Optimization of Sb2S3 Nanocrystal Concentrations in P3HT: PCBM Layers to Improve the Performance of Polymer Solar Cells
Abstract
:1. Introduction
2. Experiment
2.1. Sb2S3 Nanocrystals Fabrication
2.2. Device Fabrication
2.3. Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, G.; Adil, M.A.; Zhang, J.; Wei, Z. Large-area organic solar cells: Material requirements, modular designs, and printing methods. Adv. Mater. 2019, 31, 1805089. [Google Scholar] [CrossRef]
- Liu, G.; Jia, J.; Zhang, K.; Jia, X.E.; Yin, Q.; Zhong, W.; Li, L.; Huang, F.; Cao, Y. 15% Efficiency Tandem Organic Solar Cell Based on a Novel Highly Efficient Wide-Bandgap Nonfullerene Acceptor with Low Energy Loss. Adv. Energy Mater. 2019, 9, 1803657. [Google Scholar] [CrossRef]
- Abdallaoui, M.; Sengouga, N.; Chala, A.; Meftah, A.; Meftah, A. Comparative study of conventional and inverted p3ht:Pcbm organic solar cell. Opt. Mater. 2020, 105, 109916. [Google Scholar] [CrossRef]
- Berger, P.; Kim, M. Polymer solar cells: P3ht:Pcbm and beyond. J. Renew. Sustain. Energy 2018, 10, 013508. [Google Scholar] [CrossRef]
- Oklobia, O.; Komilian, S.; Sadat-Shafai, T. Impedance spectroscopy and capacitance–voltage measurements analysis: Impact of charge carrier life times and mapping vertical segregation in bulk heterojunction p3ht:Pcbm solar cells. Org. Electron. 2018, 61, 276–281. [Google Scholar] [CrossRef]
- Hemaprabha, E.; Pandey, U.K.; Chattopadhyay, K.; Ramamurthy, P.C. Doped silicon nanoparticles for enhanced charge transportation inorganic-inorganic hybrid solar cells. Sol. Energy 2018, 173, 744–751. [Google Scholar] [CrossRef]
