Catalytic and Photocatalytic Electrospun Nanofibers for Hydrogen Generation from Ammonia Borane Complex: A Review
Abstract
:1. Introduction
1.1. The Importance of Hydrogen
1.2. Ammonia Borane (AB) as a Valuable Source of H2
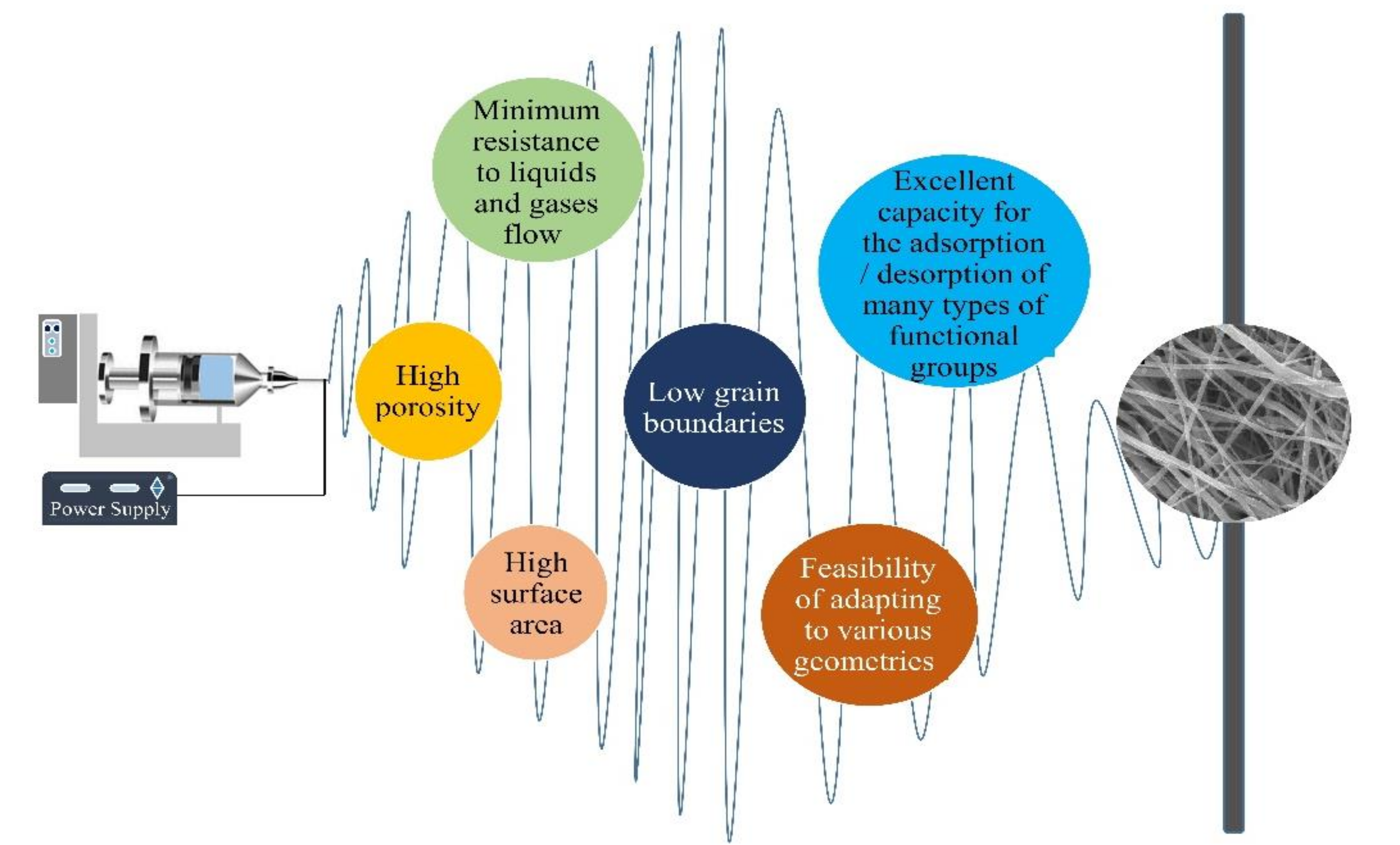
2. Electrospun Fibrous Catalysts
2.1. Characteristics of Electrospun Catalysts
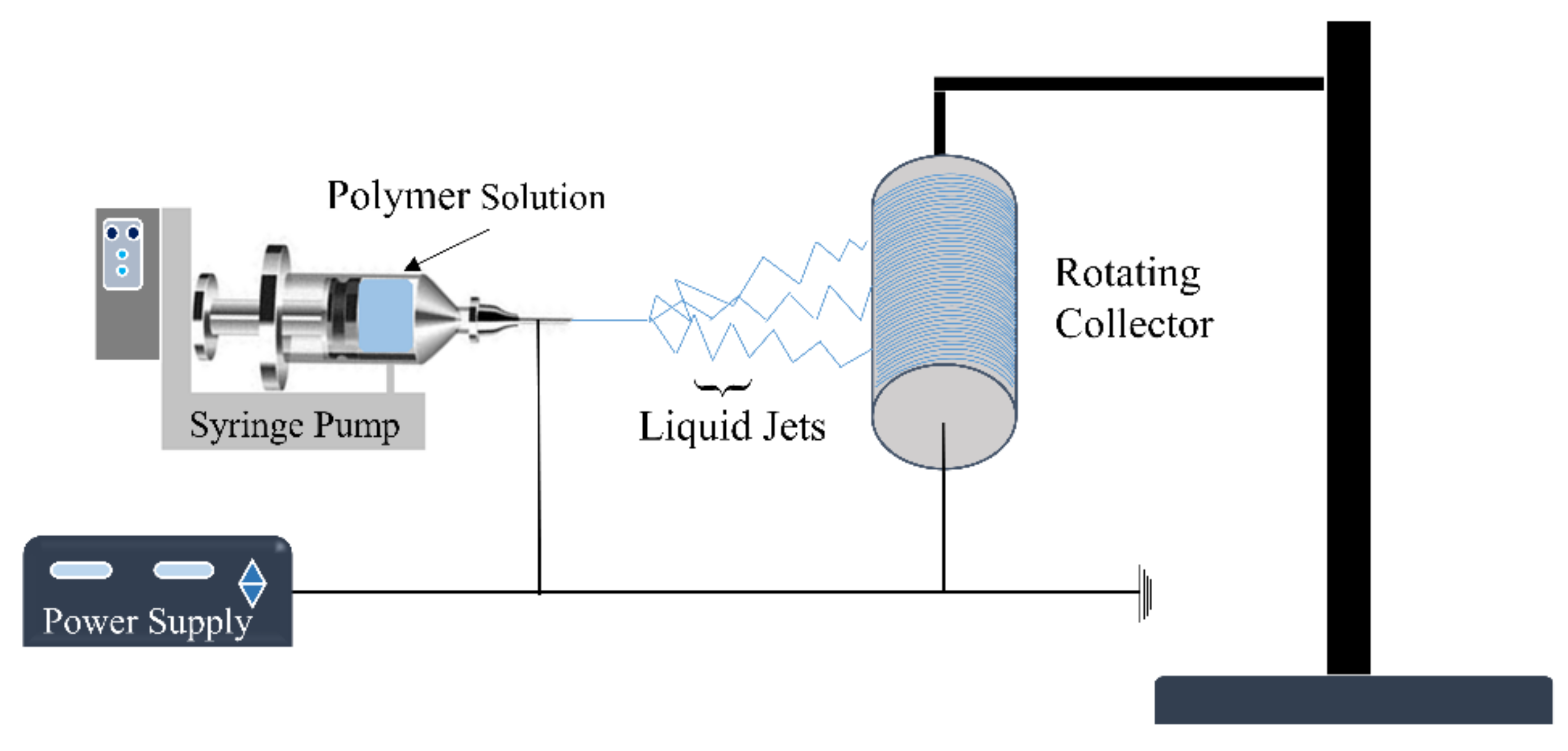
2.2. Electrospinning Technique
- i.
- Single phase ceramic NFs obtained by removing the polymer NFs via heat treatment procedure;
- ii.
- Polymer/ceramic hybrid NFs obtained without any heat treatment.
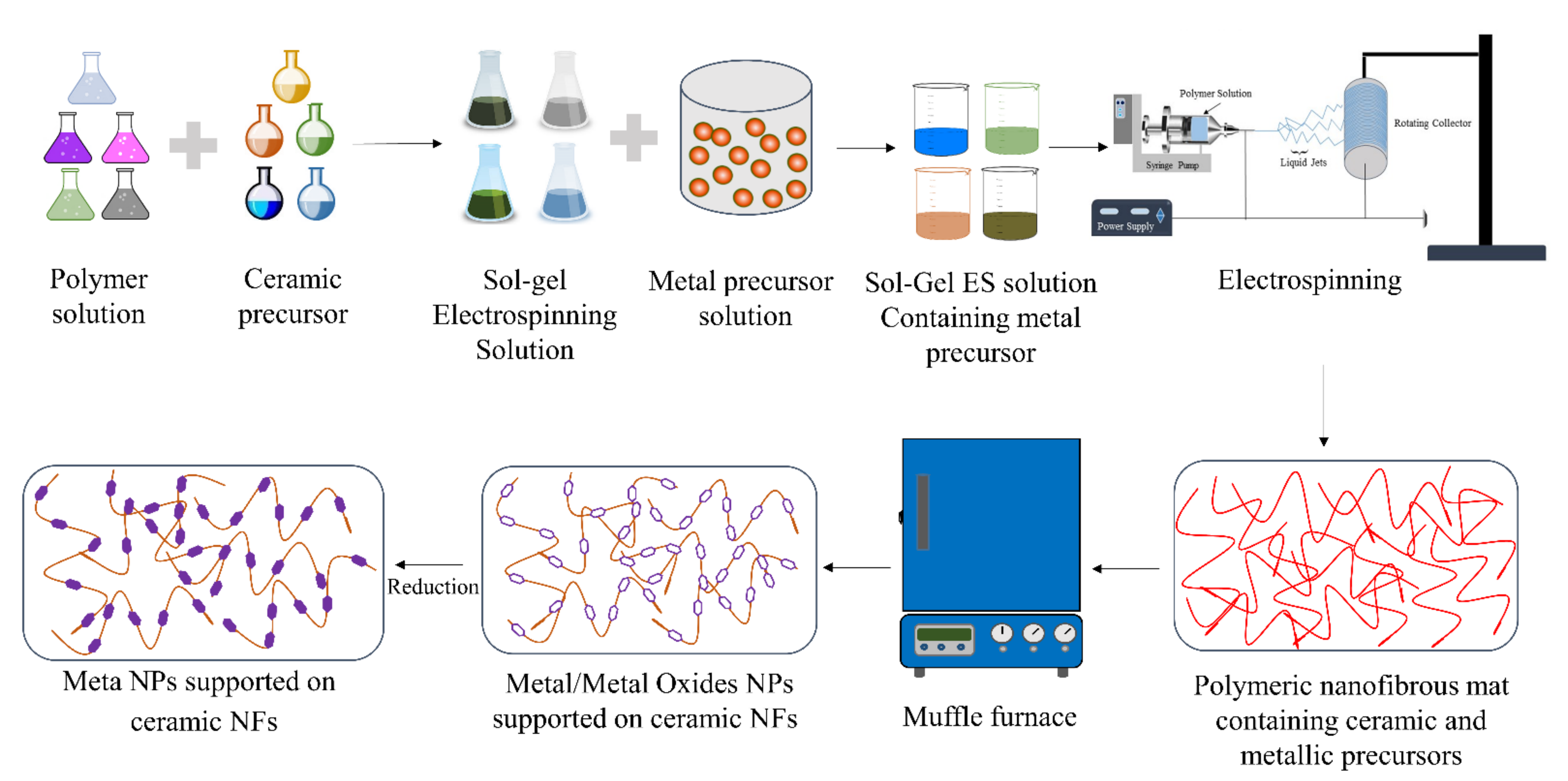
3. Fabrication of Catalytic Electrospun Nanofibers for AB Hydrolysis
- Preparation of the sol–gel solution by mixing a polymeric solution with a metal oxide precursor;
- ES of the sol–gel solution to make nanofibrous mats;
- Calcination of the fibrous mats at high temperatures to remove the polymer and convert the precursor to the desired ceramic form.
