Synergistic Effect of Bioactive Inorganic Fillers in Enhancing Properties of Dentin Adhesives—A Review
Abstract
:1. Introduction
2. Methodology
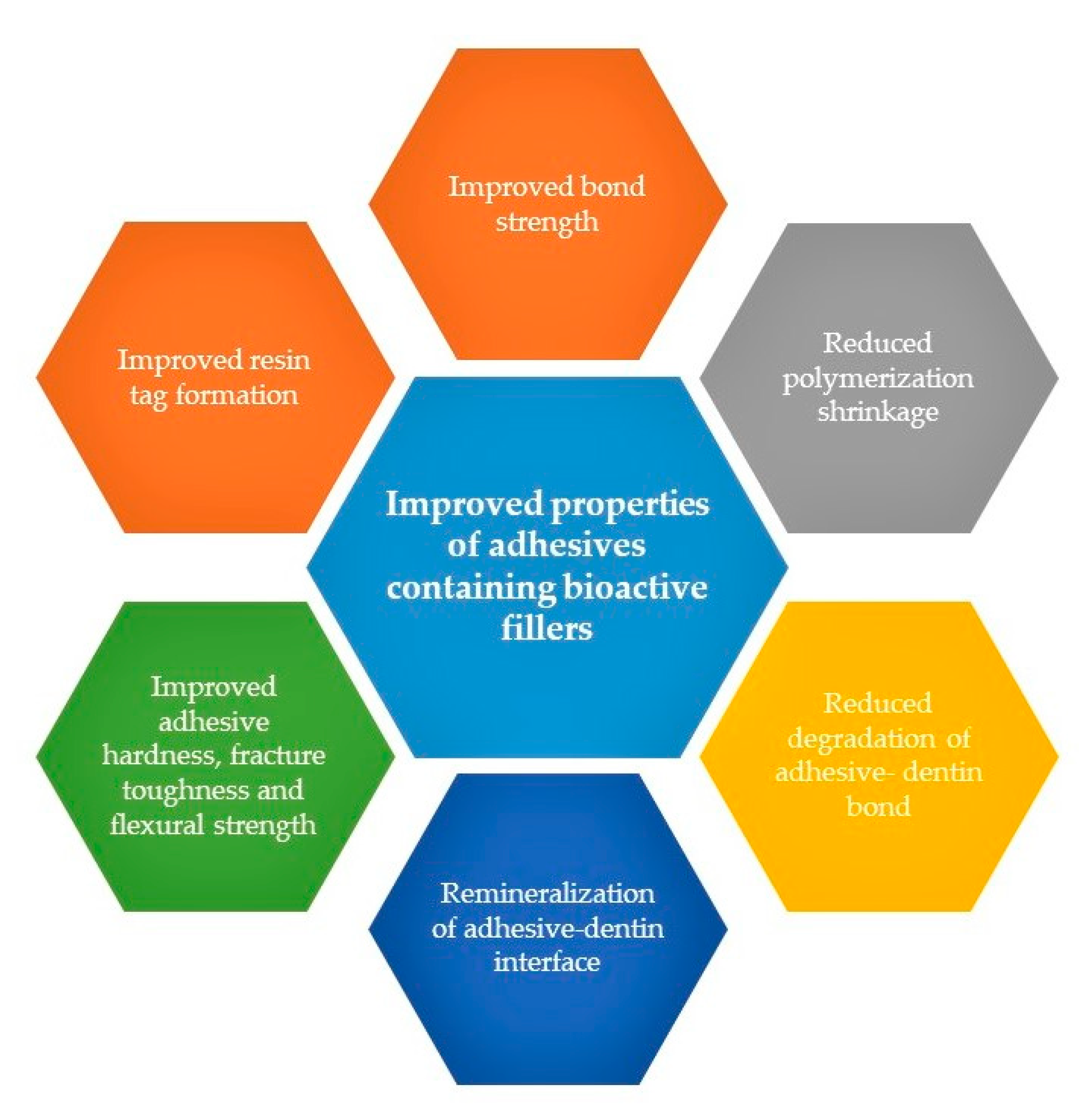
3. Bioactive Inorganic Fillers
3.1. Bioactive Glass (BG) Fillers
3.1.1. Overview
3.1.2. Improvement of DA’s Properties
3.1.3. Mechanism of Improvement of DA’s Properties
3.2. HA Fillers
3.2.1. Overview
3.2.2. Improvement of DA’s Properties
3.2.3. Mechanism of Improvement of DA’s Properties
3.3. Amorphous Calcium Phosphate (ACP) Fillers
3.3.1. Overview
3.3.2. Improvement of DA’s Properties
3.3.3. Mechanism of Improvement of DA’s Properties
3.4. Graphene Oxide (GO) Fillers
3.4.1. Overview
3.4.2. Improvement of DA’s Properties
3.4.3. Mechanism of Improvement of DA’s Properties
3.5. Calcium Fluoride (CaF2) Fillers
3.5.1. Overview
3.5.2. Improvement of DA’s Properties
3.5.3. Mechanism of Improvement of DA’s Properties
3.6. Zn Chloride (ZnCl2) Fillers
3.6.1. Overview
3.6.2. Improvement of DA’s Properties
3.6.3. Mechanism of Improvement of DA’s Properties
3.7. Silica Fillers
3.7.1. Overview
3.7.2. Improvement of DA’s Properties
3.7.3. Mechanism of Improvement of DA’s Properties
3.8. Niobium Pentoxide (Nb2O5) Fillers
3.8.1. Overview
3.8.2. Improvement of DA’s Properties
3.8.3. Mechanism of Improvement of DA’s Properties
3.9. Other Bioactive Inorganic Fillers
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pfeifer, C.S. Polymer-Based Direct Filling Materials. Dent. Clin. N. Am. 2017, 61, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.A.; Hong, J.H.; Hatton, B.D.; Finer, Y. Responsive antimicrobial dental adhesive based on drug-silica co-assembled particles. Acta Biomater. 2018, 76, 283–294. [Google Scholar] [CrossRef]
- Gajewski, V.E.; Pfeifer, C.S.; Froes-Salgado, N.R.; Boaro, L.C.; Braga, R.R. Monomers used in resin composites: Degree of conversion, mechanical properties and water sorption/solubility. Braz. Dent. J. 2012, 23, 508–514. [Google Scholar] [CrossRef]
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Resin based restorative dental materials: Characteristics and future perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Bourbia, M.; Finer, Y. Biochemical Stability and Interactions of Dental Resin Composites and Adhesives with Host and Bacteria in the Oral Cavity: A Review. J. Can. Dent. Assoc. 2018, 84, 1488–2159. [Google Scholar]
- Ferracane, J.L. Models of Caries Formation around Dental Composite Restorations. J. Dent. Res. 2017, 96, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Manuja, N.; Nagpal, R.; Pandit, I.K. Dental adhesion: Mechanism, techniques and durability. J. Clin. Pediatr. Dent. 2012, 36, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Sofan, E.; Sofan, A.; Palaia, G.; Tenore, G.; Romeo, U.; Migliau, G. Classification review of dental adhesive systems: From the IV generation to the universal type. Ann. Stomatol. 2017, 8, 1–17. [Google Scholar]
- Spencer, P.; Jonggu Park, Q.Y.; Misra, A.; Bohaty, B.S.; Singh, V.; Parthasarathy, R.; Sene, F.; de Paiva Goncalves, S.E.; Laurence, J. Durable bonds at the adhesive/dentin interface: An impossible mission or simply a moving target? Braz. Dent. Sci. 2012, 15, 4–18. [Google Scholar] [CrossRef] [Green Version]
- Chi, M.; Qi, M.; A, L.; Wang, P.; Weir, M.D.; Melo, M.A.; Sun, X.; Dong, B.; Li, C.; Wu, J.; et al. Novel Bioactive and Therapeutic Dental Polymeric Materials to Inhibit Periodontal Pathogens and Biofilms. Int. J. Mol. Sci. 2019, 20, 278. [Google Scholar] [CrossRef] [Green Version]
- Bin-Shuwaish, M.S.M.; Maawadh, A.M.; Al-Hamdan, R.S.; Alresayes, S.; Ali, T.; Almutairi, B.; Vohra, F.; Abduljabbar, T. Influence of graphene oxide filler content on the dentin bond integrity, degree of conversion and bond strength of experimental adhesive. A SEM, micro-Raman, FTIR and microtensile study. Mater. Res. Express. 2020, 7, 115403. [Google Scholar] [CrossRef]
