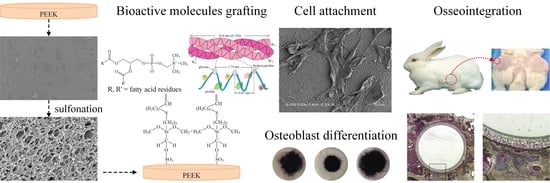
Evaluation of the Grafting Efficacy of Active Biomolecules of Phosphatidylcholine and Type I Collagen on Polyether Ether Ketone: In Vitro and In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. PEEK Control/Polishing Group Preparation
2.2. Functionalized Porous Structure of Surface-Modified PEEK
2.3. Bioactive Molecule Grafting
2.4. Surface Characterizations
2.5. Biocompatibility and Cell Morphologies
2.6. Relative Short-Term Cell Attachment
2.7. Relative Long-Term Cell Proliferation
2.8. Alkaline Phosphatase (ALP) Semi-Quantification and Staining
2.9. In Vivo Studies
2.10. Statistical Analysis
3. Results and Discussion
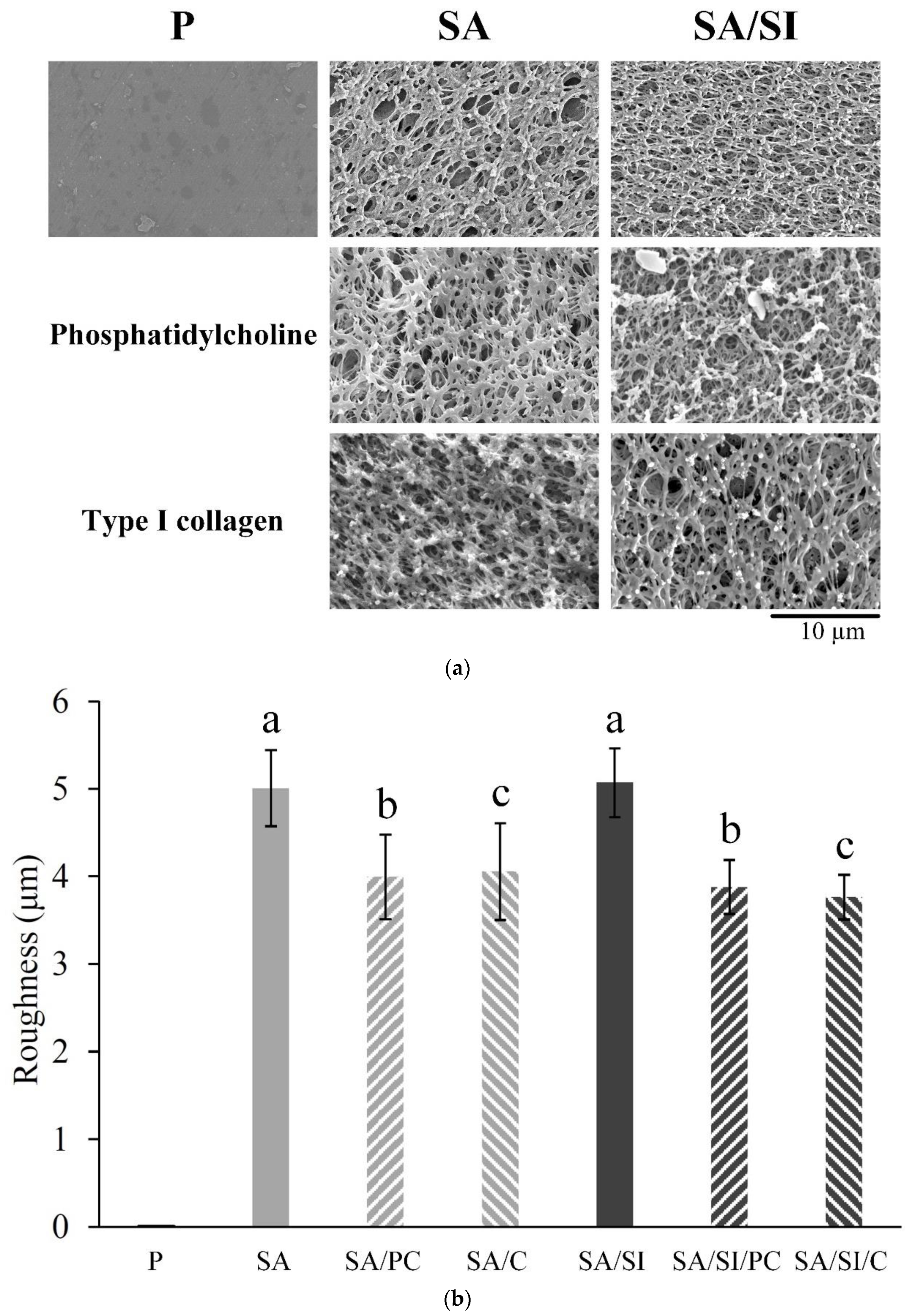
3.1. Surface-Modified Characterizations
3.1.1. Grafting Concepts, Substrate Structures, and Surface Roughness
