Fabrication of Hybrid Nanofibers from Biopolymers and Poly (Vinyl Alcohol)/Poly (ε-Caprolactone) for Wound Dressing Applications
Abstract
:1. Introduction
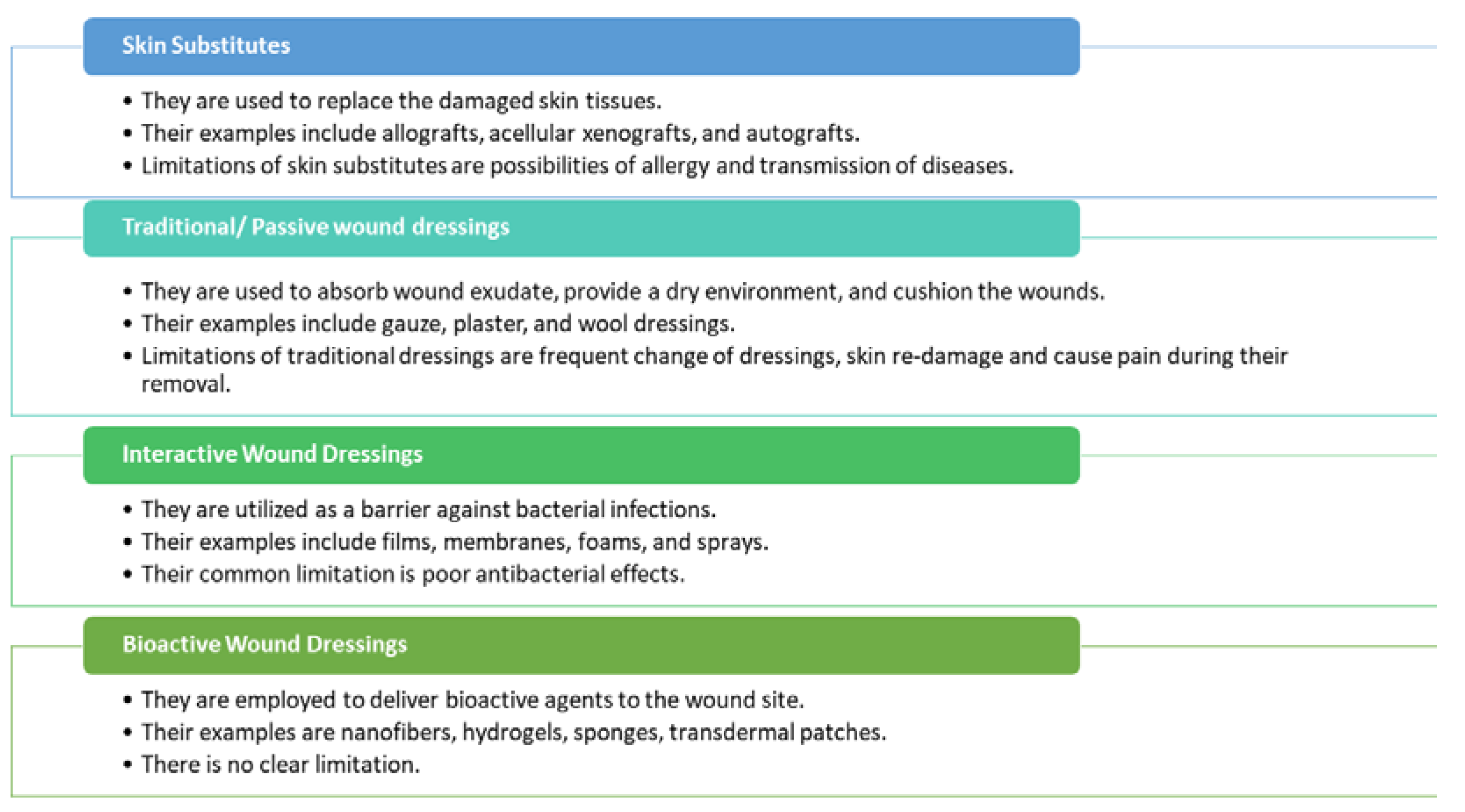
2. Classification of Wounds and the Phases of the Wound Healing Process
3. Poly (Vinyl Alcohol) and Poly (ε-Caprolactone) in Wound Healing Applications
4. Electrospinning Technique and Properties of Nanofibers
5. Fabrication of Biopolymer-Based Hybrid Nanofibers
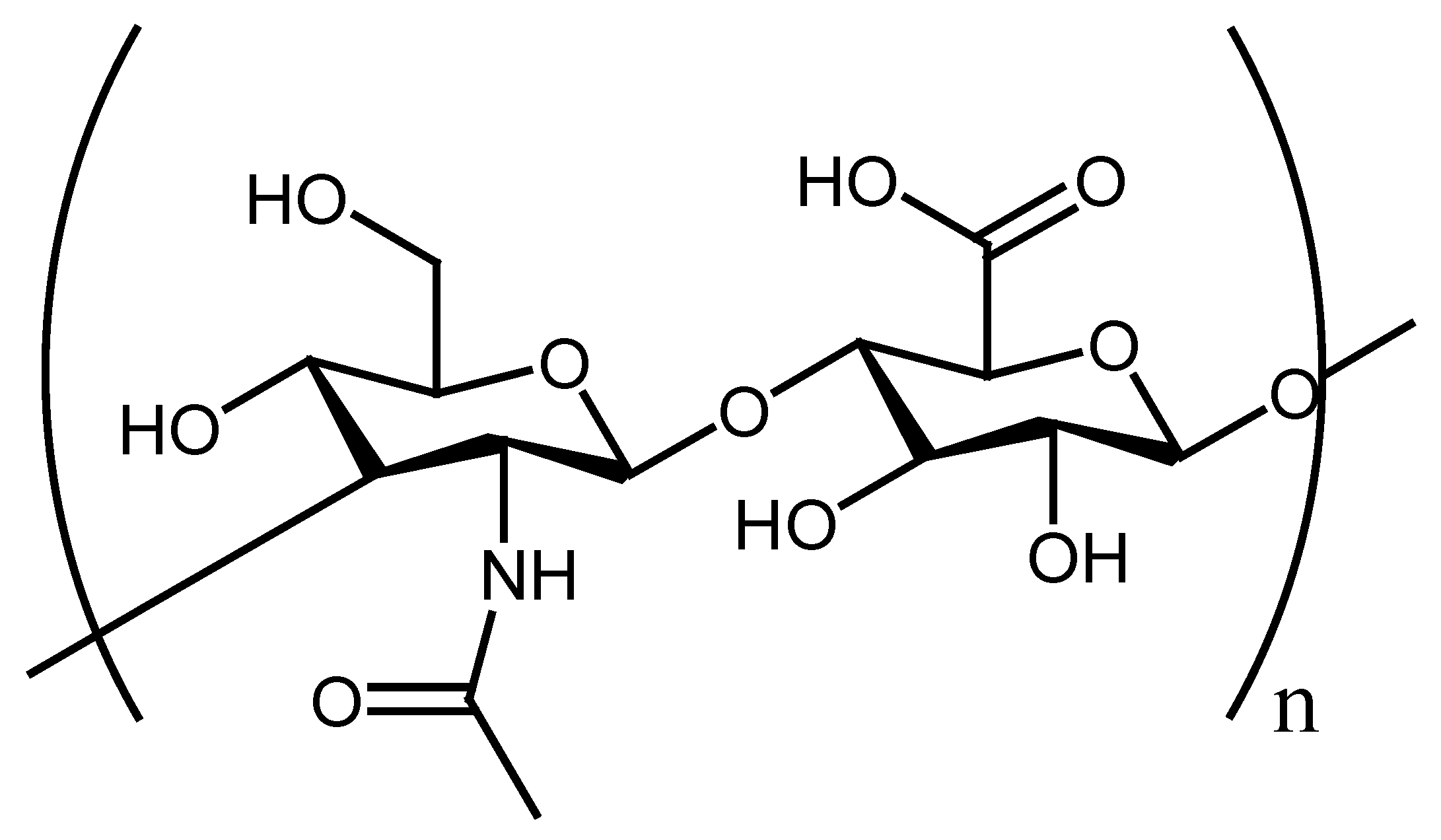
5.1. Chitosan–PVA/PCL Hybrid Nanofibers
5.2. Gelatin–PVA/PCL Hybrid Nanofibers
5.3. Alginate–PVA/PCL Hybrid Nanofibers
5.4. Cellulose–PVA/PCL Hybrid Nanofibers
5.5. Hyaluronic Acid–PVA/PCL Hybrid Nanofibers
5.6. Collagen–PVA/PCL Hybrid Nanofibers
5.7. Gum Arabic/Gum Tragacanth–PVA/PCL Hybrid Nanofibers
5.8. Silk Fibroin–PVA/PCL Hybrid Nanofibers
5.9. Lignin–PVA/PCL Hybrid Nanofibers
5.10. Other Biopolymers Combined with PVA/PCL for Hybrid Nanofiber Fabrication
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varaprasad, K.; Jayaramudu, T.; Kanikireddy, V.; Toro, C.; Sadiku, E.R. Alginate-based composite materials for wound dressing application: A mini-review. Carbohydr. Polym. 2020, 236, 116025. [Google Scholar] [CrossRef]
- Duscher, D.; Trotsyuk, A.A.; Maan, Z.N.; Hyung, S.; Rodrigues, M.; Engel, K.; Stern-buchbinder, Z.A.; Bonham, C.A.; Barrera, J.; Whittam, A.J.; et al. Optimization of transdermal deferoxamine leads to enhanced efficacy in healing skin wounds. J. Control. Releas. 2019, 308, 232–239. [Google Scholar] [CrossRef]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human Skin Wounds: A Major Snoballing Threat to Public Health and Economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [Green Version]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8. [Google Scholar] [CrossRef] [Green Version]
- Pires, A.L.R.; Motta, L.A.; Dias, A.M.A.; de Sousa, H.C.; Moraes, A.M.; Braga, M.E.M. Towards wound dressings with improved properties: Effects of poly (dimethylsiloxane) on chitosan-alginate fi lms loaded with thymol and beta-carotene. Mater. Sci. Eng. C 2018, 93, 595–605. [Google Scholar] [CrossRef]
- Alven, S.; Nqoro, X.; Aderibigbe, B.A. Polymer-Based Materials Loaded with Curcumin for Wound Healing Applications. Polymer 2020, 12, 2286. [Google Scholar] [CrossRef]
- Ng, S.; Jumaat, N. Carboxymethyl cellulose wafers containing antimicrobials: A modern drug delivery system for wound infections. Eur. J. Pharm. Sci. 2014, 1, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Dua, A.; Malik, A. Third generation materials for wound dressings. Int. J. Pharm. Sci. Res. 2014, 6, 2113–2124. [Google Scholar]
- Colobatiu, L.; Gavan, A.; Mocan, A.; Bogdan, C.; Mirel, S.; Tomuta, I. Development of bioactive compounds-loaded chitosan films by using a QbD approach—A novel and potential wound dressing material. React. Funct. Polym. 2019, 138, 46–54. [Google Scholar] [CrossRef]
- Fayemi, O.E.; Ekennia, A.C.; Katata-Seru, L.; Ebokaiwe, A.P.; Ijomone, O.M.; Onwudiwe, D.C.; Ebenso, E.E. Antimicrobial and Wound Healing Properties of Polyacrylonitrile-Moringa Extract Nanofibers. ACS Omega 2018, 3, 4791–4797. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Aderibigbe, B.A. Chitosan and Cellulose-Based Hydrogels for Wound Management. Int. J. Mol. Sci. 2020, 21, 9656. [Google Scholar] [CrossRef]
- Schreml, S.; Szeimies, R.; Prantl, L.; Karrer, S.; Landthaler, M.; Babilas, P. Oxygen in acute and chronic wound healing. Braz. J. Dermatol. 2010, 163, 257–268. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Morton, L.M.; Phillips, T.J. Wound healing and treating wounds: Differential diagnosis and evaluation of chronic wounds. J. Am. Acad. Dermatol. 2016, 74, 589–605. [Google Scholar]
- Chen, S.; Liu, B.; Carlson, M.A.; Gombart, A.F.; Reilly, D.A.; Xie, J. Recent advances in electrospun nanofibers for wound healing. Nanomed. 2017, 12, 1335–1352. [Google Scholar] [CrossRef]
- Beam, J.W. Management of superficial to partial-thickness wounds. J. Athl. Train. 2007, 42, 422–424. [Google Scholar] [PubMed]
- Dabiri, G.; Damstetter, E.; Phillips, T. Choosing a Wound Dressing Based on Common Wound Characteristics. Adv. Wound Care 2016, 5, 32–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera, A.E.; Spencer, J.M. Clinical aspects of full-thickness wound healing. Clin. Dermatol. 2007, 25, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Fontes, P.R.; Ribeiro, S.J.L.; Gaspar, A.M.M. Bacterial cellulose / phytotherapic hydrogels as dressings for wound healing. Mater. Sci. Eng. Int. J. 2019, 3, 162–173. [Google Scholar]
- Ambekar, R.S.; Kandasubramanian, B. Advancements in nanofibers for wound dressing: A review. Eur. Polym. J. 2019, 117, 304–336. [Google Scholar] [CrossRef]
- Kenawy, E.; Omer, A.M.; Tamer, T.M.; Elmeligy, M.A.; Eldin, M.S.M. Fabrication of biodegradable gelatin/ chitosan/cinnamaldehyde crosslinked membranes for antibacterial wound dressing applications. Int. J. Biol. Macromol. 2019, 139, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Carvalho, A.; Vaz, D.C.; Gil, M.H.; Mendes, A.; Bártolo, P. Development of novel alginate based hydrogel films for wound healing applications. Int. J. Biol. Macromol. 2013, 2, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Summa, M.; Russo, D.; Penna, I.; Margaroli, N.; Bayer, I.S.; Bandiera, T.; Athanassiou, A.; Bertorelli, R. A biocompatible sodium alginate / povidone iodine film enhances wound healing. Eur. J. Pharm. Biopharm. 2018, 122, 17–24. [Google Scholar] [CrossRef]
- Fredric, S.; Gowda, D.V.; Yashashwini, M. Wafers for wound healing. J. Chem. Pharm. Res. 2015, 7, 450–468. [Google Scholar]
- Freire, M.O.; Van Dyke, T.E. Natural resolution of inflammation. Periodontology 2013, 63, 149–164. [Google Scholar] [CrossRef] [Green Version]
- Hussain, Z.; Thu, H.E.; Shuid, A.N.; Katas, H.; Hussain, F. Recent Advances in Polymer-based Wound Dressings for the Treatment of Diabetic Foot Ulcer: An Overview of State-of-the-art. Curr. Drug Targets 2017, 18, 527–550. [Google Scholar] [CrossRef]
- Childs, D.R.; Murthy, A.S. An overview of Wound Healingand Management. Surg. Clin. N Am. 2017, 278, 189–207. [Google Scholar] [CrossRef]
- Ma, R.; Wang, Y.; Qi, H.; Shi, C.; Wei, G.; Xiao, L.; Huang, Z.; Liu, S.; Yu, H.; Teng, C.; et al. Nanocomposite sponges of sodium alginate/graphene oxide/polyvinyl alcohol as potential wound dressing: In vitro and in vivo evaluation. Compos. Part B 2019, 167, 396–405. [Google Scholar] [CrossRef]
