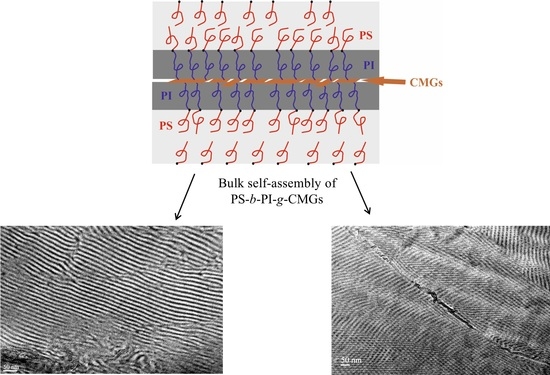
Structure/Properties Relationship of Anionically Synthesized Diblock Copolymers “Grafted to” Chemically Modified Graphene
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Synthesis of PS-b-PI-OH
2.4. Synthesis of Carboxylated Graphene Oxide (CMGs)
2.5. Synthesis of PS-b-PI-g-CMGs
3. Results and Discussion
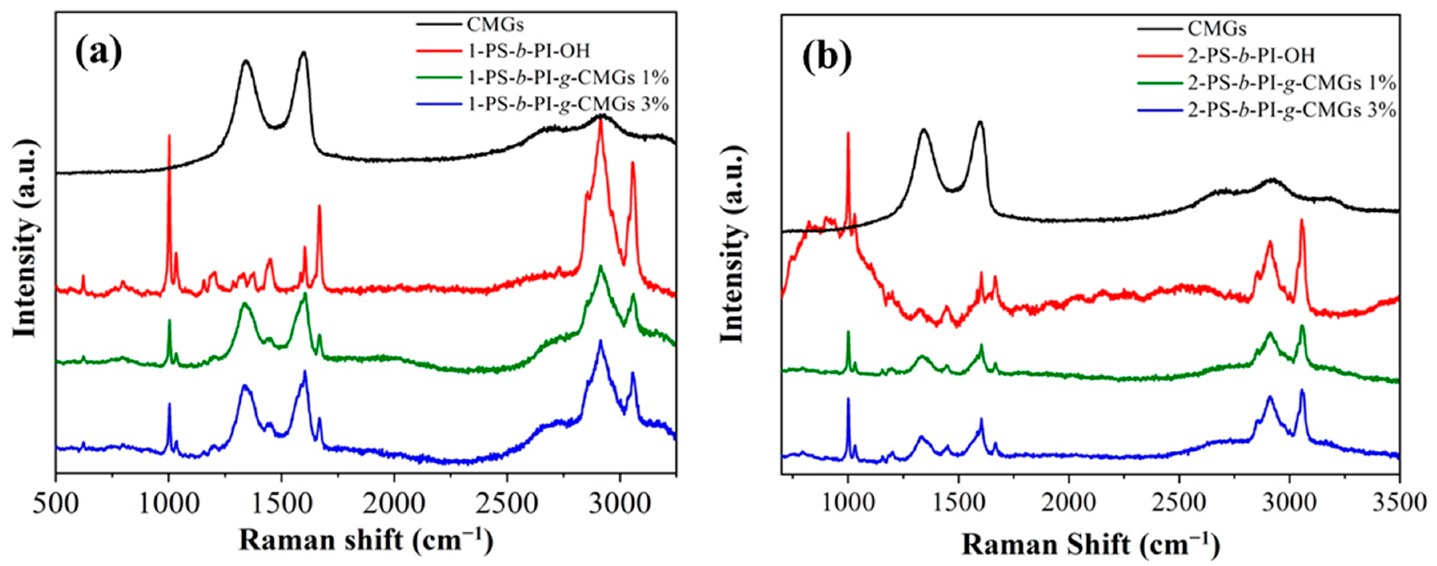
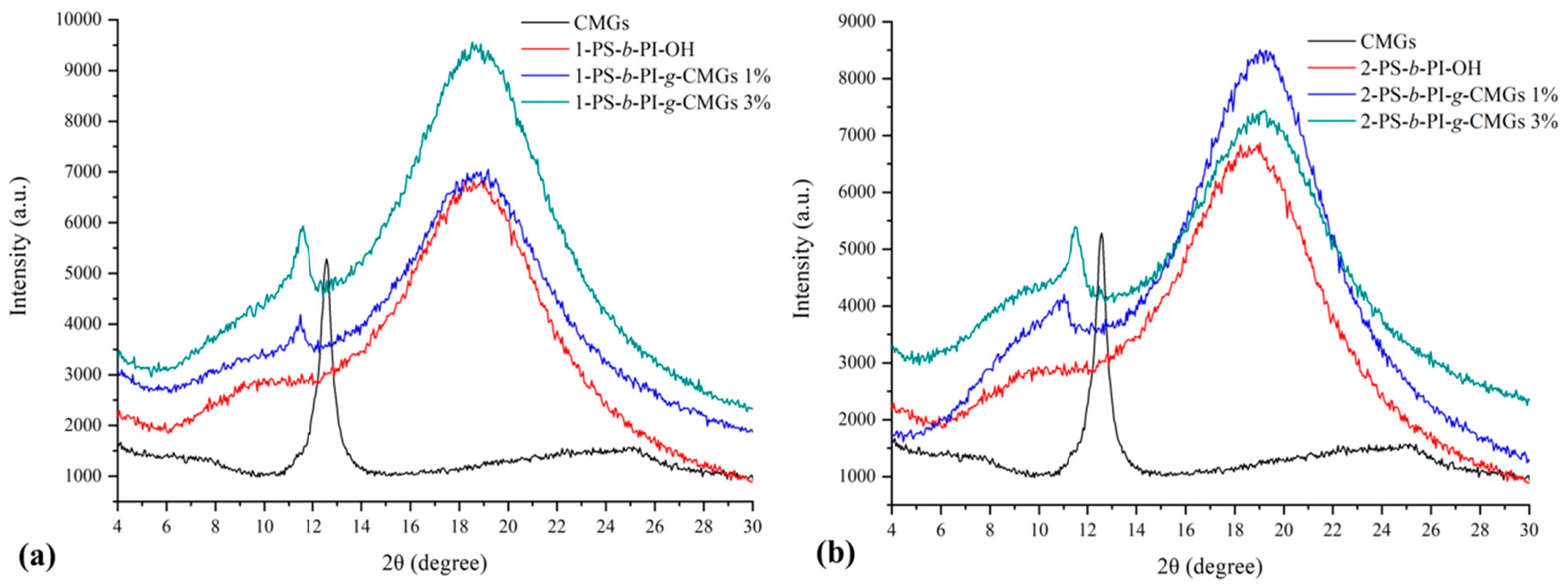
3.1. Molecular and Thermal Characterization Results
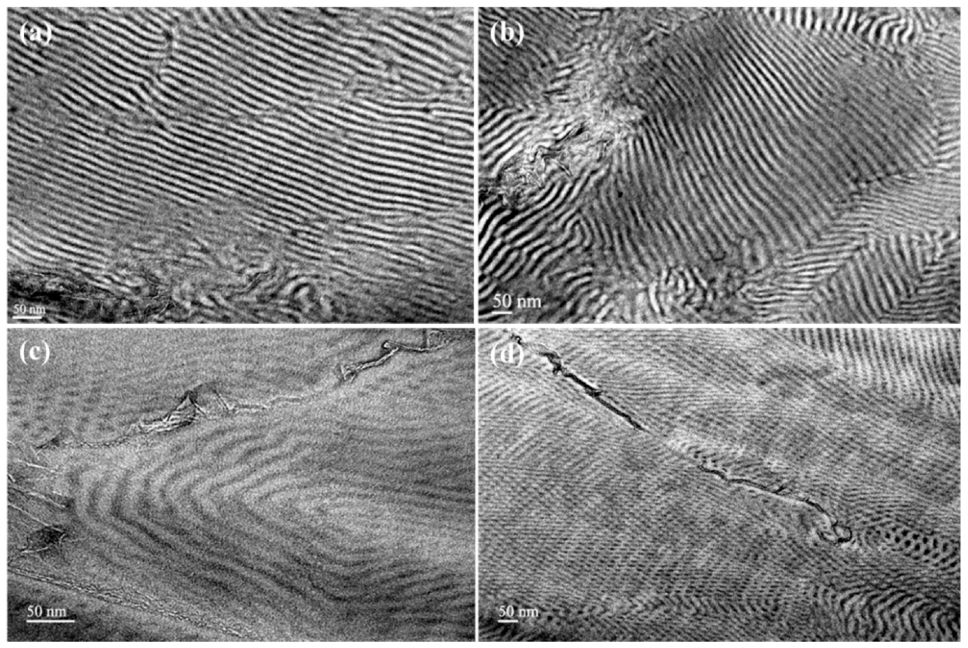
3.2. Morphological Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Kouloumpis, A.; Vourdas, N.; Zygouri, P.; Chalmpes, N.; Potsi, G.; Kostas, V.; Spyrou, K.; Stathopoulos, V.N.; Gournis, D.; Rudolf, P. Controlled Deposition of Fullerene Derivatives within a Graphene Template by Means of a Modified Langmuir-Schaefer Method. J. Colloid Interface Sci. 2018, 524, 388–398. [Google Scholar] [CrossRef]
- Kouloumpis, A.; Thomou, E.; Chalmpes, N.; Dimos, K.; Spyrou, K.; Bourlinos, A.B.; Koutselas, I.; Gournis, D.; Rudolf, P. Graphene/Carbon Dot Hybrid Thin Films Prepared by a Modified Langmuir-Schaefer Method. ACS Omega 2017, 2, 2090–2099. [Google Scholar] [CrossRef] [PubMed]
- Potsi, G.; Bourlinos, A.B.; Mouselimis, V.; Poláková, K.; Chalmpes, N.; Gournis, D.; Kalytchuk, S.; Tomanec, O.; Błoński, P.; Medved´, M.; et al. Intrinsic Photoluminescence of Amine-Functionalized Graphene Derivatives for Bioimaging Applications. Appl. Mater. Today 2019, 17, 112–122. [Google Scholar] [CrossRef]
- Brisebois, P.P.; Siaj, M. Harvesting Graphene Oxide-Years 1859 to 2019: A Review of Its Structure, Synthesis, Properties and Exfoliation. J. Mater. Chem. C 2020, 8, 1517–1547. [Google Scholar] [CrossRef]
- Chalmpes, N.; Moschovas, D.; Tantis, I.; Bourlinos, A.B.; Bakandritsos, A.; Fotiadou, R.; Patila, M.; Stamatis, H.; Avgeropoulos, A.; Karakassides, M.A.; et al. Carbon Nanostructures Derived through Hypergolic Reaction of Conductive Polymers with Fuming Nitric Acid at Ambient Conditions. Molecules 2021, 26, 1595. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.I.; Kim, T.H.; Lee, Y.; Kim, H.; Lee, H.; Yuan, G.; Satija, S.K.; Choi, J.H.; Ahn, H.; Koo, J. Perpendicular Orientation of Diblock Copolymers Induced by Confinement between Graphene Oxide Sheets. Langmuir 2018, 34, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Wang, K.; Lu, H.; Yang, Y.; Nutt, S. Single-Layer Graphene Nanosheets with Controlled Grafting of Polymer Chains. J. Mater. Chem. 2010, 20, 1982–1992. [Google Scholar] [CrossRef]
- Zhu, S.; Li, J.; Chen, Y.; Chen, Z.; Chen, C.; Li, Y.; Cui, Z.; Zhang, D. Grafting of Graphene Oxide with Stimuli-Responsive Polymers by Using ATRP for Drug Release. J. Nanopart. Res. 2012, 14, 1–11. [Google Scholar] [CrossRef]
- Ren, L.; Wang, X.; Guo, S.; Liu, T. Functionalization of Thermally Reduced Graphene by in Situ Atom Transfer Radical Polymerization. J. Nanopart. Res. 2011, 13, 6389–6396. [Google Scholar] [CrossRef]
- Cui, L.; Liu, J.; Wang, R.; Liu, Z.; Yang, W. A Facile “Graft from” Method to Prepare Molecular-Level Dispersed Graphene-Polymer Composites. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 4423–4432. [Google Scholar] [CrossRef]
- Atanase, L.I.; Riess, G. Block Copolymers as Polymeric Stabilizers in Non-Aqueous Emulsion Polymerization. Polym. Int. 2011, 60, 1563–1573. [Google Scholar] [CrossRef]
- Bessif, B.; Pfohl, T.; Reiter, G. Self-Seeding Procedure for Obtaining Stacked Block Copolymer Lamellar Crystals in Solution. Polymers 2021, 13, 1676. [Google Scholar] [CrossRef]
- Lerch, J.P.; Atanase, L.I.; Riess, G. Adsorption of Non-Ionic ABC Triblock Copolymers: Surface Modification of TiO 2 Suspensions in Aqueous and Non-Aqueous Medium. Appl. Surf. Sci. 2017, 419, 713–719. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W. Functional Silica and Carbon Nanocomposites Based on Polybenzoxazines. Macromol. Chem. Phys. 2019, 220, 1–13. [Google Scholar] [CrossRef]
- Sathirapongsasuti, N.; Panaksri, A.; Boonyagul, S.; Chutipongtanate, S. Electrospun Fibers of Polybutylene Succinate/Graphene Oxide. Polymers 2021, 13, 2042. [Google Scholar] [CrossRef]
- Barbey, R.; Lavanant, L.; Paripovic, D.; Schüwer, N.; Sugnaux, C.; Tugulu, S.; Klok, H.A. Polymer Brushes via Surface-Initiated Controlled Radical Polymerization: Synthesis, Characterization, Properties, and Applications. Chem. Rev. 2009, 109, 5437–5527. [Google Scholar] [CrossRef]