- Gao, H.; Meng, J.; Sun, J.; Deng, J. Enhanced performance of polymer solar cellsbased on p3ht:Pcbm via incorporating Au nanoparticles prepared by the micellar method. J. Mater. Sci. Mater. Electron. 2020, 31, 10760–10767. [Google Scholar] [CrossRef]
- Mousavi, S.L.; Jamali-Sheini, F.; Sabaeian, M.; Yousefi, R. Enhanced solar cell performance of p3ht:Pcbm by sns nanoparticles. Sol. Energy 2020, 199, 872–884. [Google Scholar] [CrossRef]
- Kondrotas, R.; Chen, C.; Tang, J. Sb2s3 solar cells. Joule 2018, 2, 857–878. [Google Scholar] [CrossRef] [Green Version]
- Yuan, S.; Deng, H.; Yang, X.; Hu, C.; Khan, J.; Ye, W.; Tang, J.; Song, H. Postsurface selenization for high performance sb2s3 planar thin film solar cells. ACS Photonics 2017, 4, 2862–2870. [Google Scholar] [CrossRef]
- Jiang, C.; Tang, R.; Wang, X.; Ju, H.; Chen, G.; Chen, T. Alkali metals doping for high-performance planar heterojunction sb2s3 solar cells. Sol. RRL 2019, 3, 1800272. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Yang, S.; Zhao, J.; Zou, B. Pbs quantum dots base dorganic-inorganic hybrid infrared detecting and display devices. Mater. Lett. 2017, 196, 176–178. [Google Scholar] [CrossRef]
- Kim, O.; Kwon, J.; Kim, S.; Xu, B.; Seo, K.; Park, C.; Do, W.; Bae, J.; Kang, S. Effect of pvp-capped zno nanoparticles with enhanced charge transport on the performance of p3ht/pcbm polymer solar cells. Polymers 2019, 11, 1818. [Google Scholar] [CrossRef] [Green Version]
- Parize, R.; Cossuet, T.; Chaix-Pluchery, O.; Roussel, H.; Appert, E.; Consonni, V. In situ analysis of the crystallization process of sb2s3 thin films by raman scattering and x-ray diffraction. Mater. Des. 2017, 121, 1–10. [Google Scholar] [CrossRef]