- Slaughter, B.; Shahana, S.; Fisher, O.; Khademhosseini, A.; Peppas, N. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [Green Version]
- Manju, S.; Antony, M.; Sreenivasan, K. Synthesis and evaluation of a hydrogel that binds glucose and releases ciprofloxacin. J. Mater. Sci. 2010, 45, 4006–4012. [Google Scholar] [CrossRef]
- Elbadawy, A.K.; El-Refaie, S.K.; Xin, C. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar]
- Mirela, T.; Maria, B.; Simona, M. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotech. Adv. 2019, 37, 109–131. [Google Scholar]
- Kamoun, E.A.; Kenawy, E.S.; Tamer, T.M.; El-Meligy, M.A.; Mohy, E.M.S. Poly (vinyl alcohol)-alginate physically crosslinked hydrogel membranes for wound dressing applications: Characterization and bio-evaluation. Arab J. Chem. 2015, 8, 38–47. [Google Scholar] [CrossRef]
- Kang, X.; Xie, Y.; Powell, H. Adipogenesis of murine embryonic stem cells in a three-dimensional culture system using electrospun polymer scaffolds. Biomaterials 2007, 28, 450–458. [Google Scholar] [CrossRef]
- Rub, D.; Sumit, K.; Rahu, P.; Aman, M.; Deept, N.; Dhirendra, S.K.; Divya, M. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. and Craniofac. Res. 2020, 10, 381–388. [Google Scholar]
- Dobrzański, L.A.; Hudecki, A.; Chladek, G.; Król, W.; Mertas, A. Biodegradable and antimicrobial polycaprolactone nanofibers with and without silver precipitates. Arch. Mat. Sci. Eng. 2015, 76, 5–26. [Google Scholar]
- Thomas, R.; oumya, K.R.; Mathew, S.J.; Radhakrishnan, E.K. Electrospun Polycaprolactone Membrane Incorporated with Biosynthesized Silver Nanoparticles as Effective Wound Dressing Material. Appied Biochem. Biotechnol. 2015, 176, 2213–2224. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Zagho, M.M.; Elzatahry, A.A. Polymer-based electrospun nanofibers for biomedical applications. Nanomaterials 2018, 8, 259. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhou, S.; Gao, Y.; Zhai, Y. Electrospun nanofibers as a wound dressing for treating diabetic foot ulcer. Asian J. Pharm. Sci. 2019, 14, 130–143. [Google Scholar] [CrossRef]
- Kim, S.E.; Heo, D.N.; Lee, J.B.; Kim, J.R.; Park, S.H.; Jeon, S.H.; Kwon, I.K. Electrospun gelatin/polyurethane blended nanofibers for wound healing. Biomed. Mater. 2009, 4, 044106. [Google Scholar] [CrossRef]
- Liu, G.; Gu, Z.; Hong, Y.; Cheng, L.; Li, C. Electrospun starch nanofibers: Recent advances, challenges, and strategies for potential pharmaceutical applications. J. Control. Releas. 2017, 252, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Fahimirad, S.; Ajalloueian, F. Naturally-derived electrospun wound dressings for target delivery of bio-active agents. Int. J. Pharm. 2019, 566, 307–328. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Nourbakhsh, M.S. Electrospun polycaprolactone scaffolds for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 527–539. [Google Scholar] [CrossRef]
- Sang-Myun, J.; Seul, K.M.; Ho, C.L.; Yeo, S.K.; Moon, H.J.; Hwa, S.S. Spirulina-PCL Nanofiber Wound Dressing to Improve Cutaneous Wound Healing by Enhancing Antioxidative Mechanism. J. Nanomat. 2016, 2016, 10. [Google Scholar]
- Liji, Z.; Thomas, J.W. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today 2009, 4, 66–80. [Google Scholar]
- Nadem, S.; Ziyadi, H.; Hekmati, M.; Baghali, M. Cross-linked poly(vinyl alcohol) nanofibers as drug carrier of clindamycin. Polym. Bull. 2019. [Google Scholar] [CrossRef]
- Fang, Y.; Zhu, X.; Wang, N.; Zhang, X.; Yang, D.; Nie, J.; Ma, G. Biodegradable core-shell electrospun nanofibers based on PLA and γ-PGA for wound healing. Eur. Polym. J. 2019, 116, 30–37. [Google Scholar] [CrossRef]
- Abrigo, M.; McArthur, S.L.; Kingshott, P. Electrospun nanofibers as dressings for chronic wound care: Advances, challenges, and future prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef]
- Kalantari, K.; Afifi, A.M.; Jahangirian, H.; Webster, T.J. Biomedical applications of chitosan electrospun nanofibers as a green polymer—Review. Carbohydr. Polym. 2019, 207, 588–600. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef]
- Kumar, P.S.; Sundaramurthy, J.; Sundarrajan, S.; Babu, V.J.; Singh, G.; Allakhverdiev, S.; Ramakrishna, S. Hierarchical electrospun nanofibers for energy harvesting, production, and environmental remediation. Energy Envir. Sci. 2014, 7, 3192–3222. [Google Scholar] [CrossRef]
- Xiaomin, S.; Weiping, Z.; Delong, M.; Qian, M.; Denzel, B.; Ying, M.; Anming, H. Electrospinning of Nanofibers and Their Applications for Energy Devices. J. Nanomat. 2015, 2015, 20. [Google Scholar]
- Ramakrishna, S.; Jose, R.; Archana, P.S.; Nair, A.S.; Balamurugan, R.; Teo, W.E. Science and engineering of electrospun nanofibers for advances in clean energy, water filtration, and regenerative medicine. J. Mat. Sci. 2010, 45, 6283–6312. [Google Scholar] [CrossRef]
- Dai, T.; Tanaka, M.; Huang, Y.; Hamblin, M. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti. Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, M.; Aziz, A.S.; Ubaidulla, U.; Hemalatha, P.; Saravanakumar, A.; Ravikumar, R.; Peng, M.M.; Choi, E.Y.; Jang, H.T. Sulfanilamide and silver nanoparticles-loaded polyvinyl alcohol-chitosan composite electrospun nanofibers: Synthesis and evaluation on synergism in wound healing. J. Ind. Eng. Chem. 2016, 39, 127–135. [Google Scholar] [CrossRef]
- Adeli, H.; Taghi, M.; Parvazinia, M. Wound dressing based on electrospun PVA/ chitosan/ starch nanofibrous mats: Fabrication, antibacterial and cytocompatibility evaluation and in vitro healing assay. Int. J. Biol. Macromol. 2019, 122, 238–254. [Google Scholar] [CrossRef]
- Iqbal, H.; Ali, B.; Ullah, Z.; Razzaq, A.; Ullah, N.; Menaa, B.; Menaa, F. Fabrication, physical characterizations and in vitro antibacterial activity of cefadroxil-loaded chitosan/ poly (vinyl alcohol) nanofibers against Staphylococcus aureus clinical isolates. Int. J. Biol. Macromol. 2020, 144, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Alavarse, A.C.; Silva, F.W.D.O.; Colque, J.T.; da Silva, V.M.; Prieto, T.; Venancio, E.C.; Bonvent, J. Tetracycline hydrochloride-loaded electrospun nano fi bers mats based on PVA and chitosan for wound dressing. Mater. Sci. Eng. C 2017, 77, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, H.; Liu, X.; Mao, J.; Gu, S.; Xu, W. Electrospinning of carboxyethyl chitosan/poly (vinyl alcohol)/silk fibroin nanoparticles for wound dressings. Int. J. Biol. Macromol. 2013, 53, 88–92. [Google Scholar] [CrossRef]