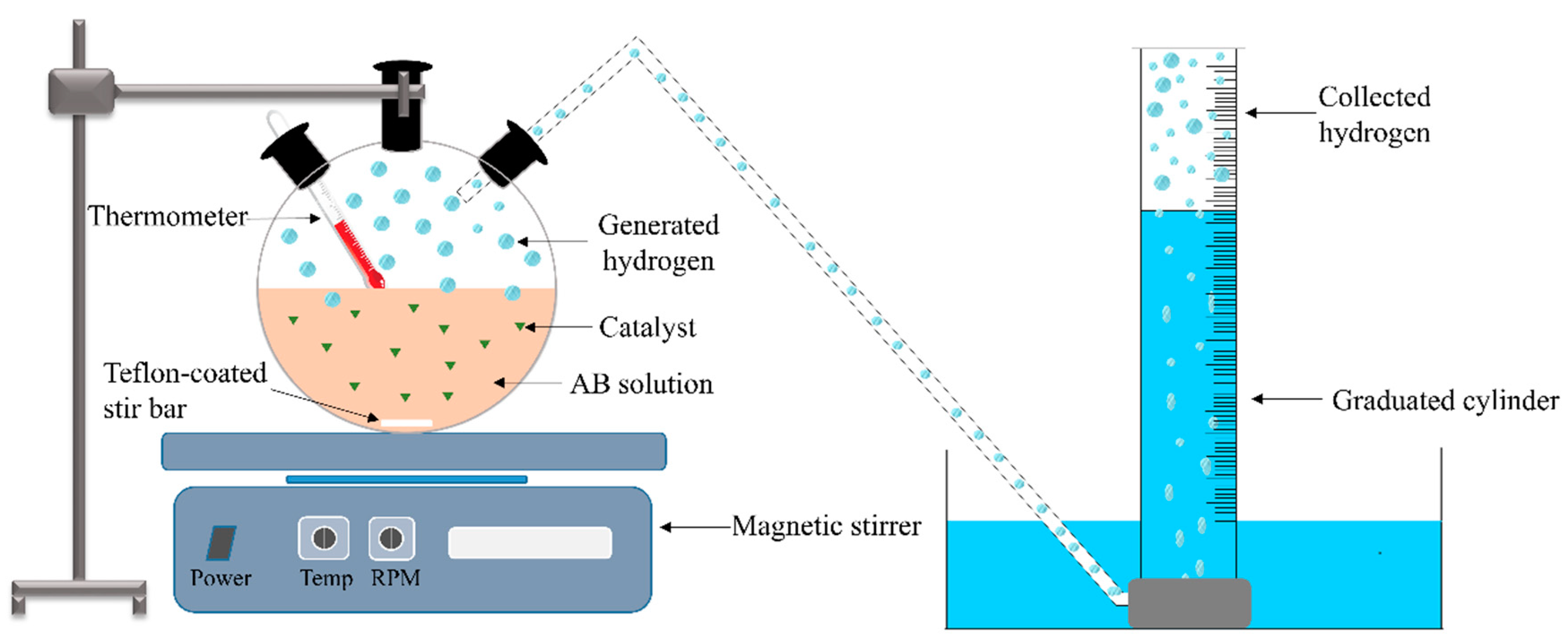
4. Reaction Setup for Testing Catalytic Performance toward AB Hydrolysis
5. Nanofibrous Ceramic Catalysts for AB Hydrolysis
6. Carbon NFs as a Catalytic Support for AB Hydrolysis Reaction
6.1. NiCu Nanorods on Carbon NFs
- i.
- To prepare NiCu NRs/C NFs, first, nickel acetate (NiAc) and copper acetate (CuAc) aqueous solutions were prepared. The aqueous solutions were mixed with a 10 wt% polyvinylpyrrolidone (PVP) aqueous solution. The sol–gel solution was mixed for 5 h at 50 °C to obtain a homogenous solution. The ES of the sol–gel solution was then conducted at 20 °C. The fabricated NFs were vacuum dried for a day at 60 °C. To remove the polymer, the NFs were calcined at 750 °C (2.3 °C/min) for 3 h in an argon (Ar) atmosphere.
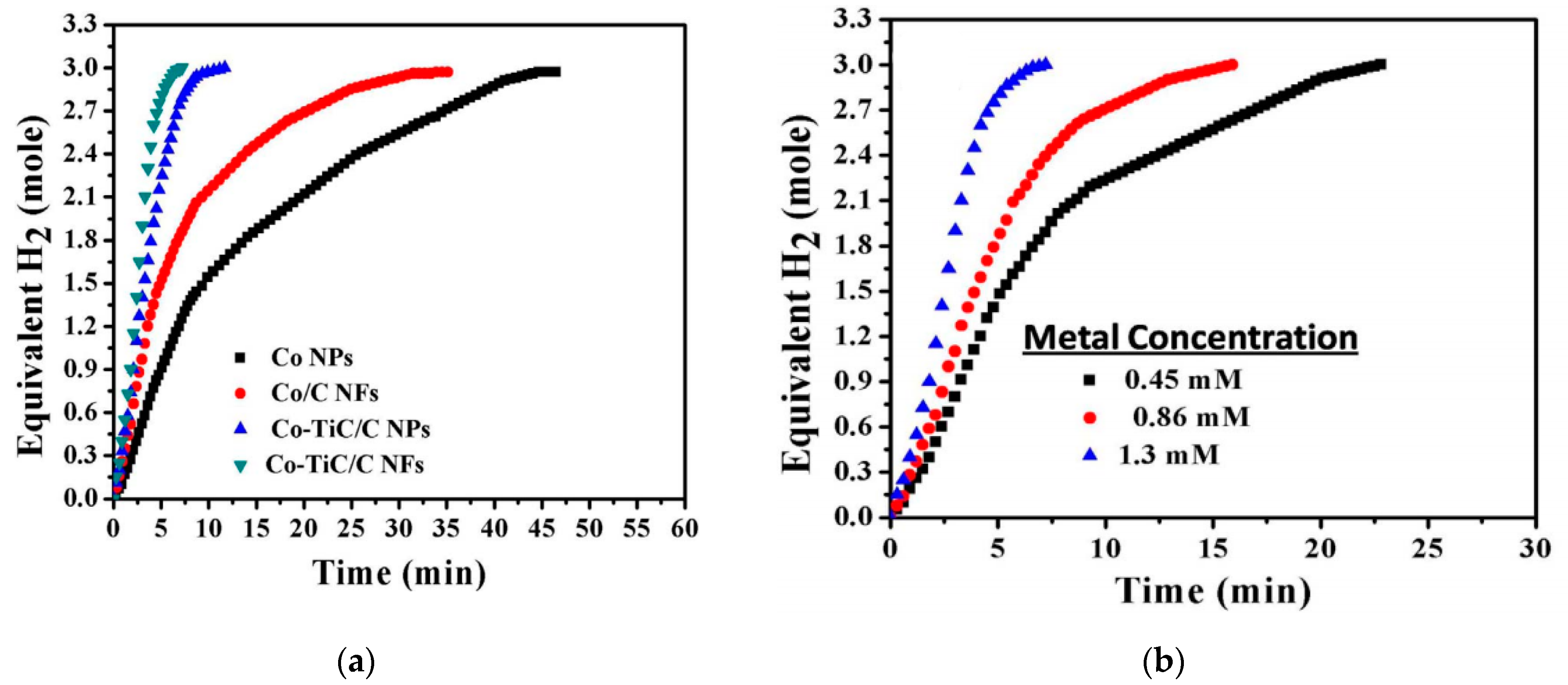
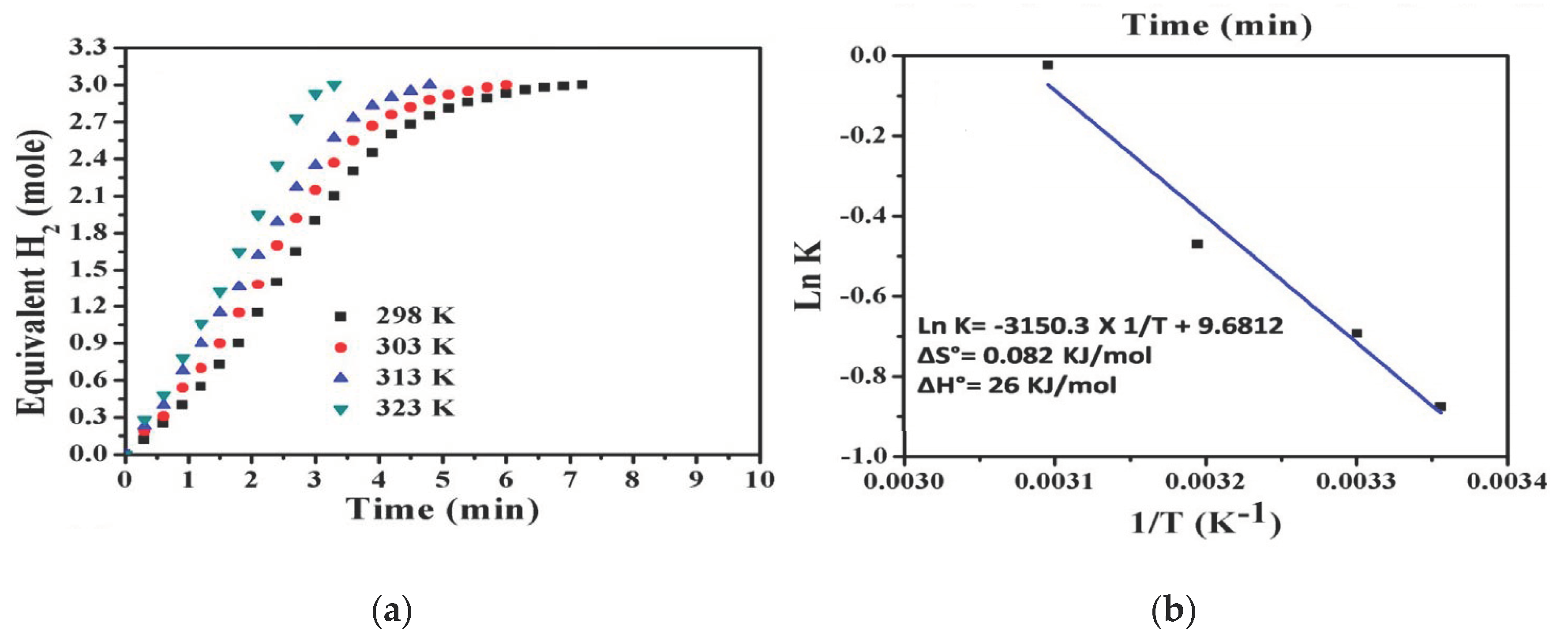
6.2. Co–TiC NP-Decorated C NFs
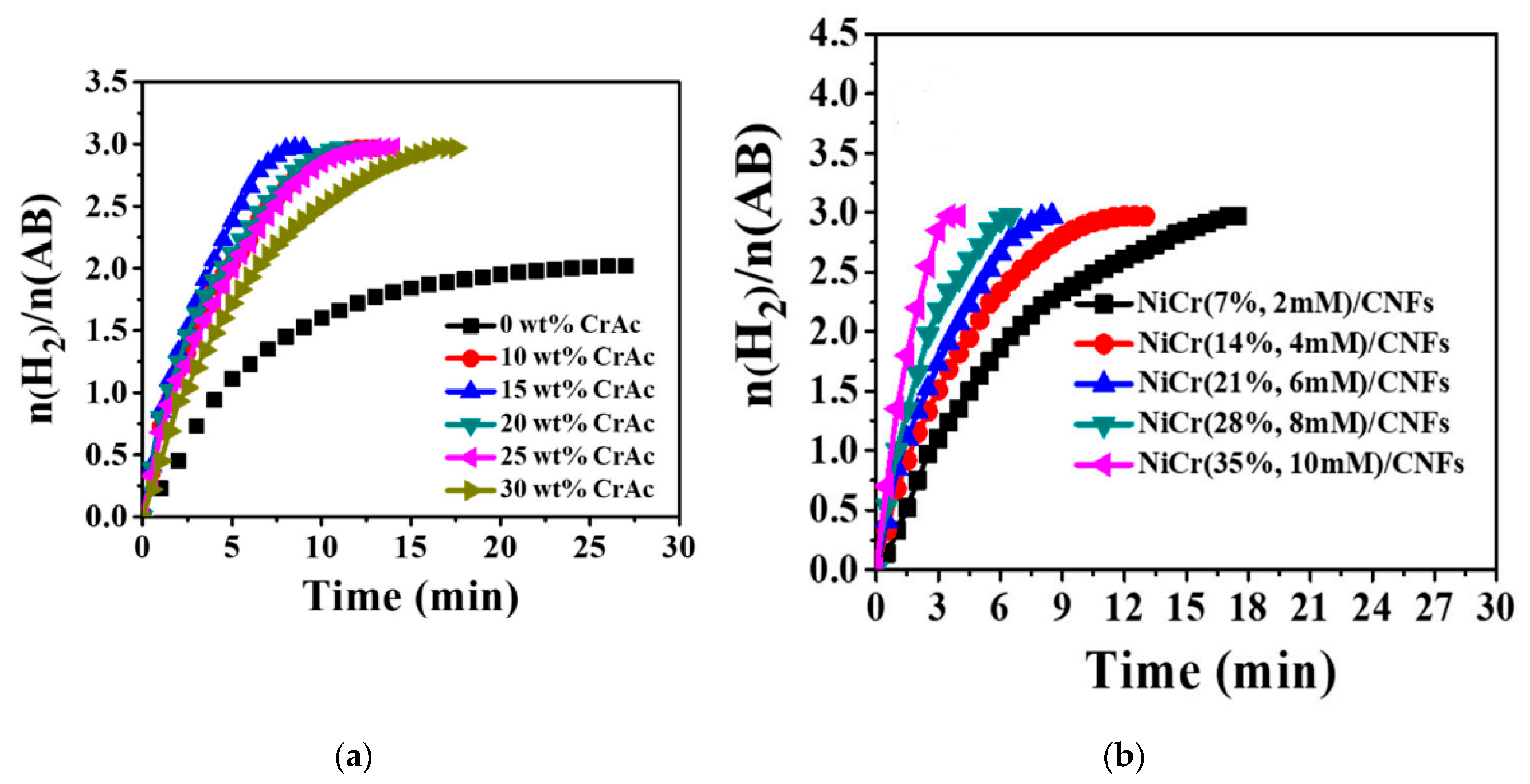
6.3. CoCr7C3-Supported C Nanofibers
6.4. NiCr NPs/C NFs
6.5. TiO2 NFs as a Catalytic Support for AB Hydrolysis Reaction
6.6. Co–B Nanoflakes (NFlks)/TiO2 NFs
7. Photocatalysis
- i.