- Medina-Montes, M.; Montiel-González, Z.; Mathews, N.; Mathew, X. The influence of film deposition temperature on the subsequent post-annealing and crystallization of sputtered sb2s3 thin films. J. Phys. Chem. Solids 2017, 111, 182–189. [Google Scholar] [CrossRef]
- Osorio Mayon, Y.; White, T.P.; Wang, R.; Yang, Z.; Catchpole, K.R. Evaporated and solution deposited planar sb2s3 solar cells: A comparison and its significance. Phys. Status Solidi 2016, 213, 108–113. [Google Scholar] [CrossRef]
- Mech, W.; Borysiuk, J.; Wincukiewicz, A.; Bożek, R.; Trautman, P.; Tokarczyk, M.; Kamińska, M.; Korona, K. Influence of active layer processing on electrical properties and efficiency of polymer—Fullerene organic solar cells. Acta Phys. Pol. A 2019, 136, 579–585. [Google Scholar] [CrossRef]
- Siddiqui, H.; Parra, M.R.; Pandey, P.; Qureshi, M.; Haque, F.Z. Combined parametric optimization of p3ht:Pc70bm films for efficient bulk-heterojunction solar cells. J. Solid State Electrochem. 2019, 23, 3267–3274. [Google Scholar] [CrossRef]
- Berriman, G.A.; Holmes, N.P.; Holdsworth, J.L.; Zhou, X.; Belcher, W.J.; Dastoor, P.C. A new model for pcbm phase segregation in p3ht:Pcbm blends. Org. Electron. 2016, 30, 12–17. [Google Scholar] [CrossRef]
- Hwang, S.; Potscavage, W.J., Jr.; Nakamichi, R.; Adachi, C. Processing and doping of thick polymer active layers for flexible organic thermoelectric modules. Org. Electron. 2016, 31, 31–40. [Google Scholar] [CrossRef]
- Untilova, V.; Biskup, T.; Biniek, L.; Vijayakumar, V.; Brinkmann, M. Control of chain alignment and crystallization helps enhance charge conductivities and thermoelectric power factors in sequentially doped p3ht:F4tcnq films. Macromolecules 2020, 53, 2441–2453. [Google Scholar] [CrossRef]