- Zdyrko, B.; Luzinov, I. Polymer Brushes by the “Grafting to” Method. Macromol. Rapid Commun. 2011, 32, 859–869. [Google Scholar] [CrossRef]
- Tsubokawa, N. Functionalization of Carbon Material by Surface Grafting of Polymers. Bull. Chem. Soc. Jpn. 2002, 75, 2115–2136. [Google Scholar] [CrossRef]
- Tsubokawa, N. Preparation and Properties of Polymer-Grafted Carbon Nanotubes and Nanofibers. Polym. J. 2005, 37, 637–655. [Google Scholar] [CrossRef]
- Tang, Z.; Kang, H.; Shen, Z.; Guo, B.; Zhang, L.; Jia, D. Grafting of Polyester onto Graphene for Electrically and Thermally Conductive Composites. Macromolecules 2012, 45, 3444–3451. [Google Scholar] [CrossRef]
- Zygo, M.; Mrlik, M.; Ilcikova, M.; Hrabalikova, M.; Osicka, J.; Cvek, M.; Sedlacik, M.; Hanulikova, B.; Munster, L.; Skoda, D.; et al. Effect of Structure of Polymers Grafted from Graphene Oxide on the Compatibility of Particles with a Silicone-Based Environment and the Stimuli-Responsive Capabilities of Their Composites. Nanomaterials 2020, 10, 591. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.R.; Phillips, D.J.; Wilson, N.R.; Gibson, M.I.; Rourke, J.P. One-Step Grafting of Polymers to Graphene Oxide. Polym. Chem. 2015, 6, 8270–8274. [Google Scholar] [CrossRef] [PubMed]
- Guimont, A.; Beyou, E.; Cassagnau, P.; Martin, G.; Sonntag, P.; D’Agosto, F.; Boisson, C. Grafting of Polyethylene onto Graphite Oxide Sheets: A Comparison of Two Routes. Polym. Chem. 2013, 4, 2828–2836. [Google Scholar] [CrossRef]
- Yang, X.; Ma, L.; Wang, S.; Li, Y.; Tu, Y.; Zhu, X. “Clicking” Graphite Oxide Sheets with Well-Defined Polystyrenes: A New Strategy to Control the Layer Thickness. Polym. Guildf. 2011, 52, 3046–3052. [Google Scholar] [CrossRef]
- Ye, Y.S.; Chen, Y.N.; Wang, J.S.; Rick, J.; Huang, Y.J.; Chang, F.C.; Hwang, B.J. Versatile Grafting Approaches to Functionalizing Individually Dispersed Graphene Nanosheets Using RAFT Polymerization and Click Chemistry. Chem. Mater. 2012, 24, 2987–2997. [Google Scholar] [CrossRef]
- Huang, W.; Wang, S.; Guo, C.; Yang, X.; Li, Y.; Tu, Y. Synthesis and Characterization of Well-Defined Poly(l-Lactide) Functionalized Graphene Oxide Sheets with High Grafting Ratio Prepared through Click Chemistry and Supramolecular Interactions. Polym. Guildf. 2014, 55, 4619–4626. [Google Scholar] [CrossRef]