3.1.2. Surface Wettability and Bioactive Molecule Grafting
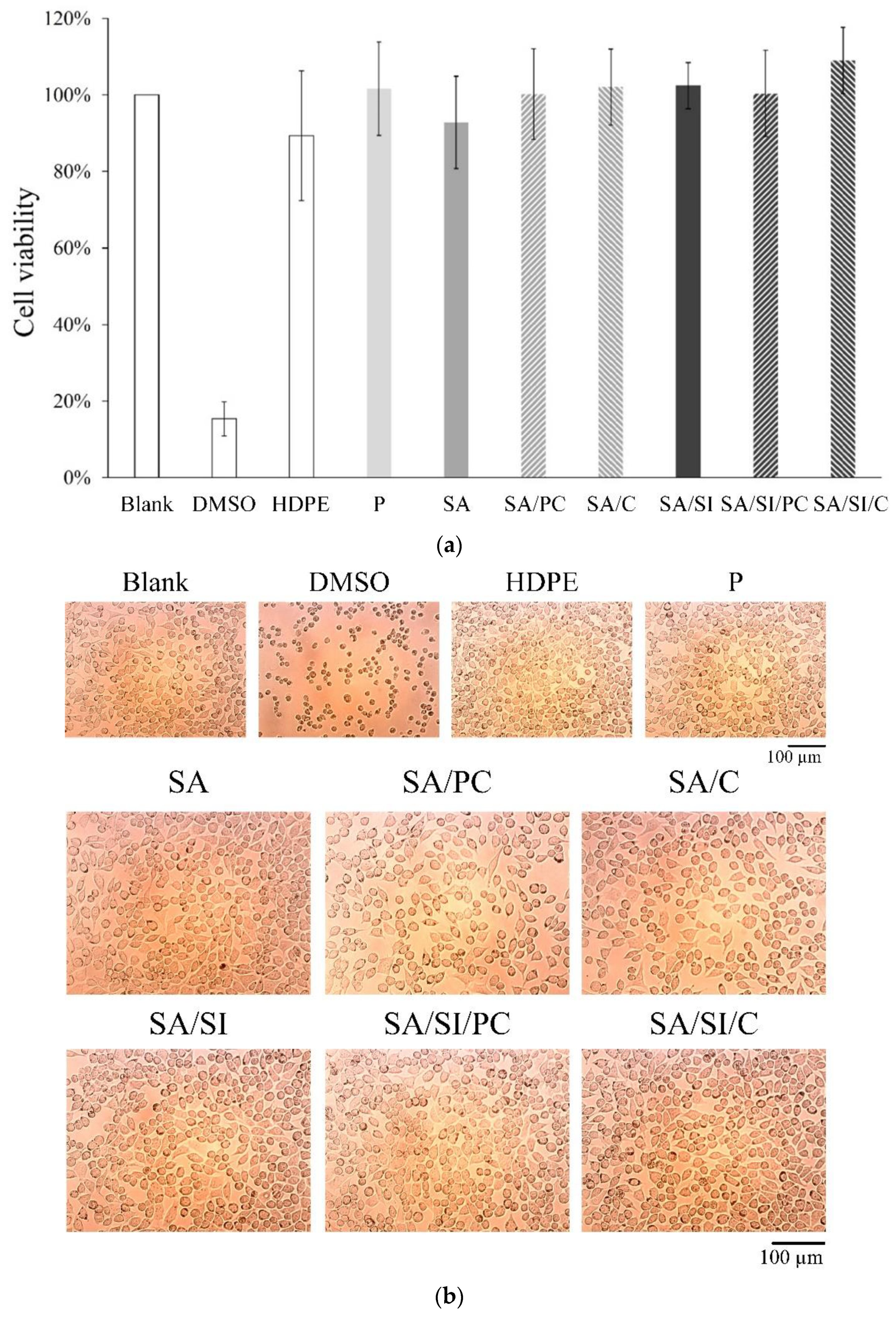
3.2. Biocompatibility and Measurements In Vitro
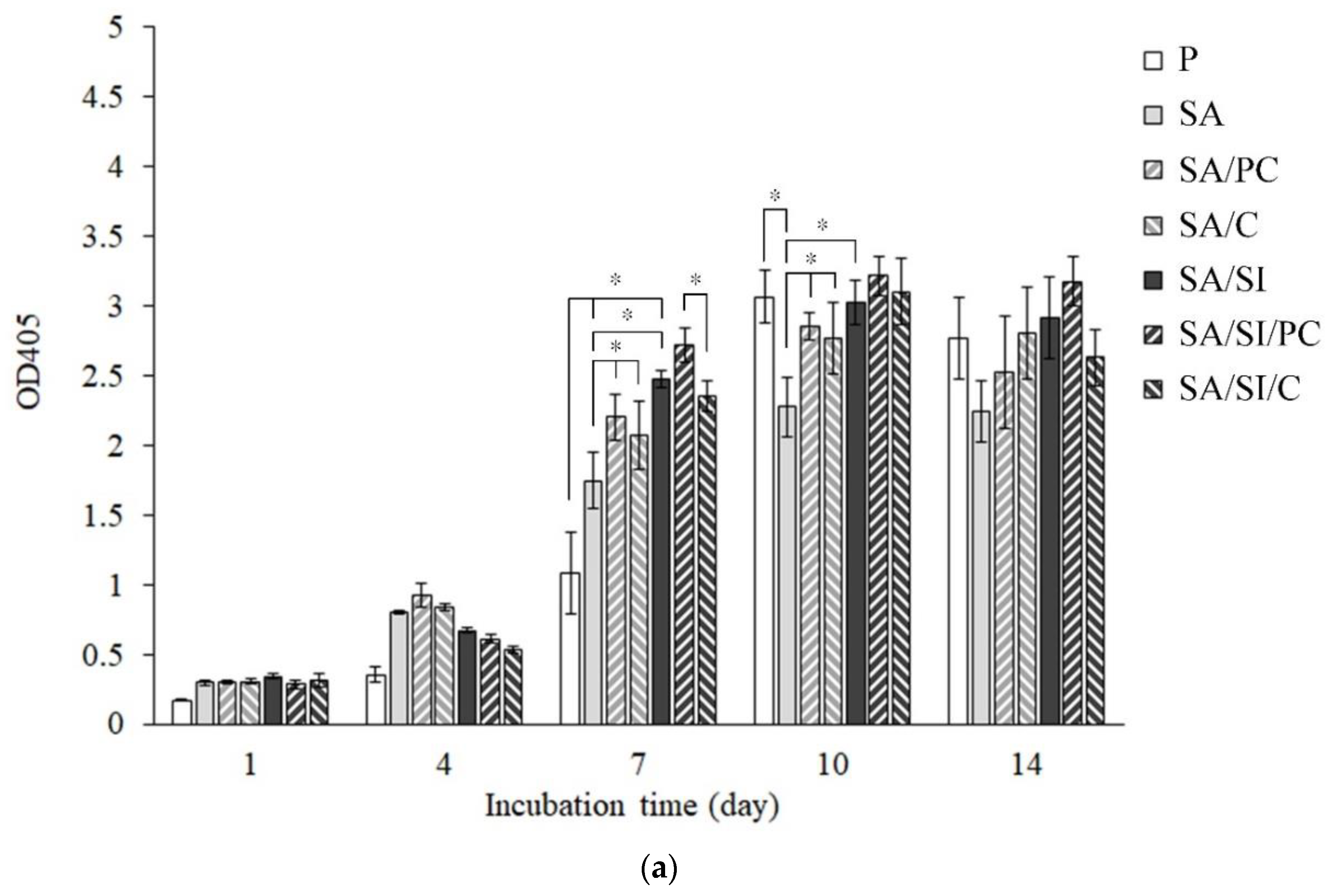
