Applied Rheology as Tool for the Assessment of Chitosan Hydrogels for Regenerative Medicine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrogels Production
2.3. Hydrogels Characterization
2.3.1. Rheological Characterization
2.3.2. Microstructural Characterization
2.4. Statistical Analyses
3. Results and Discussion
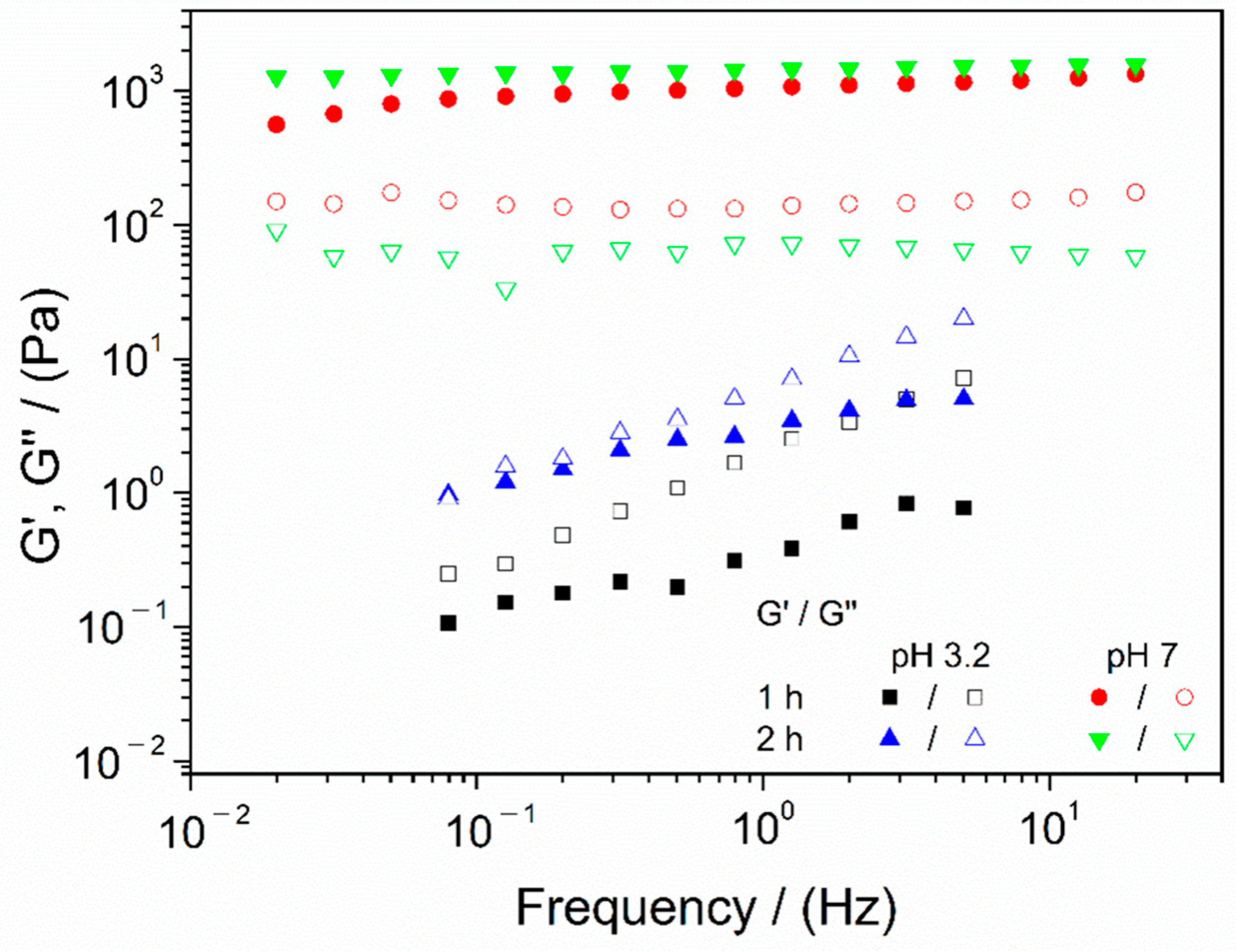
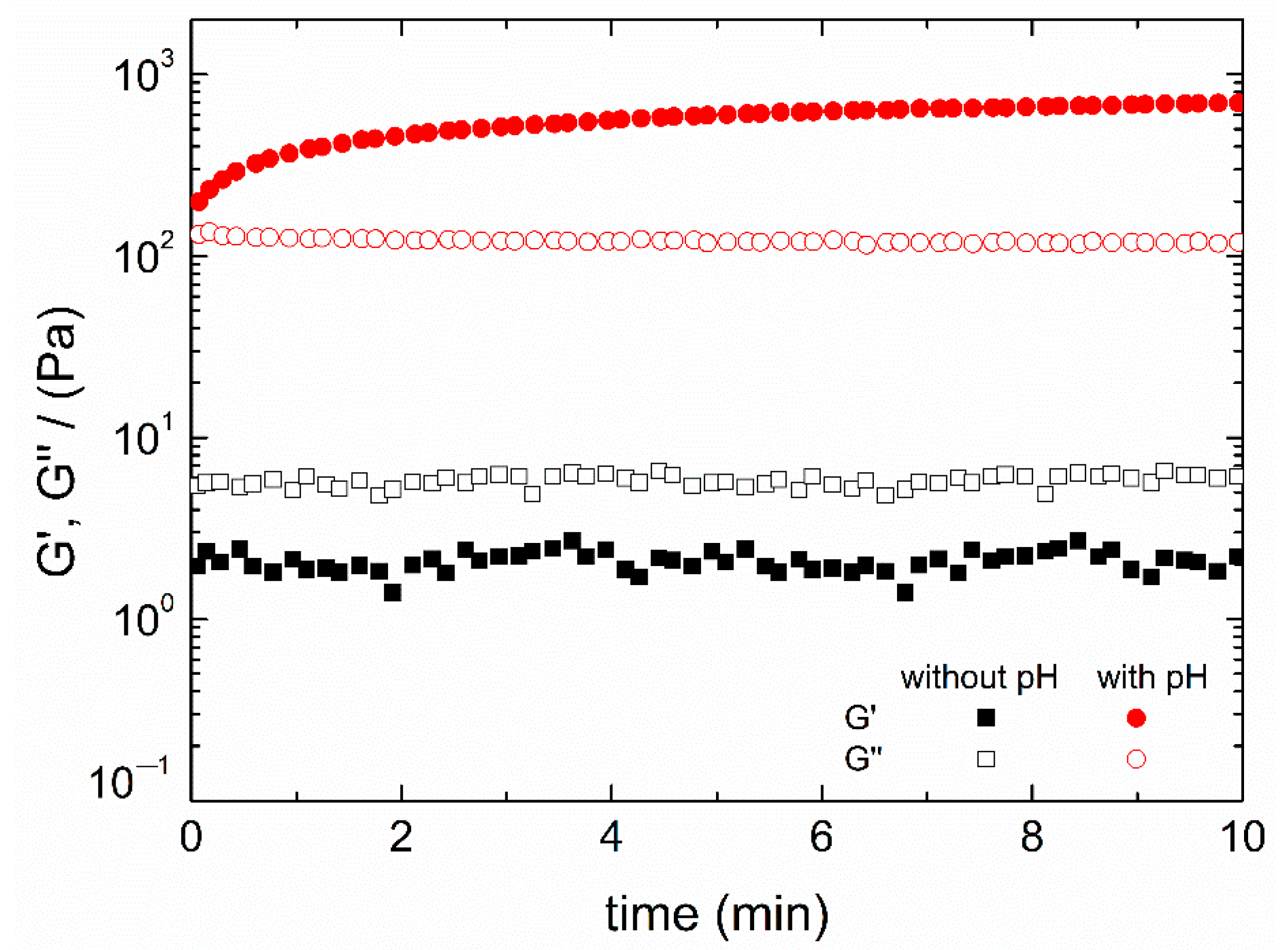
3.1. Study of the pH Change and Agitation Time
3.2. Study of the Gelation Temperature
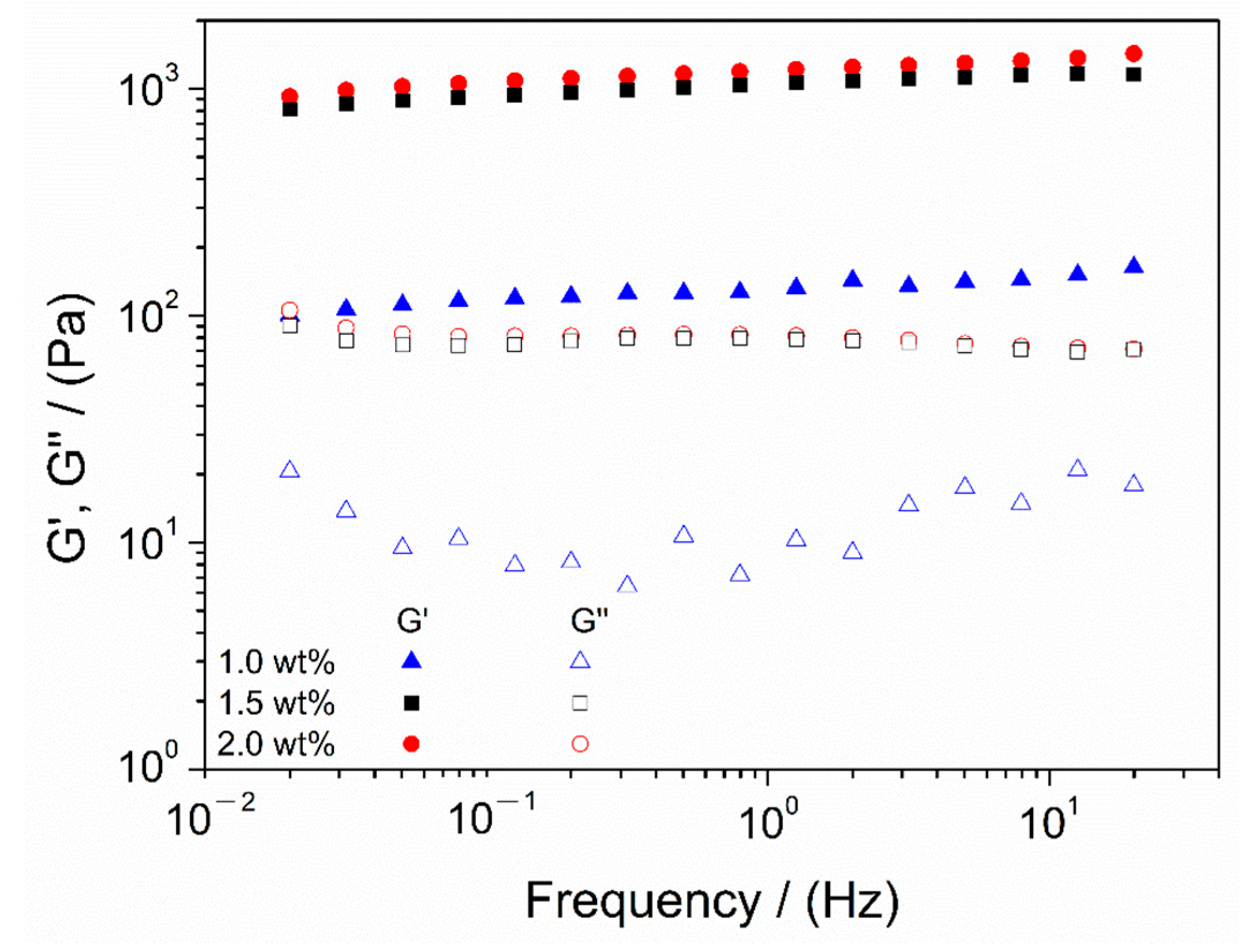
3.3. Study of Chitosan Concentration
3.4. Microstructure Evaluation
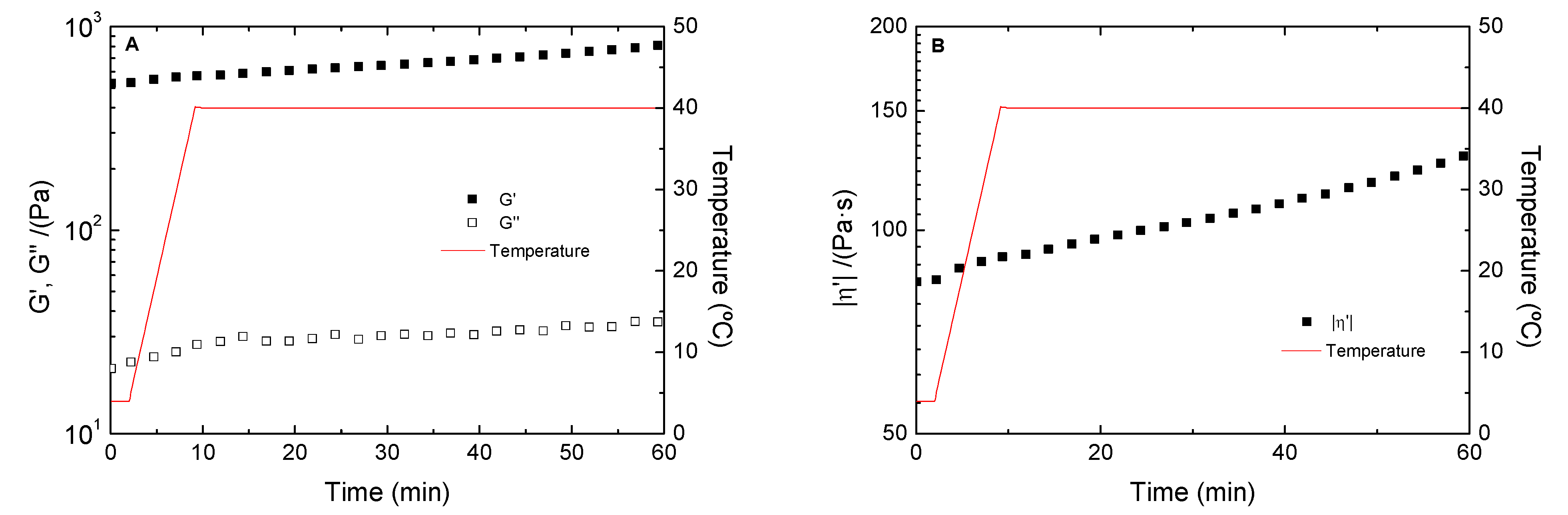
3.5. Temperature Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schultheiss, D.; Bloom, D.A.; Wefer, J.; Jonas, U. Tissue engineering from Adam to the zygote: Historical reflections. World J. Urol. 2000, 18, 84–90. [Google Scholar] [CrossRef][Green Version]
- Liu, C.; Xia, Z.; Czernuszka, J.T. Design and development of three-dimensional scaffolds for tissue engineering. Chem. Eng. Res. Des. 2007, 85, 1051–1064. [Google Scholar] [CrossRef]
- Deo, K.A.; Lokhande, G.; Gaharwar, A.K. Nanostructured Hydrogels for Tissue Engineering and Regenerative Medicine; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volumes 1–3, ISBN 9780128136997. [Google Scholar]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Y.; Jiang, Z.; Sakai, E.; Qiu, J.; Zhu, P. Highly temperature resistant cellulose nanofiber/polyvinyl alcohol hydrogel using aldehyde cellulose nanofiber as cross-linker. Cellulose 2019, 26, 5291–5303. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 1–19. [Google Scholar] [CrossRef]