- Sun, X.; Wang, W.; Wu, T.; Qiu, H.; Wang, X.; Gao, J. Grafting of Graphene Oxide with Poly(Sodium 4-Styrenesulfonate) by Atom Transfer Radical Polymerization. Mater. Chem. Phys. 2013, 138, 434–439. [Google Scholar] [CrossRef]
- Goncalves, G.; Marques, P.A.A.P.; Barros-Timmons, A.; Bdkin, I.; Singh, M.K.; Emami, N.; Grácio, J. Graphene Oxide Modified with PMMA via ATRP as a Reinforcement Filler. J. Mater. Chem. 2010, 20, 9927–9934. [Google Scholar] [CrossRef]
- Ren, L.; Huang, S.; Zhang, C.; Wang, R.; Tjiu, W.W.; Liu, T. Functionalization of Graphene and Grafting of Temperature-Responsive Surfaces from Graphene by ATRP “on Water. ” J. Nanopart. Res. 2012, 14, 1–9. [Google Scholar] [CrossRef]
- Lee, S.H.; Dreyer, D.R.; An, J.; Velamakanni, A.; Piner, R.D.; Park, S.; Zhu, Y.; Kim, S.O.; Bielawski, C.W.; Ruoff, R.S. Polymer Brushes via Controlled, Surface-Initiated Atom Transfer Radical Polymerization (ATRP) from Graphene Oxide. Macromol. Rapid Commun. 2010, 31, 281–288. [Google Scholar] [CrossRef]
- Ding, P.; Zhang, J.; Song, N.; Tang, S.; Liu, Y.; Shi, L. Growing Polystyrene Chains from the Surface of Graphene Layers via RAFT Polymerization and the Influence on Their Thermal Properties. Compos. Part A Appl. Sci. Manuf. 2015, 69, 186–194. [Google Scholar] [CrossRef]
- Gu, R.; Xu, W.Z.; Charpentier, P.A. Synthesis of Graphene-Polystyrene Nanocomposites via RAFT Polymerization. Polym. Guildf. 2014, 55, 5322–5331. [Google Scholar] [CrossRef]
- Nikdel, M.; Salami-Kalajahi, M.; Salami Hosseini, M. Synthesis of Poly(2-Hydroxyethyl Methacrylate-Co-Acrylic Acid)-Grafted Graphene Oxide Nanosheets via Reversible Addition-Fragmentation Chain Transfer Polymerization. RSC Adv. 2014, 4, 16743–16750. [Google Scholar] [CrossRef]
- Mylvaganam, K.; Zhang, L. Fabrication of Graphene-Polymer Nanocomposites through Ionic Polymerization. J. Phys. Chem. A 2016, 120, 7689–7693. [Google Scholar] [CrossRef]
- Li, B.; Hou, W.; Sun, J.; Jiang, S.; Xu, L.; Li, G.; Memon, M.A.; Cao, J.; Huang, Y.; Bielawski, C.W.; et al. Tunable Functionalization of Graphene Oxide Sheets through Surface-Initiated Cationic Polymerization. Macromolecules 2015, 48, 994–1001. [Google Scholar] [CrossRef]