3.2.1. Cell Viability of Fibroblast L929 Cells
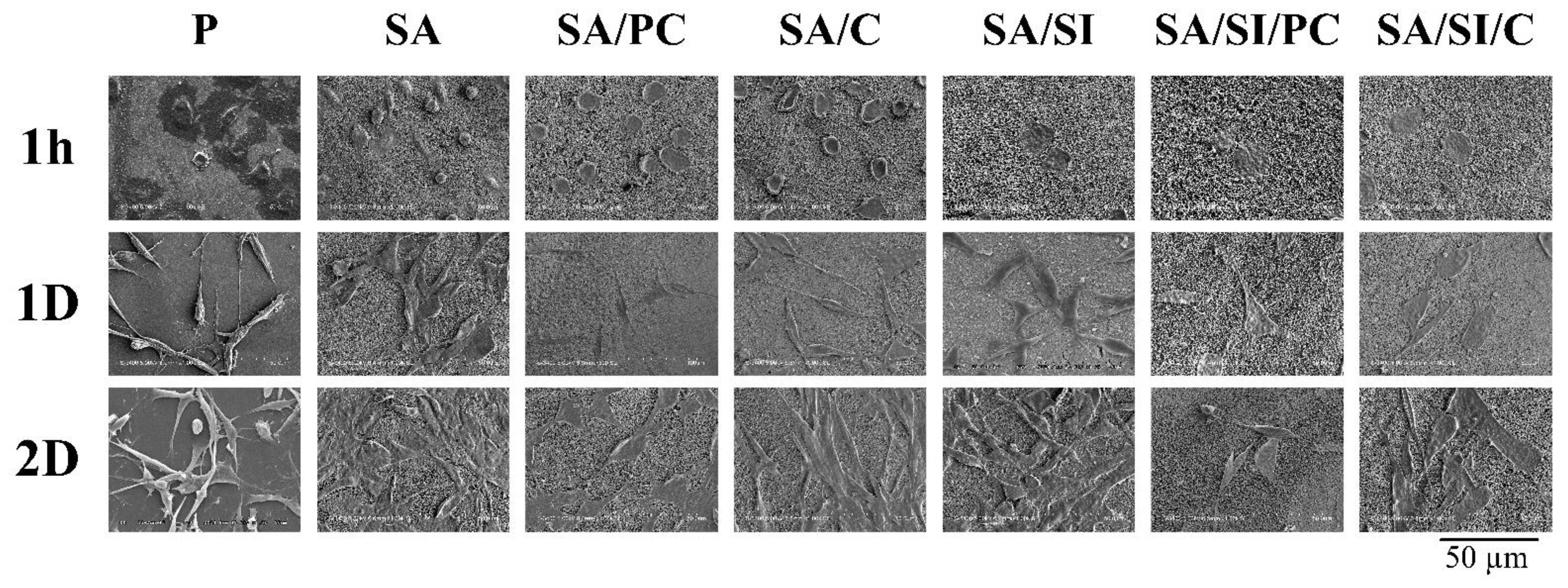
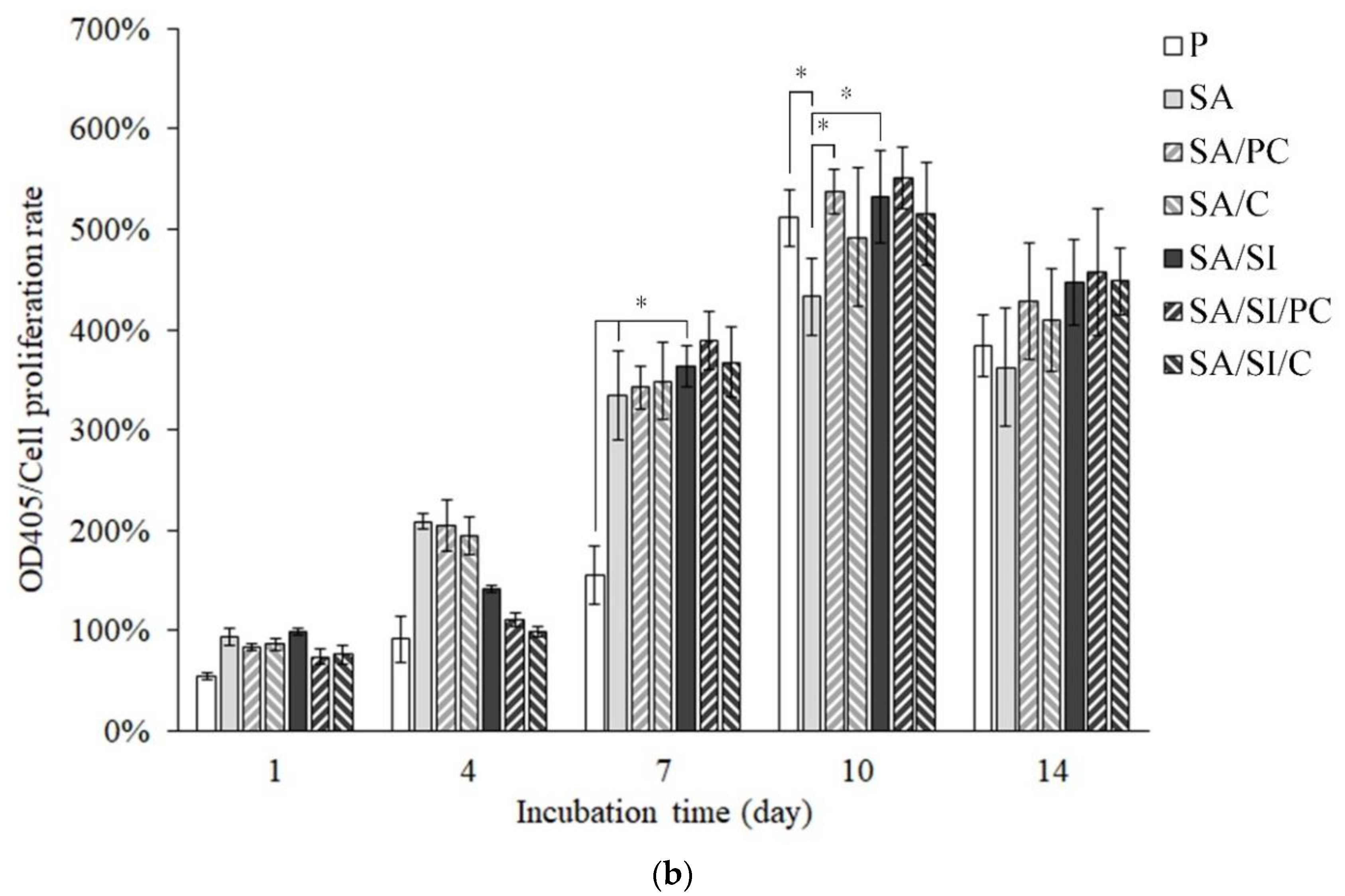
3.2.2. Cell Attachment of Bone Progenitor D1 Cells