- Majd, S.A.; Khorasgani, M.R.; Moshtaghian, S.J.; Talebi, A.; Khezri, M. Application of Chitosan/PVA Nano fiber as a potential wound dressing for streptozotocin-induced diabetic rats. Int. J. Biol. Macromol. 2016, 92, 1162–1168. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Reda, M.M.; Klingner, A. Preparation and characterization of green carboxymethylchitosan (CMCS)–Polyvinyl alcohol (PVA) electrospun nano fi bers containing gold nanoparticles (AuNPs) and its potential use as biomaterials. Int. J. Biol. Macromol. 2020, 151, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Omidi, M.; Chen, X. Development of the PVA/ CS nano fi bers containing silk protein sericin as a wound dressing: In vitro and in vivo assessment. Int. J. Biol. Macromol. 2020, 149, 513–521. [Google Scholar] [CrossRef]
- Kharaghani, D.; Khan, M.Q.; Tamada, Y.; Ogasawara, H.; Inoue, Y.; Saito, Y.; Hashmi, M.; Kim, I.S. Fabrication of electrospun antibacterial PVA / Cs nanofibers loaded with CuNPs and AgNPs by an in-situ method. Polym. Test. 2018, 17, 315–321. [Google Scholar] [CrossRef]
- Zhang, K.; Bai, X.; Yuan, Z.; Cao, X.; Jiao, X.; Li, Y.; Qin, Y.; Wen, Y.; Zhang, X. Layered nano fi ber sponge with an improved capacity for promoting blood coagulation and wound healing. Biomaterials 2019, 204, 70–79. [Google Scholar] [CrossRef]
- Sannasimuthu, A.; Ramani, M.; Ahamad, B.; Pasupuleti, M.; Al-sadoon, M.K.; Alagumuthu, T.S.; Al-Mfarij, A.R.; Arshad, A.; Mala, K.; Arockiaraj, J. Arthrospira platensis transglutaminase derived antioxidant peptide-packed electrospun chitosan / poly (vinyl alcohol) nano fi brous mat accelerates wound healing, in vitro, via inducing mouse embryonic fibroblast proliferation. Coll.Surf. B Biointerfac. 2020, 193, 111124. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, X.; Zhang, D. Electrospun Chitosan / Poly (Vinyl Alcohol) / Graphene Oxide Nanofibrous Membrane with Ciprofloxacin Antibiotic Drug for Potential Wound Dressing Application. Int. J. Mol. Sci. 2019, 20, 4395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Yan, F.; Ren, P.; Li, Y.; Wu, Q.; Fang, X.; Chen, F.; Wang, C. Incorporation of metal-organic frameworks into electrospun chitosan / poly (vinyl alcohol) nano fi brous membrane with enhanced antibacterial activity for wound dressing application. Int. J. Biol. Macromol. 2020, 158, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Shokrollahi, M.; Bahrami, S.H.; Nazarpak, M.H.; Solouk, A. Multilayer nano fi brous patch comprising chamomile loaded carboxyethyl chitosan / poly (vinyl alcohol) and polycaprolactone as a potential wound dressing. Int. J. Biol. Macromol. 2020, 147, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Tariq, M.; Ali, I.; Asghar, R.; Khanam, P.N.; Augustine, R.; Hasan, A. Novel electrospun chitosan/polyvinyl alcohol / zinc oxide nano fi brous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Biol. Macromol. 2018, 120, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Koosha, M.; Raou, M.; Moravvej, H. One-pot reactive electrospinning of chitosan / PVA hydrogel nano fi bers reinforced by halloysite nanotubes with enhanced fi broblast cell attachment for skin tissue regeneration. Coll. Surf. B Biointer. 2019, 179, 270–279. [Google Scholar] [CrossRef]
- Naeimi, A.; Payandeh, M.; Ghara, A.R.; Ghadi, F.E. In vivo evaluation of the wound healing properties of bio-nano fi ber chitosan/polyvinyl alcohol incorporating honey and Nepeta dschuparensis. Carbohydr. Polym. 2020, 240, 116315. [Google Scholar] [CrossRef]
- Sarhan, W.A.; Azzazy, H.M.E. High concentration honey chitosan electrospun nanofibers: Biocompatibility and antibacterial effects. Carbohydr. Polym. 2015, 122, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, A.M.; Hudson, S.M.; Rojas, O.J. Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver-NPs/polyvinyl alcohol) systems. Carbohydr. Polym. 2014, 100, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Fu, R.; Yu, C.; Li, Z.; Guan, H.; Hu, D.; Zhao, D.; Lu, L. Silver nanoparticle/chitosan oligosaccharide/poly (vinyl alcohol) nanofibers as wound dressings: A preclinical study. Int. J. Nanomed. 2013, 8, 4131–4145. [Google Scholar]
- Li, C.; Wang, Q.; Li, J.; Hu, M.; Shi, Z.; Wu, G.; Cui, H.; Li, Y.; Zhang, Q.; Yu, X.; et al. Silver nanoparticles / chitosan oligosaccharide/poly (vinyl alcohol) nanofiber promotes wound healing by activating TGF β 1 / Smad signaling pathway. Int. J. Nanomed. 2016, 11, 373–387. [Google Scholar]
- Kang, Y.O.; Yoon, I.; Lee, S.Y.; Kim, D.; Lee, S.J.; Park, W.H.; Hudson, S.M. Chitosan-Coated Poly (vinyl alcohol) Nanofibers For Wound Dressings. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009. [Google Scholar] [CrossRef]
- Son, B.; Yeom, B.; Song, S.; Lee, C.; Hwang, T. Antibacterial electrospun chitosan/poly (vinyl alcohol) nanofibers containing silver nitrate and titanium dioxide. J. Appl. Polym. 2008, 111, 2892–2899. [Google Scholar] [CrossRef]
- Chen, C.; Huang, S. Preparation of Reductant–Responsive N-Maleoyl-Functional Chitosan/Poly(vinyl alcohol) Nanofibers for Drug Delivery. Mol. Pharm. 2016, 12, 4152–4167. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, D.; Chen, X.; Xu§, Q.Q.; Lu, F.; Nie, J. Electrospun Water-Soluble Carboxyethyl Chitosan/Poly(vinyl alcohol) Nanofibrous Membrane as Potential Wound Dressing for Skin Regeneration. Biomacromolecules 2008, 9, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Roy, A.K.; Webster, T.J. Development of Chitosan/Poly (Vinyl Alcohol Electrospun Nanofibers for Infection Related Wound Healing. Front. Pharmacol. 2017, 7, 683. [Google Scholar] [CrossRef]
- Sundaramurthi, D.; Vasanthan, K.S.; Kuppan, P.; Krishnan, U.M.; Sethuraman, S. Electrospun nanostructured chitosan—Poly (vinyl alcohol) scaffolds: A biomimetic extracellular matrix as dermal. Biomed. Mater. 2012, 7, 055005. [Google Scholar] [CrossRef] [PubMed]
- Celebi, H.; Gurbuz, M.; Koparal, S.; Dogan, A. Development of antibacterial electrospun chitosan / poly (vinyl alcohol) nanofibers containing silver ion-incorporated HAP nanoparticles. Compos. Interf. 2013, 20, 799–812. [Google Scholar] [CrossRef]
