Elucidation of Mechanical, Physical, Chemical and Thermal Properties of Microbial Composite Films by Integrating Sodium Alginate with Bacillus subtilis sp.
Abstract
:1. Introduction
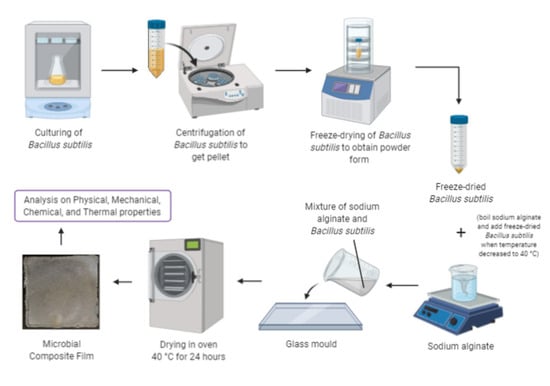
2. Materials and Methods
3. Results and Discussion
3.1. Analysis of Microbial Composite Films
3.2. Mechanical Testing
3.3. Physical Testing
3.4. Scanning Electron Microscopy (SEM)
3.5. Optimum Conditions of Microbial Composite Film for Physical and Mechanical Properties
3.6. Chemical Analysis
3.7. Fourier Transform Infrared Spectrometry (FTIR)
4. Thermal Analysis
4.1. Differential Scanning Calorimetry (DSC)
4.2. Thermogravimetric Analysis (TGA)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, R.-M.; Zheng, S.-R.; Zheng, Y.-P. Introduction to polymer matrix composites. In Polymer Matrix Composites and Technology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1–548. [Google Scholar]
- Jesson, D.; Watts, J.F. The interface and interphase in polymer matrix composites: Effect on mechanical properties and methods for identification. Polym. Rev. 2012, 52, 321–354. [Google Scholar] [CrossRef]
- Fazeli, M.; Florez, J.P.; Simão, R.A. Improvement in adhesion of cellulose fibers to the thermoplastic starch matrix by plasma treatment modification. Compos. Part B Eng. 2019, 163, 207–216. [Google Scholar] [CrossRef]
- Ho, B.; Azahari, B.; Yhaya, M.; Talebi, A.; Ng, C.; Tajarudin, H.; Ismail, N. Green technology approach for reinforcement of calcium chloride cured sodium alginate films by isolated bacteria from palm oil mill effluent (POME). Sustainability 2020, 12, 9468. [Google Scholar] [CrossRef]
- Zajdel, T.J.; Baruch, M.; Méhes, G.; Stavrinidou, E.; Berggren, M.; Maharbiz, M.M.; Simon, D.T.; Ajo-Franklin, C.M. PEDOT:PSS-based Multilayer Bacteri-al-Composite Films for Bioelectronics. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Ju, S.; Zhang, F.; Duan, J.; Jiang, J. Characterization of bacterial cellulose composite films incorporated with bulk chitosan and chitosan nanoparticles: A comparative study. Carbohydr. Polym. 2020, 237, 116167. [Google Scholar] [CrossRef] [PubMed]
- Abral, H.; Pratama, A.B.; Handayani, D.; Mahardika, M.; Aminah, I.; Sandrawati, N.; Sugiarti, E.; Muslimin, A.N.; Sapuan, S.M.; Ilyas, R.A. Antimicrobial Edible Film Prepared from Bacterial Cellulose Nanofibers/Starch/Chitosan for a Food Packaging Alternative. Int. J. Polym. Sci. 2021, 2021, 6641284. [Google Scholar] [CrossRef]
- Al-mohanna, M.T. Bacterial Introduction. Res. Gate 2017, 679–692. Available online: www.researchgate.net/publication/315948104 (accessed on 18 August 2020).