- Al-Harbi, N.; Mohammed, H.; Al-Hadeethi, Y.; Bakry, A.S.; Umar, A.; Hussein, M.A.; Abbassy, M.A.; Vaidya, K.G.; Berakdar, G.A.; Mkawi, E.M.; et al. Silica-Based Bioactive Glasses and Their Applications in Hard Tissue Regeneration: A Review. Pharmaceuticals 2021, 14, 75. [Google Scholar] [CrossRef]
- Sawant, K.; Pawar, A.M. Bioactive glass in dentistry: A systematic review. Saudi J. Oral Sci. 2020, 7, 3–10. [Google Scholar]
- Fernando, D.; Attik, N.; Pradelle-Plasse, N.; Jackson, P.; Grosgogeat, B.; Colon, P. Bioactive glass for dentin remineralization: A systematic review. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 1369–1377. [Google Scholar] [CrossRef]
- Kim, H.J.; Bae, H.E.; Lee, J.E.; Park, I.S.; Kim, H.J.; Kwon, J.; Kim, D.S. Effects of bioactive glass incorporation into glass ionomer cement on demineralized dentin. Sci. Rep. 2021, 11, 7016. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Lee, M.G.; Ferracane, J.L.; Davis, H.; Bae, H.E.; Choi, D.; Kim, D.S. Effect of bioactive glass-containing resin composite on dentin remineralization. J. Dent. 2018, 75, 58–64. [Google Scholar] [CrossRef]
- Profeta, A.C.; Mannocci, F.; Foxton, R.M.; Thompson, I.; Watson, T.F.; Sauro, S. Bioactive effects of a calcium/sodium phosphosilicate on the resin-dentine interface: A microtensile bond strength, scanning electron microscopy, and confocal microscopy study. Eur. J. Oral Sci. 2012, 120, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Profeta, A.C.; Mannocci, F.; Foxton, R.; Watson, T.F.; Feitosa, V.P.; De Carlo, B.; Mongiorgi, R.; Valdre, G.; Sauro, S. Experimental etch-and-rinse adhesives doped with bioactive calcium silicate-based micro-fillers to generate therapeutic resin-dentin interfaces. Dent. Mater. 2013, 29, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Sauro, S.; Osorio, R.; Watson, T.F.; Toledano, M. Therapeutic effects of novel resin bonding systems containing bioactive glasses on mineral-depleted areas within the bonded-dentine interface. J. Mater. Sci. Mater. Med. 2012, 23, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Kim, S.H.; Choi, S.Y.; Kim, K.M. Acid Neutralizing Ability and Shear Bond Strength Using Orthodontic Adhesives Containing Three Different Types of Bioactive Glass. Materials 2016, 9, 125. [Google Scholar] [CrossRef] [Green Version]
- de Morais, R.C.; Silveira, R.E.; Chinelatti, M.; Geraldeli, S.; de Carvalho Panzeri Pires-de-Souza, F. Bond strength of adhesive systems to sound and demineralized dentin treated with bioactive glass ceramic suspension. Clin. Oral Investig. 2018, 22, 1923–1931. [Google Scholar] [CrossRef]
- Jun, S.K.; Yang, S.A.; Kim, Y.J.; El-Fiqi, A.; Mandakhbayar, N.; Kim, D.S.; Roh, J.; Sauro, S.; Kim, H.W.; Lee, J.H.; et al. Multi-functional nano-adhesive releasing therapeutic ions for MMP-deactivation and remineralization. Sci. Rep. 2018, 8, 5663. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, K.K.; Araujo, T.P.; Carvalho, E.M.; Meier, M.M.; Tanaka, A.; Carvalho, C.N.; Bauer, J. Bioactivity and properties of an adhesive system functionalized with an experimental niobium-based glass. J. Mech. Behav. Biomed. Mater. 2018, 78, 188–195. [Google Scholar] [CrossRef]
- Tiskaya, M.; Shahid, S.; Gillam, D.; Hill, R. The use of bioactive glass (BAG) in dental composites: A critical review. Dent. Mater. 2021, 37, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Tarle, Z.; Hickel, R.; Ilie, N. Dentin Bond Strength of Experimental Composites Containing Bioactive Glass: Changes During Aging for up to 1 Year. J. Adhes. Dent. 2018, 20, 325–334. [Google Scholar] [PubMed]
- Sauro, S.; Osorio, R.; Osorio, E.; Watson, T.F.; Toledano, M. Novel light-curable materials containing experimental bioactive micro-fillers remineralise mineral-depleted bonded-dentine interfaces. J. Biomater. Sci. Polym. Ed. 2013, 24, 940–956. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Attin, T.; Tarle, Z.; Tauböck, T.T. A New Customized Bioactive Glass Filler to Functionalize Resin Composites: Acid-Neutralizing Capability, Degree of Conversion, and Apatite Precipitation. J. Clin. Med. 2020, 9, 1173. [Google Scholar] [CrossRef] [PubMed]