- Celikkin, N.; Rinoldi, C.; Costantini, M.; Trombetta, M.; Rainer, A.; Święszkowski, W. Naturally derived proteins and glycosaminoglycan scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2017, 78, 1277–1299. [Google Scholar] [CrossRef]
- Tariverdian, T.; Sefat, F.; Gelinsky, M.; Mozafari, M. 10—Scaffold for bone tissue engineering. In Woodhead Publishing Series in Biomaterials; Mozafari, M., Sefat, F., Atala, A., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 189–209. ISBN 978-0-08-102563-5. [Google Scholar]
- Kim, M.; Tang, S.; Olsen, B.D. Physics of engineered protein hydrogels. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 587–601. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-Based Hydrogels as Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.G.; Bharathi, P.; Akila, R. A comprehensive review on biopolymers. Sci. Rev. Chem. Commun. 2014, 4, 61–68. [Google Scholar]
- Rebelo, R.; Fernandes, M.; Fangueiro, R. Biopolymers in Medical Implants: A Brief Review. Procedia Eng. 2017, 200, 236–243. [Google Scholar] [CrossRef]
- Hannibal-Paci, C. Information to Users Umi. Ph.D. Thesis, University of Pennsylvania, Philadelphia, PA, USA, 1995; p. 274. [Google Scholar]
- Khan, F.; Ahmad, S.R. Polysaccharides and Their Derivatives for Versatile Tissue Engineering Application. Macromol. Biosci. 2013, 13, 395–421. [Google Scholar] [CrossRef]
