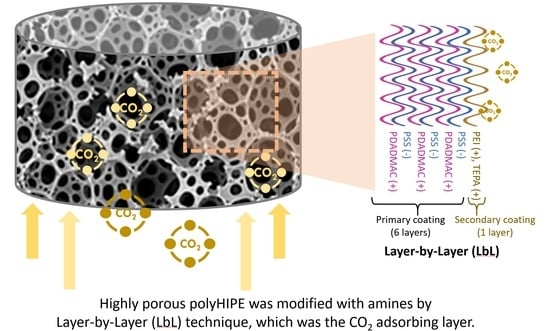
Layer-by-Layer (LbL) Surface Augmented Modification of Poly(Styrene/Divinylbenzene)High Internal Phase Emulsion for Carbon Dioxide Capture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
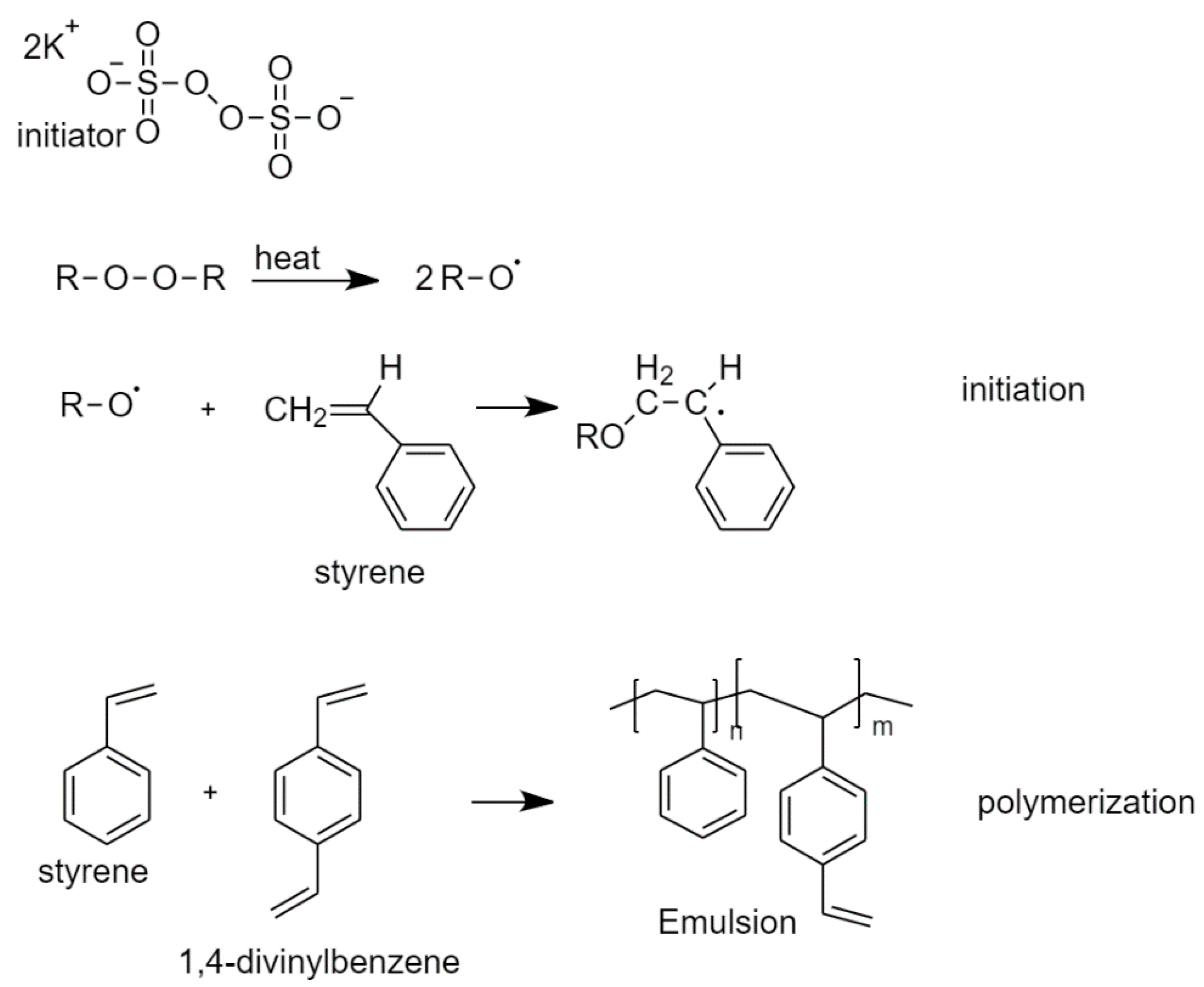
2.2. Poly(Styrene/Divinylbenzene)HIPE Polymerization
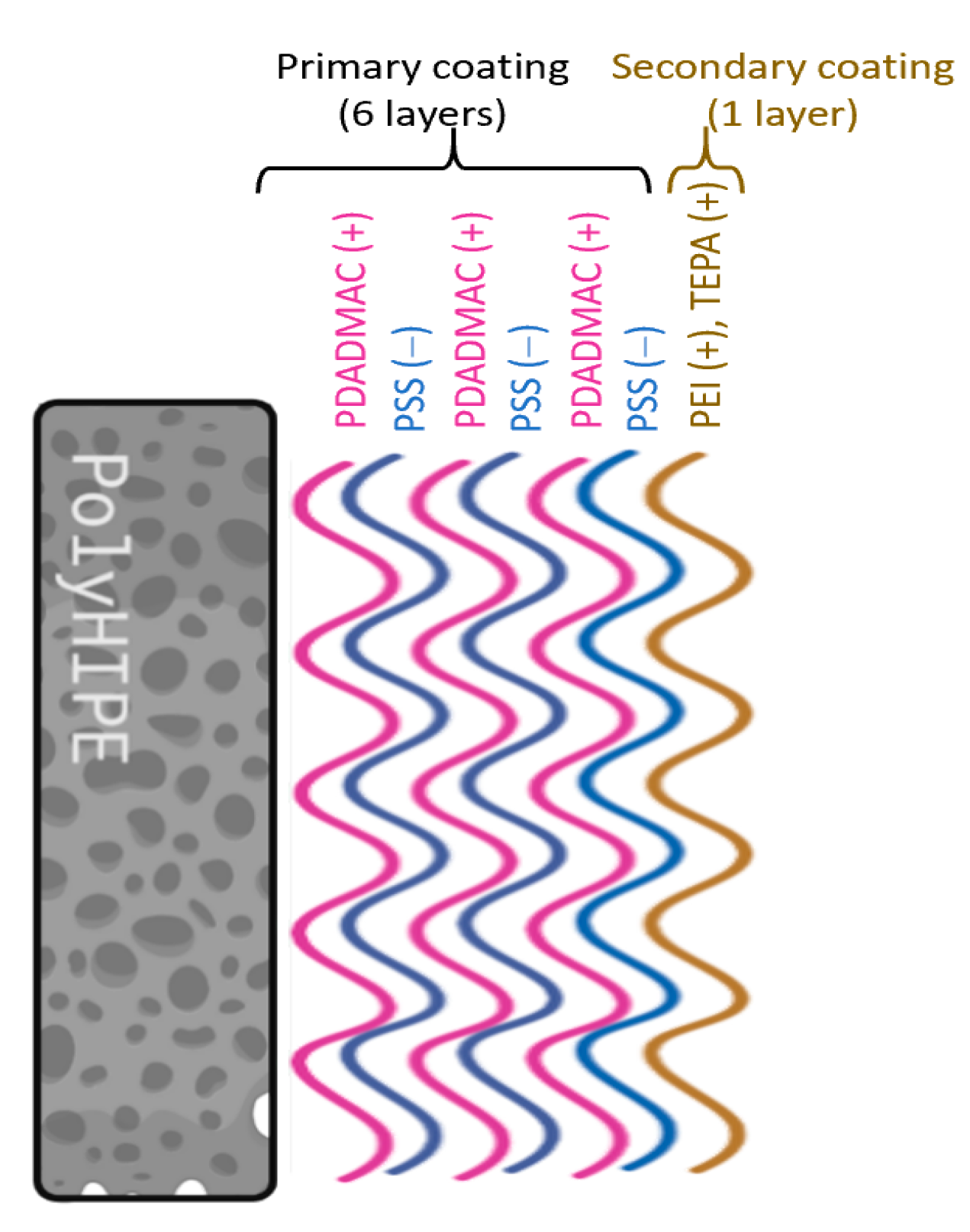
2.3. Surface Modification of Poly(S/DVB)HIPEs
2.4. Poly(S/DVB)HIPEs Characterization
3. Results
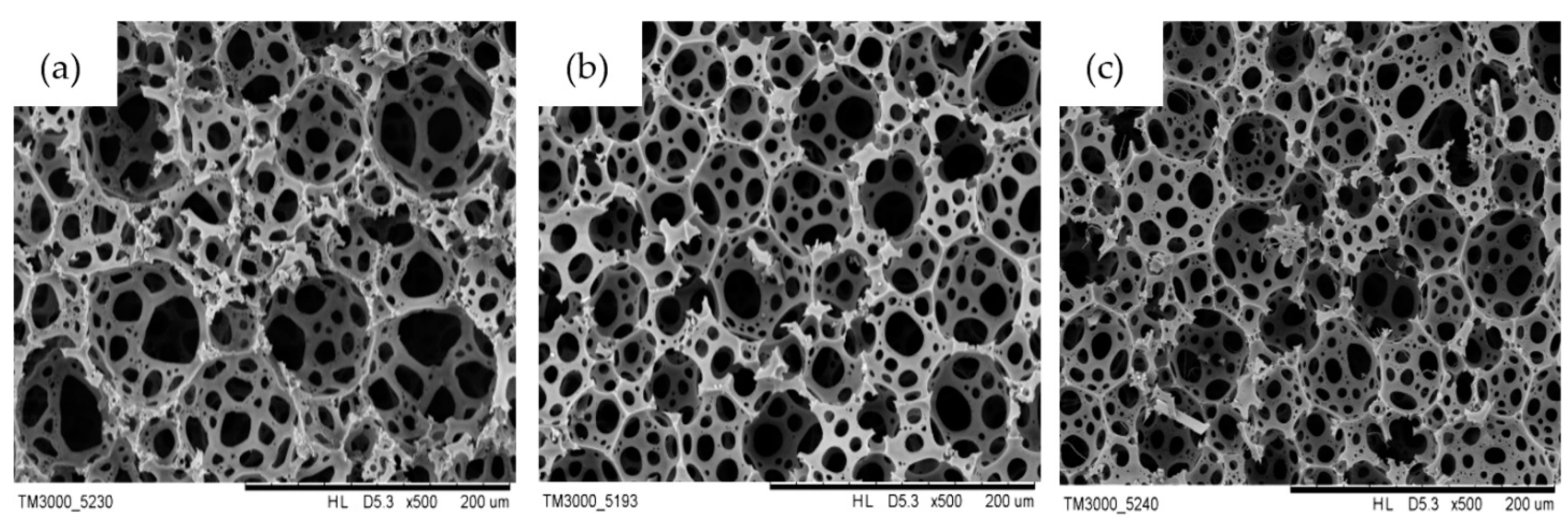
3.1. Characterization of Poly(S/DVB)HIPEs
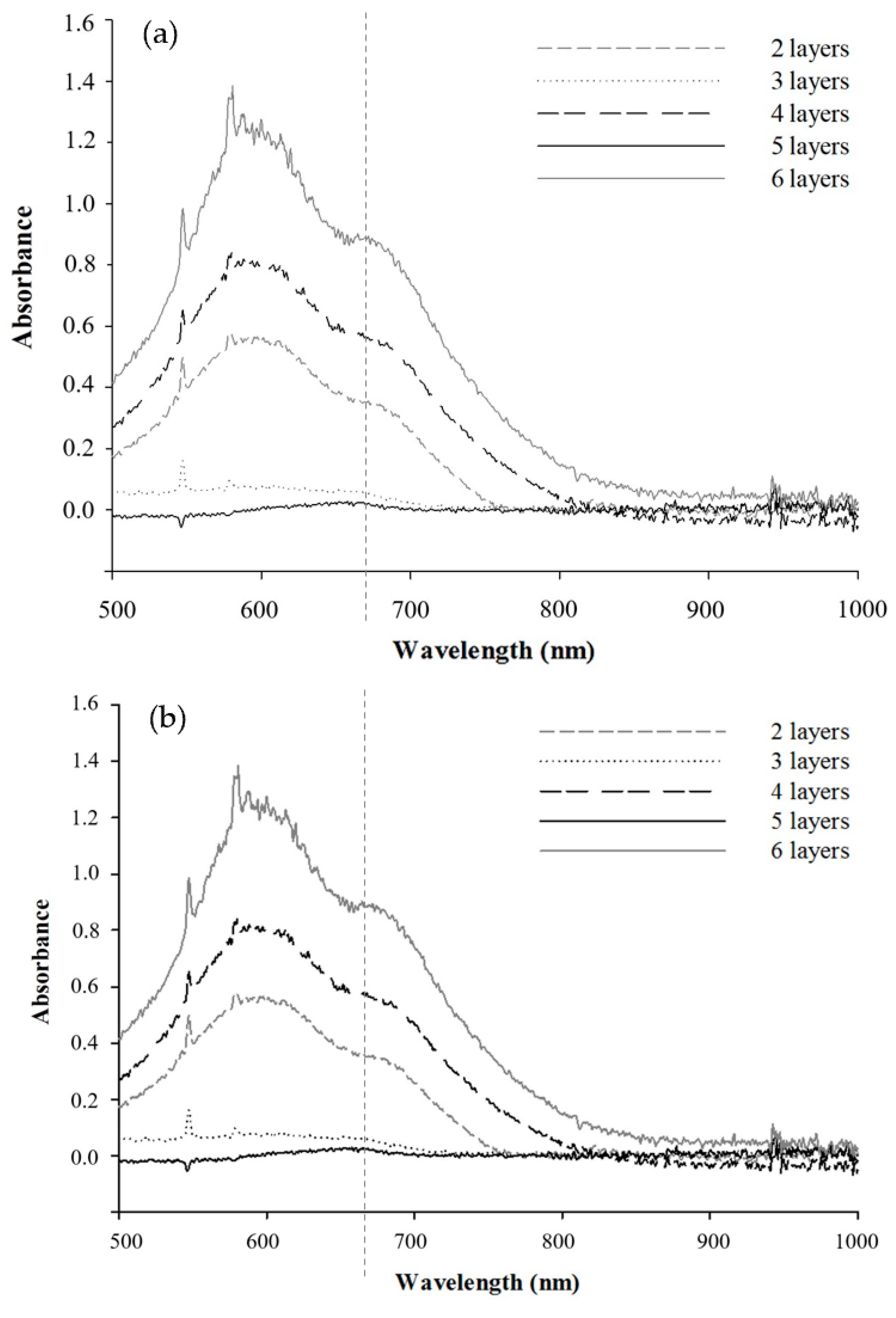
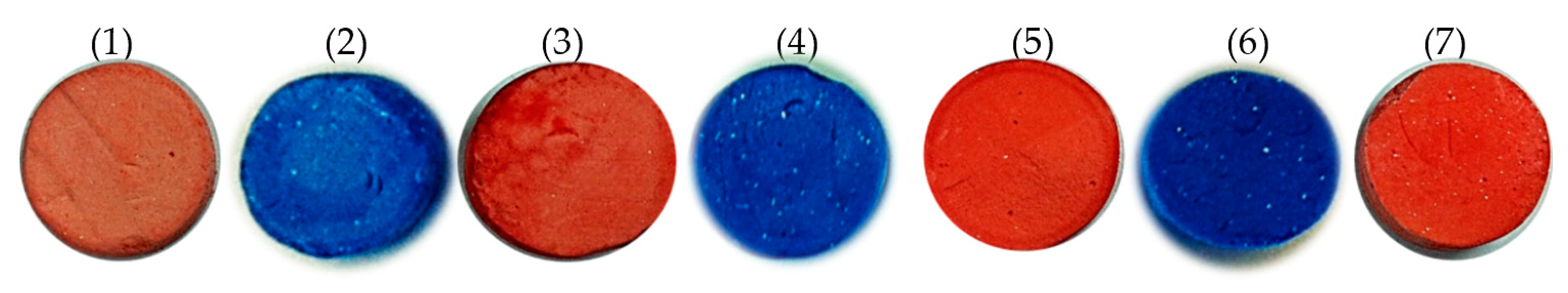
3.2. Surface Modification of Poly(S/DVB)HIPE
3.3. CO2 Adsorption Capacities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Songolzadeh, M.; Soleimani, M.; Takht Ravanchi, M.; Songolzadeh, R. Carbon Dioxide Separation From Flue Gases: A Technological, Review Emphasizing Reduction in Greenhouse Gas Emissions. Sci. World J. 2014, 2014, 828131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medlyn, B.; De Kauwe, M. Carbon dioxide and water use in forests. Nature 2013, 499, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Hamborg, E.S.; Derks, P.W.J.; van Elk, E.P.; Versteeg, G.F. Carbon dioxide removal by alkanolamines in aqueous organic solvents. A method for enhancing the desorption process. Energy Procedia 2011, 4, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.