- Tsubokawa, N.; Yamauchi, T.; Fujiki, K.; Tamesue, S. A Novel Grafting of Polymers onto the Surface of Graphene Oxide. In Graphene Materials: Structure, Properties and Modifications; IntechOpen: London, UK, 2017; pp. 3–26. [Google Scholar]
- Ramanathan, T.; Abdala, A.A.; Stankovich, S.; Dikin, D.A.; Herrera-Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniepp, H.C.; Chen, X.; Ruoff, R.S.; et al. Functionalized Graphene Sheets for Polymer Nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef]
- Politakos, N.; Liontos, G.; Karanastasis, A.; Zapsas, G.; Moschovas, D.; Avgeropoulos, A. Surface Initiated Polymerization from Graphene Oxide. Curr. Org. Chem. 2015, 19, 1757–1772. [Google Scholar] [CrossRef]
- Islam, A.; Ahmad, H.; Zaidi, N.; Kumar, S. Graphene Oxide Sheets Immobilized Polystyrene for Column Preconcentration and Sensitive Determination of Lead by Flame Atomic Absorption Spectrometry. ACS Appl. Mater. Interfaces 2014, 6, 13257–13265. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, Properties, and Applications of Graphene Oxide/Reduced Graphene Oxide and Their Nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Nagata, K.; Kawahara, T.; Hashimoto, K.; Fujiki, K.; Tamesue, S.; Yamauchi, T.; Tsubokawa, N. Grafting of Polymers onto Graphene Oxide by Cationic and Anionic Polymerization Initiated by the Surface-Initiating Groups. Compos. Interfaces 2015, 22, 25–37. [Google Scholar] [CrossRef]
- Tsubokawa, N.; Takeda, N.; Iwasa, T. Cationic Polymerization of Isobutyl Vinyl Ether Initiated by Carbon Black Surface. Polymer J. 1981, 13, 1093–1097. [Google Scholar] [CrossRef][Green Version]
- Dreyer, D.R.; Jarvis, K.A.; Ferreira, P.J.; Bielawski, C.W. Graphite Oxide as a Carbocatalyst for the Preparation of Fullerene-Reinforced Polyester and Polyamide Nanocomposites. Polym. Chem. 2012, 3, 757–766. [Google Scholar] [CrossRef]
- Zeng, M.; Wang, J.; Li, R.; Liu, J.; Chen, W.; Xu, Q.; Gu, Y. The Curing Behavior and Thermal Property of Graphene Oxide/Benzoxazine Nanocomposites. Polym. Guildf. 2013, 54, 3107–3116. [Google Scholar] [CrossRef]