- Chandrasekaran, N.; Kumar, A.; Thomsen, L.; Kabra, D.; McNeill, C.R. High performance as-cast p3ht:Pcbm solar cells: Understanding the role of molecular weight in high regioregularity p3ht. Mater. Adv. 2021, 2, 2045–2054. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, M.; Rathi, S.; Yadav, A.; Upadhyaya, A.; Gupta, S.K.; Singh, A. Study of p3ht/pcbm Morphology Using Raman Spectroscopy. AIP Conf. Proc. 2018, 1953, 100074. [Google Scholar] [CrossRef]
- Hisamuddin, S.N.; Abdullah, S.M.; Alwi, S.A.K.; Majid, S.R.; Anuar, A.; Sulaiman, K.; Tunmee, S.; Chanlek, N.; Bawazeer, T.M.; Alsoufi, M.S. Optimizing the performance of p3ht-based photodetector by tuning the composition of oxcba. Synth. Met. 2020, 268, 116506. [Google Scholar] [CrossRef]
- Xie, T.; Xie, G.; Su, Y.; Hongfei, D.; Ye, Z.; Jiang, Y. Ammonia gas sensors based on poly(3-hexylthiophene)-molybdenum disulfide film transistors. Nanotechnology 2016, 27, 065502. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-C.; Hsiao, Y.-J.; Lin, T.-C.; Li, T.-S.; Zeng, S.-A.; Yu, C.-E. Improving power conversion efficiency of p3ht/pcbm based organic solar cells by optimizing graphene doping concentration and annealing temperature. Int. J. Electrochem. Sci 2016, 11, 5819–5828. [Google Scholar] [CrossRef]
- Hamed, Z.B.; Mastour, N.; Kouki, F.; Sanhoury, M.; Bouchriha, H. Franck–condon analysis of fluorescence quenching in hybrid p3ht:Wt% tbpo-capped cdse quantum dot matrix. J. Lumin. 2016, 170, 30–36. [Google Scholar] [CrossRef]