-H.; Huang, C.-H.; Tan, C.-S. A Review of CO 2 Capture by Absorption and Adsorption. Aerosol Air Qual. Res. 2012, 12. [Google Scholar] [CrossRef] [Green Version]
- Norahim, N.; Yaisanga, P.; Faungnawakij, K.; Charinpanitkul, T.; Klaysom, C. Recent Membrane Developments for CO2 Separation and Capture. Chem. Eng. Technol. 2018, 41, 211–223. [Google Scholar] [CrossRef]
- Prasetya, N.; Himma, N.F.; Sutrisna, P.D.; Wenten, I.G.; Ladewig, B.P. A review on emerging organic-containing microporous material membranes for carbon capture and separation. Chem. Eng. J. 2020, 391, 123575. [Google Scholar] [CrossRef]
- Krishnan, A.; Gopinath, K.P.; Vo, D.-V.N.; Malolan, R.; Nagarajan, V.M.; Arun, J. Ionic liquids, deep eutectic solvents and liquid polymers as green solvents in carbon capture technologies: A review. Environ. Chem. Lett. 2020, 18, 2031–2054. [Google Scholar] [CrossRef]
- Islam, M.; Yusoff, R.; Ali, B. Degradation studies of amines and alkanolamines during CO2 absorption and stripping system. Eng. E Trans. 2010, 5, 97–109. [Google Scholar]
- Israel, S.; Gurevitch, I.; Silverstein, M.S. Carbons with a hierarchical porous structure through the pyrolysis of hypercrosslinked emulsion-templated polymers. Polymer 2015, 72, 453–463. [Google Scholar] [CrossRef]