- Chakraborty, M.; Ghosh, A.; Ghosh, U.U.; DasGupta, S. Enhanced cooling by an oscillating droplet on DMF platform. In Proceedings of the 2015 AIChE Annual Meeting, Salt Lake City, UT, USA, 8–13 November 2015; Volume 1, pp. 162–169. [Google Scholar]
- Rodríguez-Pedroso, A.T.; Ramírez-Arrebato, M.A.; Rivero-González, D.; Bosquez-Molina, E.; Barrera-Necha, L.L.; Bautista-Baños, S. Propiedades Químico-Estructurales Y Actividad Biológica De La Quitosana. Rev. Chapingo Ser. Hortic. 2009, 15, 307–317. [Google Scholar]
- Hu, J.; Andablo-Reyes, E.; Soltanahmadi, S.; Sarkar, A. Synergistic Microgel-Reinforced Hydrogels as High-Performance Lubricants. ACS Macro Lett. 2020, 9, 1726–1731. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Zou, Y.; Wu, S.; Zou, X. Self-healing and tough hydrogels with conductive properties prepared through an interpenetrating polymer network strategy. Polymer 2020, 206, 122907. [Google Scholar] [CrossRef]
- Tsirigotis-Maniecka, M.; Szyk-Warszyńska, L.; Maniecki, Ł.; Szczęsna, W.; Warszyński, P.; Wilk, K.A. Tailoring the composition of hydrogel particles for the controlled delivery of phytopharmaceuticals. Eur. Polym. J. 2021, 151. [Google Scholar] [CrossRef]
- Rivero Berti, I.; Islan, G.A.; Castro, G.R. Enzymes and biopolymers. The opportunity for the smart design of molecular delivery systems. Bioresour. Technol. 2021, 322, 124546. [Google Scholar] [CrossRef]