- Ng, C.W.C.; Ismail, A.F.; Makhtar, M.M.Z.; Jamaluddin, M.N.F.; Tajarudin, H.A. Conversion of food waste via two-stage fermentation to controllable chicken feed nutrients by local isolated microorganism. Int. J. Recycl. Org. Waste Agric. 2020, 9, 33–47. [Google Scholar]
- Mohd Zaini Makhtar, M.; Tajarudin, H.A. Electricity generation using membrane-less microbial fuel cell powered by sludge supplemented with lignocellulosic waste. Int. J. Energy Res. 2020, 44, 3260–3265. [Google Scholar] [CrossRef]
- Othman, M.F.; Tamat, M.R.; Wan Nadiah, W.A.; Serri, N.A.; Aziz, H.A.; Tajarudin, H.A. Bioconversion of leachate to acetic and butyric acid by Clostridium butyricum NCIMB 7423 in membrane fermentor. Pertanika J. Sci. Technol. 2017, 25, 39–48. [Google Scholar]
- Aziz, H.A.; Tajarudin, H.A.; Wei, T.H.L.; Alazaiza, M.Y.D. Iron and manganese removal from groundwater using limestone filter with iron-oxidized bacteria. Int. J. Environ. Sci. Technol. 2020, 17, 2667–2680. [Google Scholar] [CrossRef]
- Shahid, S.; Aslam, M.A.; Ali, S.; Zameer, M.; Faisal, M. Self-Healing of Cracks in Concrete Using Bacillus Strains Encapsulated in Sodium Alginate Beads. ChemistrySelect 2020, 5, 312–323. [Google Scholar] [CrossRef]
- Rhim, J.-W. Physical and mechanical properties of water resistant sodium alginate films. LWT Food Sci. Technol. 2004, 37, 323–330. [Google Scholar] [CrossRef]
- Vicente, A.; Cerqueira, M.; Hilliou, L.; Rocha, C.M.R. Protein-based resins for food packaging. In Multifunctional and Nanoreinforced Polymers for Food Packaging; Woodhead Publishing: Cambridge, UK, 2011; pp. 610–648. [Google Scholar]