- Irradiation of the semiconductor catalyst;
- ii.
- Electronic transition of electrons from the valance band to the conduction band of the semiconductor;
- iii.
- Creation of holes in the valance band because of the electronic transition of electrons;
- iv.
- Generation of radicals from electrons and holes;
- v.
- Reaction of AB with radicals to generate H2.
7.1. Titanium Dioxide Photocatalysts
7.2. Modification of TiO2 for Higher Photocatalytic Activity under Visible Light
8. TiO2 NFs as a Catalytic Support for AB Hydrolysis Reaction
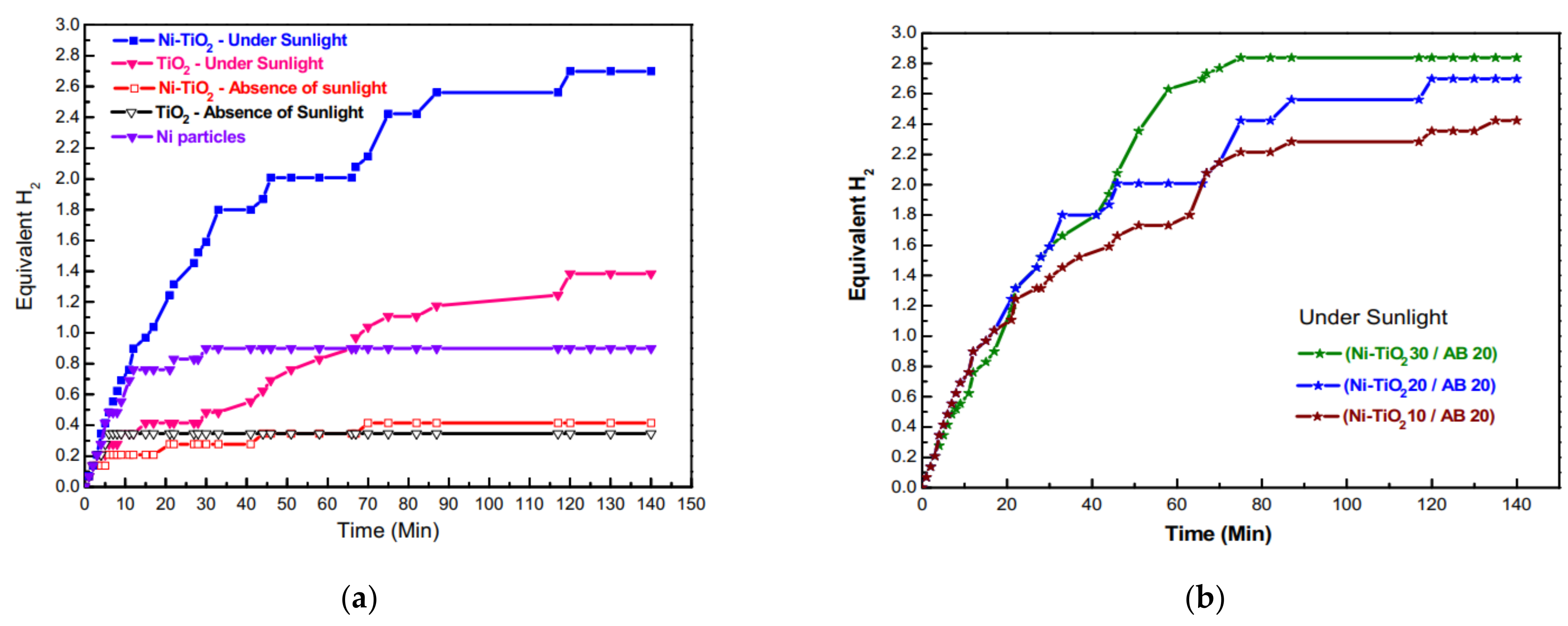
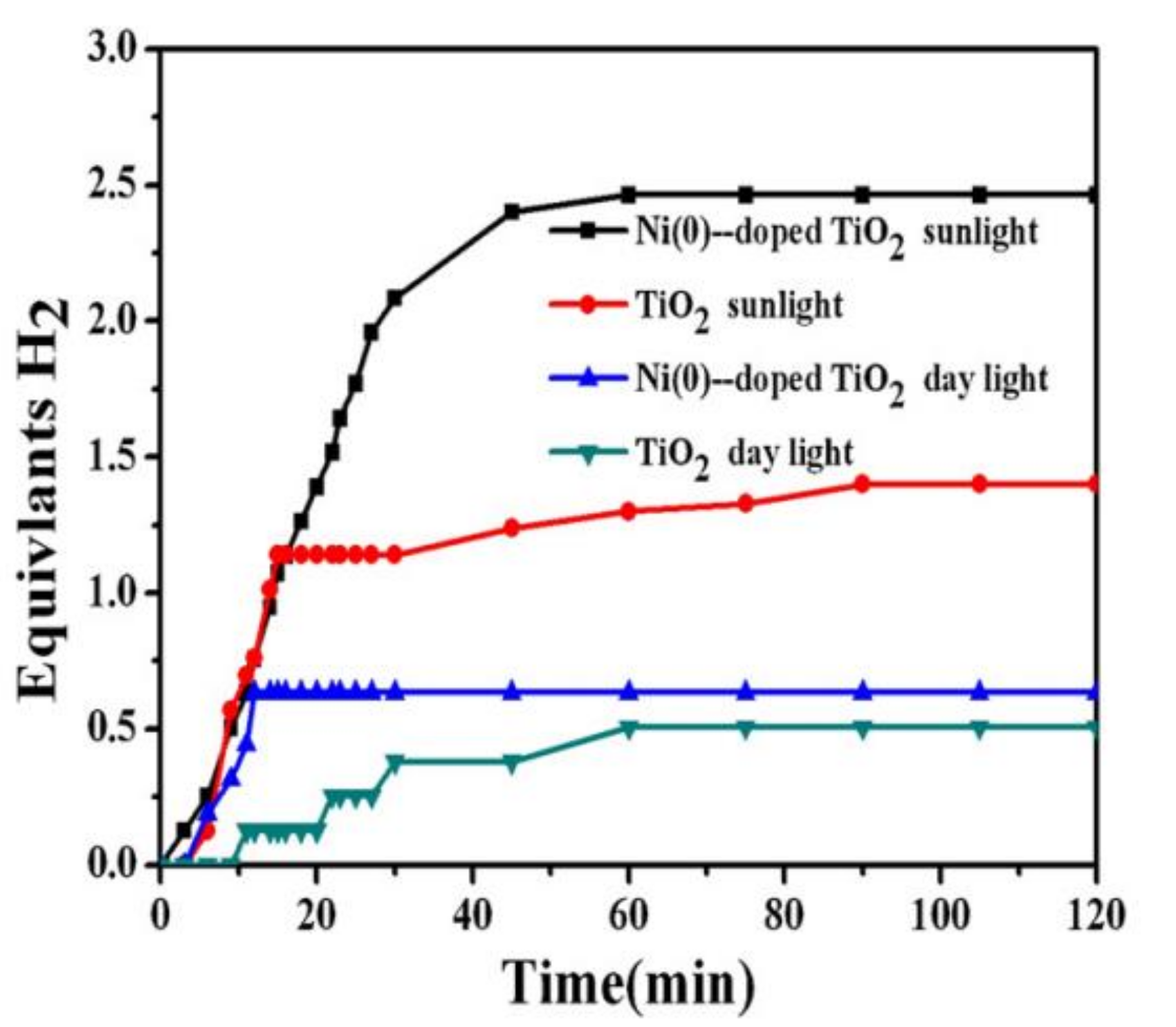
8.1. Ni-Doped TiO2 NFs
8.2. Ni(0)-Doped TiO2/C NFs
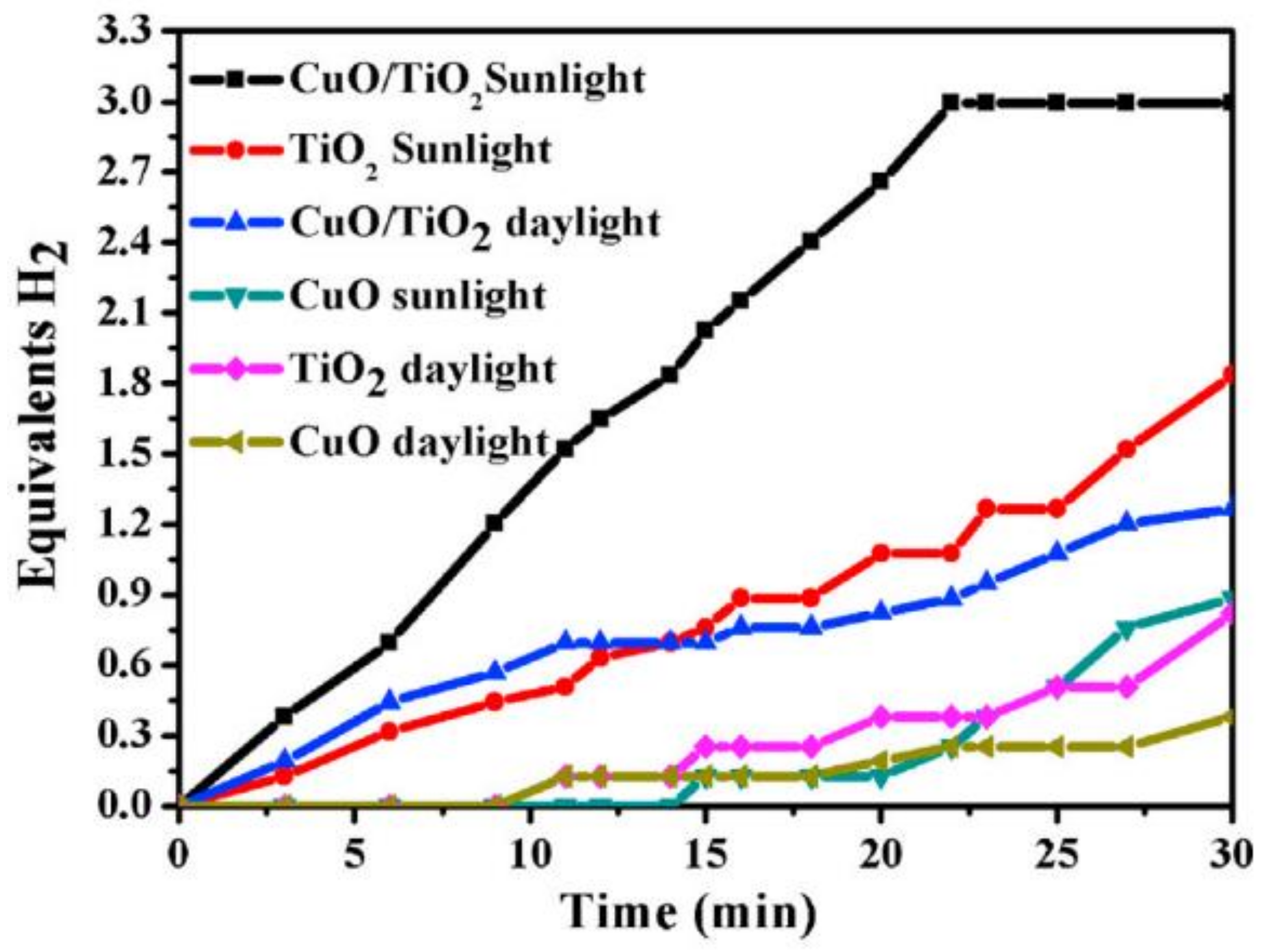