- Yang, S.; Lei, P.; Shan, Y.; Zhang, D. Preparation and characterization of antibacterial electrospun chitosan/ poly (vinyl alcohol)/ graphene oxide composite nanofibrous membrane. Appl. Surf. Sci. 2018, 435, 832–840. [Google Scholar] [CrossRef]
- Gholipour-Kanani, A.; Bahrami, S.H.; Samadi-kochaksaraie, A.; Ahmadi-Tafti, H.; Rabbani, S.; Kororian, A.; Erfani, E. Effect of tissue-engineered chitosan-poly (vinyl alcohol) nanofibrous scaffolds on healing of burn wounds of rat skin. IET Nanobiotechnol. 2012, 1–7. [Google Scholar] [CrossRef]
- Kegere, J.; Ouf, A.; Siam, R.; Mamdouh, W. Fabrication of Poly (vinyl alcohol)/Chitosan/Bidens pilosa Composite Electrospun Nano fi bers with Enhanced Antibacterial Activities. ACS OMEGA 2019, 4, 8778–8785. [Google Scholar] [CrossRef] [Green Version]
- Levengood, S.L.; Erickson, A.E.; Chang, F.; Zhang, M. Chitosan-Poly(caprolactone) Nanofibers for Skin Repair. J. Mater. Chem. B Mater. Biol. Med. 2018, 5, 1822–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Liang, Y.; Shi, M.; Guo, B. Anti-oxidant electroactive and antibacterial nano fi brous wound dressings based on poly (ε-caprolactone)/quaternized chitosan-graft-polyaniline for full-thickness skin wound healing. Chem. Eng. J. 2020, 385, 123464. [Google Scholar] [CrossRef]
- Afshar, S.; Rashedi, S.; Nazockdast, H.; Ghazalian, M. Preparation and characterization of electrospun poly (lactic acid)—Chitosan core-shell nano fi bers with a new solvent system. Int. J. Biol. Macromol. 2019, 138, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Gholipour-kanani, A.; Bahrami, S.H.; Joghataie, M.T.; Samadikuchaksaraei, A.; Ahmadi-Taftie, H.; Rabbani, S.; Kororian, A.; Erfan, E.; Erfani, E. Tissue engineered poly (caprolactone)-chitosan-poly (vinyl alcohol) nanofibrous scaffolds for burn and cutting wound healing. IET Nanobiotechnol. 2014, 8, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Xu, L. Batch preparation of electrospun polycaprolactone/chitosan/aloe vera blended nanofiber membranes for novel wound dressing. Int. J. Biol. Macromol. 2020, 160, 352–363. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, H.; Zhang, J.; Li, X.; Wu, Y.; Wei, Y.; Ji, S.; Kong, D.; Zhao, Q. Functional poly(e-caprolactone)/chitosan dressings with nitric oxide- releasing property improve wound healing. Acta Biomater. 2017, 54, 128–137. [Google Scholar] [CrossRef]
- Lemraski, E.G.; Jahangirian, H.; Dashti, M.; Khajehali, E.; Sharafinia, S.; Rafiee-Moghaddam, R.; Webster, T.J. Antimicrobial Double-Layer Wound Dressing Based on Chitosan/Polyvinyl Alcohol/Copper: In vitro and in vivo Assessment. Int. J. Nanomed. 2021, 16, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Chanda, A.; Adhikari, J.; Ghosh, A.; Roy, S.; Thomas, S.; Datta, P.; Saha, P. Electrospun chitosan / polycaprolactone-hyaluronic acid bilayered scaffold for potential wound healing applications. Int. J. Biol. Macromol. 2018, 116, 774–785. [Google Scholar] [CrossRef]
- Poornima, B.; Korrapati, P.S. Fabrication of chitosan-polycaprolactone composite nanofibrous scaffold for simultaneous delivery of ferulic acid and resveratrol. Carbohydr. Polym. 2017, 157, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Yoon, G.H.; Lee, H.C.; Shin, H.S. Chitosan nanoparticle/PCL nanofiber composite for wound dressing and drug delivery. J. Biomater. Sci. Polym. Ed. 2015, 26, 252–263. [Google Scholar] [CrossRef]
- Zhong, S.P.; Zhang, Y.; Lim, C.T. Tissue scaffolds for skin wound healing and dermal reconstruction. Nanomed. Nanobiotechnol. 2010, 2, 510–525. [Google Scholar] [CrossRef]
- Kawai, K.; Suzuki, S.; Tabata, Y.; Nishimura, Y. Accelerated wound healing through the incorporation of basic fibroblast growth factorimpregnated gelatin microspheres into artificial dermis using a pressure-induced decubitus ulcer model in genetically diabetic mice. Br. J. Plast. Surg. 2005, 58, 1115–1123. [Google Scholar] [CrossRef]
- Torabi, N.; Khorram, M.; Zomorodian, K.; Yazdanpanah, S.; Veisi, H.; Veisi, H. Evaluation of electrospun poly (vinyl alcohol)-based nanofiber mats incorporated with Zataria multiflora essential oil as potential wound dressing. Int. J. Biol. Macromol. 2019, 125, 743–750. [Google Scholar]
- Ahlawat, J.; Kumar, V.; Gopinath, P. Carica papaya loaded poly (vinyl alcohol) -gelatin nano fibrous scaffold for potential application in wound dressing. Mater. Sci. Eng. C 2019, 103, 109834. [Google Scholar] [CrossRef]
- Alishahi, M.; Khorram, M.; Asgari, Q.; Davani, F.; Goudarzi, F.; Emami, A.; Arastehfar, A.; Zomorodian, K. Glucantime-loaded electrospun core-shell nano fi bers composed of poly (ethylene oxide)/gelatin-poly (vinyl alcohol)/chitosan as dressing for cutaneous leishmaniasis. Int. J. Biol. Macromol. 2020, 163, 288–297. [Google Scholar] [CrossRef]
- Ajmal, G.; Bonde, G.V.; Mittal, P.; Khan, G.; Pandey, V.K.; Bakade, B.V.; Mishra, B. Biomimetic PCL-gelatin based nano fi bers loaded with cipro fl oxacin hydrochloride and quercetin: A potential antibacterial and anti-oxidant dressing material for accelerated healing of a full thickness wound. Int. J. Pharm. 2019, 567, 118480. [Google Scholar] [CrossRef]
- Ghaee, A.; Bagheri-khoulenjani, S.; Amir, H.; Bogheiri, H. Biomimetic nanocomposite scaffolds based on surface modified PCL-nanofibers containing curcumin embedded in chitosan/gelatin for skin regeneration. Compos. Part B 2019, 177, 107339. [Google Scholar] [CrossRef]
- Shi, R.; Geng, H.; Gong, M.; Ye, J.; Wu, C.; Hu, X. Long-acting broad-spectrum antimicrobial electrospun poly (e-caprolactone)/gelatin micro/nanofibers for wound dressing. J. Coll. Interf. Sci. 2018, 509, 275–284. [Google Scholar] [CrossRef]
- Jafari, A.; Amirsadeghi, A.; Hassanajili, S.; Azarpira, N. Bioactive antibacterial bilayer PCL/gelatin nanofibrous scaffold promotes full-thickness wound healing. Int. J. Pharm. 2020. [Google Scholar] [CrossRef] [PubMed]
- Basar, A.O.; Castro, S.; Torres-giner, S.; Lagaron, J.M.; Sasmazel, H.T. Novel poly (ε-caprolactone)/gelatin wound dressings prepared by emulsion electrospinning with controlled release capacity of Ketoprofen anti- inflammatory drug. Mater. Sci. Eng. C 2017, 81, 459–468. [Google Scholar] [CrossRef]