- Nimje, V.R.; Chen, C.Y.; Chen, C.C.; Jean, J.S.; Reddy, A.S.; Fan, C.W.; Pan, K.Y.; Liu, H.T.; Chen, J.L. Corrigendum to “Stable and high energy generation by a strain of Bacillus subtilis in a microbial fuel cell”. J. Power Sources 2010, 195, 5427–5428. [Google Scholar] [CrossRef]
- Husain, M.; Husain, Q. Applications of redox mediators in the treatment of organic pollutants by using oxidoreductive en-zymes: A review. Crit. Rev. Environ. Sci. Technol. 2008, 38, 1–42. [Google Scholar] [CrossRef]
- Voulhoux, R.; Bos, M.P.; Geurtsen, J.; Mols, M.; Tommassen, J. Role of a Highly Conserved Bacterial Protein in Outer Membrane Protein Assembly. Science 2003, 299, 262–265. [Google Scholar] [CrossRef]
- Kamiya, N.; Tazawa, M. Studies on water permeability of a single plant cell by means of transcellular osmosis. Protoplasma 1956, 46, 394–422. [Google Scholar] [CrossRef]
- Stubbendieck, R.; Vargas-Bautista, C.; Straight, P.D. Bacterial Communities: Interactions to Scale. Front. Microbiol. 2016, 7, 1234. [Google Scholar] [CrossRef] [Green Version]
- Mishima, Y.; Momma, K.; Hashimoto, W.; Mikami, B.; Murata, K. Crystal structure of AlgQ2, a macromolecule (algi-nate)-binding protein of Sphingomonas sp. A1, complexed with an alginate tetrasaccharide at 1.6-Å resolution. J. Biol. Chem. 2003, 278, 6552–6559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Guo, J.; Yu, Y.; An, Q.; Wang, L.; Li, S.; Huang, X.; Mu, S.; Qi, S. Hydrogen bonds of sodium alginate/Antarctic krill protein composite material. Carbohydr. Polym. 2016, 142, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Abulateefeh, S.R.; Taha, M.O. Enhanced drug encapsulation and extended release profiles of calcium-alginate nanoparticles by using tannic acid as a bridging cross-linking agent. J. Microencapsul. 2015, 32, 96–105. [Google Scholar] [CrossRef]