8.3. CuO/TiO2 NFs
8.4. Cu(0) NPs/TiO2 NFs
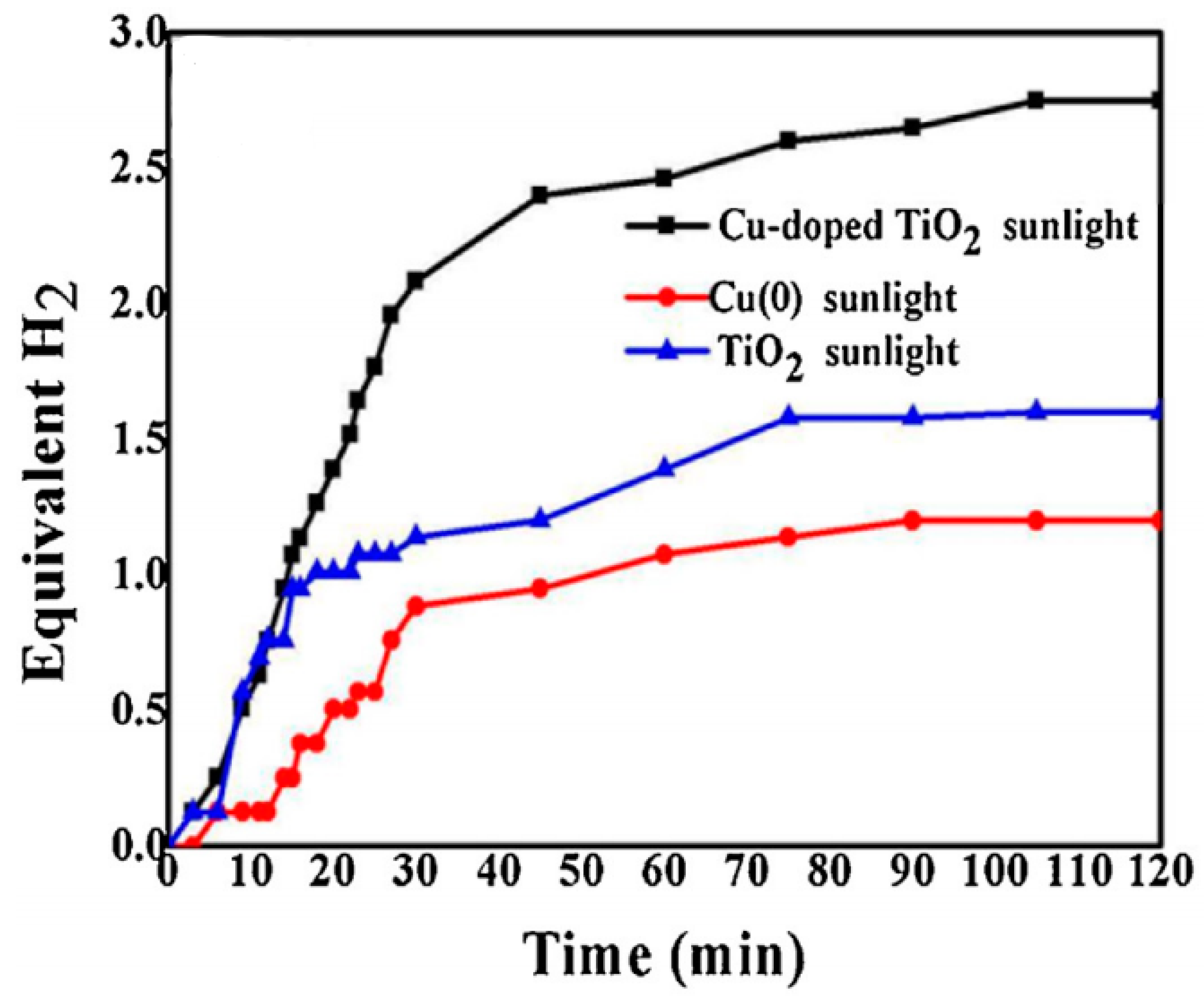
8.5. Cu-Doped TiO2/C NFs
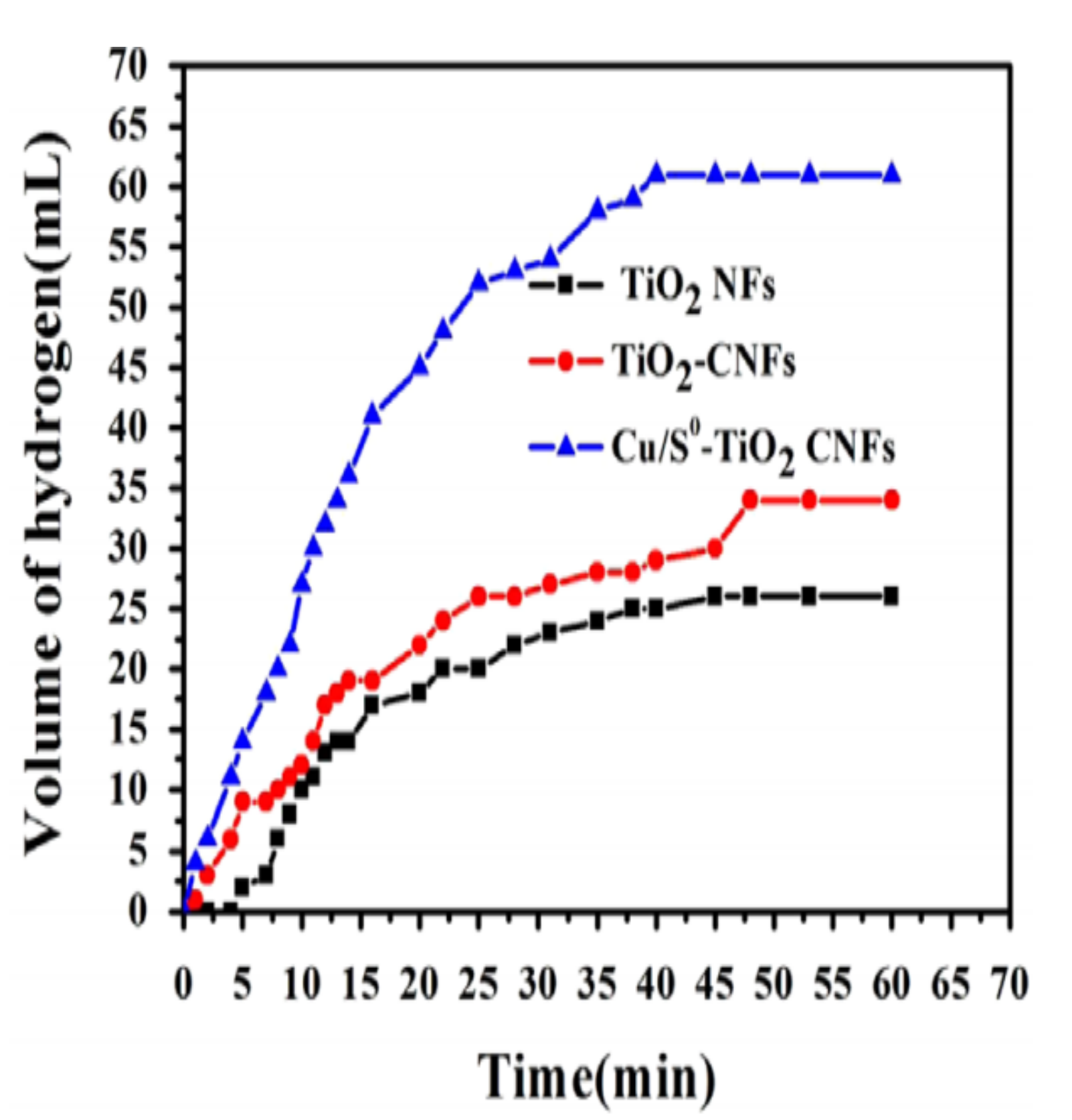
8.6. Cu0/S-Doped TiO2 NPs/C NFs
- i.
- The generation of electrons during the photocatalytic process.
- ii.
- The transfer of electrons from S-doped TiO2 NPs to the vacant Cu d-orbital, leaving holes in the S-doped TiO2 NPs.
- iii.
- The adoption of AB by C NFs and the transfer of electrons in Cu to H3N2+, according to Equation (2).
- iv.
- Positive charges in the S-doped TiO2 NPs attacked H3B2- forming three moles of H2.
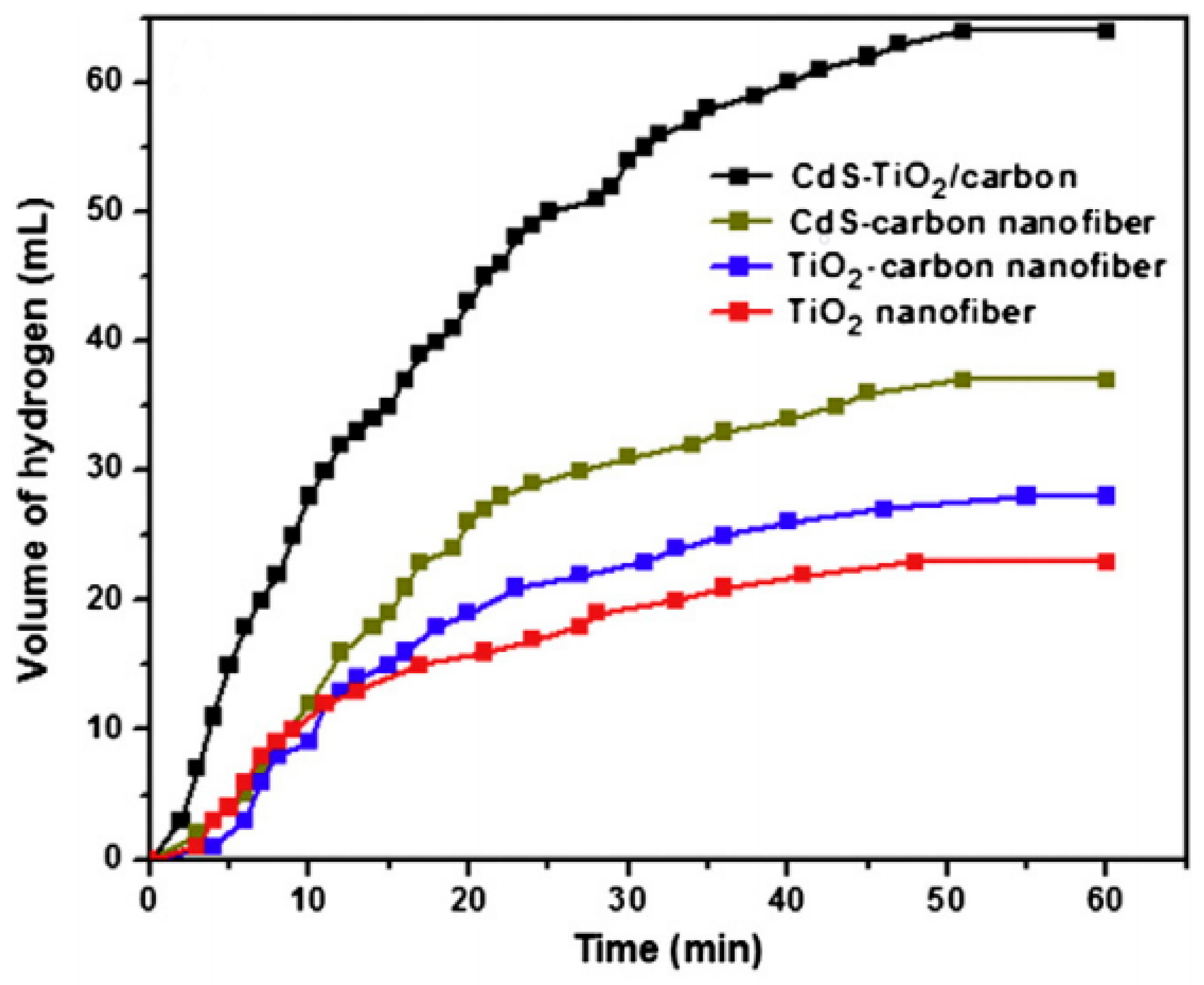
8.7. CdS–TiO2-Doped C NFs
8.8. CdS NPs/CdTiO3 NFs
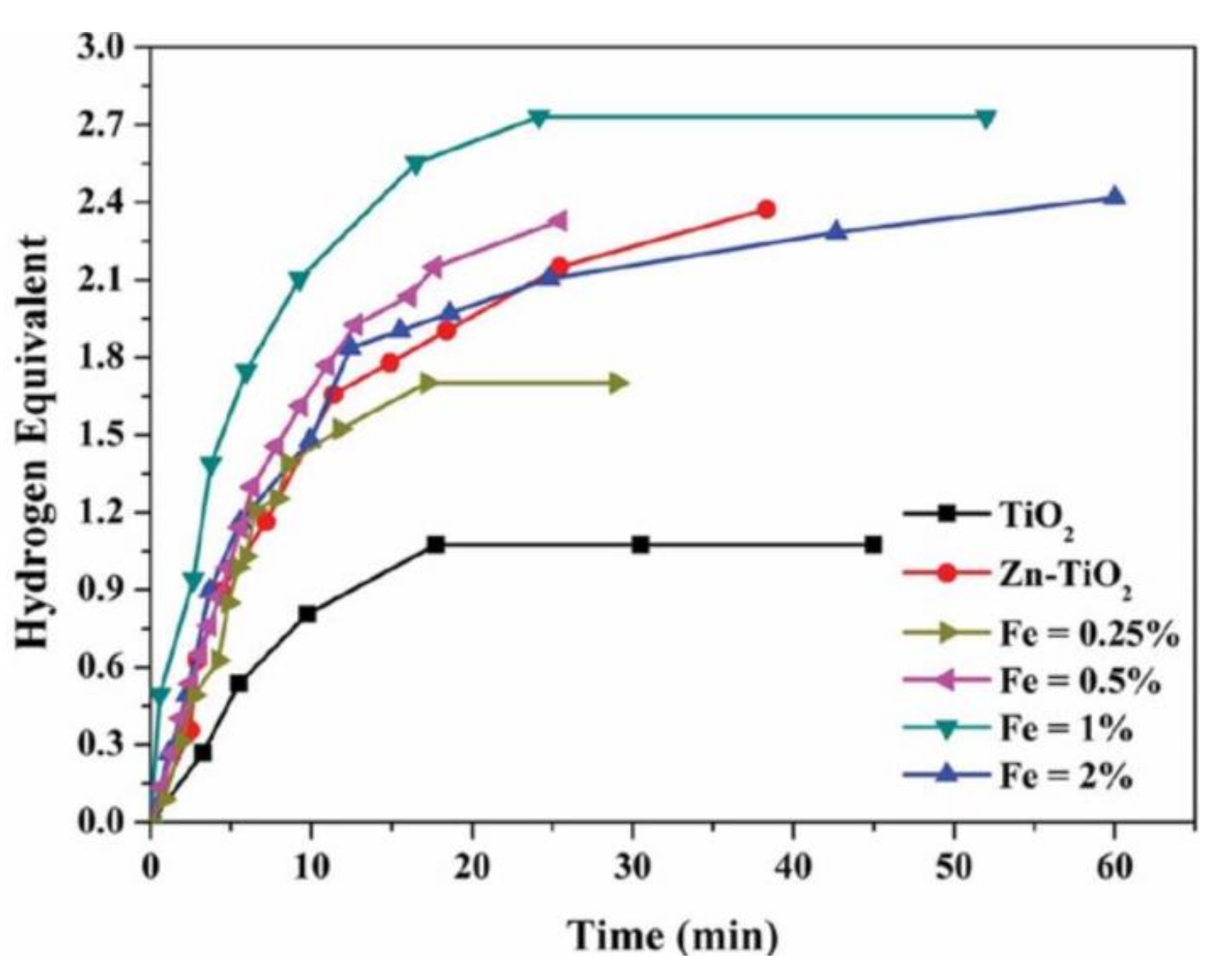