- Kozlov, O.V.; Luponosov, Y.N.; Solodukhin, A.N.; Flament, B.; Douhéret, O.; Viville, P.; Beljonne, D.; Lazzaroni, R.; Cornil, J.; Ponomarenko, S.A. Simple donor-acceptor molecule with long exciton diffusion length for organic photovoltaics. Org. Electron. 2018, 53, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Dinçalp, H.; Saltan, G.M.; Zafer, C.; Kıymaz, D.A. Bromo-substituted cibalackrot backbone, a versatile donor or acceptor main core for organic optoelectronic devices. J. Mol. Struct. 2018, 1173, 512–520. [Google Scholar] [CrossRef]
- Otieno, F.; Mutuma, B.K.; Airo, M.; Ranganathan, K.; Erasmus, R.; Coville, N.; Wamwangi, D. Enhancement of organic photovoltaic device performance via p3ht:Pcbm solution heat treatment. Thin Solid Film. 2017, 625, 62–69. [Google Scholar] [CrossRef]
- Song, C.; Wang, Z.; Li, J.; Chen, Y.; Zhao, F.; Zhang, H. Extension of π-conjugation and enhancement of electron-withdrawing ability at terminal indanedione for a-π-d-π-a small molecules for application in organic solar cells. Org. Electron. 2020, 81, 105679. [Google Scholar] [CrossRef]
- Ahmad, Z.; Touati, F.; Shakoor, R.; Al-Thani, N. Study of a ternary blend system for bulk heterojunction thin film solar cells. Chin. Phys. B 2016, 25, 080701. [Google Scholar] [CrossRef]
- Lefrançois, A.; Luszczynska, B.; Pepin-Donat, B.; Lombard, C.; Bouthinon, B.; Verilhac, J.-M.; Gromova, M.; Faure-Vincent, J.; Pouget, S.; Chandezon, F. Enhanced charge separation in ternary p3ht/pcbm/cuins 2 nanocrystals hybrid solar cells. Sci. Rep. 2015, 5, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gollu, S.R.; Sharma, R.; Srinivas, G.; Kundu, S.; Gupta, D. Incorporation of silver and gold nanostructures for performance improvement in p3ht:Pcbm inverted solar cell with rgo/zno nanocomposite as an electron transport layer. Org. Electron. 2016, 29, 79–87. [Google Scholar] [CrossRef]