- Wardhono, E.Y.; Kanani, N.; Alfirano, A.; Rahmayetti, R. Development of polylactic acid (PLA) bio-composite films reinforced with bacterial cellulose nanocrystals (BCNC) without any surface modification. J. Dispers. Sci. Technol. 2019, 41, 1488–1495. [Google Scholar] [CrossRef]
- Bredin ALarcher AMullins, B. NOTICE: This is the author’s version of a work that was accepted for publication in Computers and Geosciences. Changes resulting from the publishing process, such as peer review, editing, corrections, structural formatting, and other quality. Comput. Geosci. 2005, 31, 846–864. [Google Scholar]
- Wong, D.W.S.; Gregorski, K.S.; Hudson, J.S.; Pavláth, A.E. Calcium Alginate Films: Thermal Properties and Permeability to Sorbate and Ascorbate. J. Food Sci. 1996, 61, 337–341. [Google Scholar] [CrossRef]







| Mass of Bacillus subtilis (g) | Tensile Strength (MPa) | Breaking Strain (%) | Toughness (MJ/m3) | |
|---|---|---|---|---|
| Sodium Alginate Film | 0 | 0.611 | 84.372 | 0.016 |
| Microbial Composite Films | 0.1 | 0.620 | 84.849 | 0.016 |
| 0.2 | 0.635 | 85.092 | 0.018 | |
| 0.3 | 0.709 | 85.169 | 0.024 | |
| 0.4 | 0.736 | 85.192 | 0.024 | |
| 0.5 | 0.858 | 87.406 | 0.045 | |
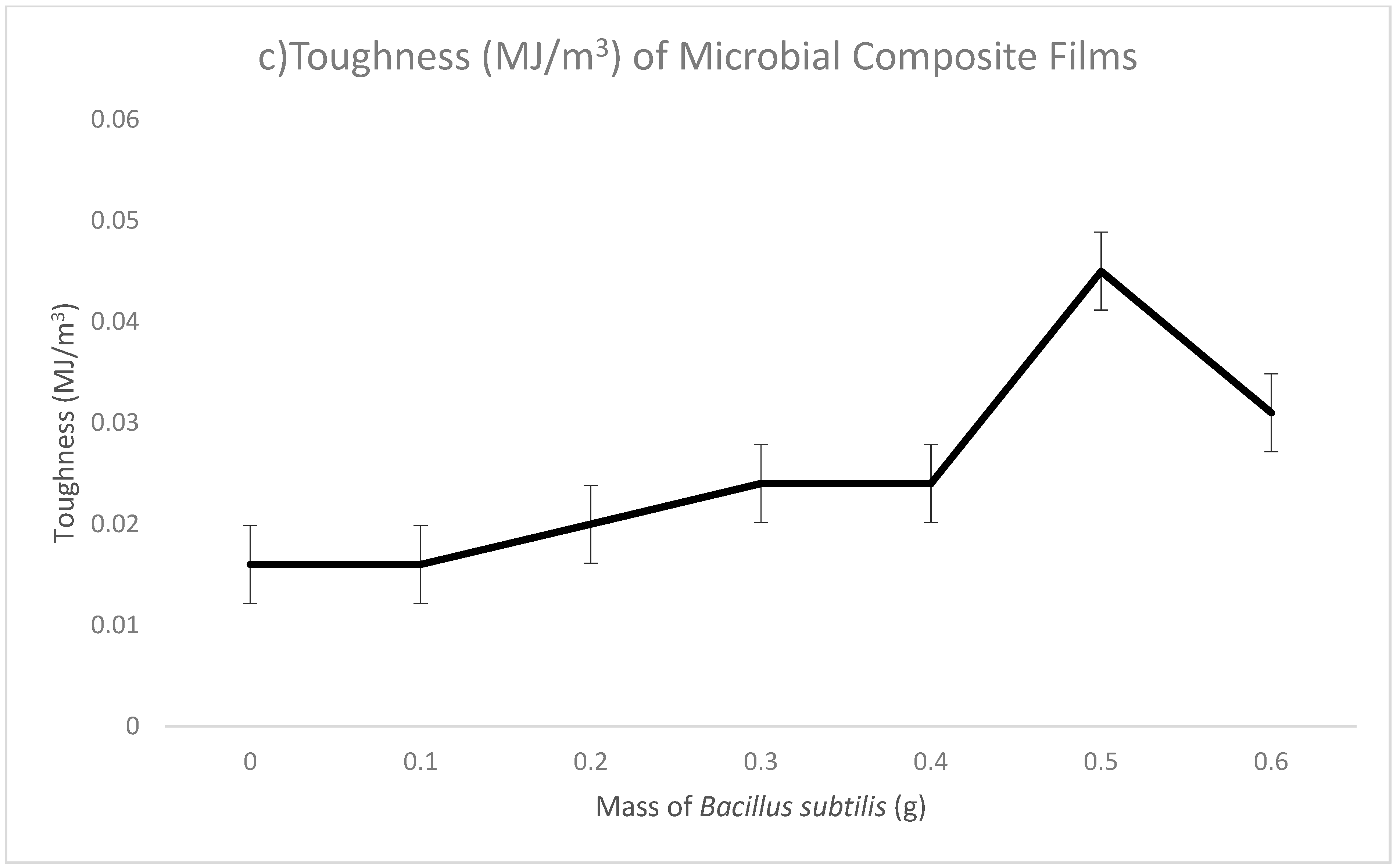
| 0.6 | 0.831 | 86.155 | 0.310 | |
| Conclusions | When mass of Bacillus subtilis increased, tensile strength increased. | When mass of Bacillus subtilis increased, breaking strain increased. | When mass of Bacillus subtilis increased, toughness increased. | |