8.9. Zn–Fe-Doped TiO2 NFs
9. Cobalt NFs as a Catalytic Support for AB Hydrolysis Reaction
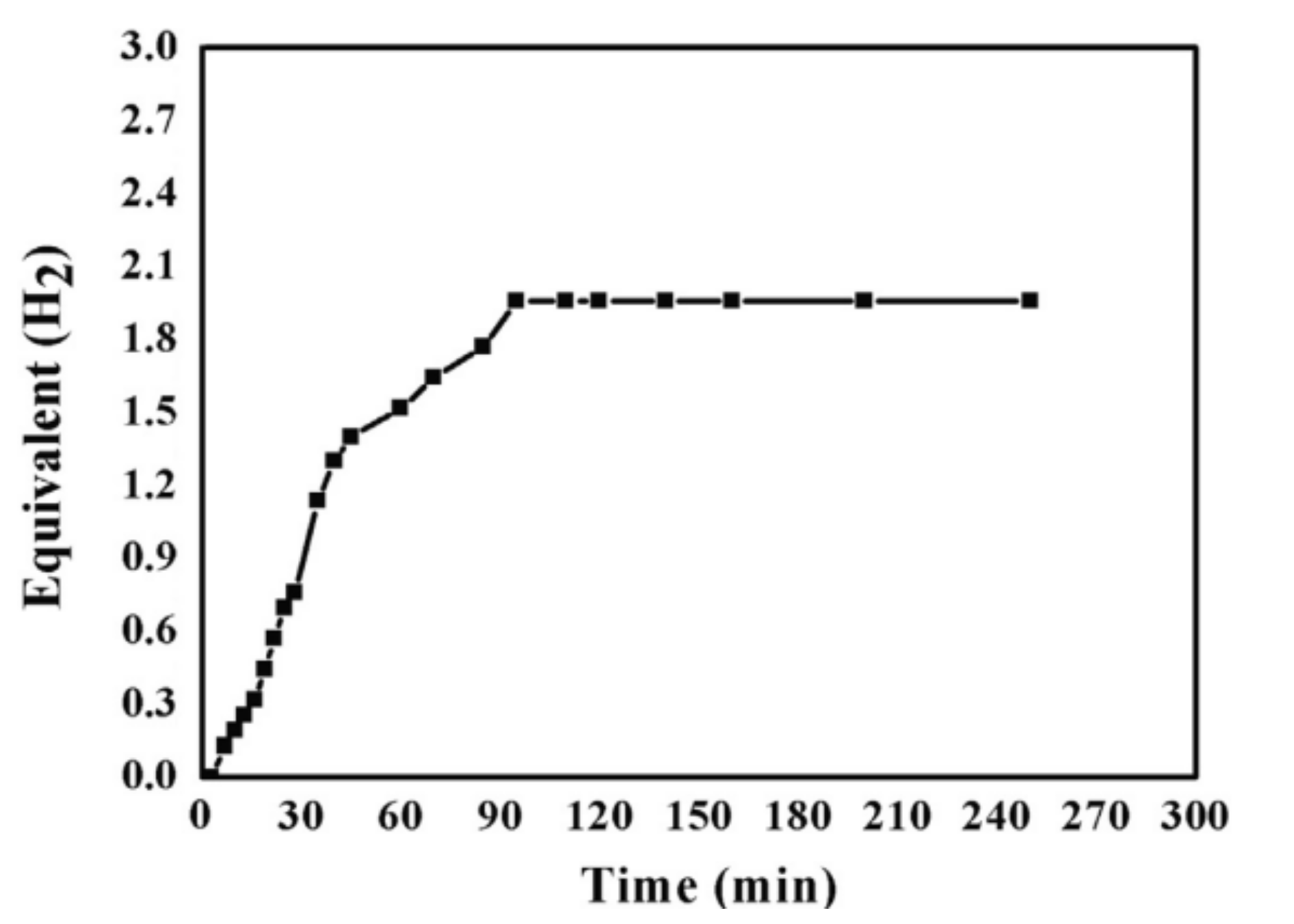
9.1. Co, Ni, and Cu Oxide NFs
9.2. Co–Mn–O NFs
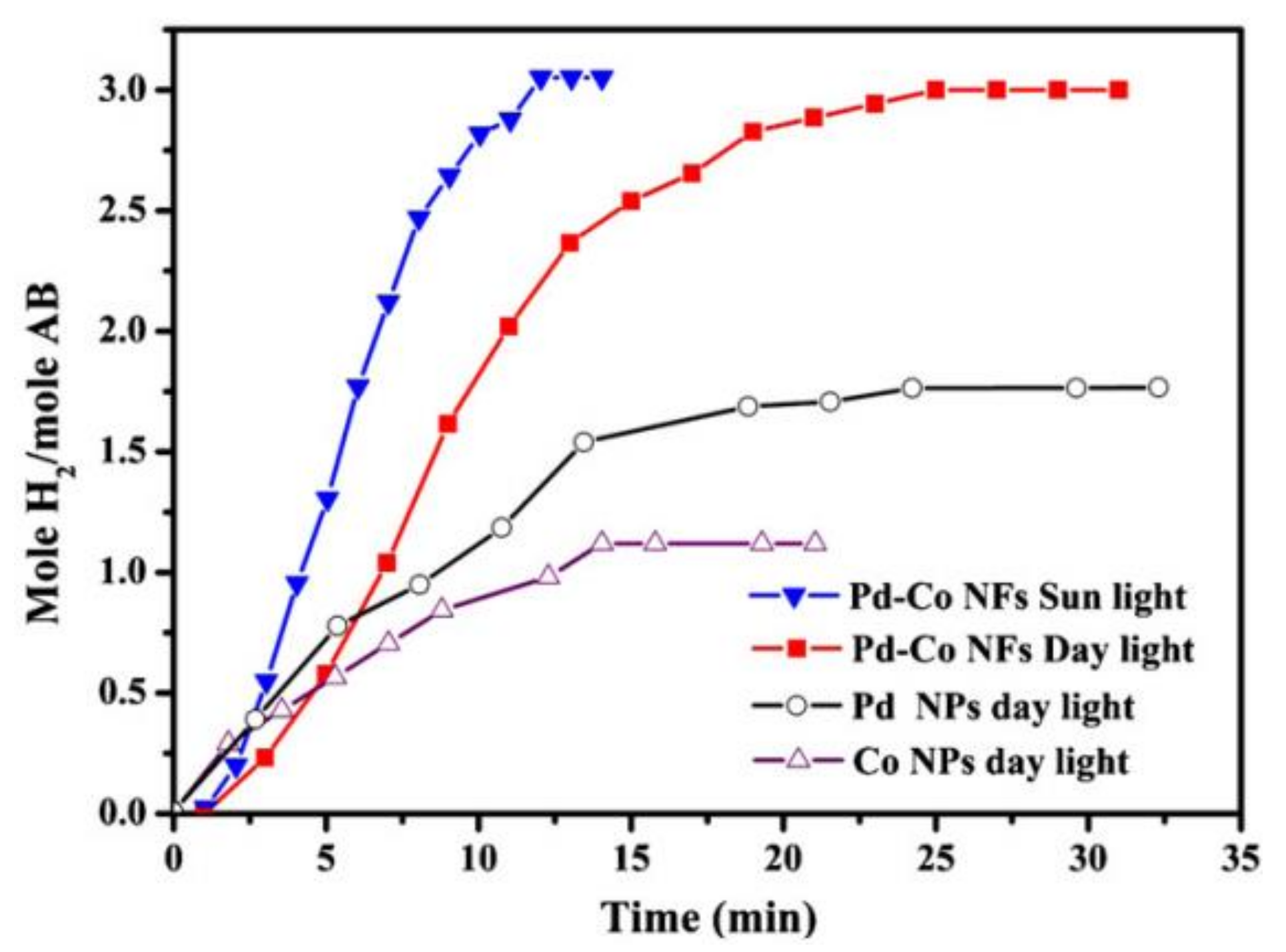
9.3. Pd-Doped Co NFs
10. Recommendation
- Studying and improving the flow system operations of AB hydrolysis using electrospun ceramic catalysts;
- Studying and testing aluminum oxide NFs (Al2O3) as catalysts or catalyst supports;
- Conducting research to study the effect of trimetalic alloying;
- Studying and developing new electrospun biocatalysts;
- Modeling and simulation should be investigated to bring some conclusion that might help in saving energy and time to improve new catalysts.
11. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shang, J.; Xu, X.; Liu, K.; Bao, Y.; Yangyang; He, M. LSPR-driven upconversion enhancement and photocatalytic H2 evolution for Er-Yb:TiO2/MoO3-x nano-semiconductor heterostructure. Ceram. Int. 2019, 45, 16625–16630. [Google Scholar] [CrossRef]
- Lv, B.; Lu, L.; Feng, X.; Wu, X.; Wang, X.; Zou, X.; Zhang, F. Efficient photocatalytic hydrogen production using an NH4TiOF3/TiO2/g-C3N4 composite with a 3D camellia-like Z-scheme heterojunction structure. Ceram. Int. 2020, 46, 26689–26697. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, S.; Zhang, Y.; Zhu, Z.; Su, W.; Sheng, M. Fabrication of porous Cu supported Ni-P/CeO2 composite coatings for enhanced hydrogen evolution reaction in alkaline solution. Ceram. Int. 2020, 46, 20871–20877. [Google Scholar] [CrossRef]
- Nazir, H.; Muthuswamy, N.; Louis, C.; Jose, S.; Prakash, J.; Buan, M.E.; Flox, C.; Chavan, S.; Shi, X.; Kauranen, P.; et al. Is the H2 economy realizable in the foreseeable future? Part II: H2 storage, transportation, and distribution. Int. J. Hydrogen Energy 2020, 45, 20693–20708. [Google Scholar] [CrossRef]
- Park, K.; Koo, J. Optimal design of the distributed H2 production system with cost and safety considerations. Int. J. Hydrogen Energy 2020, 45, 34316–34325. [Google Scholar] [CrossRef]
- Brooks, R.M.; Maafa, I.M.; Al-Enizi, A.M.; El-Halwany, M.M.; Ubaidullah, M.; Yousef, A. Electrospun Bimetallic NiCr Nanoparticles@Carbon Nanofibers as an Efficient Catalyst for Hydrogen Generation from Ammonia Borane. Nanomaterials 2019, 9, 1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barakat, N.A.M.; Motlak, M.; Taha, A.; Nassar, M.M.; Mahmoud, M.S.; Fouad, H.; Moaaed, M. Super effective Zn-Fe-doped TiO2nanofibers as photocatalyst for ammonia borane hydrolysis. Int. J. Green Energy 2015, 13, 642–649. [Google Scholar] [CrossRef]
- Yousef, A.; Brooks, R.M.; El-Halwany, M.; El-Newehy, M.H.; Al-Deyab, S.S.; Barakat, N.A. Cu0/S-doped TiO2 nanoparticles-decorated carbon nanofibers as novel and efficient photocatalyst for hydrogen generation from ammonia borane. Ceram. Int. 2016, 42, 1507–1512. [Google Scholar] [CrossRef]
- Yousef, A.; Brooks, R.M.; El-Halwany, M.; Abutaleb, A.; El-Newehy, M.H.; Al-Deyab, S.S.; Kim, H.Y. Electrospun CoCr7C3 -supported C nanofibers: Effective, durable, and chemically stable catalyst for H2 gas generation from ammonia borane. Mol. Catal. 2017, 434, 32–38. [Google Scholar] [CrossRef]
- Barakat, N.A. Catalytic and photo hydrolysis of ammonia borane complex using Pd-doped Co nanofibers. Appl. Catal. A Gen. 2013, 451, 21–27. [Google Scholar] [CrossRef]
- Frueh, S.; Kellett, R.; Mallery, C.; Molter, T.; Willis, W.S.; King’Ondu, C.; Suib, S.L. Pyrolytic Decomposition of Ammonia Borane to Boron Nitride. Inorg. Chem. 2011, 50, 783–792. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, L.; Luo, X.; Wan, C.; Xu, L. Recent Advances in Noble Metal Catalysts for Hydrogen Production from Ammonia Borane. Catalysts 2020, 10, 788. [Google Scholar] [CrossRef]
- Barakat, N.A. Effective Co–Mn–O nanofibers for ammonia borane hydrolysis. Mater. Lett. 2013, 106, 229–232. [Google Scholar] [CrossRef]
- Yousef, A.; Barakat, N.; El-Newehy, M.H.; Ahmed, D.; Kim, H.Y. Catalytic hydrolysis of ammonia borane for hydrogen generation using Cu(0) nanoparticles supported on TiO2 nanofibers. Colloids Surf. A Physicochem. Eng. Asp. 2015, 470, 194–201. [Google Scholar] [CrossRef]
- Yousef, A.; Barakat, N.; Khalil, K.A.; Unnithan, A.R.; Panthi, G.; Pant, B.; Kim, H.Y. Photocatalytic release of hydrogen from ammonia borane-complex using Ni(0)-doped TiO2/C electrospun nanofibers. Colloids Surf. A Physicochem. Eng. Asp. 2012, 410, 59–65. [Google Scholar] [CrossRef]
- Yousef, A.; Barakat, N.A.; Kim, H.Y. Electrospun Cu-doped titania nanofibers for photocatalytic hydrolysis of ammonia borane. Appl. Catal. A Gen. 2013, 467, 98–106. [Google Scholar] [CrossRef]
- Filiz, B.C.; Figen, A.K. Fabrication of electrospun nanofiber catalysts and ammonia borane hydrogen release efficiency. Int. J. Hydrogen Energy 2016, 41, 15433–15442. [Google Scholar] [CrossRef]
- Tonbul, Y.; Akbayrak, S.; Özkar, S. Palladium(0) nanoparticles supported on ceria: Highly active and reusable catalyst in hydrogen generation from the hydrolysis of ammonia borane. Int. J. Hydrogen Energy 2016, 41, 11154–11162. [Google Scholar] [CrossRef]
- Fiorenza, R.; Scirè, S.; Venezia, A. Carbon supported bimetallic Ru-Co catalysts for H2 production through NaBH4 and NH3 BH3 hydrolysis. Int. J. Energy Res. 2018, 42, 1183–1195. [Google Scholar] [CrossRef]
- Wang, L. Functional Nanofibre: Enabling Material for the Next Generation Smart Textiles. J. Fiber Bioeng. Inform. 2008, 1, 81–92. [Google Scholar] [CrossRef]
- Almessiere, M.; Slimani, Y.; Güner, S.; Baykal, A.; Ercan, I. Effect of dysprosium substitution on magnetic and structural properties of NiFe2O4 nanoparticles. J. Rare Earths 2019, 37, 871–878. [Google Scholar] [CrossRef]
- Koblischka, M.; Koblischka-Veneva, A.; Zeng, X.; Hannachi, E.; Slimani, Y. Microstructure and Fluctuation-Induced Conductivity Analysis of Bi2Sr2CaCu2O8δ (Bi-2212) Nanowire Fabrics+. Crystals 2020, 10, 986. [Google Scholar] [CrossRef]
- Albetran, H.; Slimani, Y.; Almessiere, M.; AlAhmari, F.; Shirsath, S.E.; Akhtar, S.; Low, I.M.; Baykal, A.; Ercan, I.; Alhamari, F. Synthesis, characterization and magnetic investigation of Er-substituted electrospun NiFe2O4 nanofibers. Phys. Scr. 2020, 95, 075801. [Google Scholar] [CrossRef]
- Alahmari, F.; Almessiere, M.; Ünal, B.; Slimani, Y.; Baykal, A. Electrical and optical properties of Ni0.5Co0.5-xCdxNd0.02Fe1.78O4 (x ≤ 0.25) spinel ferrite nanofibers. Ceram. Int. 2020, 46, 24605–24614. [Google Scholar] [CrossRef]
- Alahmari, F.; Almessiere, M.; Slimani, Y.; Güngüneş, H.; Shirsath, S.E.; Akhtar, S.; Jaremko, M.; Baykal, A. Synthesis and characterization of electrospun Ni0.5Co0.5−xCdxNd0.02Fe1.78O4 nanofibers. Nano Struct. Nano Objects 2020, 24, 100542. [Google Scholar] [CrossRef]
- Liao, H.-S.; Lin, J.; Liu, Y.; Huang, P.; Jin, A.; Chen, X.S. Self-assembly mechanisms of nanofibers from peptide amphiphiles in solution and on substrate surfaces. Nanoscale 2016, 8, 14814–14820. [Google Scholar] [CrossRef] [Green Version]
- Nayak, R.; Padhye, R.; Kyratzis, I.L.; Truong, Y.B.; Arnold, L. Recent advances in nanofibre fabrication techniques. Text. Res. J. 2011, 82, 129–147. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Hu, X.; Cheng, B.; Liu, H. Preparation of Hollow Fiber Membranes by Nonsolvent Induced Phase Separation along with Hydrogen Gas Formation Using a Single Orifice Spinneret. Macromol. Mater. Eng. 2017, 302, 1700282. [Google Scholar] [CrossRef]
- Lolla, D.; Lolla, M.; Abutaleb, A.; Shin, H.U.; Reneker, D.H.; Chase, G.G. Fabrication, Polarization of Electrospun Polyvinylidene Fluoride Electret Fibers and Effect on Capturing Nanoscale Solid Aerosols. Materials 2016, 9, 671. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.U.; Abutaleb, A.; Lolla, D.; Chase, G.G. Effect of Calcination Temperature on NO–CO Decomposition by Pd Catalyst Nanoparticles Supported on Alumina Nanofibers. Fibers 2017, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Abutaleb, A.; Lolla, D.; Aljuhani, A.; Shin, H.U.; Rajala, J.W.; Chase, G.G. Effects of Surfactants on the Morphology and Properties of Electrospun Polyetherimide Fibers. Fibers 2017, 5, 33. [Google Scholar] [CrossRef] [Green Version]
- Yousef, A.; Brooks, R.M.; Abutaleb, A.; Al-Deyab, S.S.; El-Newehy, M.H. Low Temperature Synthesis of Cobalt–Chromium Carbide Nanoparticles-Doped Carbon Nanofibers. J. Nanosci. Nanotechnol. 2018, 18, 2938–2942. [Google Scholar] [CrossRef]
- Abutaleb, A. Electrochemical Oxidation of Urea on NiCu Alloy Nanoparticles Decorated Carbon Nanofibers. Catalysts 2019, 9, 397. [Google Scholar] [CrossRef] [Green Version]
- Lolla, D.; Abutaleb, A.; Kashfipour, M.A.; Chase, G.G. Polarized Catalytic Polymer Nanofibers. Materials 2019, 12, 2859. [Google Scholar] [CrossRef] [Green Version]
- Abutaleb, A.; Lolla, D.; Aljuhani, A.; Shin, H.U.; Ali, M.A.; Hassan, A.A.Y.; Maafa, I.M.H.; Chase, G.G. Liquid Phase Selective Hydrogenation of Phenol to Cyclohexanone over Electrospun Pd/PVDF-HFP Catalyst. Fibers 2019, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Rodaev, V.V.; Zhigachev, A.O.; Golovin, Y.I. Fabrication and characterization of electrospun ZrO2/Al2O3 nanofibers. Ceram. Int. 2017, 43, 16023–16026. [Google Scholar] [CrossRef]
- Yuan, K.; Jin, X.; Yu, Z.; Gan, X.; Wang, X.; Zhang, G.; Zhu, L.; Xu, N. Electrospun mesoporous zirconia ceramic fibers for catalyst supporting applications. Ceram. Int. 2018, 44, 282–289. [Google Scholar] [CrossRef]
- Asadi-Pakdel, K.; Aghdam, R.M.; Asl, M.S.; Sani, M.A.F. Synthesis and morphology optimization of electrospun SiBNC nanofibers. Ceram. Int. 2020, 46, 6052–6059. [Google Scholar] [CrossRef]
- Contreras-Cáceres, R.; Cabeza, L.; Perazzoli, G.; Díaz, A.; López-Romero, J.M.; Melguizo, C.; Prados, J. Electrospun Nanofibers: Recent Applications in Drug Delivery and Cancer Therapy. Nanomaterials 2019, 9, 656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ognibene, G.; Gangemi, C.M.A.; Spitaleri, L.; Gulino, A.; Purrello, R.; Cicala, G.; Fragalà, M.E. Role of the surface composition of the polyethersulfone–TiiP–H2T4 fibers on lead removal: From electrostatic to coordinative binding. J. Mater. Sci. 2019, 54, 8023–8033. [Google Scholar] [CrossRef]
- Jung, J.-W.; Lee, C.-L.; Yu, S.; Kim, I.-D. Electrospun nanofibers as a platform for advanced secondary batteries: A comprehensive review. J. Mater. Chem. A 2016, 4, 703–750. [Google Scholar] [CrossRef]
- Esfahani, H.; Jose, R.; Ramakrishna, S. Electrospun Ceramic Nanofiber Mats Today: Synthesis, Properties, and Applications. Materials 2017, 10, 1238. [Google Scholar] [CrossRef] [Green Version]
- Gangemi, C.M.A.; Iudici, M.; Spitaleri, L.; Randazzo, R.; Gaeta, M.; D’Urso, A.; Gulino, A.; Purrello, R.; Fragalà, M.E. Polyethersulfone Mats Functionalized with Porphyrin for Removal of Para-nitroaniline from Aqueous Solution. Molecules 2019, 24, 3344. [Google Scholar] [CrossRef] [Green Version]
- Yousefa, A.; Hameed, R.A.; Shaikh, S.F.; Abutalebd, A.; Halwanye, M.-; Al-Enizi, A.M. Enhanced electro-adsorption desalination performance of graphene by TiC. Sep. Purif. Technol. 2021, 254, 117602. [Google Scholar] [CrossRef]
- Su, Z.; Ding, J.; Wei, G. Electrospinning: A facile technique for fabricating polymeric nanofibers doped with carbon nanotubes and metallic nanoparticles for sensor applications. RSC Adv. 2014, 4, 52598–52610. [Google Scholar] [CrossRef] [Green Version]
- Salmeri, M.; Ognibene, G.; Saitta, L.; Lombardo, C.; Genovese, C.; Barcellona, M.; D’Urso, A.; Spitaleri, L.; Blanco, I.; Cicala, G.; et al. Optimization of ZnO Nanorods Growth on Polyetheresulfone Electrospun Mats to Promote Antibacterial Properties. Molecules 2020, 25, 1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zafar, M.; Najeeb, S.; Khurshid, Z.; Vazirzadeh, M.; Zohaib, S.; Najeeb, B.; Sefat, F. Potential of Electrospun Nanofibers for Biomedical and Dental Applications. Materials 2016, 9, 73. [Google Scholar] [CrossRef]
- Qasim, S.B.; Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Shah, A.H.; Husain, S.; Rehman, I.U. Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, M.A.; Albetran, H.M.; Alheshibri, M.H.; Timoumi, A.; Algarou, N.A.; Akhtar, S.; Slimani, Y.; Almessiere, M.A.; AlAhmari, F.S.; Baykal, A.; et al. Synthesis of Electrospun TiO2 Nanofibers and Characterization of Their Antibacterial and Antibiofilm Potential against Gram-Positive and Gram-Negative Bacteria. Antibiotics 2020, 9, 572. [Google Scholar] [CrossRef] [PubMed]
- AlAhmari, F.; Rehman, S.; Almessiere, M.; Khan, F.; Slimani, Y.; Baykal, A. Synthesis of Ni0.5Co0.5-xCdxFe1.78Nd0.02O4 (x ≤ 0.25) nanofibers by using electrospinning technique induce anti-cancer and anti-bacterial activities. J. Biomol. Struct. Dyn. 2020, 39, 1–8. [Google Scholar] [CrossRef]
