 | - |
| Sample Codes | Sol:Oil Ratio | Drying Method | SBET (m2/g) | Vp (cm3/g) | Dp (nm) |
|---|---|---|---|---|---|
| S7 | 1:1 | SCD | 483 | 1.39 | 9.9 |
| APD | 27 | 0.03 | - | ||
| S6 | 1:2 | SCD | 543 | 1.75 | 11.4 |
| APD | 134 | 0.16 | - | ||
| S2 | 1:3 | SCD | 426 | 1.31 | 12.7 |
| APD | 45 | 0.05 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamal Mohamed, S.M.; Heinrich, C.; Milow, B. Effect of Process Conditions on the Properties of Resorcinol-Formaldehyde Aerogel Microparticles Produced via Emulsion-Gelation Method. Polymers 2021, 13, 2409. https://doi.org/10.3390/polym13152409
Kamal Mohamed SM, Heinrich C, Milow B. Effect of Process Conditions on the Properties of Resorcinol-Formaldehyde Aerogel Microparticles Produced via Emulsion-Gelation Method. Polymers. 2021; 13(15):2409. https://doi.org/10.3390/polym13152409
Chicago/Turabian StyleKamal Mohamed, Seeni Meera, Charlotte Heinrich, and Barbara Milow. 2021. "Effect of Process Conditions on the Properties of Resorcinol-Formaldehyde Aerogel Microparticles Produced via Emulsion-Gelation Method" Polymers 13, no. 15: 2409. https://doi.org/10.3390/polym13152409
APA StyleKamal Mohamed, S. M., Heinrich, C., & Milow, B. (2021). Effect of Process Conditions on the Properties of Resorcinol-Formaldehyde Aerogel Microparticles Produced via Emulsion-Gelation Method. Polymers, 13(15), 2409. https://doi.org/10.3390/polym13152409