- Khvostenko, D.; Hilton, T.J.; Ferracane, J.L.; Mitchell, J.C.; Kruzic, J.J. Bioactive glass fillers reduce bacterial penetration into marginal gaps for composite restorations. Dent. Mater. 2016, 32, 73–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pajor, K.; Pajchel, L.; Kolmas, J. Hydroxyapatite and Fluorapatite in Conservative Dentistry and Oral Implantology-A Review. Materials 2019, 12, 2683. [Google Scholar] [CrossRef] [Green Version]
- Moheet, I.A.; Luddin, N.; Rahman, I.A.; Kannan, T.P.; Nik Abd Ghani, N.R.; Masudi, S.M. Modifications of Glass Ionomer Cement Powder by Addition of Recently Fabricated Nano-Fillers and Their Effect on the Properties: A Review. Eur. J. Dent. 2019, 13, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Bordea, I.R.; Candrea, S.; Alexescu, G.T.; Bran, S.; Baciut, M.; Baciut, G.; Lucaciu, O.; Dinu, C.M.; Todea, D.A. Nano-hydroxyapatite use in dentistry: A systematic review. Drug Metab. Rev. 2020, 52, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Giannini, M.; Makishi, P.; Ayres, A.P.; Vermelho, P.M.; Fronza, B.M.; Nikaido, T.; Tagami, J. Self-etch adhesive systems: A literature review. Braz. Dent. J. 2015, 26, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Hamdan, R.S.; Almutairi, B.; Kattan, H.F.; Alsuwailem, N.A.; Farooq, I.; Vohra, F.; Abduljabbar, T. Influence of Hydroxyapatite Nanospheres in Dentin Adhesive on the Dentin Bond Integrity and Degree of Conversion: A Scanning Electron Microscopy (SEM), Raman, Fourier Transform-Infrared (FTIR), and Microtensile Study. Polymers 2020, 12, 2948. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamdan, R.S.; Almuthairi, B.; Kattan, H.F.; Alresayes, S.; Abduljabbar, T.; Vohra, F. Assessment of Hydroxyapatite Nanospheres Incorporated Dentin Adhesive. A SEM/EDX, Micro-Raman, Microtensile and Micro-Indentation Study. Coatings 2020, 10, 1181. [Google Scholar] [CrossRef]
- Kavrik, F.; Kucukyilmaz, E. The effect of different ratios of nano-sized hydroxyapatite fillers on the micro-tensile bond strength of an adhesive resin. Microsc. Res. Tech. 2019, 82, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Belli, R.; Stotzel, C.; Hilpert, A.; Muller, F.A.; Lohbauer, U. Biomimetically- and hydrothermally-grown HAp nanoparticles as reinforcing fillers for dental adhesives. J. Adhes. Dent. 2013, 15, 413–422. [Google Scholar]
- Pepla, E.; Besharat, L.K.; Palaia, G.; Tenore, G.; Migliau, G. Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: A review of literature. Ann. Stomatol. 2014, 5, 108–114. [Google Scholar] [CrossRef]
- Melo, M.A.; Cheng, L.; Zhang, K.; Weir, M.D.; Rodrigues, L.K.; Xu, H.H. Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dent. Mater. 2013, 29, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Liu, S.; Zhou, X.; Hannig, M.; Rupf, S.; Feng, J.; Peng, X.; Cheng, L. Modifying Adhesive Materials to Improve the Longevity of Resinous Restorations. Int. J. Mol. Sci. 2019, 20, 723. [Google Scholar] [CrossRef] [Green Version]
- Hou, A.; Luo, J.; Zhang, M.; Li, J.; Chu, W.; Liang, K.; Yang, J.; Li, J. Two-in-one strategy: A remineralizing and anti-adhesive coating against demineralized enamel. Int. J. Oral Sci. 2020, 12, 1–11. [Google Scholar] [CrossRef]
- Gupta, N.; Mohan Marya, C.; Nagpal, R.; Singh Oberoi, S.; Dhingra, C. A Review of Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP) and Enamel Remineralization. Compend. Contin. Educ. Dent. 2016, 37, 36–39. [Google Scholar]