- Oseni, S.O.; Mola, G.T. Bimetallic nanocomposites and the performance of inverted organic solar cell. Compos. Part B Eng. 2019, 172, 660–665. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Tiwari, D.; Tripathi, S.K.; Dwivedi, P.K.; Dipak, P.; Chandel, T.; Prasad, N.E. Fabrication and properties of p3ht:Pcbm/cu2snse3(ctse) nanocrystals based inverted hybrid solar cells. Sol. Energy 2019, 187, 167–174. [Google Scholar] [CrossRef]
- Qu, S.; Yao, Q.; Wang, L.; Chen, Z.; Xu, K.; Zeng, H.; Shi, W.; Zhang, T.; Uher, C.; Chen, L. Highly anisotropic p3ht films with enhanced thermoelectric performance via organic small molecule epitaxy. NPG Asia Mater. 2016, 8, e292. [Google Scholar] [CrossRef]
- Aoyama, Y.; Douhéret, O.; Leclère, P.; Moerman, D.; Mizukado, J.; Suda, H.; Lazzaroni, R.; Yoshida, Y. On the influence of the photo-oxidation of p3ht on the conductivity of photoactive film of p3ht:Pcbm bulk heterojunctions. Org. Electron. 2017, 43, 142–147. [Google Scholar] [CrossRef]
- Corzo, D.; Almasabi, K.; Bihar, E.; Macphee, S.; Rosas-Villalva, D.; Gasparini, N.; Inal, S.; Baran, D. Digital inkjet printing of high-efficiency large-area nonfullerene organic solar cells. Adv. Mater. Technol. 2019, 4, 1900040. [Google Scholar] [CrossRef]
- Hernández-Martínez, D.; Nicho, M.; Alvarado-Tenorio, G.; García-Carvajal, S.; Castillo-Ortega, M.; Vásquez-López, C. Elaboration and characterization of p3ht–peo–swcnt fibers by electrospinning technique. SN Appl. Sci. 2020, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Brambilla, L.; CapelFerrón, C.; Tommasini, M.; Hong, K.; López Navarrete, J.; Hernández, V.; Zerbi, G. Infrared and multi-wavelength raman spectroscopy of regio-regular p3ht and its deuteron derivatives. J. Raman Spectrosc. 2018, 49, 569–580. [Google Scholar] [CrossRef]