- Shin, H.U.; Lolla, D.; Abutaleb, A.; Hwang, S.Y.; Chase, G.G. CO Oxidation over Pd-Au Alloy Nanoparticle Doped Fibrous TiO2-Support Media. Int. J. Nanosci. Nanoeng. 2018, 4, 12–23. [Google Scholar]
- Hameed, R.A.; Mohamed, I.M.; Al-Enizi, A.M.; Abutaleb, A.; Shaikh, S.F.; Yousef, A. Fabrication of electrospun nickel sulphide nanoparticles onto carbon nanofibers for efficient urea electro-oxidation in alkaline medium. Int. J. Hydrogen Energy 2021, 46, 12944–12960. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Nafady, A.; El-Halwany, M.; Brooks, R.M.; Abutaleb, A.; Yousef, A. Electrospun carbon nanofiber-encapsulated NiS nanoparticles as an efficient catalyst for hydrogen production from hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2019, 44, 21716–21725. [Google Scholar] [CrossRef]
- Yousef, A.; Barakat, N.; El-Newehy, M.; Kim, H.Y. Chemically stable electrospun NiCu nanorods@carbon nanofibers for highly efficient dehydrogenation of ammonia borane. Int. J. Hydrogen Energy 2012, 37, 17715–17723. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Wade, R.J.; Burdick, J.A. Advances in nanofibrous scaffolds for biomedical applications: From electrospinning to self-assembly. Nano Today 2014, 9, 722–742. [Google Scholar] [CrossRef]
- Bhushani, J.A.; Anandharamakrishnan, C. Electrospinning and electrospraying techniques: Potential food based applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Sun, B.; Long, Y.; Zhang, H.; Li, M.; Duvail, J.-L.; Jiang, X.; Yin, H. Advances in three-dimensional nanofibrous macrostructures via electrospinning. Prog. Polym. Sci. 2014, 39, 862–890. [Google Scholar] [CrossRef]
- Braghirolli, D.I.; Steffens, D.; Pranke, P. Electrospinning for regenerative medicine: A review of the main topics. Drug Discov. Today 2014, 19, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.; Sundaramurthy, J.; Sundarrajan, S.; Babu, V.J.; Singh, G.; Allakhverdiev, S.; Ramakrishna, S. Hierarchical electrospun nanofibers for energy harvesting, production and environmental remediation. Energy Environ. Sci. 2014, 7, 3192–3222. [Google Scholar] [CrossRef]
- Jiang, T.; Carbone, E.J.; Lo, K.W.-H.; Laurencin, C.T. Electrospinning of polymer nanofibers for tissue regeneration. Prog. Polym. Sci. 2015, 46, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Ghorani, B.; Tucker, N. Fundamentals of electrospinning as a novel delivery vehicle for bioactive compounds in food nanotechnology. Food Hydrocoll. 2015, 51, 227–240. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Moheman, A.; Alam, M.S.; Mohammad, A. Recent trends in electrospinning of polymer nanofibers and their applications in ultra thin layer chromatography. Adv. Colloid Interface Sci. 2016, 229, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.D.; Dalton, P.D.; Hutmacher, D.W. Melt electrospinning today: An opportune time for an emerging polymer process. Prog. Polym. Sci. 2016, 56, 116–166. [Google Scholar] [CrossRef]
- Ray, S.S.; Chen, S.-S.; Li, C.-W.; Nguyen, N.C.; Nguyen, H.T. A comprehensive review: Electrospinning technique for fabrication and surface modification of membranes for water treatment application. RSC Adv. 2016, 6, 85495–85514. [Google Scholar] [CrossRef]
- Sarbatly, R.; Krishnaiah, D.; Kamin, Z. A review of polymer nanofibres by electrospinning and their application in oil–water separation for cleaning up marine oil spills. Mar. Pollut. Bull. 2016, 106, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.; Mali, S.S.; Kamble, A.S.; Hong, C.K.; Kim, J.H.; Patil, P. Electrospinning: A versatile technique for making of 1D growth of nanostructured nanofibers and its applications: An experimental approach. Appl. Surf. Sci. 2017, 423, 641–674. [Google Scholar] [CrossRef]
- Wen, P.; Zong, M.-H.; Linhardt, R.J.; Feng, K.; Wu, H. Electrospinning: A novel nano-encapsulation approach for bioactive compounds. Trends Food Sci. Technol. 2017, 70, 56–68. [Google Scholar] [CrossRef]
- Khalf, A.; Madihally, S.V. Recent advances in multiaxial electrospinning for drug delivery. Eur. J. Pharm. Biopharm. 2017, 112, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Jun, Y.; Qin, J.; Lee, S.-H. Electrospinning versus microfluidic spinning of functional fibers for biomedical applications. Biomaterials 2017, 114, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Kitsara, M.; Agbulut, O.; Kontziampasis, D.; Chen, Y.; Menasché, P. Fibers for hearts: A critical review on electrospinning for cardiac tissue engineering. Acta Biomater. 2017, 48, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Han, J.; Wang, F.; Shao, W.; Xiong, R.; Zhang, Q.; Pan, H.; Yang, Y.; Samal, S.K.; Zhang, F.; et al. Electrospun Nanofibers Membranes for Effective Air Filtration. Macromol. Mater. Eng. 2017, 302, 1600353. [Google Scholar] [CrossRef]
- Mercante, L.; Scagion, V.P.; Migliorini, F.L.; Mattoso, L.H.; Correa, D. Electrospinning-based (bio)sensors for food and agricultural applications: A review. TrAC Trends Anal. Chem. 2017, 91, 91–103. [Google Scholar] [CrossRef]
- Aruna, S.; Balaji, L.; Kumar, S.S.; Prakash, B.S. Electrospinning in solid oxide fuel cells—A review. Renew. Sustain. Energy Rev. 2017, 67, 673–682. [Google Scholar] [CrossRef]
- Lee, J.K.Y.; Chen, N.; Peng, S.; Li, L.; Tian, L.; Thakor, N.; Ramakrishna, S. Polymer-based composites by electrospinning: Preparation & functionalization with nanocarbons. Prog. Polym. Sci. 2018, 86, 40–84. [Google Scholar] [CrossRef]
- Chinnappan, A.; Baskar, C.; Baskar, S.; Ratheesh, G.; Ramakrishna, S. An overview of electrospun nanofibers and their application in energy storage, sensors and wearable/flexible electronics. J. Mater. Chem. C 2017, 5, 12657–12673. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, J.; Zhang, L.; Qiu, Y. Recent advances in energy materials by electrospinning. Renew. Sustain. Energy Rev. 2018, 81, 1825–1858. [Google Scholar] [CrossRef]
- Rauf, M.; Wang, J.-W.; Zhang, P.; Iqbal, W.; Qu, J.; Li, Y. Non-precious nanostructured materials by electrospinning and their applications for oxygen reduction in polymer electrolyte membrane fuel cells. J. Power Sources 2018, 408, 17–27. [Google Scholar] [CrossRef]
- Zong, H.; Xia, X.; Liang, Y.; Dai, S.; Alsaedi, A.; Hayat, T.; Kong, F.; Pan, J.H. Designing function-oriented artificial nanomaterials and membranes via electrospinning and electrospraying techniques. Mater. Sci. Eng. C 2018, 92, 1075–1091. [Google Scholar] [CrossRef]
- Chen, S.; Li, R.; Li, X.; Xie, J. Electrospinning: An enabling nanotechnology platform for drug delivery and regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 188–213. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, F.; Zhang, H. Emulsion electrospinning: Fundamentals, food applications and prospects. Trends Food Sci. Technol. 2018, 80, 175–186. [Google Scholar] [CrossRef]
- Soares, R.M.; Siqueira, N.; Prabhakaram, M.P.; Ramakrishna, S. Electrospinning and electrospray of bio-based and natural polymers for biomaterials development. Mater. Sci. Eng. C 2018, 92, 969–982. [Google Scholar] [CrossRef]
- Hemamalini, T.; Dev, V.R.G. Comprehensive review on electrospinning of starch polymer for biomedical applications. Int. J. Biol. Macromol. 2018, 106, 712–718. [Google Scholar] [CrossRef]
- Ghosal, K.; Agatemor, C.; Špitálsky, Z.; Thomas, S.; Kny, E. Electrospinning tissue engineering and wound dressing scaffolds from polymer-titanium dioxide nanocomposites. Chem. Eng. J. 2019, 358, 1262–1278. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, Y.; Duan, G.; Mei, C.; Greiner, A.; Agarwal, S. Electrospun nanofiber reinforced composites: A review. Polym. Chem. 2018, 9, 2685–2720. [Google Scholar] [CrossRef]
- Pascariu, P.; Homocianu, M. ZnO-Based ceramic nanofibers: Preparation, properties and applications. Ceram. Int. 2019, 45, 11158–11173. [Google Scholar] [CrossRef]
- Huang, W.; Tong, Z.; Wang, R.; Liao, Z.; Bi, Y.; Chen, Y.; Ma, M.; Lyu, P.; Ma, Y. A review on electrospinning nanofibers in the field of microwave absorption. Ceram. Int. 2020, 46, 26441–26453. [Google Scholar] [CrossRef]
- Chen, L.-F.; Lu, Y.; Yu, L.; Lou, X.W. Designed formation of hollow particle-based nitrogen-doped carbon nanofibers for high-performance supercapacitors. Energy Environ. Sci. 2017, 10, 1777–1783. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, C.; Zhou, M.; Fu, Q.; Zhao, C.; Wu, M.; Lei, Y. Highly nitrogen doped carbon nanofibers with superior rate capability and cyclability for potassium ion batteries. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Levitt, A.S.; Alhabeb, M.; Hatter, C.B.; Sarycheva, A.; Dion, G.; Gogotsi, Y. Electrospun MXene/carbon nanofibers as supercapacitor electrodes. J. Mater. Chem. A 2019, 7, 269–277. [Google Scholar] [CrossRef]
- Roy, A.; Talarposhti, M.R.; Normile, S.J.; Zenyuk, I.V.; De Andrade, V.; Artyushkova, K.; Serov, A.; Atanassov, P. Nickel–copper supported on a carbon black hydrogen oxidation catalyst integrated into an anion-exchange membrane fuel cell. Sustain. Energy Fuels 2018, 2, 2268–2275. [Google Scholar] [CrossRef]
- Zhang, H.M.; Wang, Y.F.; Kwok, Y.H.; Wu, Z.C.; Xia, D.H.; Leung, D.Y.C. A Direct Ammonia Microfluidic Fuel Cell using NiCu Nanoparticles Supported on Carbon Nanotubes as an Electrocatalyst. ChemSusChem 2018, 11, 2889–2897. [Google Scholar] [CrossRef]
- Guisbiers, G.; Khanal, S.; Ruiz-Zepeda, F.; de la Puente, J.R.; José-Yacaman, M. Cu–Ni nano-alloy: Mixed, core–shell or Janus nano-particle? Nanoscale 2014, 6, 14630–14635. [Google Scholar] [CrossRef] [PubMed]
- Al-Enizi, A.M.; Brooks, R.M.; Abutaleb, A.; El-Halwany, M.; El-Newehy, M.H.; Yousef, A. Electrospun carbon nanofibers containing Co-TiC nanoparticles-like superficial protrusions as a catalyst for H2 gas production from ammonia borane complex. Ceram. Int. 2017, 43, 15735–15742. [Google Scholar] [CrossRef]
- Patel, N.; Fernandes, R.; Miotello, A. Promoting effect of transition metal-doped Co–B alloy catalysts for hydrogen production by hydrolysis of alkaline NaBH4 solution. J. Catal. 2010, 271, 315–324. [Google Scholar] [CrossRef]
- Yang, K.; Yao, Q.; Huang, W.; Chen, X.; Lu, Z.-H. Enhanced catalytic activity of NiM (M = Cr, Mo, W) nanoparticles for hydrogen evolution from ammonia borane and hydrazine borane. Int. J. Hydrogen Energy 2017, 42, 6840–6850. [Google Scholar] [CrossRef]
- Yousef, A.; Brooks, R.M.; El-Halwany, M.; Obaid, M.; El-Newehy, M.H.; Al-Deyab, S.S.; Barakat, N. A novel and chemical stable Co–B nanoflakes-like structure supported over titanium dioxide nanofibers used as catalyst for hydrogen generation from ammonia borane complex. Int. J. Hydrogen Energy 2016, 41, 285–293. [Google Scholar] [CrossRef]
- Wang, T.; Gao, Y.; Tang, T.; Bian, H.; Zhang, Z.; Xu, J.; Xiao, H.; Chu, X. Preparation of ordered TiO2 nanofibers/nanotubes by magnetic field assisted electrospinning and the study of their photocatalytic properties. Ceram. Int. 2019, 45, 14404–14410. [Google Scholar] [CrossRef]
- Mkhalid, I.; Fierro, J.; Mohamed, R.; Alshahri, A. Visible light driven photooxidation of imazapyr herbicide over highly efficient mesoporous Ag/Ag2O–TiO2 p-n heterojunction photocatalysts. Ceram. Int. 2020, 46, 25822–25832. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, R.; Kim, H.Y.; Yi, C.; Barakat, N.; Navamathavan, R.; El-Newehy, M. Electrospun nickel doped titanium dioxide nanofibers as an effective photocatalyst for the hydrolytic dehydrogenation of ammonia borane. Int. J. Hydrogen Energy 2012, 37, 10036–10045. [Google Scholar] [CrossRef]
- Bakri, A.S.; Sahdan, M.Z.; Adriyanto, F.; Raship, N.A.; Said, N.D.M.; Abdullah, S.A.; Rahim, M.S. Effect of Annealing Temperature of Titanium Dioxide Thin Films on Structural and Electrical Properties; AIP Publishing: Melville, NY, USA, 2017; p. p. 30030. [Google Scholar]