| Remarks | The highest tensile strength recorded in 0.5 g. | The highest breaking strain recorded in 0.5 g. | The highest toughness recorded in 0.5 g. | |
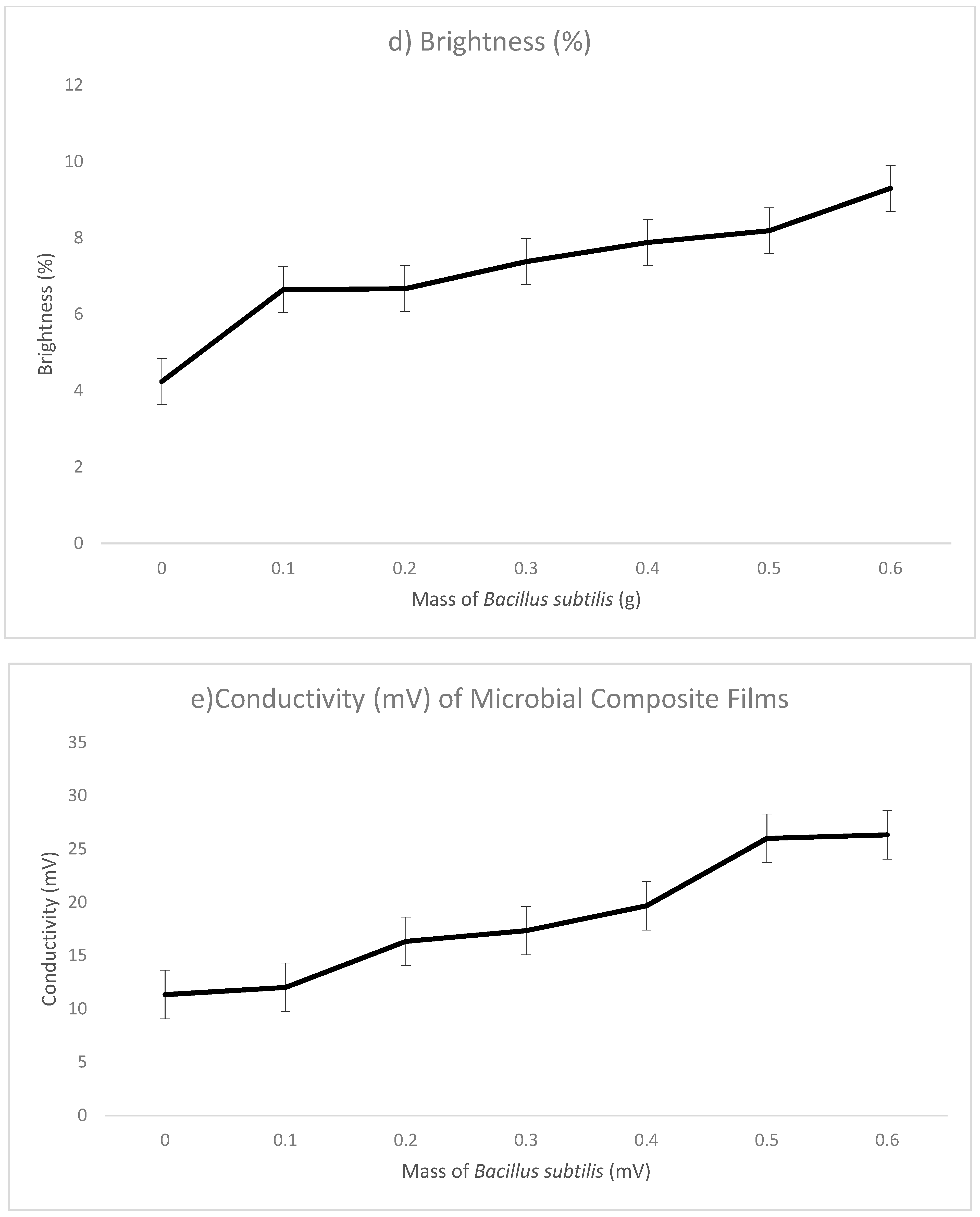
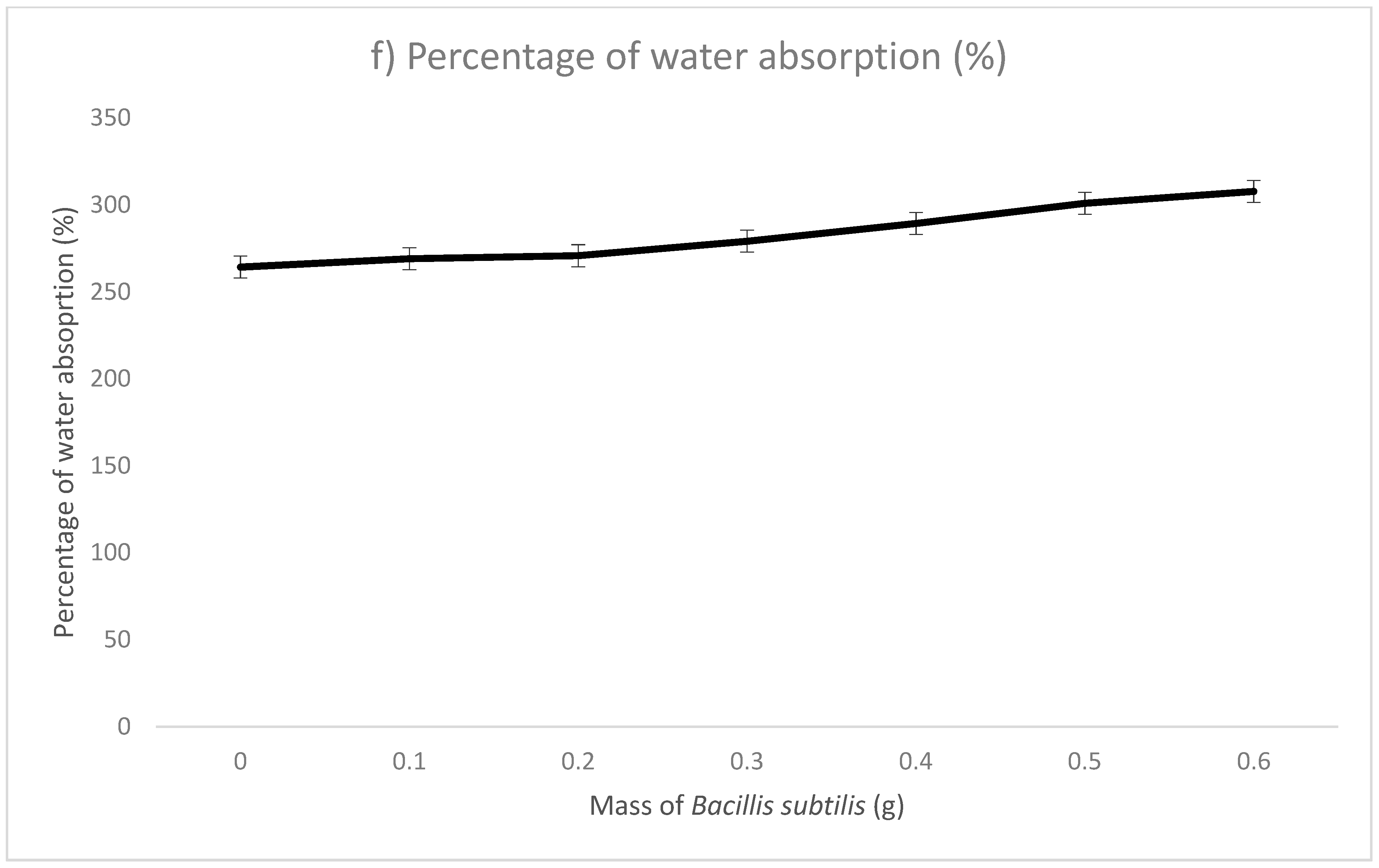
| Mass of Bacillus subtilis (g) | Thickness (mm) | Opacity (%) | Brightness (%) | Conductivity | Water Absorption (%) | Scanning Electron Microscopy (SEM) | |||
|---|---|---|---|---|---|---|---|---|---|
| White Light | Black Light | mV | S/m | ||||||
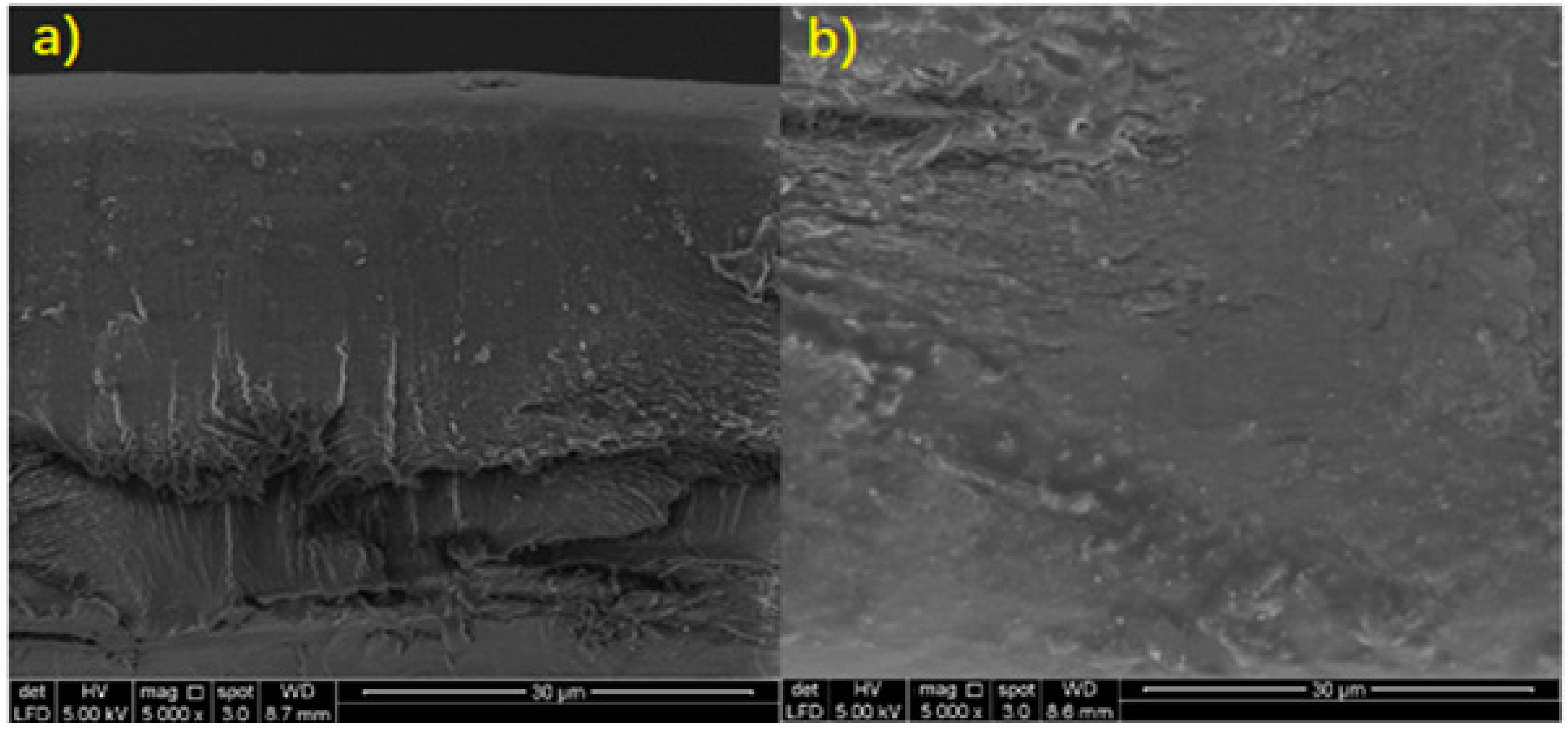
| Sodium Alginate Film | 0 | 0.578 | 7.52 | 38.41 | 4.24 | 11.33 | 0.74 | 264.29 | Rough surface Voids on cross-sectional diagram |
| Microbial Composite Films | 0.1 | 0.665 | 8.1 | 37.86 | 6.65 | 12 | 0.24 | 269.05 | Smooth surface Filled voids on cross-sectional diagram |
| 0.2 | 0.761 | 8.39 | 38.61 | 6.67 | 16.33 | 0.33 | 270.83 | ||
| 0.3 | 0.892 | 9.19 | 39.29 | 7.38 | 17.33 | 0.35 | 279.17 | ||
| 0.4 | 1.002 | 12.16 | 41.69 | 7.88 | 19.67 | 0.39 | 289.35 | ||
| 0.5 | 1.057 | 13.65 | 40.55 | 8.19 | 37 | 0.74 | 300.93 | ||
| 0.6 | 1.059 | 16.45 | 41.18 | 9.3 | 26.33 | 0.53 | 307.78 | ||
| Conclusions | When mass of Bacillus subtilis increased, thickness increased. | When mass of Bacillus subtilis increased, opacity increased. | When mass of Bacillus subtilis increased, brightness increased. | When mass of Bacillus subtilis increased, conductivity increased. | When mass of Bacillus subtilis increased, water absorption increased. | When mass of Bacillus subtilis increased, conductivity increased. | |||