- de Antoni, L.O.; de Menezes, E.W.; Loguercio, L.F.; Rodrigues, M.R.F.; de Andrade, R.L.; Costa, T.M.; Benvenutti, E.V.; Santos, J.F.L.; Santos, M.J.L. Ionic silsesquioxane-capped au nanoparticle powders: Application in p3ht/pcbm solar cells and the effect of the capping layer on surface plasmon dumping. Mater. Chem. Phys. 2018, 206, 204–212. [Google Scholar] [CrossRef]
- Agbolaghi, S. Optical/thermal studies on nanostructures of poly(3-hexylthiophene) and carbon nanotube/graphene precursors. Fuller. Nanotub. Carbon Nanostruct. 2019, 27, 572–581. [Google Scholar] [CrossRef]
- Sharma, T.; Singhal, R.; Vishnoi, R.; Sharma, P.; Patra, A.; Chand, S.; Lakshmi, G.; Biswas, S. Electronic excitation induced modifications of optical and morphological properties of pcbm thin films. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2016, 379, 176–180. [Google Scholar] [CrossRef]
- Subhashini, R.; Sathya, D.; Sivashankar, V.; Mageshwari, P.L.; Arjunan, S. Growth and characterization of bis(l-threonine) copper (ii) monohydrate single crystals: A semiorganic second order nonlinear optical material. Opt. Mater. 2016, 62, 357–365. [Google Scholar] [CrossRef]
- Sakthiguru, N.; Sithique, M.A. Preparation and in vitro biological evaluation of lawsone loaded o-carboxymethyl chitosan/zinc oxide nanocomposite for wound-healing application. Chem. Sel. 2020, 5, 2710–2718. [Google Scholar] [CrossRef]
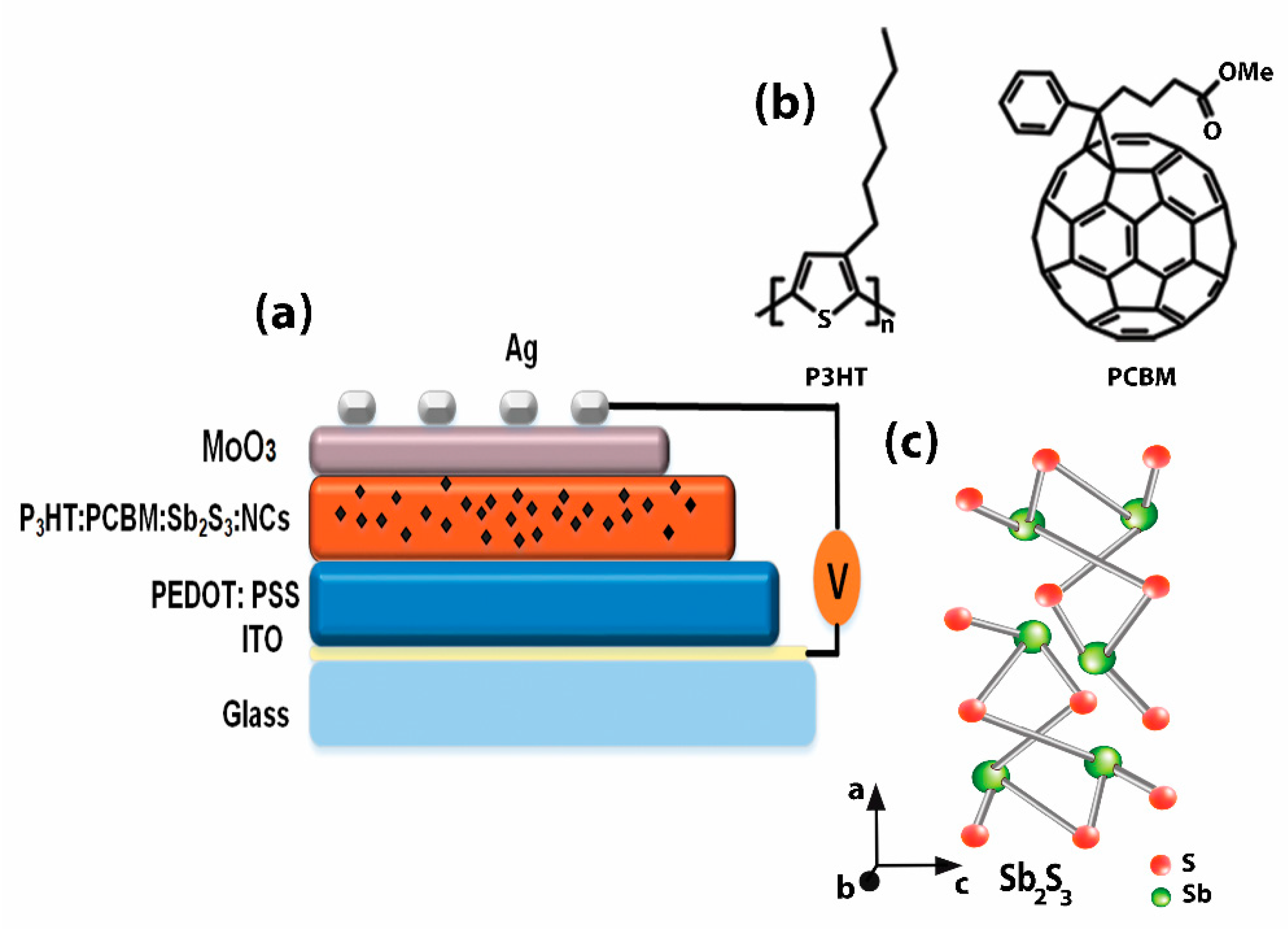
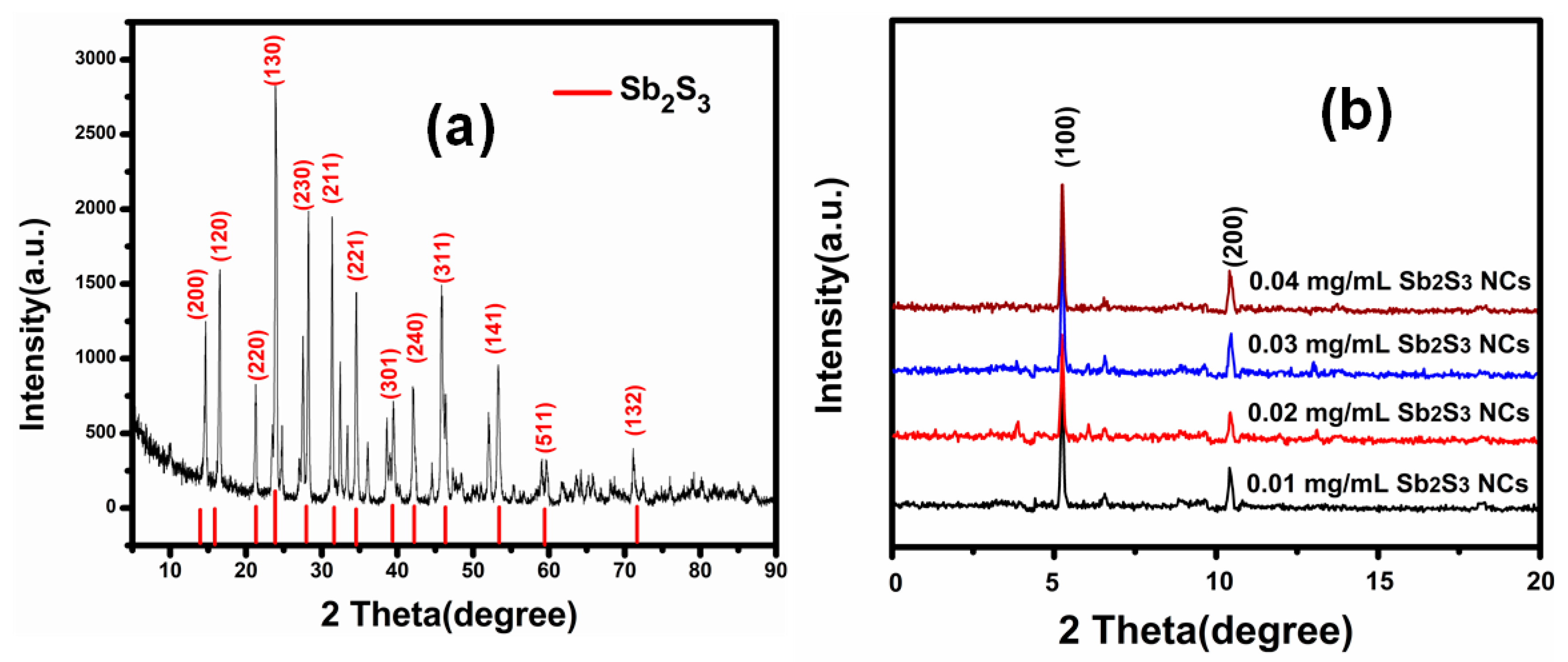

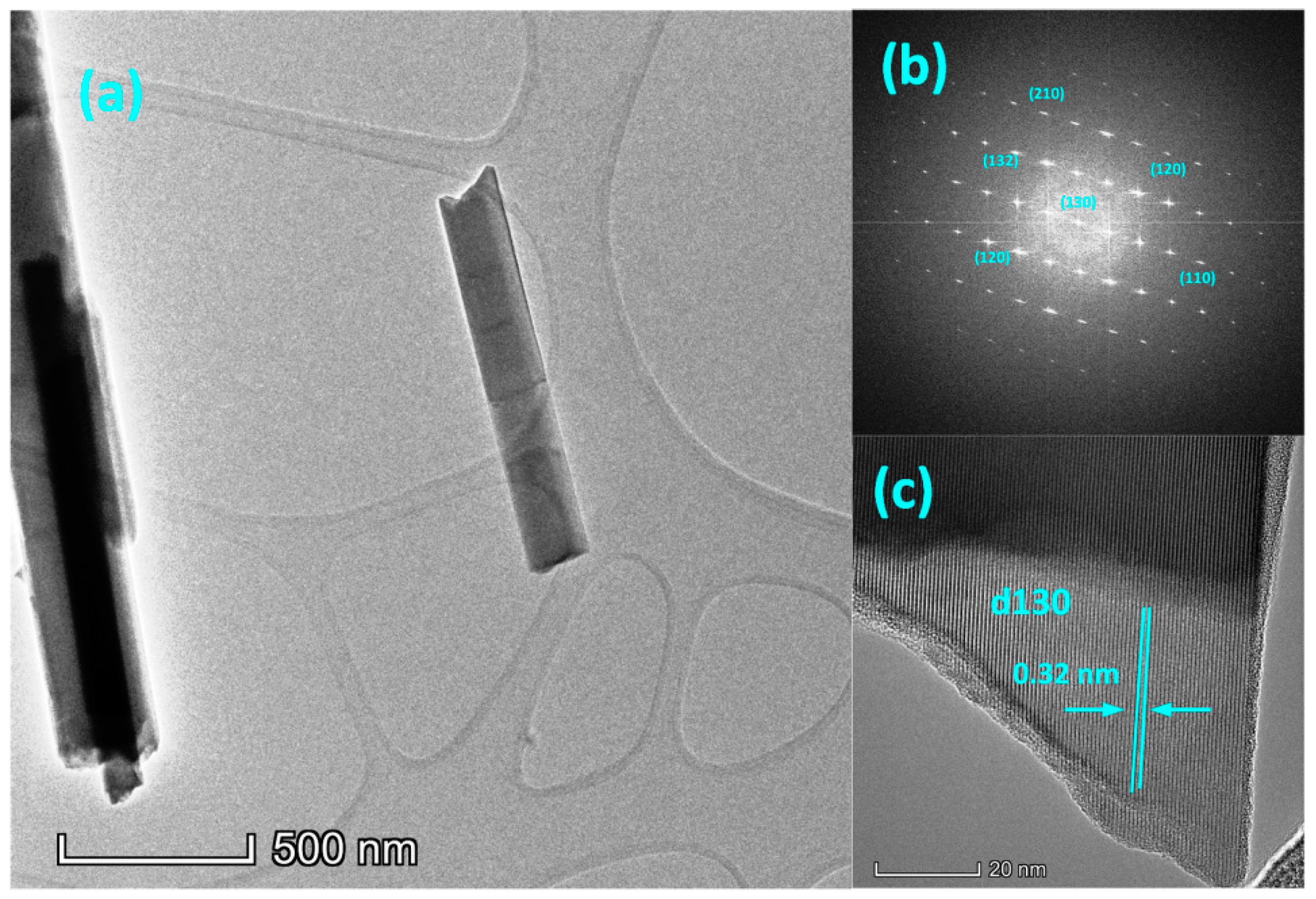
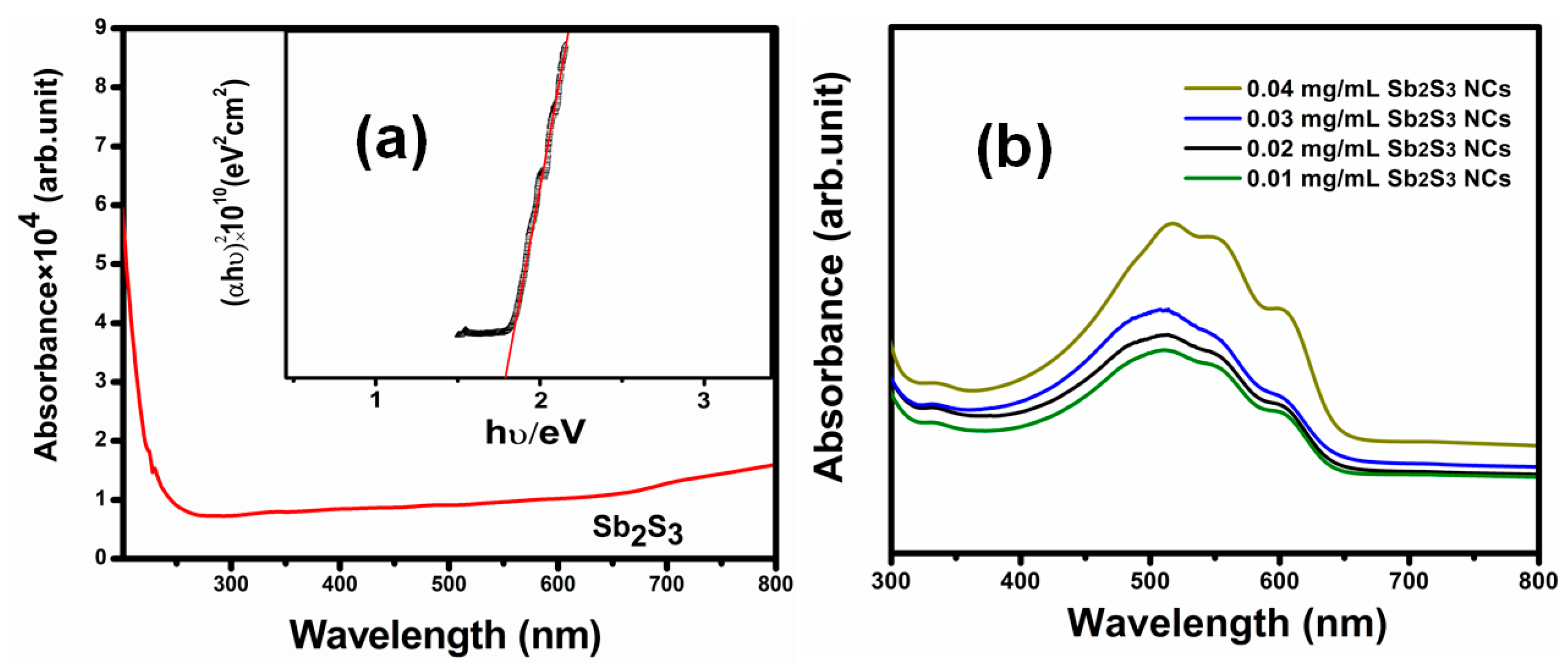

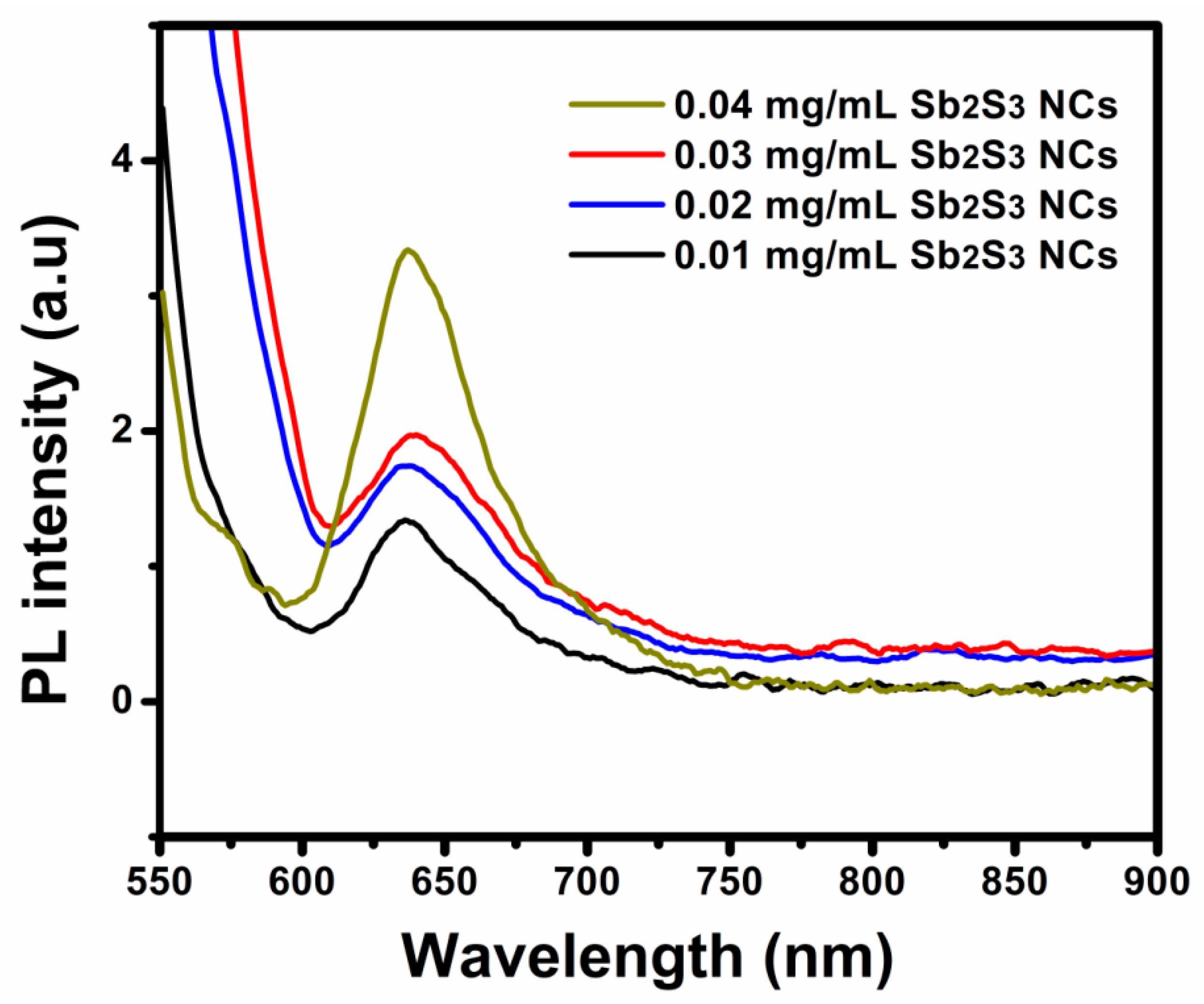
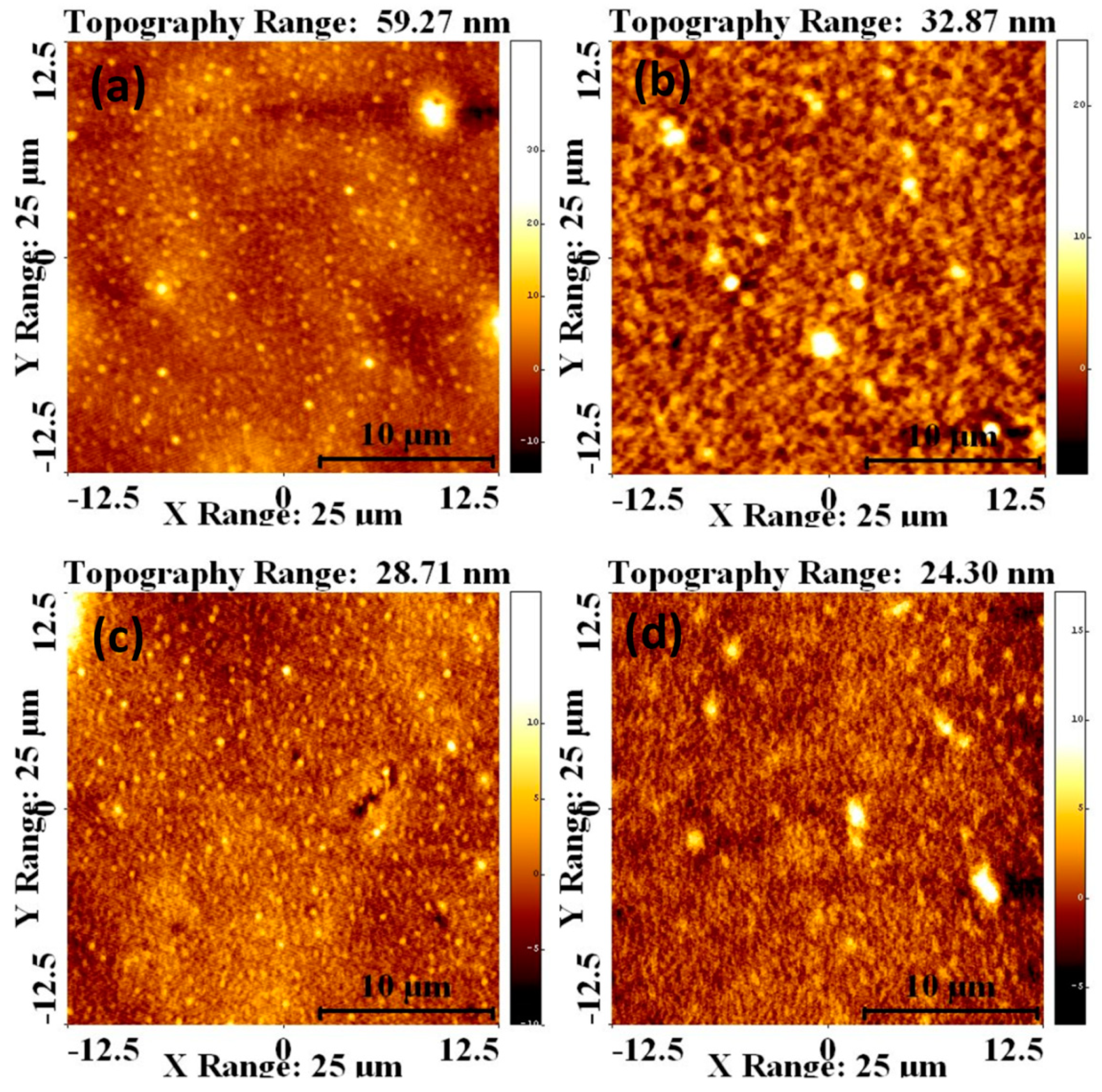
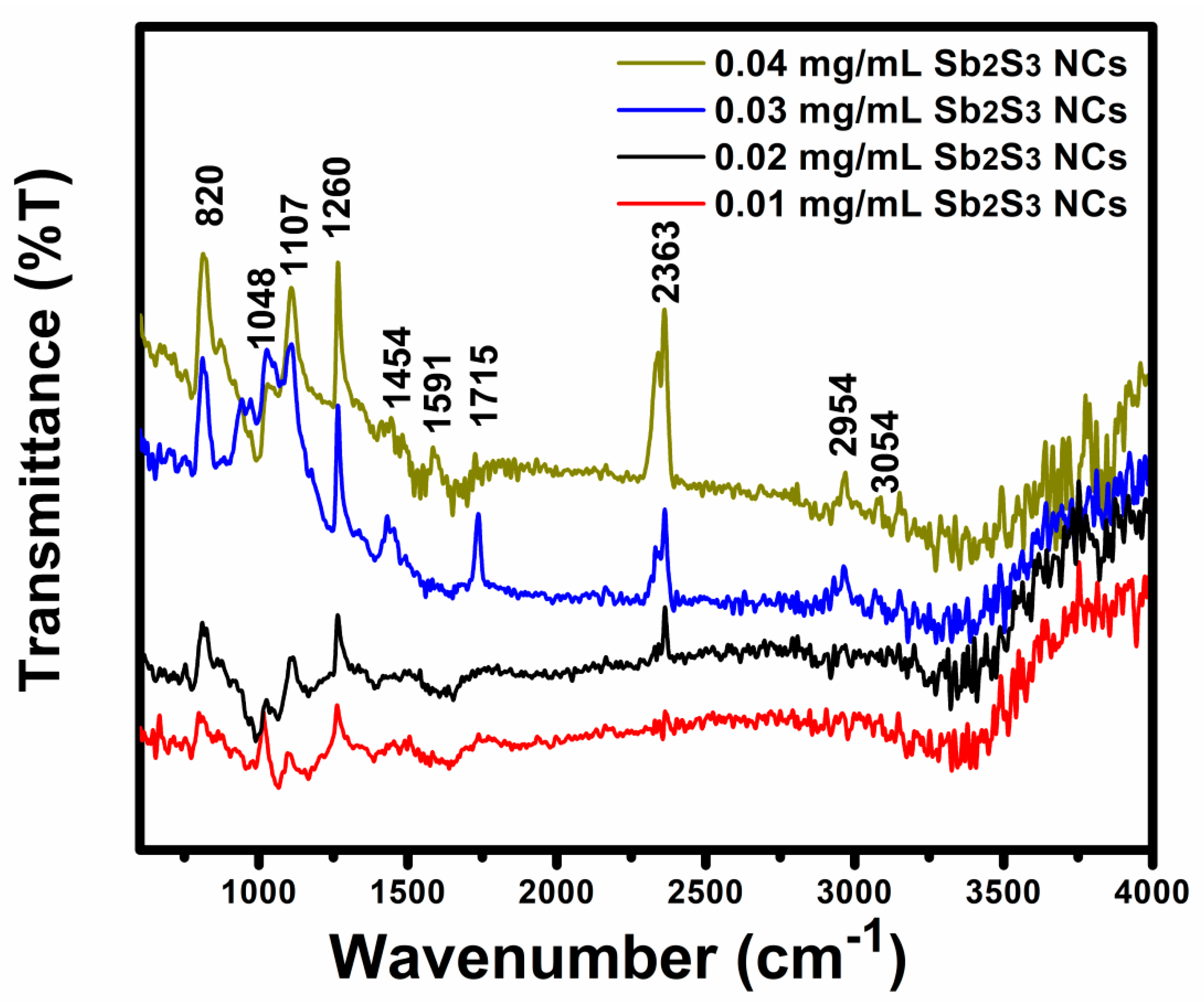