- Iafisco, M.; Degli Esposti, L.; Ramirez-Rodriguez, G.B.; Carella, F.; Gomez-Morales, J.; Ionescu, A.C.; Brambilla, E.; Tampieri, A.; Delgado-Lopez, J.M. Fluoride-doped amorphous calcium phosphate nanoparticles as a promising biomimetic material for dental remineralization. Sci. Rep. 2018, 8, 17016. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Weir, M.D.; Cheng, L.; Lin, N.J.; Lin-Gibson, S.; Chow, L.C.; Zhou, X.; Xu, H.H. Antibacterial activity and ion release of bonding agent containing amorphous calcium phosphate nanoparticles. Dent. Mater. 2014, 30, 891–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Weir, M.D.; Chow, L.C.; Reynolds, M.A.; Xu, H.H. Rechargeable calcium phosphate orthodontic cement with sustained ion release and re-release. Sci. Rep. 2016, 6, 36476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marovic, D.; Sariri, K.; Demoli, N.; Ristic, M.; Hiller, K.A.; Skrtic, D.; Rosentritt, M.; Schmalz, G.; Tarle, Z. Remineralizing amorphous calcium phosphate based composite resins: The influence of inert fillers on monomer conversion, polymerization shrinkage, and microhardness. Croat. Med. J. 2016, 57, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Marovic, D.; Skenderovic, H.; Gamulin, O.; Klaric, E.; Tarle, Z. Light transmittance and polymerization kinetics of amorphous calcium phosphate composites. Clin. Oral Investig. 2017, 21, 1173–1182. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Sun, W.B.; Zhang, H. Amorphous calcium phosphate and its application in dentistry. Chem. Cent. J. 2011, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Moreau, J.L.; Sun, L.; Chow, L.C.; Xu, H.H. Mechanical and acid neutralizing properties and bacteria inhibition of amorphous calcium phosphate dental nanocomposite. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 98, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Balhaddad, A.A.; Kansara, A.A.; Hidan, D.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Toward dental caries: Exploring nanoparticle-based platforms and calcium phosphate compounds for dental restorative materials. Bioact. Mater. 2019, 4, 43–55. [Google Scholar] [CrossRef]
- Baig, M.S.; Fleming, G.J. Conventional glass-ionomer materials: A review of the developments in glass powder, polyacid liquid and the strategies of reinforcement. J. Dent. 2015, 43, 897–912. [Google Scholar] [CrossRef]
- Ge, Z.; Yang, L.; Xiao, F.; Wu, Y.; Yu, T.; Chen, J.; Lin, J.; Zhang, Y. Graphene Family Nanomaterials: Properties and Potential Applications in Dentistry. Int. J. Biomater. 2018, 2018, 1539678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, N.; Lv, C.; Xu, Z. Wetting of graphene oxide: A molecular dynamics study. Langmuir 2014, 30, 3572–3578. [Google Scholar] [CrossRef]
- Bregnocchi, A.; Zanni, E.; Uccelletti, D.; Marra, F.; Cavallini, D.; De Angelis, F.; De Bellis, G.; Bossu, M.; Ierardo, G.; Polimeni, A.; et al. Graphene-based dental adhesive with anti-biofilm activity. J. Nanobiotechnol. 2017, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- AlFawaz, Y.F.; Almutairi, B.; Kattan, H.F.; Zafar, M.S.; Farooq, I.; Naseem, M.; Vohra, F.; Abduljabbar, T. Dentin Bond Integrity of Hydroxyapatite Containing Resin Adhesive Enhanced with Graphene Oxide Nano-Particles—An SEM, EDX, Micro-Raman, and Microtensile Bond Strength Study. Polymers 2020, 12, 2978. [Google Scholar] [CrossRef]
- Khan, A.A.; Al-Khureif, A.A.; Saadaldin, S.A.; Mohamed, B.A.; Musaibah, A.S.O.; Divakar, D.D.; Eldwakhly, E. Graphene oxide-based experimental silane primers enhance shear bond strength between resin composite and zirconia. Eur. J. Oral Sci. 2019, 127, 570–576. [Google Scholar] [CrossRef]
- Aguiar, T.R.; de Oliveira, M.; Arrais, C.A.; Ambrosano, G.M.; Rueggeberg, F.; Giannini, M. The effect of photopolymerization on the degree of conversion, polymerization kinetic, biaxial flexure strength, and modulus of self-adhesive resin cements. J. Prosthet. Dent. 2015, 113, 128–134. [Google Scholar] [CrossRef]
- Alshahrani, A.; Bin-Shuwaish, M.S.; Al-Hamdan, R.S.; Almohareb, T.; Maawadh, A.M.; Al Deeb, M.; Alhenaki, A.M.; Abduljabbar, T.; Vohra, F. Graphene oxide nano-filler based experimental dentine adhesive. A SEM/EDX, Micro-Raman and microtensile bond strength analysis. J. Appl. Biomater. Funct. Mater. 2020, 18. [Google Scholar] [PubMed]