- Daud, H.; Ghani, A.; Iqbal, D.N.; Ahmad, N.; Nazir, S.; Muhammad, M.J.; Hussain, E.A.; Nazir, A.; Iqbal, M. Preparation and characterization of guar gum based biopolymeric hydrogels for controlled release of antihypertensive drug. Arab. J. Chem. 2021, 14, 103111. [Google Scholar] [CrossRef]
- Crompton, K.E.; Prankerd, R.J.; Paganin, D.M.; Scott, T.F.; Horne, M.K.; Finkelstein, D.I.; Gross, K.A.; Forsythe, J.S. Morphology and gelation of thermosensitive chitosan hydrogels. Biophys. Chem. 2005. [Google Scholar] [CrossRef]
- Fu, J.; Yang, F.; Guo, Z. The chitosan hydrogels: From structure to function. New J. Chem. 2018, 42, 17162–17180. [Google Scholar] [CrossRef]
- Li, C.-P.; Weng, M.-C.; Huang, S.-L. Preparation and Characterization of pH Sensitive Chitosan/3-Glycidyloxypropyl Trimethoxysilane (GPTMS) Hydrogels by Sol-Gel Method. Polymers 2020, 12, 1326. [Google Scholar] [CrossRef]
- Lavanya, K.; Chandran, S.V.; Balagangadharan, K.; Selvamurugan, N. Temperature- and pH-responsive chitosan-based injectable hydrogels for bone tissue engineering. Mater. Sci. Eng. C 2020, 111, 110862. [Google Scholar] [CrossRef]
- Ahmad, A.M. Potential pharmacokinetic interactions between antiretrovirals and medicinal plants used as complementary and African traditional medicines. Biopharm. Drug Dispos. 2007, 28, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Calero, N.; Muñoz, J.; Ramírez, P.; Guerrero, A. Flow behaviour, linear viscoelasticity and surface properties of chitosan aqueous solutions. Food Hydrocoll. 2010, 24, 659–666. [Google Scholar] [CrossRef]