- Laurens, L.M.L.; Lane, M.; Nelson, R.S. Sustainable Seaweed Biotechnology Solutions for Carbon Capture, Composition, and Deconstruction. Trends Biotechnol. 2020, 38, 1232–1244. [Google Scholar] [CrossRef]
- Aslam, Z.; Anait, U.; Abbas, A.; Ihsanullah, I.; Irshad, U.; Mahmood, N. Adsorption of carbon dioxide onto activated carbon prepared from lawn grass. Biomass Convers. Biorefinery 2020. [Google Scholar] [CrossRef]
- Barbieri, G.; Brunetti, A.; Scura, F.; Drioli, E. CO2 Separation by Membrane Technologies: Applications and Potentialities. Chem. Eng. Trans. 2011. [Google Scholar] [CrossRef]
- Pakeyangkoon, P.; Magaraphan, R.; Malakul, P.; Nithitanakul, M. Effect of Soxhlet Extraction and Surfactant System on Morphology and Properties of Poly(DVB)PolyHIPE. Macromol. Symp. 2008, 264, 149–156. [Google Scholar] [CrossRef]
- Cameron, N. High Internal Phase Emulsion Templating as a Route to Well-defined Porous Polymers. Polymer 2005, 46, 1439–1449. [Google Scholar] [CrossRef] [Green Version]
- Silverstein, M.S. Emulsion-templated porous polymers: A retrospective perspective. Polymer 2014, 55, 304–320. [Google Scholar] [CrossRef]
- Yue, M.; Sun, L.-B.; Cao, Y.; Wang, Z.; Wang, Y.; Yu, Q.; Zhu, J. Promoting the CO 2 adsorption in the amine-containing SBA15 by hydroxyl group. Microporous Mesoporous Mater. 2008, 114, 74–81. [Google Scholar] [CrossRef]