- Dai, Z.; Liu, M.; Ma, Y.; Cao, L.; Xu, H.H.K.; Zhang, K.; Bai, Y. Effects of Fluoride and Calcium Phosphate Materials on Remineralization of Mild and Severe White Spot Lesions. Biomed Res. Int. 2019, 2019, 1271523. [Google Scholar] [CrossRef] [PubMed]
- Kulshrestha, S.; Khan, S.; Hasan, S.; Khan, M.E.; Misba, L.; Khan, A.U. Calcium fluoride nanoparticles induced suppression of Streptococcus mutans biofilm: An in vitro and in vivo approach. Appl. Microbiol. Biotechnol. 2016, 100, 1901–1914. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Weir, M.D.; Xu, H.H.; Kraigsley, A.M.; Lin, N.J.; Lin-Gibson, S.; Zhou, X. Antibacterial and physical properties of calcium-phosphate and calcium-fluoride nanocomposites with chlorhexidine. Dent. Mater. 2012, 28, 573–583. [Google Scholar] [CrossRef] [Green Version]
- Mitwalli, H.; Balhaddad, A.A.; AlSahafi, R.; Oates, T.W.; Melo, M.A.S.; Xu, H.H.K.; Weir, M.D. Novel CaF2 Nanocomposites with Antibacterial Function and Fluoride and Calcium Ion Release to Inhibit Oral Biofilm and Protect Teeth. J. Funct. Biomater. 2020, 11, 56. [Google Scholar] [CrossRef]
- Fei, X.; Li, Y.; Weir, M.D.; Baras, B.H.; Wang, H.; Wang, S.; Sun, J.; Melo, M.A.S.; Ruan, J.; Xu, H.H.K. Novel pit and fissure sealant containing nano-CaF2 and dimethylaminohexadecyl methacrylate with double benefits of fluoride release and antibacterial function. Dent. Mater. 2020, 36, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Dai, Q.; Weir, M.D.; Melo, M.A.S.; Lynch, C.D.; Oates, T.W.; Zhang, K.; Zhao, Z.; Xu, H.H.K. A nano-CaF2-containing orthodontic cement with antibacterial and remineralization capabilities to combat enamel white spot lesions. J. Dent. 2019, 89, 103172. [Google Scholar] [CrossRef] [PubMed]
- Mahrous, A.; Radwan, M.M.; Kamel, S.M. Micro-Shear Bond Strength of Novel MDP Calcium-Fluoride-Releasing Self-Adhesive Resin Cement After Thermocycling. Int. J. Periodontics Restor. Dent. 2020, 40, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Essam, M.N.; Niazy, M.A.; Farouk, H.; Mostafa, A.A. The Remineralizing Effect of Incorporating Ca-Phosphate and Ca-Fluoride Nanoparticles into the Self-Etch Adhesives Used in Restoring Class I Cavities. Al-Azhar Dent. J. Girls 2019, 6, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Weir, M.D.; Moreau, J.L.; Levine, E.D.; Strassler, H.E.; Chow, L.C.; Xu, H.H. Nanocomposite containing CaF(2) nanoparticles: Thermal cycling, wear and long-term water-aging. Dent. Mater. 2012, 28, 642–652. [Google Scholar] [CrossRef] [Green Version]
- Lukomska-Szymanska, M.; Kleczewska, J.; Nowak, J.; Prylinski, M.; Szczesio, A.; Podlewska, M.; Sokolowski, J.; Lapinska, B. Mechanical Properties of Calcium Fluoride-Based Composite Materials. Biomed Res. Int. 2016, 2016, 2752506. [Google Scholar] [CrossRef] [PubMed]
- Firoozmand, L.M.; Noleto, L.E.; Gomes, I.A.; Bauer, J.R.; Ferreira, M.C. Effect of Fluoride and Simplified Adhesive Systems on the Bond Strength of Primary Molars and Incisors. Braz. Dent. J. 2015, 26, 368–373. [Google Scholar] [CrossRef] [Green Version]
- Rosin-Grget, K.; Peros, K.; Sutej, I.; Basic, K. The cariostatic mechanisms of fluoride. Acta Med. Acad. 2013, 42, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Koeser, J.; Carvalho, T.S.; Pieles, U.; Lussi, A. Preparation and optimization of calcium fluoride particles for dental applications. J. Mater. Sci. Mater. Med. 2014, 25, 1671–1677. [Google Scholar] [CrossRef] [Green Version]
- Abou Neel, E.A.; Bozec, L.; Perez, R.A.; Kim, H.W.; Knowles, J.C. Nanotechnology in dentistry: Prevention, diagnosis, and therapy. Int. J. Nanomed. 2015, 10, 6371–6394. [Google Scholar] [CrossRef] [Green Version]
- Toledano, M.; Yamauti, M.; Ruiz-Requena, M.E.; Osorio, R. A ZnO-doped adhesive reduced collagen degradation favouring dentine remineralization. J. Dent. 2012, 40, 756–765. [Google Scholar] [CrossRef]