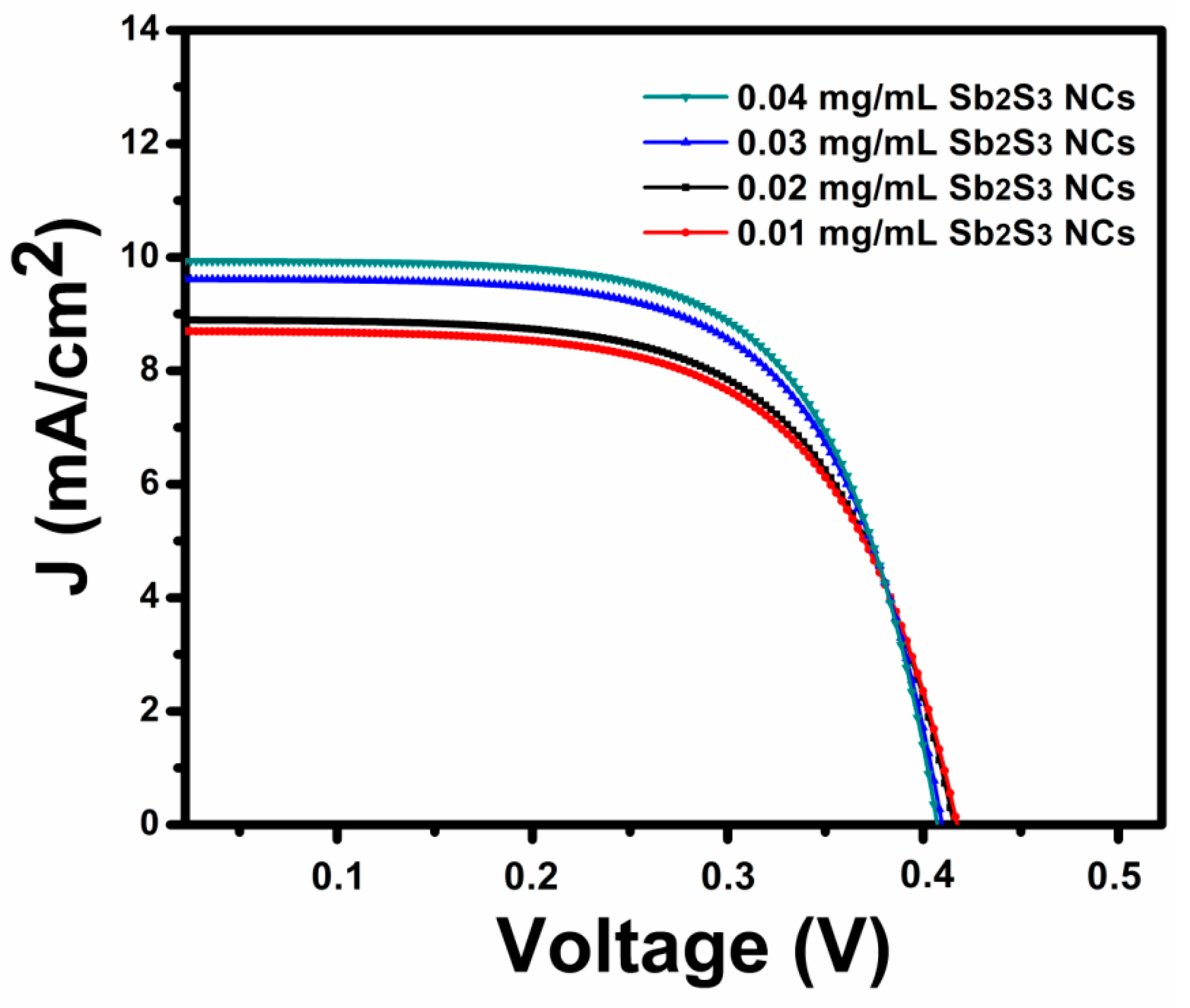
| Sample | Voc (mV) | Jsc (mA/cm2) | FF (%) | |||
|---|---|---|---|---|---|---|
| 0.01 mg/mL | 423 | 8.67 | 59.8 | 2.18 | 47.7 | 489.7 |
| 0.02 mg/mL | 421 | 8.91 | 62.6 | 2.34 | 33.6 | 552.6 |
| 0.03 mg/mL | 416 | 9.57 | 64.4 | 2.56 | 26.6 | 742.1 |
| 0.04 mg/mL | 412 | 10.04 | 66.0 | 2.72 | 23.2 | 945.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mkawi, E.M.; Al-Hadeethi, Y.; Bazuhair, R.S.; Yousef, A.S.; Shalaan, E.; Arkook, B.; Abdeldaiem, A.M.; Almalki, R.; Bekyarova, E. Optimization of Sb2S3 Nanocrystal Concentrations in P3HT: PCBM Layers to Improve the Performance of Polymer Solar Cells. Polymers 2021, 13, 2152. https://doi.org/10.3390/polym13132152
Mkawi EM, Al-Hadeethi Y, Bazuhair RS, Yousef AS, Shalaan E, Arkook B, Abdeldaiem AM, Almalki R, Bekyarova E. Optimization of Sb2S3 Nanocrystal Concentrations in P3HT: PCBM Layers to Improve the Performance of Polymer Solar Cells. Polymers. 2021; 13(13):2152. https://doi.org/10.3390/polym13132152
Chicago/Turabian StyleMkawi, E. M., Y. Al-Hadeethi, R. S. Bazuhair, A. S. Yousef, E. Shalaan, B. Arkook, A. M. Abdeldaiem, Rahma Almalki, and E. Bekyarova. 2021. "Optimization of Sb2S3 Nanocrystal Concentrations in P3HT: PCBM Layers to Improve the Performance of Polymer Solar Cells" Polymers 13, no. 13: 2152. https://doi.org/10.3390/polym13132152
APA StyleMkawi, E. M., Al-Hadeethi, Y., Bazuhair, R. S., Yousef, A. S., Shalaan, E., Arkook, B., Abdeldaiem, A. M., Almalki, R., & Bekyarova, E. (2021). Optimization of Sb2S3 Nanocrystal Concentrations in P3HT: PCBM Layers to Improve the Performance of Polymer Solar Cells. Polymers, 13(13), 2152. https://doi.org/10.3390/polym13132152