- Martín, A.; García, R.; Karaman, D.S.; Rosenholm, J. Polyethyleneimine-functionalized large pore ordered silica materials for poorly water-soluble drug delivery. J. Mater. Sci. 2014, 49, 1437–1447. [Google Scholar] [CrossRef]
- Jiang, B.; Kish, V.; Fauth, D.J.; Gray, M.L.; Pennline, H.W.; Li, B. Performance of amine-multilayered solid sorbents for CO2 removal: Effect of fabrication variables. Int. J. Greenh. Gas Control 2011, 5, 1170–1175. [Google Scholar] [CrossRef]
- Plaza, M.G.; Pevida, C.; Arias, B.; Fermoso, J.; Arenillas, A.; Rubiera, F.; Pis, J.J. Application of thermogravimetric analysis to the evaluation of aminated solid sorbents for CO2 capture. J. Therm. Anal. Calorim. 2008, 92, 601–606. [Google Scholar] [CrossRef]
- Saiwan, C.; Muchan, P.; deMontigny, D.; Tontiwachwutikul, P. New Poly(Vinylbenzylchloride/Divinylbenzene) Adsorbent for Carbon Dioxide Adsorption. II. Effect of Amine Functionalization. Energy Procedia 2014, 63, 2317–2322. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Cao, S.; Wu, Y.; Liang, F.; Sun, Y.; Lin, Z.; Sun, L. Simultaneously efficient light absorption and charge transport of phosphate and oxygen-vacancy confined in bismuth tungstate atomic layers triggering robust solar CO2 reduction. Nano Energy 2017, 32, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Wright, A.J.; Main, M.J.; Cooper, N.J.; Blight, B.A.; Holder, S.J. Poly High Internal Phase Emulsion for the Immobilization of Chemical Warfare Agents. ACS Appl. Mater. Interfaces 2017, 9, 31335–31339. [Google Scholar] [CrossRef] [PubMed]
- Barbetta, A.; Cameron, N.R. Morphology and Surface Area of Emulsion-Derived (PolyHIPE) Solid Foams Prepared with Oil-Phase Soluble Porogenic Solvents: Three-Component Surfactant System. Macromolecules 2004, 37, 3202–3213. [Google Scholar] [CrossRef]
- Erbay, E.; Okay, O. Pore memory of macroporous styrene-divinylbenzene copolymers. J. Appl. Polym. Sci. 1999, 71, 1055–1062. [Google Scholar] [CrossRef]
- Jin, J.M.; Yang, S.H.; Han, S.T.; Choe, S.J. Highly crosslinked poly(acrylamide-co-divinylbenzene) microspheres by precipitation polymerization. J. Ind. Eng. Chem. 2006, 12, 268–274. [Google Scholar]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [Green Version]
- Lei, L.; Zhang, Q.; Shi, S.; Zhu, S. Highly Porous Poly(high internal phase emulsion) Membranes with “Open-Cell” Structure and CO2-Switchable Wettability Used for Controlled Oil/Water Separation. Langmuir 2017, 33. [Google Scholar] [CrossRef]
- Volzone, C. Retention of pollutant gases: Comparison between clay minerals and their modified products. Appl. Clay Sci. 2007, 36, 191–196. [Google Scholar] [CrossRef]