- Toledano, M.; Sauro, S.; Cabello, I.; Watson, T.; Osorio, R. A Zn-doped etch-and-rinse adhesive may improve the mechanical properties and the integrity at the bonded-dentin interface. Dent. Mater. 2013, 29, e142–e152. [Google Scholar] [CrossRef]
- Barcellos, D.C.; Fonseca, B.M.; Pucci, C.R.; Cavalcanti, B.; Persici Ede, S.; Goncalves, S.E. Zn-doped etch-and-rinse model dentin adhesives: Dentin bond integrity, biocompatibility, and properties. Dent. Mater. 2016, 32, 940–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feitosa, V.P.; Pomacondor-Hernandez, C.; Ogliari, F.A.; Leal, F.; Correr, A.B.; Sauro, S. Chemical interaction of 10-MDP (methacryloyloxi-decyl-dihydrogen-phosphate) in zinc-doped self-etch adhesives. J. Dent. 2014, 42, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Aguilera, F.S.; Osorio, E.; Cabello, I.; Toledano-Osorio, M.; Osorio, R. Bond strength and bioactivity of Zn-doped dental adhesives promoted by load cycling. Microsc. Microanal. 2015, 21, 214–230. [Google Scholar] [CrossRef]
- Almeida, G.S.; da Silva, E.M.; Guimaraes, J.G.A.; da Silva, R.N.L.; Dos Santos, G.B.; Poskus, L.T. ZnCl2 Incorporated into Experimental Adhesives: Selected Physicochemical Properties and Resin-Dentin Bonding Stability. Biomed Res. Int. 2017, 2017, 5940479. [Google Scholar] [CrossRef] [Green Version]
- Pomacondor-Hernandez, C.; Osorio, R.; Aguilera, F.S.; Cabello, I.; De Goes, M.; Toledano, M. Effect of zinc-doping in physicochemical properties of dental adhesives. Am. J. Dent. 2015, 28, 292–296. [Google Scholar] [PubMed]
- Toledano, M.; Osorio, R.; Osorio, E.; Cabello, I.; Toledano-Osorio, M.; Aguilera, F.S. A zinc chloride-doped adhesive facilitates sealing at the dentin interface: A confocal laser microscopy study. J. Mech. Behav. Biomed. Mater. 2017, 74, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, C.A.; Campos, R.M.; Macedo, J.P.; Silva, A.R.; Maximo, L.N.; Silva, T.M.; Franca, F.M.; Turssi, C.P.; Basting, R.T.; Goncalves, S.E.P.; et al. Incorporation of ZnCl2 into an etch-and-rinse adhesive system on flexural strength, degree of conversion and bond durability to caries-affected dentin. Am. J. Dent. 2019, 32, 299–305. [Google Scholar]
- Campos, R.M.P.; Oliveira, C.A.R.; Macedoa, J.P.C.; Françaa, F.M.G.; Basting, R.T.; Turssia, C.P.; Silva, T.M.; Gonçalves, S.E.P.; Amaral, F.L.B. Effect of zinc chloride added to self-etching primer on bond strength to caries-affected dentin and chemical-physical-mechanical properties of adhesives. Int. J. Adhes. Adhes. 2019, 95, 102412. [Google Scholar] [CrossRef]
- Navarra, C.O.; Breschi, L.; Turco, G.; Diolosa, M.; Fontanive, L.; Manzoli, L.; Di Lenarda, R.; Cadenaro, M. Degree of conversion of two-step etch-and-rinse adhesives: In situ micro-Raman analysis. J. Dent. 2012, 40, 711–717. [Google Scholar] [CrossRef] [PubMed]
- AlShaafi, M.M. Factors affecting polymerization of resin-based composites: A literature review. Saudi Dent. J. 2017, 29, 48–58. [Google Scholar] [CrossRef]
- Osorio, R.; Yamauti, M.; Osorio, E.; Ruiz-Requena, M.E.; Pashley, D.H.; Tay, F.R.; Toledano, M. Zinc reduces collagen degradation in demineralized human dentin explants. J. Dent. 2011, 39, 148–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toledano, M.; Aguilera, F.S.; Osorio, E.; Cabello, I.; Toledano-Osorio, M.; Osorio, R. Self-etching zinc-doped adhesives improve the potential of caries-affected dentin to be functionally remineralized. Biointerphases 2015, 10, 031002. [Google Scholar] [CrossRef] [PubMed]
- Osorio, R.; Osorio, E.; Cabello, I.; Toledano, M. Zinc induces apatite and scholzite formation during dentin remineralization. Caries Res. 2014, 48, 276–290. [Google Scholar] [CrossRef]
- Duarte, M.A.H.; Marciano, M.A.; Vivan, R.R.; Tanomaru Filho, M.; Tanomaru, J.M.G.; Camilleri, J. Tricalcium silicate-based cements: Properties and modifications. Braz. Oral Res. 2018, 32, e70. [Google Scholar] [CrossRef] [Green Version]