- Naseri-nosar, M.; Farzamfar, S.; Sahrapeyma, H.; Ghorbani, S.; Bastami, F.; Vaez, A.; Salehi, M. Cerium oxide nanoparticle-containing poly (ε-caprolactone)/gelatin electrospun fi lm as a potential wound dressing material: In vitro and in vivo evaluation. Mater. Sci. Eng. C 2017, 81, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Pavli, V.; Fohlerová, Z.; Pavli, D.; Khunová, V.; Vojtová, L. Effect of halloysite nanotube structure on physical, chemical, structural and biological properties of elastic polycaprolactone/gelatin nano fi bers for wound healing applications. Mater. Sci. Eng. C 2018, 91, 94–102. [Google Scholar]
- Farzamfar, S.; Naseri-Nosar, M.; Samadian, H.; Mahakizadeh, S.; Tajerian, R.; Rahmati, M.; Vaez, A.; Salehi, M. Taurine-loaded poly (ε-caprolactone)/gelatin electrospun mat as a potential wound dressing material: In vitro and in vivo evaluation. J. Bioact. Compat. Polym. 2018, 33, 282–294. [Google Scholar] [CrossRef]
- Unalan, I.; Endlein, S.J.; Slavik, B.; Buettner, A.; Goldmann, W.H.; Detsch, R.; Boccaccini, A.R. Evaluation of Electrospun Poly (ε-Caprolactone)/ Gelatin Nanofiber Mats Containing Clove Essential Oil for Antibacterial Wound Dressing. Pharmaceuticals 2019, 11, 570. [Google Scholar] [CrossRef] [Green Version]
- Ramalingam, R.; Dhand, C.; Leung, C.M.; Ezhilarasu, H.; Prasannan, M.; Ong, S.T.; Subramanian, S.; Kamruddin, M.; Lekshminarayanan, R.; Ramakrishna, S.; et al. Poly-ε-Caprolactone/ Gelatin Hybrid Electrospun Composite Nanofibrous Mats Containing Ultrasound Assisted Herbal Extract: Antimicrobial and Cell Proliferation Study. Nanomaterials 2019, 9, 462. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Guan, J.; Guo, S.; Guo, F.; Niu, X.; Liu, Q.; Zhang, C.; Nie, H.; Wang, Y. Human urine-derived stem cells in combination with polycaprolactone/gelatin nanofibrous membranes enhance wound healing by promoting angiogenesis. J. Transl. Med. 2014, 12, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeli-sardou, M.; Mehdi, M.; Torkzadeh-mahani, M.; Dodel, M. Controlled release of lawsone from polycaprolactone / gelatin electrospun nano fi bers for skin tissue regeneration. Int. J. Biol. Macromol. 2019, 124, 478–491. [Google Scholar] [CrossRef] [PubMed]
- d’Ayala, G.G.; Malinconico, M.; Laurienzo, P. Marine derived polysaccharides for biomedical applications: Chemical modification approaches. Molecules 2008, 13, 2069–2106. [Google Scholar] [CrossRef] [Green Version]
- Naja, M.; Osfouri, S.; Azin, R.; Zaeri, S. Alginate-based electrospun core / shell nano fi bers containing dexpanthenol: A good candidate for wound dressing. J. Drug Deliv. Sci. Technol. 2020, 57, 101708. [Google Scholar]
- Zhu, L.; Liu, X.; Du, L.; Jin, Y. Preparation of asiaticoside-loaded coaxially electrospinning nano fi bers and their effect on deep partial-thickness burn injury. Biomed. Pharmacother. 2016, 83, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Shalumon, K.T.; Anulekha, K.H.; Nair, S.V.; Nair, S.V.; Chennazhi, K.P.; Jayakumar, R. Sodium alginate / poly (vinyl alcohol)/ nano ZnO composite nanofibers for antibacterial wound dressings. Int. J. Biol. Macromol. 2011, 49, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Arthanari, S.; Mani, G.; Jang, J.H.; Choi, J.O.; Cho, Y.H.; Lee, G.H.; Cha, S.E.; Oh, H.S.; Kwon, D.H.; Jang, H.T. Preparation and characterization of gatifloxacin- loaded alginate/ poly (vinyl alcohol) electrospun nanofibers Preparation and characterization of gatifloxacin-loaded alginate/ poly (vinyl alcohol) electrospun nanofibers. Artif. Cells Nanomedicine Biotechnol. 2016, 44, 847–852. [Google Scholar]
- Fu, R.; Li, C.; Yu, C.; Xie, H.; Shi, S.; Li, Z.; Lu, L. A novel electrospun membrane based on moxifloxacin hydrochloride/poly (vinyl alcohol)/sodium alginate for antibacterial wound dressings in practical application A novel electrospun membrane based on moxifloxacin hydrochloride/ poly (vinyl alcohol). Drug Deliv. 2016, 3, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Rashtchian, M.; Hivechi, A.; Bahrami, S.H.; Milan, P.B.; Simorgh, S. Fabricating alginate/poly (caprolactone) nano fi bers with enhanced bio-mechanical properties via cellulose nanocrystal incorporation. Carbohydr. Polym. 2020, 233, 115873. [Google Scholar] [CrossRef]
- Udaseen, S.; Asthana, S.; Raveendran, N.T.; Kumar, K.; Samal, A.; Pal, K.; Pramanik, K.; Ray, S.S. Optimization of process parameters for nozzle—Free electrospinning of poly (vinyl alcohol) and alginate blend nano-fibrous scaffolds. Int. J. Enhanc. Res. Sci. Technol. Eng. 2014, 3, 405–411. [Google Scholar]
- Coskun, G.; Karaca, E.; Ozyurtlu, M.; Özbek, S.; Yermezler, A.; Cavysoglu, I. Histological evaluation of wound healing performance of electrospun poly(vinyl alcohol)/sodium alginate as wound dressing in vivo. Biomed. Mater. Eng. 2014, 24, 1527–1536. [Google Scholar] [PubMed]
- Üstündağ, G.; Karaca, E.; Özbek, S.; Çavuşoğlu, L. In vivo evaluation of electrospun poly (vinyl alcohol)/ sodium alginate nanofibrous mat as wound dressing. Ref. Res. 2010, 4, 90–298. [Google Scholar]
- Mano, J.F.; Silva, G.A.; Azevedo, H.S. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R. Soc. Interf. 2007, 4, 999–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, X.; Yu, H.; Zhang, C. Nano-porous nitrocellulose liquid bandage modulates cell and cytokine response and accelerates cutaneous wound healing in a mouse model. Carbohydr. Polym. 2016, 136, 618–629. [Google Scholar] [CrossRef]
- Shefa, A.A.; Sultana, T.; Ki, M.; Lee, S.Y.; Gwon, J.; Lee, B. Curcumin incorporation into an oxidized cellulose nanofiber-polyvinyl alcohol hydrogel system promotes wound healing. Mater. Des. 2020, 186, 108313. [Google Scholar] [CrossRef]
- Zulkifli, F.H.; Shahitha, F.; Hussain, J. Improved cellular response of chemically crosslinked collagen in corporated hydroxyethyl cellulose / poly (vinyl) alcohol nanofibers scaffold. J. Biomater. Appl. 2015, 29, 1014–1027. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Menazea, A.A.; Abdelghany, A.M. Blend biopolymeric nano fi brous scaffolds of cellulose acetate/ ε-polycaprolactone containing metallic nanoparticles prepared by laser ablation for wound disinfection applications. Int. J. Biol. Macromol. 2020, 155, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Doostan, M.; Khorramizadeh, M.R. Antibacterial and antioxidant assessment of cellulose acetate/ polycaprolactone nano fi brous mats impregnated with propolis. Int. J. Biol. Macromol. 2019, 140, 1260–1268. [Google Scholar] [CrossRef]