| Remarks | The highest thickness recorded with 0.6 g. | The highest opacity recorded with 0.6 g. | The highest brightness recorded with 0.6 g. | The highest conductivity recorded with 0.5 g. | The highest water absorption recorded with 0.6 g. | The highest conductivity recorded with 0.5 g. | |||
| Physical and Mechanical Analysis | Value |
|---|---|
| (1) Tensile strength (MPa) | 0.858 |
| (2) Breaking strain (%) | 87.406 |
| (3) Toughness (MJ/m3) | 0.045 |
| (4) Thickness (mm) | 1.057 |
| (5) Opacity (white light, %) | 13.650 |
| (6) Opacity (black light, %) | 40.550 |
| (7) Brightness (%) | 8.190 |
| (8) Conductivity (mV) | 37.000 |
| (9) Water absorption (%) | 300.930 |
| (10) Scanning electron microscopy (SEM) | Microbial composite film has smoother surface and filled voids. |
| Mass of Bacillus subtilis (g) | Fourier Transform Infrared Spectrometry (FTIR) | |
|---|---|---|
| Sodium alginate film | 0 | Bonds: OH stretching, C–H symmetrical stretching, OH bending of absorbed water, HCH and OCH in-plane bending vibration, CH2 rocking vibration at C6, C–C, C–OH, C–H ring and side group vibrations, COC, CCO and CCH deformation and stretching. |
| Microbial composite films | 0.1 | Bonds: OH stretching, C–H symmetrical stretching, OH bending of absorbed water, HCH and OCH in-plane bending vibration, CH2 rocking vibration at C6, C–C, C–OH, C–H ring and side group vibrations, COC, CCO and CCH deformation and stretching, C–OH out-of-plane bending. |
| 0.2 | ||
| 03 | ||
| 0.4 | ||
| 0.5 | ||
| 0.6 | ||
| Conclusions | Microbial composite films possessed more chemical bonds compared to sodium alginate films, which contributed to stronger properties. | |