- Barakat, N.A.; Kanjwal, M.A.; Chronakis, I.S.; Kim, H.Y. Influence of temperature on the photodegradation process using Ag-doped TiO2 nanostructures: Negative impact with the nanofibers. J. Mol. Catal. A Chem. 2013, 366, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yu, W.; Han, C.; Guo, J.; Zhang, Q.; Xie, H.; Shao, Q.; Sun, Z.; Guo, Z. Large Scaled Synthesis of Heterostructured Electrospun TiO2/SnO2Nanofibers with an Enhanced Photocatalytic Activity. J. Electrochem. Soc. 2017, 164, H651–H656. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Prakash, J.; Misra, M.; Sharma, A.; Gupta, R.K. Dual Functional Ta-Doped Electrospun TiO2 Nanofibers with Enhanced Photocatalysis and SERS Detection for Organic Compounds. ACS Appl. Mater. Interfaces 2017, 9, 28495–28507. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.; Leung, D.Y.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. New Non-Oxide Photocatalysts Designed for Overall Water Splitting under Visible Light. J. Phys. Chem. C 2007, 111, 7851–7861. [Google Scholar] [CrossRef]
- Pant, B.; Barakat, N.; Pant, H.R.; Park, M.; Saud, P.S.; Kim, J.-W.; Kim, H.-Y. Synthesis and photocatalytic activities of CdS/TiO2 nanoparticles supported on carbon nanofibers for high efficient adsorption and simultaneous decomposition of organic dyes. J. Colloid Interface Sci. 2014, 434, 159–166. [Google Scholar] [CrossRef]
- Yousef, A.; El-Halwany, M.; Barakat, N.A.; Kim, H.Y. One pot synthesis of Cu-doped TiO2 carbon nanofibers for dehydrogenation of ammonia borane. Ceram. Int. 2015, 41, 6137–6140. [Google Scholar] [CrossRef]
- Pant, B.; Pant, H.R.; Park, M.; Liu, Y.; Choi, J.-W.; Barakat, N.A.M.; Kim, H.Y. Electrospun CdS–TiO2 doped carbon nanofibers for visible-light-induced photocatalytic hydrolysis of ammonia borane. Catal. Commun. 2014, 50, 63–68. [Google Scholar] [CrossRef]
- Pant, B.; Pant, H.R.; Barakat, N.; Park, M.; Han, T.-H.; Lim, B.H.; Kim, H.Y. Incorporation of cadmium sulfide nanoparticles on the cadmium titanate nanofibers for enhanced organic dye degradation and hydrogen release. Ceram. Int. 2014, 40, 1553–1559. [Google Scholar] [CrossRef]
- Wu, M.-C.; Wu, P.-Y.; Lin, T.-H.; Lin, T.-F. Photocatalytic performance of Cu-doped TiO2 nanofibers treated by the hydrothermal synthesis and air-thermal treatment. Appl. Surf. Sci. 2018, 430, 390–398. [Google Scholar] [CrossRef]










| Review | Covered Application | Year | Reference |
|---|---|---|---|
| Electrospinning of polymeric nanofibers for drug delivery applications. | Drug delivery | 2014 | [55] |
| Advances in nanofibrous scaffolds for biomedical applications: From electrospinning to self-assembly. | Biomedical. | 2014 | [56] |
| Electrospinning and electrospraying techniques: Potential food-based applications. | Food Industry. | 2014 | [57] |
| Advances in three-dimensional nanofibrous macrostructures via electrospinning. | Tissue engineering. Energy harvesting and Storage, Filtration. | 2014 | [58] |
| Electrospinning for regenerative medicine: a review of the main topics. | Tissue engineering. | 2014 | [59] |
| Hierarchical electrospun nanofibers for energy harvesting, production, and environmental remediation. | Photovoltaics and photocatalysis. Hydrogen energy Harvesting, Fuel cells. | 2014 | [60] |
| Electrospinning of polymer nanofibers for tissue regeneration. | Medical. | 2015 | [61] |
| Fundamentals of electrospinning as a novel delivery vehicle for bioactive compounds in food nanotechnology. | Food technology. | 2015 | [62] |
| A review on electrospinning for membrane fabrication: Challenges and applications. | Water treatment. | 2015 | [63] |
| A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. | Biomedical and biotechnology. | 2015 | [64] |
| Recent trends in electrospinning of polymer nanofibers and their applications in ultra-thin layer chromatography. | Chromatography. | 2016 | [65] |
| Melt electrospinning today: An opportune time for an emerging polymer process. | Energy, environment, filtration, and separation. Biomedical. | 2016 | [66] |
| A comprehensive review: electrospinning technique for fabrication and surface modification of membranes for water treatment application. | Water treatment. | 2016 | [67] |
| A review of polymer nanofibres by electrospinning and their application in oil-water separation for cleaning up marine oil spills. | Oil–water separation. | 2016 | [68] |
| Electrospinning: A versatile technique for making of 1D growth of nanostructured nanofibers and its applications: An experimental approach. | Energy conversion and storage. Environmental. Biomedical. | 2017 | [69] |
| Electrospinning: A novel nano-encapsulation approach for bioactive compounds. | Encapsulation of different types of bioactive compounds by biopolymer matrixes. | 2017 | [70] |
| Recent advances in multiaxial electrospinning for drug delivery. | Drug delivery. | 2017 | [71] |
| Electrospinning versus microfluidic spinning of functional fibers for biomedical applications. | Tissue engineering. Organ function regeneration. Drug delivery. | 2017 | [72] |
| Fibers for hearts: A critical review on electrospinning for cardiac tissue engineering. | Cardiac tissue engineering. | 2017 | [73] |
| Electrospun Nanofibers Membranes for Effective Air Filtration | Air Filtration. | 2017 | [74] |
| Electrospinning-based (bio)sensors for food and agricultural applications: A review. | Biosensor (Analysis of food/and agricultural products). | 2017 | [75] |
| Electrospinning in solid oxide fuel cells–A review. | Solid oxide fuel cells. | 2017 | [76] |
| Polymer-based composites by electrospinning: Preparation & functionalization with nanocarbons | Tissue engineering Chemical Biosensors Environmental remediation. | 2018 | [77] |
| An overview of electrospun nanofibers and their application in energy storage, sensors, and wearable/flexible electronics. | Wearable/flexible electronics. | 2017 | [78] |
| Recent advances in energy materials by electrospinning. | Energy-related devices. | 2018 | [79] |
| Non-precious nanostructured materials by electrospinning and their applications for oxygen reduction in polymer electrolyte membrane fuel cells. | Fuel cell (Oxygen reduction reaction in a fuel cell). | 2018 | [80] |
| Designing function-oriented artificial nanomaterials and membranes via electrospinning and electrospraying techniques. | Tissue engineering and medicine. Membrane filtration. Lithium battery. | 2018 | [81] |
| Electrospinning: An enabling nanotechnology platform for drug delivery and regenerative medicine. | Biomedical. Regenerative medicine. | 2018 | [82] |
| Emulsion electrospinning: Fundamentals, food applications, and prospects. | Food. | 2018 | [83] |
| Electrospinning and electrospray of bio-based and natural polymers for biomaterials development. | Food Industry. Enzyme immobilization. Tissue engineering. Drug delivery. Wound dressing. | 2018 | [84] |
| Comprehensive review on the electrospinning of starch polymer for biomedical applications. | Biomedical applications. | 2018 | [85] |
| Electrospinning tissue engineering and wound dressing scaffolds from polymer-titanium dioxide nanocomposites. | Tissue engineering. Wound dressing. | 201 | [86] |
| Electrospun nanofiber reinforced composites: a review. | Reinforced composites. | 2018 | [87] |
| ZnO-based ceramic nanofibers: Preparation, properties and applications | ZnO-based CNF applications. | 2019 | [88] |
| A review on electrospinning nanofibers in the field of microwave absorption | Microwave Absorption. | 2020 | [89] |
| Catalyst | Catalytic Preparation Procedure | Catalytic Characterization Equipment | Reactor Type and Reaction Temperature (°C) | AB Concentration (mM) | TOF (mol H2/mol of Metal. min) | Reaction Order | Ea kJ/mol | Reference |
|---|---|---|---|---|---|---|---|---|
| NiCu NRs/C NFs | ES followed by calcination | SEM, FE-SEM, XRD, XPS, TGA and TEM-EDX | Batch 25, 30, 35, 40 | 13.33 | - | - | 28.9 | [54] |
| Co-TiC NPs decorated C NFs | ES followed by calcination | SEM, FE-SEM/EDX, TEM, HR-TEM, TEM-EDX | Batch 25 | 100 | 32.18 | Pseudo-1st with respect to the catalyst concentration | 26.19 | [9] |
| CoCr7C3- supported C NFs | ES followed by calcination | SEM, FE-SEM, XRD, TEM, HR-TEM, EDX, ICP-OES | Batch 25, 30, 40, 50 | 100 | 25.78 | Pseudo-zero-order with respect to AB Pseudo-1st concerning the catalyst | 24.2 | [9] |
| NiCr NPs/C NFs | ES followed by calcination | SEM, FESEM, XRD, TEM, HR-TEM, TEM-EDX | Batch 25, 30, 35, 40, 45, 50 | 100 | 5.78 | Pseudo-zero-order for AB Pseudo-1st to the catalyst | 37.6 | [6] |
| Technique | Purposes | Example | References |
|---|---|---|---|
| Doping with transition metals | Control the band gap of TiO2 that effectively helps to generate electron and hole pairs when using visible light instead of ultraviolet light. Formation of Schottky barrier at the metal and TiO2 interface that works as efficient electron trap to reduce the recombination rate of electrons/holes. | Ni(0)-TiO2/C NFs Cu(0) NPs/TiO2 NFs | [14,108,109] |
| Loading a metal oxide | Reduction of recombination rate of electrons/holes. | CuO/TiO2 | [8] |
| Surface modification by forming a composite system from combing two semiconductors | The synergistic effect can give better charge separation and chemical stability. | CdS–TiO2-doped C NFs | [110] |
| Catalyst | Catalytic Preparation Procedure | Catalytic Characterization Equipment | Reactor Type and Reaction Temperature (°C) | AB Concentration (mM) | Reaction Order | Ea kJ/mol | Reference |
|---|---|---|---|---|---|---|---|
| Ni-doped TiO2 NFs | ES followed by calcination | FE-SEM, HR-TEM, XRD, EDX, XPS, TGA, UV-visible spectrophotometer and photoluminescence (PL) spectroscopy | Batch Sunlight | 8.4 | - | - | [103] |
| Ni(0)-doped TiO2/C NFs | ES followed by calcination | XRD, TEM, TEM-EDX | Batch Sunlight | 13.33 | - | - | [15] |
| CuO/TiO2 NFs | ES followed by calcination | SEM, FE-SEM, EDX, XRD, TEM, HR-TEM, XPS | Batch Sunlight Day light | 13.33 | - | - | [16] |
| Cu(0) NPs/TiO2 NFs | ES followed by Hydrothermal | SEM, FE-SEM, XRD, TEM, HR-TEM, EDX, XPS | Batch Sunlight | 13.33 | - | - | [14] |
| Cu-doped TiO2/C NFs | ES followed by calcination | XRD, FE-SEM, TEM, HR-TEM, EDX | Batch Sunlight | 13.33 | - | - | [111] |
| Cu(0)/S-doped TiO2 NPs/C NFs | ES followed by calcination | FE-SEM, EDX, TEM, HR-TEM, XRD | Batch Sunlight by Mercury lamp | 13.33 | - | - | [8] |
| CdS–TiO2-doped C NFs | ES followed by calcination | FE-SEM, TEM, TEM, HR-EDX, XRD, TGA | Batch Sunlight by Mercury lamp | 13.33 | - | - | [112] |
| CdS NPs/CdTiO3 NFs | ES followed by calcination | FE-SEM, EDS, TEM, HR-TEM, XRD | Batch Sunlight | 13.33 | - | - | [113] |
| Zn–Fe-doped TiO2 NFs | ES followed by calcination | SEM, FE-SEM, EDX, XRD, TEM, HR-TEM | Batch Visible light irradiation using by Mercury lamp | 13.33 | Pseudo-zero-order for AB | Negative value | [16] |
| Catalyst | Catalytic Preparation Procedure | Catalytic Characterization Equipment | Reactor Type and Reaction Temperature (°C) | AB Concentration (mM) | Reaction Order | Ea kJ/mol | Reference |
|---|---|---|---|---|---|---|---|
| CoO NFs | ES followed by calcination | XRD, FT-IR, BET, SEM | Batch 22–80 | 13.33 | zero-order reaction | 35.4 | [17] |
| Co-Mn-O NFs | ES followed by calcination | XRD, SEM, TEM, TGA | Batch | 13.33 | - | - | [13] |
| Pd-doped Co NFs | ES followed by calcination | SEM, FE-SEM, EDX, XRD, TGA, TEM, HR-TEM, Raman | Batch Sunlight | 13.33 | - | - | [10] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abutaleb, A. Catalytic and Photocatalytic Electrospun Nanofibers for Hydrogen Generation from Ammonia Borane Complex: A Review. Polymers 2021, 13, 2290. https://doi.org/10.3390/polym13142290
Abutaleb A. Catalytic and Photocatalytic Electrospun Nanofibers for Hydrogen Generation from Ammonia Borane Complex: A Review. Polymers. 2021; 13(14):2290. https://doi.org/10.3390/polym13142290
Chicago/Turabian StyleAbutaleb, Ahmed. 2021. "Catalytic and Photocatalytic Electrospun Nanofibers for Hydrogen Generation from Ammonia Borane Complex: A Review" Polymers 13, no. 14: 2290. https://doi.org/10.3390/polym13142290
APA StyleAbutaleb, A. (2021). Catalytic and Photocatalytic Electrospun Nanofibers for Hydrogen Generation from Ammonia Borane Complex: A Review. Polymers, 13(14), 2290. https://doi.org/10.3390/polym13142290