- Liao, Y.H.; Jones, S.A.; Forbes, B.; Martin, G.P.; Brown, M.B. Hyaluronan pharmaceutical characterization and drug delivery. Drug Deliv. 2005, 12, 327–342. [Google Scholar] [CrossRef]
- Uppal, R.; Ramanswamy, G.N.; Arnold, C.; Goodband, R.; Wang, Y. Hyaluronic acid nanofiber wound dressing-production, characterization, and in vivo behavior. J Biomed. Mater. Res. 2011, 97B, 20–29. [Google Scholar] [CrossRef]
- Séon-Lutz, M.; Couffin, A.C.; Vignoud, S.; Schlatter, G.; Hébraud, A. Electrospinning in water and in situ crosslinking of hyaluronic acid/ cyclodextrin nanofibers: Towards wound dressing with controlled drug release. Carbohydr. Polym. 2019, 207, 276–287. [Google Scholar] [CrossRef]
- Chen, X.; Lu, B.; Zhou, D.; Shao, M.; Xu, W.; Zhou, Y. Photocrosslinking maleilated hyaluronate/methacrylated poly (vinyl alcohol) nanofibrous mats for hydrogel wound dressings. Int. J. Biol. Macromol. 2020, 155, 903–910. [Google Scholar] [CrossRef]
- Wang, Z.; Qian, Y.; Li, L.; Pan, L.; Njunge, L.W.; Dong, L.; Yang, L. Evaluation of emulsion electrospun polycaprolactone/hyaluronan/epidermal growth factor nanofibrous scaffolds for wound healing. J. Biomater. Appl. 2016, 30, 686–698. [Google Scholar] [CrossRef]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The use of natural polymers in tissue engineering: A focus on electrospun extracellular matrix analogues. Polym. Adv. Technol. 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Arul, V.; Kartha, R.; Jayakumar, R. A therapeutic approach for diabetic wound healing using biotinylated GHK incorporated collagen matrices. Life Sci. 2007, 80, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Singh, O.; Gupta, S.S.; Soni, M.; Moses, S.; Shukla, S.; Mathur, R.K. Collagen dressing versus conventional dressings in burn and chronic wounds: A retrospective study. J. Cutan. Aesthet. Surg. 2011, 4, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Senthil, R.; Berly, R.; Ram, T.B.; Gobi, N. Electrospun poly(Vinyl) alcohol/collagen nanofibrous scaffold hybridized by graphene oxide for accelerated wound healing. Int. J. Artif. Organs 2018, 41, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Sobhanian, P.; Khorram, M.; Hashemi, S.S.; Mohammadi, A. Development of nanofibrous collagen-grafted poly (vinyl alcohol)/gelatin/alginate scaffolds as potential skin substitute. Int. J. Biol. Macromol. 2019, 130, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wang, H. Spatial arrangement of polycaprolactone/collagen nanofiber scaffolds regulates the wound healing related behaviors of human adipose stromal cells. Tissue Eng. Part A 2012, 18, 631–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, J.; Chen, L.; Liu, Z.; Li, J.; Yang, J.; Zhong, A.; Zhou, M.; Sun, Y.; Guo, L.; Yang, Y.; et al. Sustained release of N-acetylcysteine by sandwich structured polycaprolactone/collagen scaffolds for wound healing. J. Biomed. Mater. Res. Part A 2019. [Google Scholar] [CrossRef]
- Tort, S.; Acartürk, F.; Beşikci, A. Evaluation of three-layered doxycycline-collagen loaded nanofiber wound dressing. Int. J. Pharm. 2017, 529, 642–653. [Google Scholar] [CrossRef]
- Guo, S.; Di Pietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Bahrami, S.H.; Joghataei, M.T. Fabrication of novel nanofiber scaffolds from gum tragacanth/poly(vinyl alcohol) for wound dressing application: In vitro evaluation and antibacterial properties. Mater. Sci. Eng. C 2013, 33, 4935–4943. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Kargozar, S.; Bahrami, S.H.; Joghataei, M.T. Fabrication of curcumin-loaded gum tragacanth/poly(vinyl alcohol) nanofibers with optimized electrospinning parameters. J. Ind. Text. 2017, 46, 1170–1192. [Google Scholar] [CrossRef]
- Eghbalifam, N.; Shojaosadati, S.A.; Hashemi-najafabadi, S.; Khorasani, A.C. Synthesis and characterization of antimicrobial wound dressing material based on silver nanoparticles loaded gum Arabic nanofibers. Int. J. Biol. Macromol. 2020, 155, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Merrell, J.; McLaughlin, S.; Tie, L.; Laurencin, C.; Chen, A.; Nair, L. Curcumin-loaded poly (ε-caprolactone) nanofibres: Diabetic wound dressing withanti-oxidant and anti-inflammatory properties. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1149–1156. [Google Scholar] [CrossRef] [Green Version]
- Ranjbar-Mohammadi, M.; Bahrami, S.H. Electrospun curcumin loaded poly(ε-caprolactone)/gum tragacanth nanofibers for biomedical application. Int. J. Biol. Macromol. 2016, 84, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-mohammadi, M. Characteristics of aloe vera incorporated poly (e-caprolactone)/gum tragacanth nanofibers as dressings for wound care. J. Ind. Eng. Chem. 2018, 47, 1464–1477. [Google Scholar] [CrossRef]
- Ranjbar-mohammadi, M.; Rabbani, S.; Bahrami, S.H.; Joghataei, M.T.; Moayer, F. Antibacterial performance and in vivo diabetic wound healing of curcumin loaded gum tragacanth/poly (ε-caprolactone) electrospun nanofibers. Mater. Sci. Eng. C 2016, 69, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Inpanya, P.; Faikrua, A.; Ounaroon, A.; Sittichokechaiwut, A.; Viyoch, J. Effects of the blended fibroin/aloe gel film on wound healing in streptozotocin-induced diabetic rats. Biomed. Mater. 2012, 7, 035008. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kundu, S.C. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials 2009, 30, 2956–2965. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Wang, G.; Tseng, C.; Su, W. Epidermal cells differentiated from stem cells from human exfoliated deciduous teeth and seeded onto polyvinyl alcohol / silk fi broin nano fiber dressings accelerate wound repair. Mater. Sci. Eng. C 2019, 104, 109986. [Google Scholar] [CrossRef]
- Kheradvar, S.A.; Nourmohammadi, J.; Tabesh, H.; Bagheri, B. Starch nanoparticle as a vitamin E-TPGS carrier loaded in silk fibroin-poly(vinyl alcohol)-Aloe vera nanofibrous dressing. Coll. Surf. B Biointerf. 2018, 166, 9–16. [Google Scholar] [CrossRef]
- Ojah, N.; Saikia, D.; Gogoi, D.; Baishya, P.; Ahmed, G.A.; Ramteke, A.; Choudhury, A.J. Surface modification of core-shell silk/PVA nanofibers by oxygen dielectric barrier discharge plasma: Studies of physico-chemical properties and drug release behavior. Appl. Surf. Sci. 2019, 475, 219–229. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.; Li, D.; Qin, J.; Zhang, M.; Wang, K.; Zhao, J.; Zhang, L. LBL deposition of chitosan/heparin bilayers for improving biological ability and reducing infection of nanofibers. Int. J. Biol. Macromol. 2020, 154, 999–1006. [Google Scholar] [CrossRef]