- Tsubokawa, N.; Hamada, H.; Sone, Y. Grafting of Polyesters from Carbon Fiber. Anionic Ring-Opening Copolymerization of Epoxides with Cyclic Acid Anhydrides Initiated by Cook Groups on the Surface of Carbon Fiber. Polym. Plast. Technol. Eng. 1989, 28, 201–214. [Google Scholar] [CrossRef]
- Tsubokawa, N.; Yamada, A.; Sone, Y. Grafting of Polyesters on Carbon Black V. Preparation of Polyester-Grafted Carbon Black with a Higher Grafting Ratio by the Copolymerization of Epoxide with Cyclic Acid Anhydrides Using Cook Groups on Carbon Black as the Initiator. Polym. J. 1984, 16, 333–340. [Google Scholar] [CrossRef][Green Version]
- Hadjichristidis, N.; Iatrou, H.; Pispas, S.; Pitsikalis, M. Anionic Polymerization: High Vacuum Techniques. J. Polym. Sci. Part. A Polym. Chem. 2000, 38, 3211–3234. [Google Scholar] [CrossRef]
- Avgeropoulos, A.; Paraskeva, S.; Hadjichristidis, N.; Thomas, E.L. Synthesis and Microphase Separation of Linear Triblock Terpolymers of Polystyrene, High 1,4-Polybutadiene, and High 3,4-Polyisoprene. Macromolecules 2002, 35, 4030–4035. [Google Scholar] [CrossRef]
- Park, K.W.; Jung, J.H. Spectroscopic and Electrochemical Characteristics of a Carboxylated Graphene-ZnO Composites. J. Power Sources 2012, 199, 379–385. [Google Scholar] [CrossRef]
- Neises, B.; Steglich, W. Simple Method for the Esterification of Carboxylic Acids. Angew. Chemie Int. Ed. Engl. 1978, 17, 522–524. [Google Scholar] [CrossRef]
- Chalmpes, N.; Spyrou, K.; Vasilopoulos, K.C.; Bourlinos, A.B.; Moschovas, D.; Avgeropoulos, A.; Gioti, C.; Karakassides, M.A.; Gournis, D. Hypergolics in Carbon Nanomaterials Synthesis: New Paradigms and Perspectives. Molecules 2020, 25, 2207. [Google Scholar] [CrossRef]
- Arjunan, V.; Subramanian, S.; Mohan, S. Fourier transform infrared and Raman spectral analysis of trans-1,4-polyisoprene. Spectrochim. Acta Part A 2001, 57, 2547–2554. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, M.; Kumar, A.; Singh, R.; Kashyap, R.; Rani, S.; Kumar, D. Surface Modification of Graphene Oxide Using Esterification. Mater. Today Proc. 2019, 18, 1556–1561. [Google Scholar] [CrossRef]