- Milosavljević, N.B.; Ristić, M.Đ.; Perić-Grujić, A.A.; Filipović, J.M.; Štrbac, S.B.; Rakočević, Z.L.; Krušić, M.T.K. Removal of Cu2+ ions using hydrogels of chitosan, itaconic and methacrylic acid: FTIR, SEM/EDX, AFM, kinetic and equilibrium study. Colloids Surf. A Physicochem. Eng. Asp. 2011, 388, 59–69. [Google Scholar] [CrossRef]
- Huang, B.; Liu, M.; Zhou, C. Chitosan composite hydrogels reinforced with natural clay nanotubes. Carbohydr. Polym. 2017, 175, 689–698. [Google Scholar] [CrossRef] [PubMed]



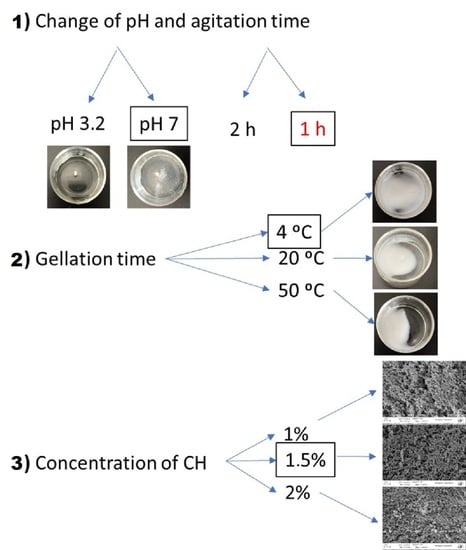
| System | Chitosan Concentration (wt.%) | Agitation Time (h) | pH Change | Final Temperature (°C) |
|---|---|---|---|---|
| 1 | 1.5 | 1 | Yes | 50 |
| 2 | 1.5 | 1 | No | 50 |
| 3 | 1.5 | 2 | Yes | 50 |
| 4 | 1.5 | 2 | No | 50 |
| 5 | 1.5 | 1 | Yes | 20 |
| 6 | 1.5 | 1 | Yes | 4 |
| 7 | 1.0 | 1 | Yes | 4 |
| 8 | 2.0 | 1 | Yes | 4 |
| Systems | Critical Strain (%) | G′1 (Pa) | tan(δ)1 (-) | η*1 (Pa·s) | |
|---|---|---|---|---|---|
| Agitation Time | pH Change | ||||
| 1 h | No | 1.01 ± 0.05 I | 3.47 ± 0.42 a | 2.04 ± 0.03 A | 0.99 ± 0.26 α |
| Yes | 0.48 ± 0.08 II | 1117 ± 12 b | 0.11 ± 0.01 B | 184 ± 29 β | |
| 2 h | No | 0.42 ± 0.08 II | 0.38 ± 0.07 c | 2.93 ± 0.05 C | 0.57 ± 0.17 α |
| Yes | 0.48 ± 0.11 II | 1456 ± 22 d | 0.04 ± 0.01 D | 191 ± 43 β | |
| Gelation Temperature | Critical Strain (%) | G′1 (Pa) | tan(δ)1 (-) | η*1 (Pa·s) |
|---|---|---|---|---|
| 4 °C | 1.01 ± 0.11 I | 1122 ± 15 a | 0.05 ± 0.02 A | 142 ± 17 α |
| 20 °C | 1.01 ± 0.05 I | 720 ± 12 b | 0.06 ± 0.03 A | 91 ± 16 β |