| Remarks | Microbial composite films had extra wavenumbers at ~800 and ~662cm−1 compared to sodium alginate films. | |
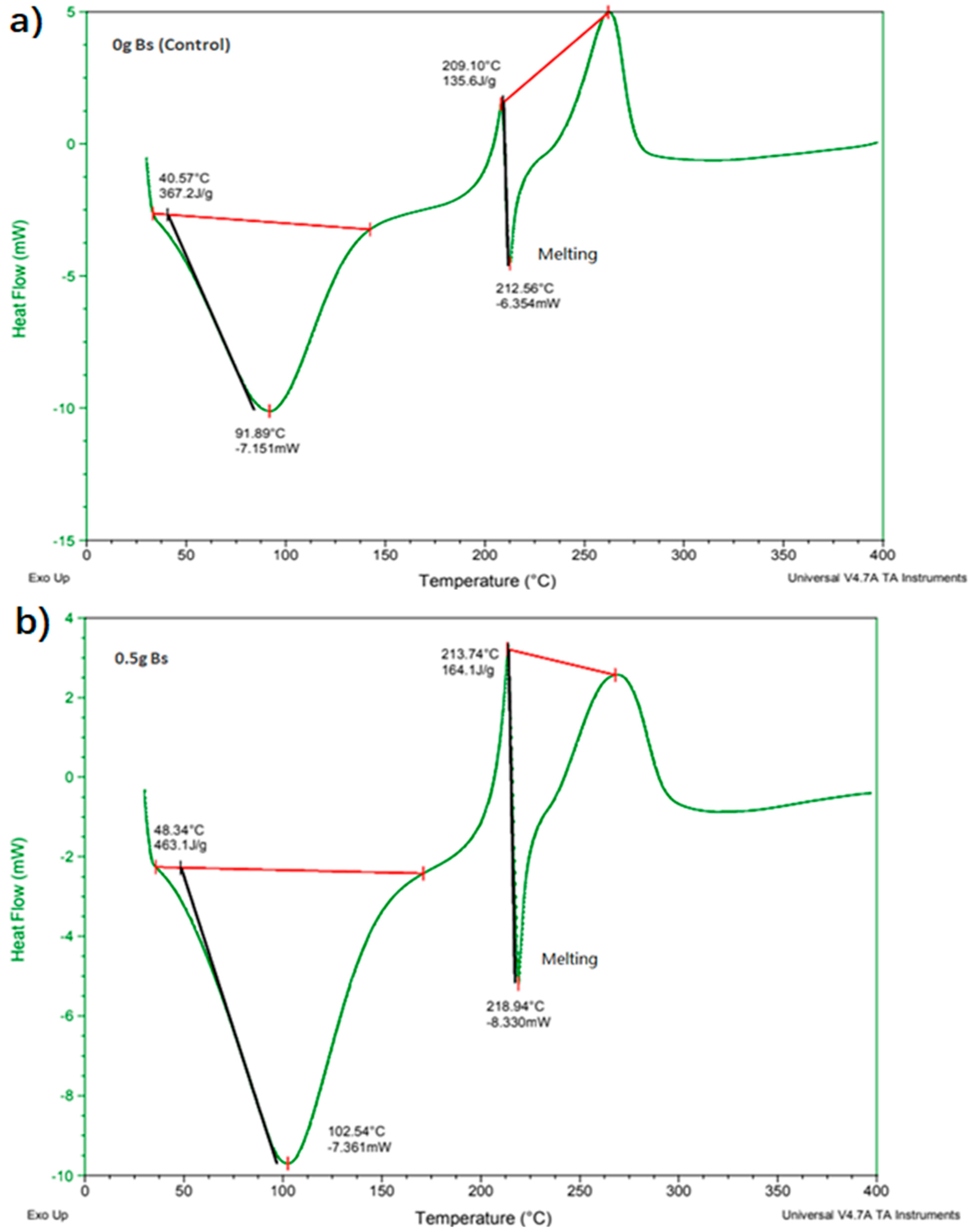
| Mass of Bacillus subtilis (g) | Differential Scanning Calorimetry (DSC) | Thermogravimetric Analysis (TGA) | |
|---|---|---|---|
| Sodium alginate film | 0 | 212.56 | 248.02 |
| Microbial composite films | 0.1 | 216.01 | 249.88 |
| 0.2 | 216.55 | 249.64 | |
| 03 | 217.69 | 250.72 | |
| 0.4 | 217.87 | 251.12 | |
| 0.5 | 218.94 | 252.69 | |
| 0.6 | 218.58 | 252.52 | |
| Conclusions | When mass of Bacillus subtilis increased, melting point increased. | When mass of Bacillus subtilis increased, decomposition temperature increased. | |
| Remarks | The highest melting point recorded with 0.5 g. | The highest decomposition temperature recorded with 0.6 g. | |
| Physical, mechanical and chemical Analysis | Sodium Alginate Film (Control) | 0.5 g Microbial Composite Film |
|---|---|---|
| (1) Tensile strength (MPa) | 0.611 | 0.858 |
| (2) Breaking strain (%) | 84.372 | 87.406 |
| (3) Toughness (MJ/m3) | 0.016 | 0.045 |
| (4) Thickness (mm) | 0.578 | 1.057 |
| (5) Opacity (white light, %) | 7.520 | 13.650 |
| (6) Opacity (black light, %) | 38.410 | 40.550 |
| (7) Brightness (%) | 4.240 | 8.190 |
| (8) Conductivity (mV) | 11.220 | 37.000 |
| (9) Water absorption (%) | 264.290 | 300.930 |
| (10) Differential scanning calorimetry, DSC (°C) | 212.560 | 218.940 |
| (11) Thermogravimetric analysis, TGA (°C) | 248.020 | 252.690 |