- Chouhan, D.; Janani, G.; Chakraborty, B.; Samit, S.; Nandi, K.; Mandal, B. Functionalized PVA–silk blended nanofibrous mats promote diabetic wound healing via regulation of extracellular matrix and tissue remodelling. J. Tissue Eng. Regen. Med. 2017, 12, e1559–e1570. [Google Scholar] [CrossRef] [PubMed]
- Rathur, H.M.; Bloulton, A.J.M. Recent advances in the diagnosis and management of diabetic neuropathy. J. Bone Jt. Surg. 2005, 87, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Aadil, K.R.; Mussatto, S.I.; Jha, H. Synthesis and characterization of silver nanoparticles loaded poly (vinyl alcohol) -lignin electrospun nano fi bers and their antimicrobial activity. Int. J. Biol. Macromol. 2018, 120, 763–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.S.; Kim, Y.O.; Ha, Y.M.; Lim, D.; Hwang, J.Y.; Kim, J.; Park, M.; Cho, J.W.; Jung, Y.C. Antimicrobial properties of lignin-decorated thin multi-walled carbon nanotubes in poly(vinyl alcohol) nanocomposites. Eur. Polym. J. 2018, 105, 79–84. [Google Scholar] [CrossRef]
- Balakrishnan, S.B.; Thambusamy, S. Preparation of silver nanoparticles and riboflavin embedded electrospun polymer nanofibrous sca ff olds for in vivo wound dressing application. Process Biochem. 2020, 88, 148–158. [Google Scholar] [CrossRef]
- Azarian, M.H.; Boochathum, P.; Kongsema, M. Biocompatibility and biodegradability of filler encapsulated chloroacetated natural rubber/polyvinyl alcohol nanofiber for wound dressing. Mater. Sci. Eng. C 2019, 103, 109829. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, Y.; Li, Z.; Tang, P.; Tang, Y.; Zhang, Y.; Nie, X.; Fang, C.; Li, X.; Zhang, H. Konjac glucomannan/polyvinyl alcohol nanofibers with enhanced skin healing properties by improving fibrinogen adsorption. Mater. Sci. Eng. C 2020, 110, 110718. [Google Scholar] [CrossRef] [PubMed]
- Alipour, R.; Khorshidi, A.; Shojaei, A.F.; Mashayekhi, F.; Moghaddam, M.J.M. Skin wound healing acceleration by Ag nanoparticles embedded in PVA/PVP/Pectin/Mafenide acetate composite nanofibers. Polym. Test. 2019, 79, 106022. [Google Scholar] [CrossRef]

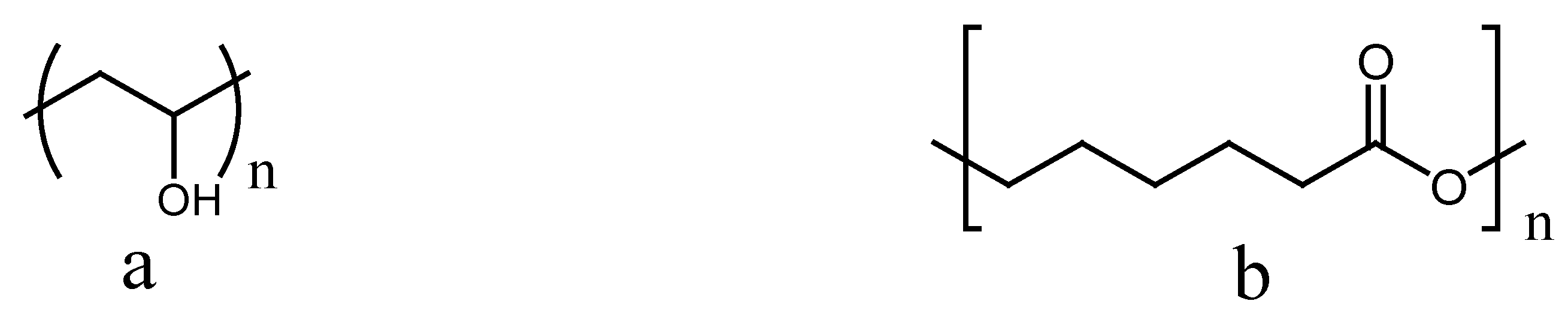



| Polymers Used | Loaded Bioactive Agents | Study Outcomes | Reference |
|---|---|---|---|
| Chitosan and PVA | Ag nanoparticles | High swelling capacity and accelerated wound healing | [56] |
| Chitosan and PVA | - | Good mechanical performance and excellent biocompatibility with high antibacterial effects | [57] |
| Chitosan and PVA | Cefadroxil | Sustained drug release and good antibacterial activity | [58] |
| Chitosan and PVA | Tetracycline | Good antibacterial efficacy and fast wound recovery | [59] |
| N-carboxyethyl chitosan and PVA | - | Non-toxicity | [60] |
| Chitosan and PVA | - | The accelerated diabetic wound healing process | [61] |
| Carboxymethyl chitosan and PVA | Au nanoparticles | Non-toxicity and high antibacterial effects | [62] |
| Chitosan and PVA | Silk protein sericin | Excellent biocompatibility and accelerated wound healing process | [63] |
| Chitosan and PVA | Ag and Au nanoparticles | Superior antimicrobial activity | [64] |
| Chitosan and PVA | - | Accelerated wound healing mechanism | [65] |
| Chitosan and PVA | Arthrospira platensis | High cell viability and potential wound healing process | [66] |
| Chitosan and PVA | Graphene oxide | Good bactericidal activity | [67] |
| Chitosan and PVA | Cu metal-organic frameworks | Excellent cell adhesion and proliferation with a fast wound healing process | [68] |
| Carboxyethyl chitosan and PVA | Chamomile | Good antioxidant and antibacterial activity | [69] |
| Chitosan and PVA | ZnO nanoparticles | Good antibacterial and fast diabetic wound healing | [70] |
| Chitosan and PVA | Halloysite nanotubes | Good biocompatibility and cell attachment | [71] |
| Chitosan and PVA | Nepeta dschuparensis and honey | Faster burn wound healing process | [2] |
| Chitosan and PVA | Honey | Superior antibacterial efficacy | [73] |
| Chitosan and PVA | Ag nanoparticles | Superior synergistic antibacterial effects | [74] |
| Chitosan oligosaccharides and PVA | Ag nanoparticles | High antibacterial efficacy and fast wound closure | [75] |
| Chitosan oligosaccharide and PVA | Ag nanoparticles | Accelerated wound healing process | [76] |
| Chitosan and PVA | - | Good wound healing properties | [77] |
| Chitosan and PVA | Ag nitrate and titanium | Excellent antibacterial activity | [78] |
| N-Maleoyl-functional chitosan and PVA | Tetracycline hydrochloride | Good wound-healing effects and superior antibacterial effects | [79] |
| Chitosan and PVA | - | Good cell adhesion and proliferation | [80] |
| Chitosan and PVA | - | Potential wound healing management | [81] |
| Chitosan and PVA | - | Accelerated wound healing process | [82] |
| Chitosan and PVA | Ag ions | Excellent antibacterial activity | [83] |
| Chitosan and PVA | Graphene oxide and ciprofloxacin | Good antibacterial efficacy and excellent cytocompatibility | [84] |
| Chitosan and PVA | - | Rapid burn wound healing process [74] | [85] |
| Chitosan and PVA | Bidens pilosa | Good antimicrobial activity | [86] |
| Chitosan and PVA | - | Fast wound healing recovery | [87] |
| Chitosan-graft polyaniline and PCL | - | Good mechanical properties and accelerated wound closure | [88] |
| Chitosan and PLA | Curcumin | Initial burst drug release followed by sustained release | [89] |
| Chitosan, PCL, and PVA | - | The rapid wound healing process | [90] |
| Chitosan and PCL | Aloe vera | Moderate WVTR and Excellent antibacterial activity | [91] |
| Chitosan and PCL | Nitric acid | Fast wound healing mechanism | [92] |
| Chitosan-g-polyaniline and PCL | - | Excellent antibacterial activity and good wound closure | [93] |
| Chitosan, PCL, and HA | - | Good biocompatibility and non-toxicity, | [94] |
| Chitosan and PCL | Resveratrol and ferulic acid | Faster wound contraction rate | [95] |
| Chitosan and PCL | - | Non-toxicity | [96] |
| Chitosan and PCL | Aloe vera | Good mechanical and biological properties | |
| Gelatin and PVA | ZM essential oil | Good cytocompatibility and antibacterial effects | [99] |