| 50 °C | 0.48 ± 0.08 II | 1117 ± 12 a | 0.11 ± 0.01 B | 184 ± 29 γ |
| Biopolymer Concentration | Critical Strain (%) | G′1 (Pa) | tan(δ)1 (-) | η*1 (Pa·s) |
|---|---|---|---|---|
| 1.0 wt.% | 0.48 ± 0.08 I | 130 ± 12 a | 0.08 ± 0.01 A | 21 ± 6 α |
| 1.5 wt.% | 1.01 ± 0.11 II | 1122 ± 15 b | 0.05 ± 0.02 A | 142 ± 17 β |
| 2.0 wt.% | 1.01 ± 0.11 II | 1049 ± 97 b | 0.06 ± 0.04 A | 201 ± 21 γ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Cid, P.; Jiménez-Rosado, M.; Alonso-González, M.; Romero, A.; Perez-Puyana, V. Applied Rheology as Tool for the Assessment of Chitosan Hydrogels for Regenerative Medicine. Polymers 2021, 13, 2189. https://doi.org/10.3390/polym13132189
Sánchez-Cid P, Jiménez-Rosado M, Alonso-González M, Romero A, Perez-Puyana V. Applied Rheology as Tool for the Assessment of Chitosan Hydrogels for Regenerative Medicine. Polymers. 2021; 13(13):2189. https://doi.org/10.3390/polym13132189
Chicago/Turabian StyleSánchez-Cid, Pablo, Mercedes Jiménez-Rosado, María Alonso-González, Alberto Romero, and Victor Perez-Puyana. 2021. "Applied Rheology as Tool for the Assessment of Chitosan Hydrogels for Regenerative Medicine" Polymers 13, no. 13: 2189. https://doi.org/10.3390/polym13132189
APA StyleSánchez-Cid, P., Jiménez-Rosado, M., Alonso-González, M., Romero, A., & Perez-Puyana, V. (2021). Applied Rheology as Tool for the Assessment of Chitosan Hydrogels for Regenerative Medicine. Polymers, 13(13), 2189. https://doi.org/10.3390/polym13132189