- Tsirka, K.; Katsiki, A.; Chalmpes, N.; Gournis, D.; Paipetis, A.S. Mapping of Graphene Oxide and Single Layer Graphene Flakes—Defects Annealing and Healing. Front. Mater. 2018, 5, 37. [Google Scholar] [CrossRef]
- Chalmpes, N.; Asimakopoulos, G.; Spyrou, K.; Vasilopoulos, K.C.; Bourlinos, A.B.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Functional Carbon Materials Derived through Hypergolic Reactions at Ambient Conditions. Nanomaterials 2020, 10, 566. [Google Scholar] [CrossRef]
| Sample Number | Samples | (g/mol) | (kg/mol) | (kg/mol) | Đtotal a | fPS b | φPS c |
|---|---|---|---|---|---|---|---|
| 1 | PS-b-PI-OH | 21,000 | 17,500 | 38,500 | 1.04 | 0.51 | 0.52 |
| 2 | PS-b-PI-OH | 31,000 | 10,500 | 41,500 | 1.06 | 0.73 | 0.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsigiannopoulos, D.; Grana, E.; Tsitoni, K.; Moutsios, I.; Manesi, G.-M.; Nikitina, E.A.; Chalmpes, N.; Moschovas, D.; Gournis, D.; Ivanov, D.A.; et al. Structure/Properties Relationship of Anionically Synthesized Diblock Copolymers “Grafted to” Chemically Modified Graphene. Polymers 2021, 13, 2308. https://doi.org/10.3390/polym13142308
Katsigiannopoulos D, Grana E, Tsitoni K, Moutsios I, Manesi G-M, Nikitina EA, Chalmpes N, Moschovas D, Gournis D, Ivanov DA, et al. Structure/Properties Relationship of Anionically Synthesized Diblock Copolymers “Grafted to” Chemically Modified Graphene. Polymers. 2021; 13(14):2308. https://doi.org/10.3390/polym13142308
Chicago/Turabian StyleKatsigiannopoulos, Dimitrios, Eftychia Grana, Konstantina Tsitoni, Ioannis Moutsios, Gkreti-Maria Manesi, Evgeniia A. Nikitina, Nikolaos Chalmpes, Dimitrios Moschovas, Dimitrios Gournis, Dimitri A. Ivanov, and et al. 2021. "Structure/Properties Relationship of Anionically Synthesized Diblock Copolymers “Grafted to” Chemically Modified Graphene" Polymers 13, no. 14: 2308. https://doi.org/10.3390/polym13142308
APA StyleKatsigiannopoulos, D., Grana, E., Tsitoni, K., Moutsios, I., Manesi, G.-M., Nikitina, E. A., Chalmpes, N., Moschovas, D., Gournis, D., Ivanov, D. A., & Avgeropoulos, A. (2021). Structure/Properties Relationship of Anionically Synthesized Diblock Copolymers “Grafted to” Chemically Modified Graphene. Polymers, 13(14), 2308. https://doi.org/10.3390/polym13142308