| (12) Scanning electron microscopy (SEM) | Rougher cross-sectional and surface | Filled cross-sectional and smoother surface |
| (13) Fourier transform infrared spectrometry (FTIR) | Lack of values at 800 and 662 cm−1 wavenumbers. | Extra values at 800 and 662 cm−1 wavenumbers for C–C, C–OH, C–H ring and side group vibrations and C–OH out-of-plane bending. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wai Chun, C.N.; Tajarudin, H.A.; Ismail, N.; Azahari, B.; Mohd Zaini Makhtar, M. Elucidation of Mechanical, Physical, Chemical and Thermal Properties of Microbial Composite Films by Integrating Sodium Alginate with Bacillus subtilis sp. Polymers 2021, 13, 2103. https://doi.org/10.3390/polym13132103
Wai Chun CN, Tajarudin HA, Ismail N, Azahari B, Mohd Zaini Makhtar M. Elucidation of Mechanical, Physical, Chemical and Thermal Properties of Microbial Composite Films by Integrating Sodium Alginate with Bacillus subtilis sp. Polymers. 2021; 13(13):2103. https://doi.org/10.3390/polym13132103
Chicago/Turabian StyleWai Chun, Charles Ng, Husnul Azan Tajarudin, Norli Ismail, Baharin Azahari, and Muaz Mohd Zaini Makhtar. 2021. "Elucidation of Mechanical, Physical, Chemical and Thermal Properties of Microbial Composite Films by Integrating Sodium Alginate with Bacillus subtilis sp." Polymers 13, no. 13: 2103. https://doi.org/10.3390/polym13132103
APA StyleWai Chun, C. N., Tajarudin, H. A., Ismail, N., Azahari, B., & Mohd Zaini Makhtar, M. (2021). Elucidation of Mechanical, Physical, Chemical and Thermal Properties of Microbial Composite Films by Integrating Sodium Alginate with Bacillus subtilis sp. Polymers, 13(13), 2103. https://doi.org/10.3390/polym13132103