- Philpotts, C.J.; Cariddi, E.; Spradbery, P.S.; Joiner, A. In vitro evaluation of a silica whitening toothpaste containing blue covarine on the colour of teeth containing anterior restoration materials. J. Dent. 2017, 67S, S29–S33. [Google Scholar] [CrossRef]
- Monteiro, P.; Brito, P.; Pereira, J.; Alves, R. The importance of the optical properties in dental silica-based ceramics. J. Calif. Dent. Assoc. 2012, 40, 476–481. [Google Scholar]
- Yan, H.; Wang, S.; Han, L.; Peng, W.; Yi, L.; Guo, R.; Liu, S.; Yang, H.; Huang, C. Chlorhexidine-encapsulated mesoporous silica-modified dentin adhesive. J. Dent. 2018, 78, 83–90. [Google Scholar] [CrossRef]
- Alhenaki, A.M.; Attar, E.A.; Alshahrani, A.; Farooq, I.; Vohra, F.; Abduljabbar, T. Dentin Bond Integrity of Filled and Unfilled Resin Adhesive Enhanced with Silica Nanoparticles—An SEM, EDX, Micro-Raman, FTIR and Micro-Tensile Bond Strength Study. Polymers 2021, 13, 1093. [Google Scholar] [CrossRef]
- Guo, G.; Fan, Y.; Zhang, J.F.; Hagan, J.L.; Xu, X. Novel dental composites reinforced with zirconia-silica ceramic nanofibers. Dent. Mater. 2012, 28, 360–368. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Lin, C.; Wang, Y.; Ma, J.; Wang, X.; Yao, X.; Tang, B. Preparation of Zn doped mesoporous silica nanoparticles (Zn-MSNs) for the improvement of mechanical and antibacterial properties of dental resin composites. Dent. Mater. 2020, 36, 794–807. [Google Scholar] [CrossRef]
- Fallahzadeh, F.; Safarzadeh-Khosroshahi, S.; Atai, M. Dentin bonding agent with improved bond strength to dentin through incorporation of sepiolite nanoparticles. J. Clin. Exp. Dent. 2017, 9, e738–e742. [Google Scholar] [CrossRef] [Green Version]
- Timpe, N.; Fullriede, H.; Borchers, L.; Stiesch, M.; Behrens, P.; Menzel, H. Nanoporous silica nanoparticles with spherical and anisotropic shape as fillers in dental composite materials. BioNanoMaterials 2014, 15, 89–99. [Google Scholar] [CrossRef]
- Watson, T.F.; Atmeh, A.R.; Sajini, S.; Cook, R.J.; Festy, F. Present and future of glass-ionomers and calcium-silicate cements as bioactive materials in dentistry: Biophotonics-based interfacial analyses in health and disease. Dent. Mater. 2014, 30, 50–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Profeta, A.C. Dentine bonding agents comprising calcium-silicates to support proactive dental care: Origins, development and future. Dent. Mater. J. 2014, 33, 443–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, H.A.; Casanova, H. Effects of silica nanoparticles and silica-zirconia nanoclusters on tribiological properties of dental resin composites. J. Nanotechnol. 2018, 2018, 7589051. [Google Scholar] [CrossRef] [Green Version]
- Jansto, S.G. The integration of process and product metallurgy in niobium bearing steels. Metals 2018, 8, 671. [Google Scholar] [CrossRef] [Green Version]
- Leitune, V.C.; Takimi, A.; Collares, F.M.; Santos, P.D.; Provenzi, C.; Bergmann, C.P.; Samuel, S.M. Niobium pentoxide as a new filler for methacrylate-based root canal sealers. Int. Endod. J. 2013, 46, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Leitune, V.C.; Collares, F.M.; Takimi, A.; de Lima, G.B.; Petzhold, C.L.; Bergmann, C.P.; Samuel, S.M. Niobium pentoxide as a novel filler for dental adhesive resin. J. Dent. 2013, 41, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balbinot, G.S.; Leitune, V.C.B.; Ogliari, F.A.; Collares, F.M. Niobium silicate particles promote in vitro mineral deposition on dental adhesive resins. J. Dent. 2020, 101, 103449. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.M.; Leitune, V.C.B.; Balbinot, G.S.; Balhaddad, A.R.A.; Melo, M.A.S.; Samuel, S.M.W.; Collares, F.M. Physicochemical Effects of Niobic Acid Addition Into Dental Adhesives. Front. Mater. 2021, 7, 601708. [Google Scholar] [CrossRef]