| Poly(S/DVB) HIPE | Surface Area (m2/g) | Compressive Strength (MPa) | Young’s Modulus (MPa) | Td (°C) | Residue Yield (%) |
|---|---|---|---|---|---|
| 80:20 | 22.39 ± 10.30 | 0.13 ± 0.02 | 1.79 ± 0.44 | 373.79 | 8.18 |
| 20:80 | 189.40 ± 16.14 | 0.29 ± 0.09 | 3.59 ± 1.12 | 432.80 | 24.81 |
| 0:100 | 363.06 ± 149.77 | 0.30 ± 0.03 | 5.41 ± 1.29 | 440.98 | 32.51 |
| (S/DVB) Ratio | Mass Fraction of Nitrogen (%) | Adsorption Capacity (mmol/g) | |||
|---|---|---|---|---|---|
| Modified polyHIPE with PEI | Modified polyHIPE with TEPA | Unmodified Surface | Modified polyHIPE with PEI | Modified polyHIPE with TEPA | |
| 0:100 | 0.81 | 1.54 | 0.71 ± 0.19 | 1.01 ± 0.27 | 0.72 ± 0.11 |
| 20:80 | 0.59 | 0.52 | 0.64 ± 0.12 | 0.82 ± 0.62 | 0.72 ± 0.13 |
| 80:20 | 0.52 | 0.56 | 0.63 ± 0.15 | 0.68 ± 0.16 | 0.64 ± 0.04 |
| Materials | CO2 Adsorption Capacity (mmol/g) | Ref. |
|---|---|---|
| polyHIPE | 0.71 ± 0.19 | This paper |
| Modified polyHIPE with PEI | 1.01 ± 0.27 | This paper |
| Modified polyHIPE with TEPA | 0.72 ± 0.11 | This paper |
| Natural bentonite clay | 0.15 mmol/g | [28] |
| Acid- natural bentonite clay | 0.38 mmol/g | [28] |
| Activated carbon prepared from lawn grass | 0.12 mmol/g | [11] |
| Ionic liquids | 4.72 mol of CO2/mol of solvent | [7] |
| Deep eutectic solvents | 4.29 g CO2 per g of solvent | [7] |