| Gelatin and PVA | Carica papaya | good biocompatibility and bactericidal activity | [100] |
| Gelatin, PVA, and chitosan | Glucantime | Good properties for the treatment of Leishmania wounds | [101] |
| Gelatin and PCL | Quercetin and ciprofloxacin | Initial burst drug release followed by sustained frug release with fast wound closure | [102] |
| Gelatin, PCL, and chitosan | Curcumin | High cell attachment and biocompatibility with good antioxidant efficacy | [103] |
| Gelatin and PCL | QAS | Excellent mechanical properties and high antibacterial activity | [104] |
| Gelatin and PCL | Amoxicillin and Zn nanoparticles | Sustained drug release profile, good antibacterial efficacy, and accelerated wound healing | [105] |
| Gelatin and PCL | Ketoprofen | High cell viability indicating good biocompatibility | [106] |
| Gelatin and PCL | Cerium oxide | Moderate WVTR and fast wound recovery | [107] |
| Gelatin and PCL | Halloysite nanotubes | Non-toxicity | [108] |
| Gelatin and PCL | Taurine | Accelerated wound healing process | [109] |
| Gelatin and PCL | Clove essential oil | Superior antibacterial activity | [110] |
| Gelatin and PCL | Gymnema sylvestre | Initial burst release that can contribute to good antibacterial effects | [111] |
| Gelatin and PCL | Human urine-derived stem cells | Improved wound healing properties and increased re-epithelization | [112] |
| Gelatin and PCL | Lawsone | Good mechanical properties, superior antibacterial efficacy, and accelerated wound healing process | [113] |
| Sodium Alginate and PVA | Dexpanthenol | Controlled drug release and good cytocompatibility | [115] |
| Alginate, PVA, and Chitosan | Asiaticoside | Improved wound healing mechanism | [116] |
| Sodium Alginate and PVA | ZnO nanoparticles | Excellent antibacterial activity | [117] |
| Alginate and PVA | Gatifloxacin | Continuous controlled drug release mechanism | [118] |
| Sodium Alginate and PVA | Moxifloxacin | High swelling capacity, good antibacterial efficacy, and superior wound healing process | [119] |
| Alginate and PCL | Nanocrystal cellulose | Non-toxicity | [120] |
| Alginate and PVA | - | Surface morphology that mimics ECM | [121] |
| Alginate and PVA | - | Superior wound healing mechanism | [122] |
| Sodium Alginate and PVA | - | Accelerated wound healing process | [123] |
| Cellulose and PVA | Curcumin | Excellent biocompatibility and fast wound healing process | [126] |
| Hydroxyethyl cellulose and PVA | - | Good mechanical properties and non-toxicity | [127] |
| Cellulose acetate and PCL | Metallic nanoparticles (Ag, CuO, and ZnO nanoparticles) | Good antibacterial activity | [128] |
| Cellulose acetate and PCL | Propolis | Excellent antioxidant and antimicrobial efficacy | [129] |
| HA and PVA | - | Good cytocompatibility and fast wound healing | [132] |
| Hyaluronate-methacrylated and PVA | - | Non-toxicity and high cell adhesion | [133] |
| Hyaluronan and PCL | Epidermal growth factors | Accelerated wound healing | [134] |
| Collagen and PVA | Graphene oxide | Excellent biocompatibility and improved wound healing process | [138] |
| Collagen and PVA | - | High swelling capacity and good cytocompatibility | [139] |
| Collagen and PCL | - | Higher cell proliferation and migration rate | [140] |
| Collagen and PCL | N-acetylcysteine | Initial rapid drug release followed by sustained release, and a fast wound healing process | [141] |
| Collagen and PCL | Doxycycline | Good biocompatibility | [142] |
| Gum tragacanth and PVA | - | High cell adhesion and proliferation, and good antibacterial efficacy | [144] |
| Gum tragacanth and PVA | Curcumin | High cell attachment and proliferation | [145] |
| Gum Arabic and PVA | Ag nanoparticles | Non-toxicity and excellent antimicrobial activity | [146] |
| Gum tragacanth–PCL–PVA | - | Good mechanical performance and accelerated diabetic wound closure | [147] |
| Gum tragacanth and PCL | Curcumin | Initial burst drug release followed by a sustained release | [148] |
| Gum tragacanth and PCL | Aloe vera | High cell proliferation | [149] |
| Gum tragacanth and PCL | Curcumin | Accelerated diabetic wound healing process | [150] |
| Silk fibroin and PVA | Epidermal cells | Fast wound recovery | [153] |
| Silk fibroin and PVA | Starch nanoparticles and Aloe vera | High encapsulation efficiency and good antioxidant efficacy | [154] |
| Silk fibroin and PVA | Amoxicillin trihydrate | Improved mechanical properties and excellent antibacterial activity | [155] |
| Silk fibroin and PCL | - | High antibacterial effects | [156] |
| Silk fibroin and PVA | - | Accelerated diabetic wound healing process | [157] |
| Lignin and PVA | Ag nanoparticles | Good antimicrobial efficacy | [159] |
| Lignin and PVA | - | Enhanced mechanical properties and good antibacterial activity | [160] |
| β-Cyclodextrin and PVA | Ag nanoparticles and riboflavin | Non-toxicity, excellent antagonistic bactericidal activity, and fast wound healing process | [161] |
| Chloroacetated natural rubber and PVA | Kaolin and starch | Excellent cytocompatibility | [162] |
| Konjac glucomannan and PVA | - | Accelerated wound healing mechanism | [163] |
| Pectin, PVA, and PVP | Ag nanoparticles | Good cytocompatibility, higher antibacterial efficacy, and accelerated wound healing process | [164] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alven, S.; Aderibigbe, B.A. Fabrication of Hybrid Nanofibers from Biopolymers and Poly (Vinyl Alcohol)/Poly (ε-Caprolactone) for Wound Dressing Applications. Polymers 2021, 13, 2104. https://doi.org/10.3390/polym13132104
Alven S, Aderibigbe BA. Fabrication of Hybrid Nanofibers from Biopolymers and Poly (Vinyl Alcohol)/Poly (ε-Caprolactone) for Wound Dressing Applications. Polymers. 2021; 13(13):2104. https://doi.org/10.3390/polym13132104
Chicago/Turabian StyleAlven, Sibusiso, and Blessing Atim Aderibigbe. 2021. "Fabrication of Hybrid Nanofibers from Biopolymers and Poly (Vinyl Alcohol)/Poly (ε-Caprolactone) for Wound Dressing Applications" Polymers 13, no. 13: 2104. https://doi.org/10.3390/polym13132104
APA StyleAlven, S., & Aderibigbe, B. A. (2021). Fabrication of Hybrid Nanofibers from Biopolymers and Poly (Vinyl Alcohol)/Poly (ε-Caprolactone) for Wound Dressing Applications. Polymers, 13(13), 2104. https://doi.org/10.3390/polym13132104