- Crystal, Y.O.; Niederman, R. Evidence-Based Dentistry Update on Silver Diamine Fluoride. Dent. Clin. N. Am. 2019, 63, 45–68. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, K.; Weir, M.D.; Liu, H.; Zhou, X.; Xu, H.H. Effects of antibacterial primers with quaternary ammonium and nano-silver on Streptococcus mutans impregnated in human dentin blocks. Dent. Mater. 2013, 29, 462–472. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Melo, M.A.; Cheng, L.; Weir, M.D.; Bai, Y.; Xu, H.H. Effect of quaternary ammonium and silver nanoparticle-containing adhesives on dentin bond strength and dental plaque microcosm biofilms. Dent. Mater. 2012, 28, 842–852. [Google Scholar] [CrossRef] [Green Version]
- Nyvad, B.; Crielaard, W.; Mira, A.; Takahashi, N.; Beighton, D. Dental caries from a molecular microbiological perspective. Caries Res. 2013, 47, 89–102. [Google Scholar] [CrossRef]
- Garcia, I.M.; Leitune, V.C.B.; Takimi, A.S.; Bergmann, C.P.; Samuel, S.M.W.; Melo, M.A.; Collares, F.M. Cerium Dioxide Particles to Tune Radiopacity of Dental Adhesives: Microstructural and Physico-Chemical Evaluation. J. Funct. Biomater. 2020, 11, 7. [Google Scholar] [CrossRef] [Green Version]
- Garcia, I.M.; Leitune, V.C.B.; Ferreira, C.J.; Collares, F.M. Tantalum oxide as filler for dental adhesive resin. Dent. Mater. J. 2018, 37, 897–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, G.C.; Meimer, M.M.; Loguercio, A.D.; Cecchin, F.; Gomes, O.M.M.; Reis, A. Effects of zirconia nanoparticles addition to experimental adhesives on radiopacity and microhardness. Braz. J. Oral Sci. 2013, 12, 319–322. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Weir, M.D.; Antonucci, J.M.; Schumacher, G.E.; Zhou, X.D.; Xu, H.H. Evaluation of three-dimensional biofilms on antibacterial bonding agents containing novel quaternary ammonium methacrylates. Int. J. Oral Sci. 2014, 6, 77–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
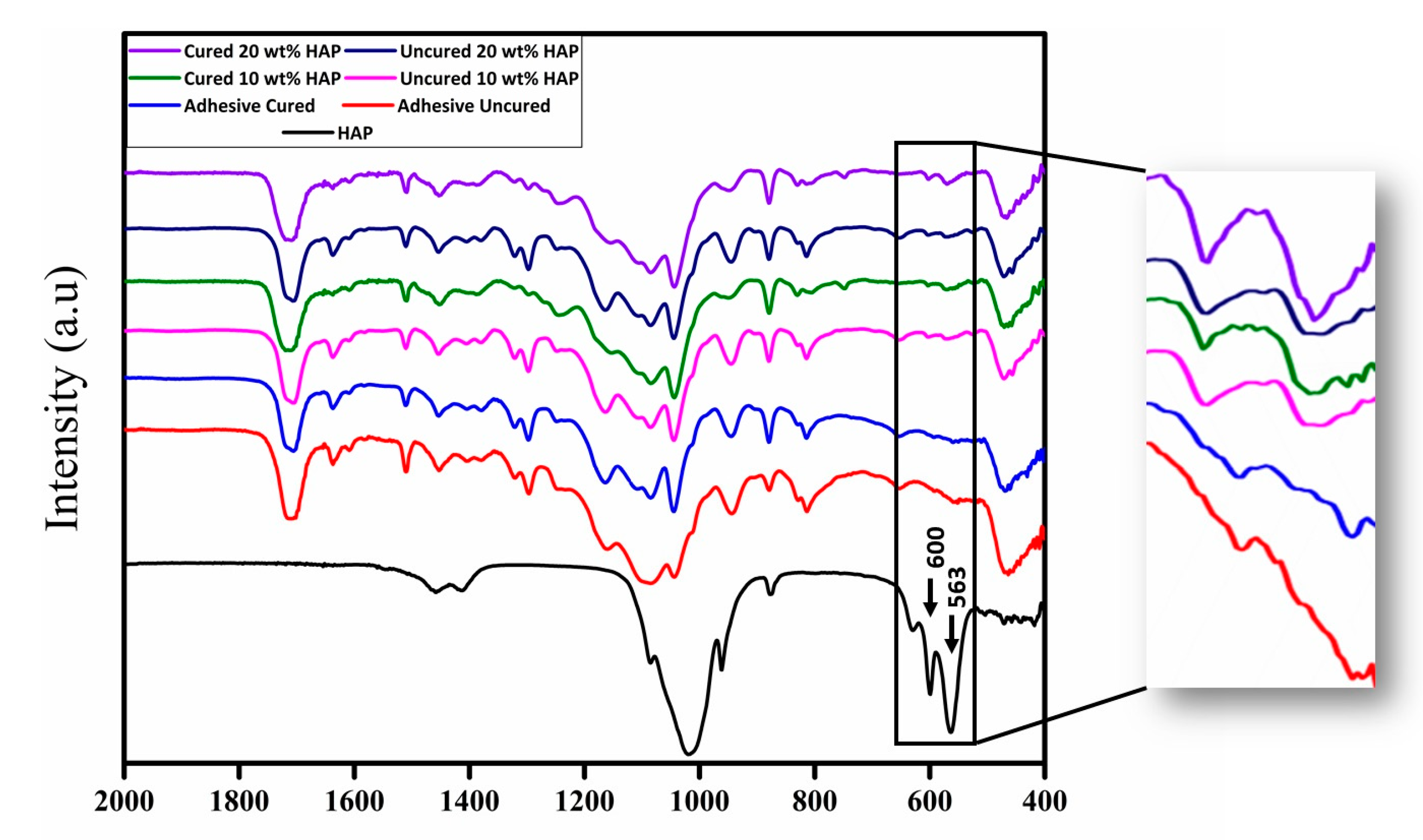
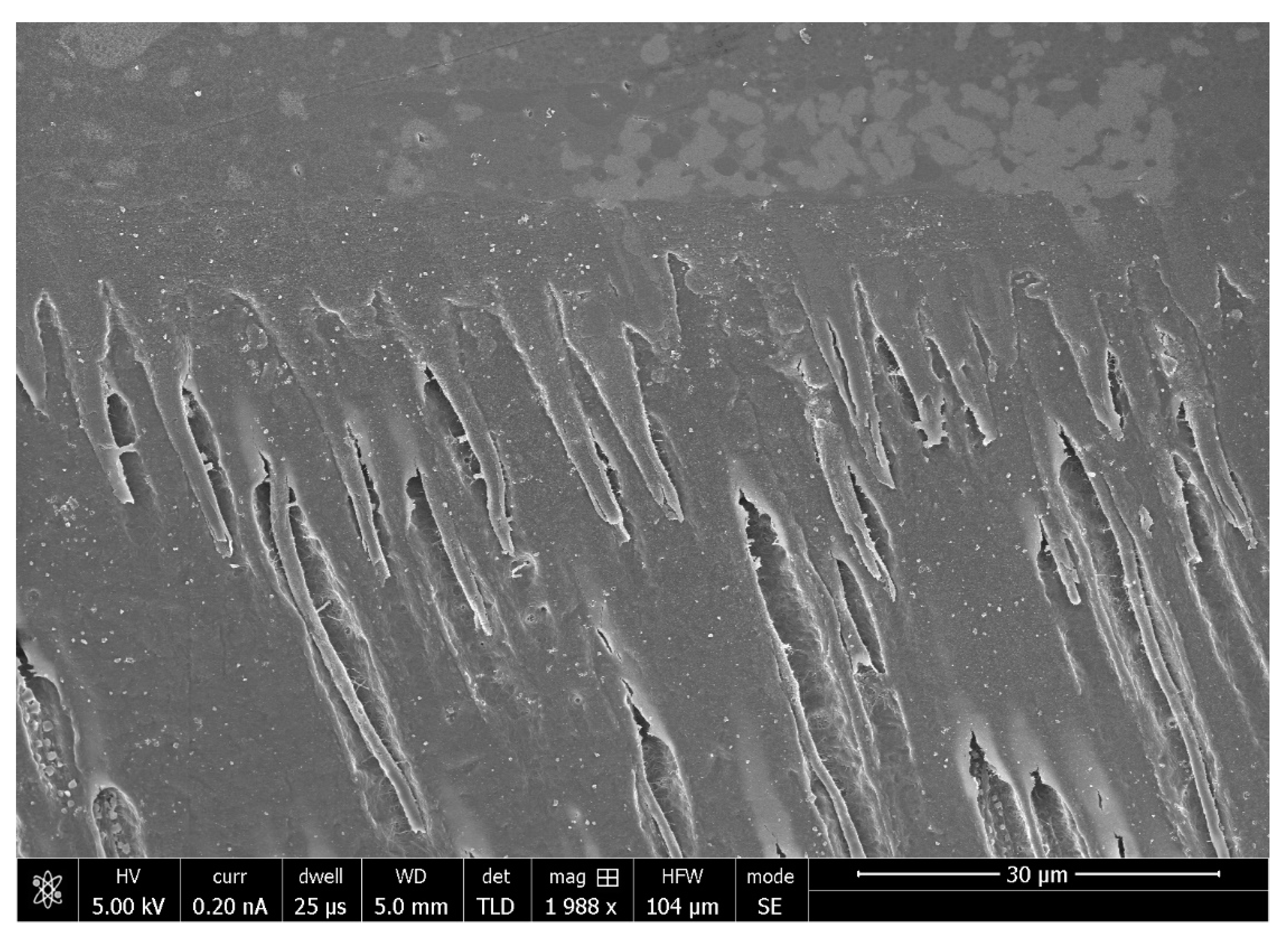
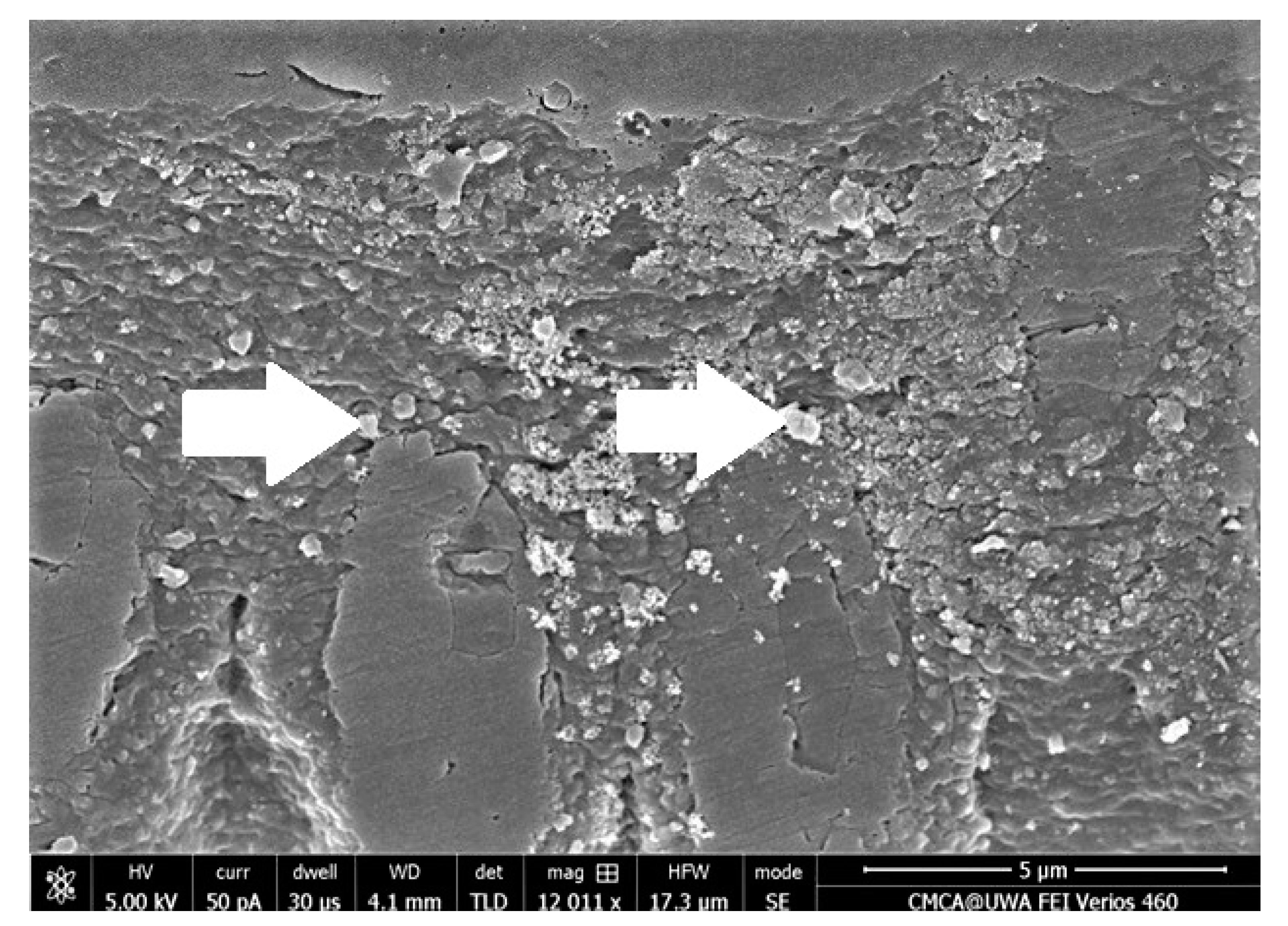

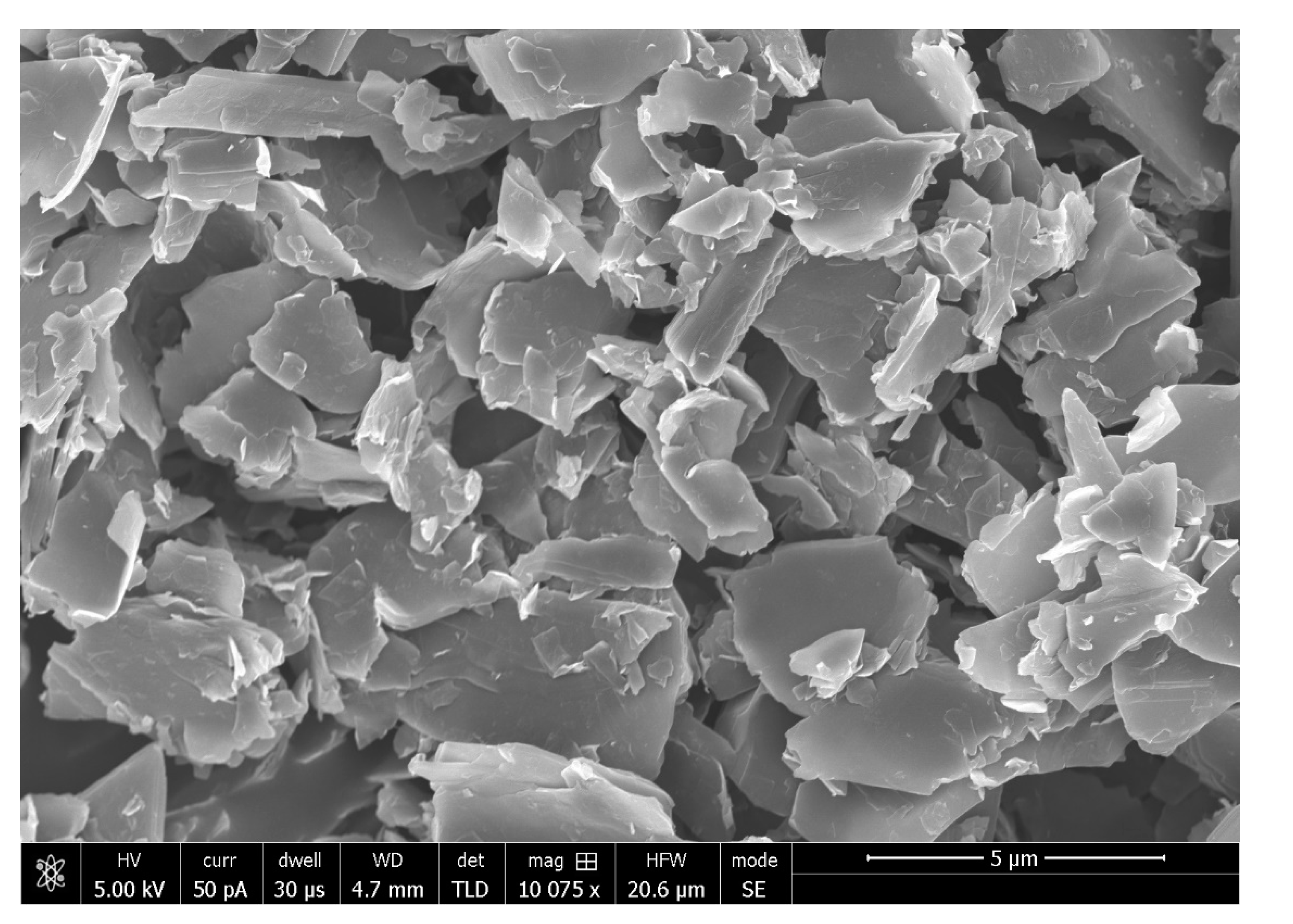
| S.No. | Bioactive Fillers | Improves Adhesive’s Properties | Reason(s) for the Improved Properties | Selected Reference(s) |
|---|---|---|---|---|
| 1. | Silver (Ag) based fillers | √ | Antibacterial property, remineralizing effect, high surface area | [104,105,106] |
| 2. | Niobic acid (Nb2O5·n H2O) | √ | Improved resistance against solvents, bioactive inorganic nature | [103] |
| 3. | Chitosan | √ | Antibacterial | [39] |
| 4. | Zn based fillers | √ | Interference with the matrix metalloproteinases (MMPs)-mediated collagen degradation, remineralizing effect due to slow Zn liberation resulting in ZnO rich layer | [75,107] |
| 5. | Cerium dioxide (CeO2) filler | √ | Improved radiopacity, sufficient dispersion in the DA | [108] |
| 6. | Tantalum oxide (Ta2O5) filler | √ | Improved radiopacity, improvement of attraction between polymer chains and solvent molecules (resulting in less degradation of adhesive-dentin bond) | [109] |
| 7. | Zirconia (Zr) based fillers | √ | Improved radiopacity and micro-hardness | [110] |
| 8. | Quaternary ammonium salts (QAS) | √ | Antibacterial effect | [111] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, I.; Ali, S.; Al-Saleh, S.; AlHamdan, E.M.; AlRefeai, M.H.; Abduljabbar, T.; Vohra, F. Synergistic Effect of Bioactive Inorganic Fillers in Enhancing Properties of Dentin Adhesives—A Review. Polymers 2021, 13, 2169. https://doi.org/10.3390/polym13132169
Farooq I, Ali S, Al-Saleh S, AlHamdan EM, AlRefeai MH, Abduljabbar T, Vohra F. Synergistic Effect of Bioactive Inorganic Fillers in Enhancing Properties of Dentin Adhesives—A Review. Polymers. 2021; 13(13):2169. https://doi.org/10.3390/polym13132169
Chicago/Turabian StyleFarooq, Imran, Saqib Ali, Samar Al-Saleh, Eman M. AlHamdan, Mohammad H. AlRefeai, Tariq Abduljabbar, and Fahim Vohra. 2021. "Synergistic Effect of Bioactive Inorganic Fillers in Enhancing Properties of Dentin Adhesives—A Review" Polymers 13, no. 13: 2169. https://doi.org/10.3390/polym13132169