| Liquid polymers | 1.36 mol of CO2/mol of solvent | [7] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azman, M.I.; Chungprempree, J.; Preechawong, J.; Sapsrithong, P.; Nithitanakul, M. Layer-by-Layer (LbL) Surface Augmented Modification of Poly(Styrene/Divinylbenzene)High Internal Phase Emulsion for Carbon Dioxide Capture. Polymers 2021, 13, 2247. https://doi.org/10.3390/polym13142247
Azman MI, Chungprempree J, Preechawong J, Sapsrithong P, Nithitanakul M. Layer-by-Layer (LbL) Surface Augmented Modification of Poly(Styrene/Divinylbenzene)High Internal Phase Emulsion for Carbon Dioxide Capture. Polymers. 2021; 13(14):2247. https://doi.org/10.3390/polym13142247
Chicago/Turabian StyleAzman, Muhammad Imran, Jirasuta Chungprempree, Jitima Preechawong, Pornsri Sapsrithong, and Manit Nithitanakul. 2021. "Layer-by-Layer (LbL) Surface Augmented Modification of Poly(Styrene/Divinylbenzene)High Internal Phase Emulsion for Carbon Dioxide Capture" Polymers 13, no. 14: 2247. https://doi.org/10.3390/polym13142247
APA StyleAzman, M. I., Chungprempree, J., Preechawong, J., Sapsrithong, P., & Nithitanakul, M. (2021). Layer-by-Layer (LbL) Surface Augmented Modification of Poly(Styrene/Divinylbenzene)High Internal Phase Emulsion for Carbon Dioxide Capture. Polymers, 13(14), 2247. https://doi.org/10.3390/polym13142247