3.2.3. Cell Proliferation of Bone Progenitor D1 Cells
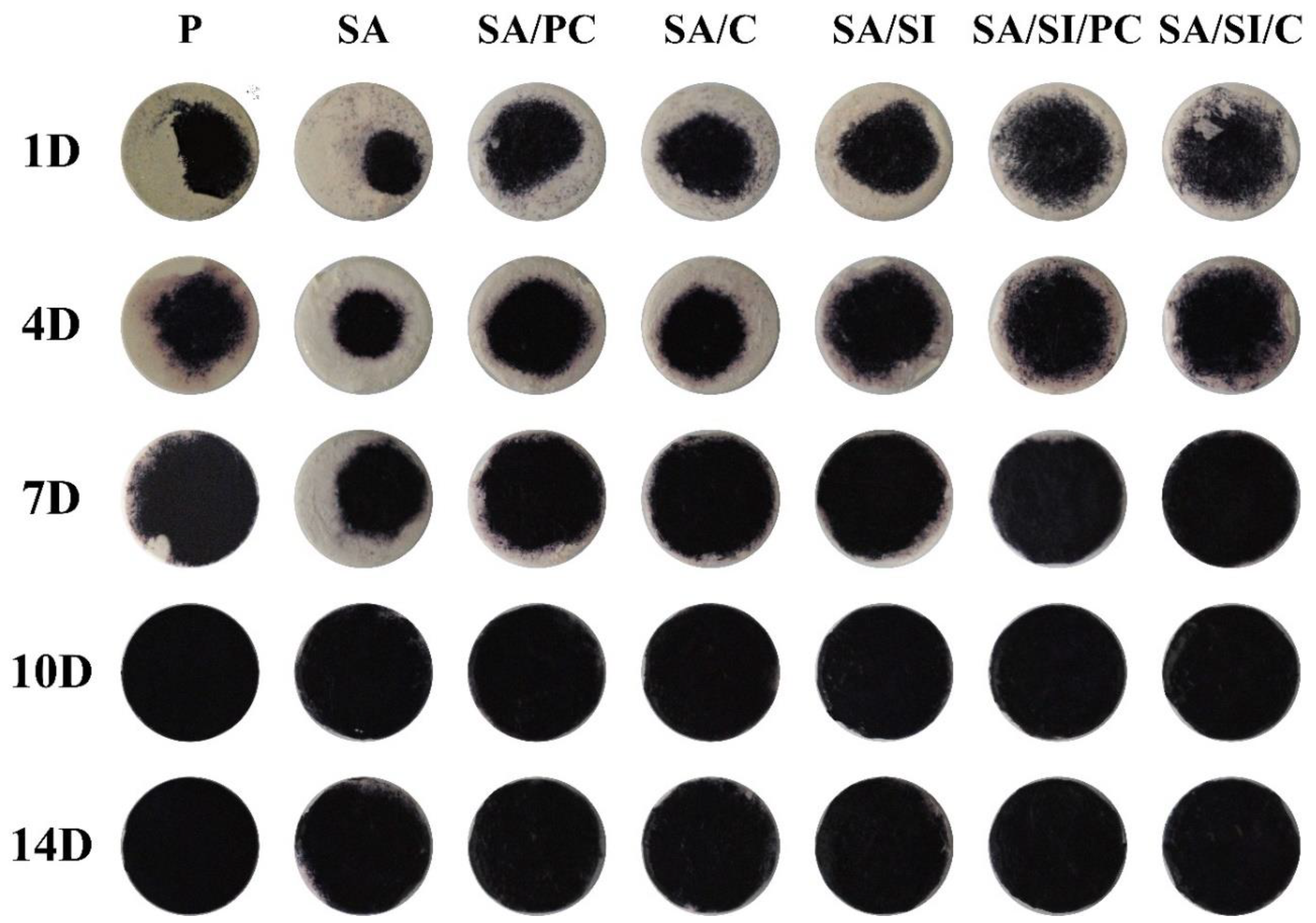
3.2.4. ALP Activity and Staining for the Mineralization of D1 Cells
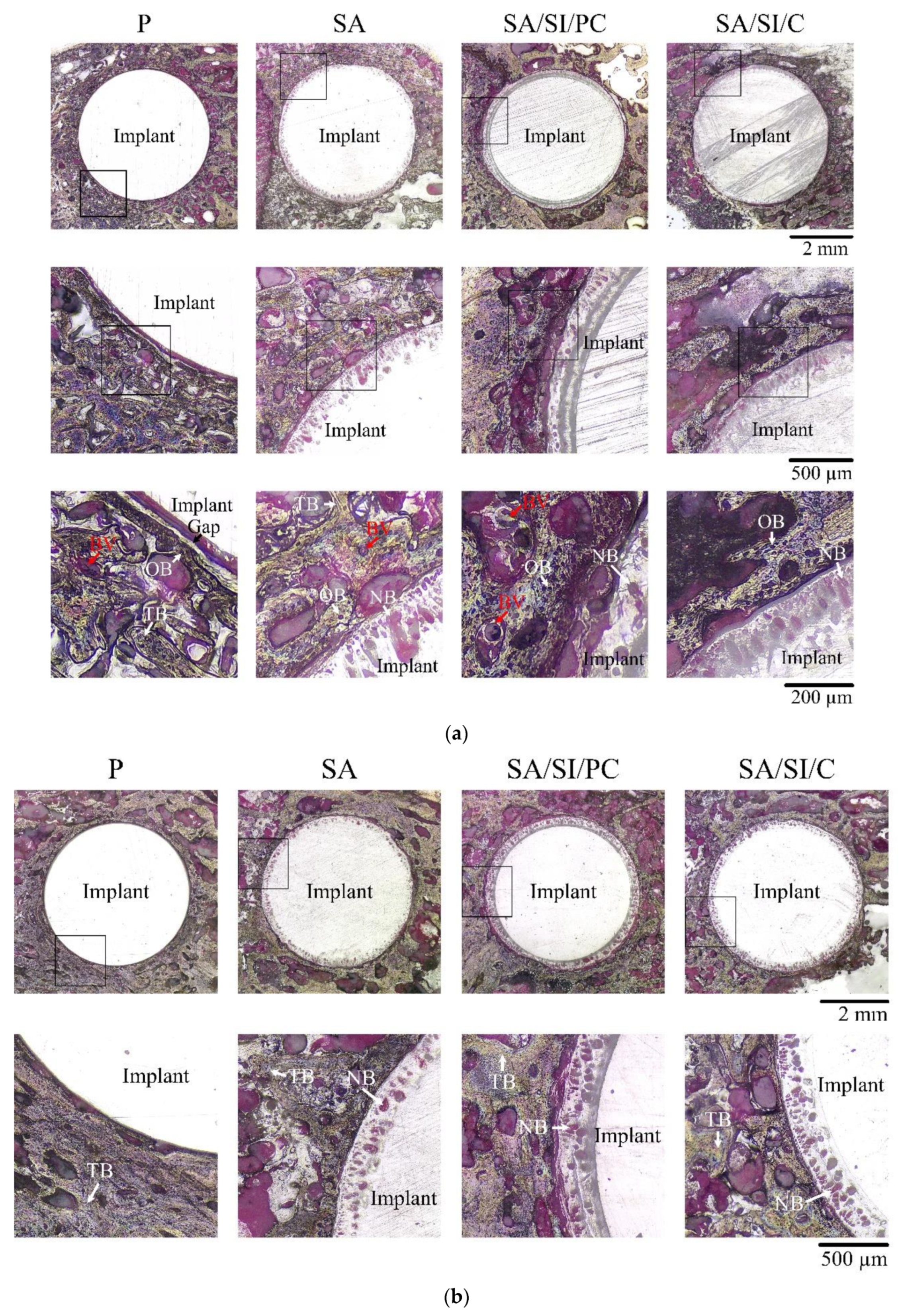
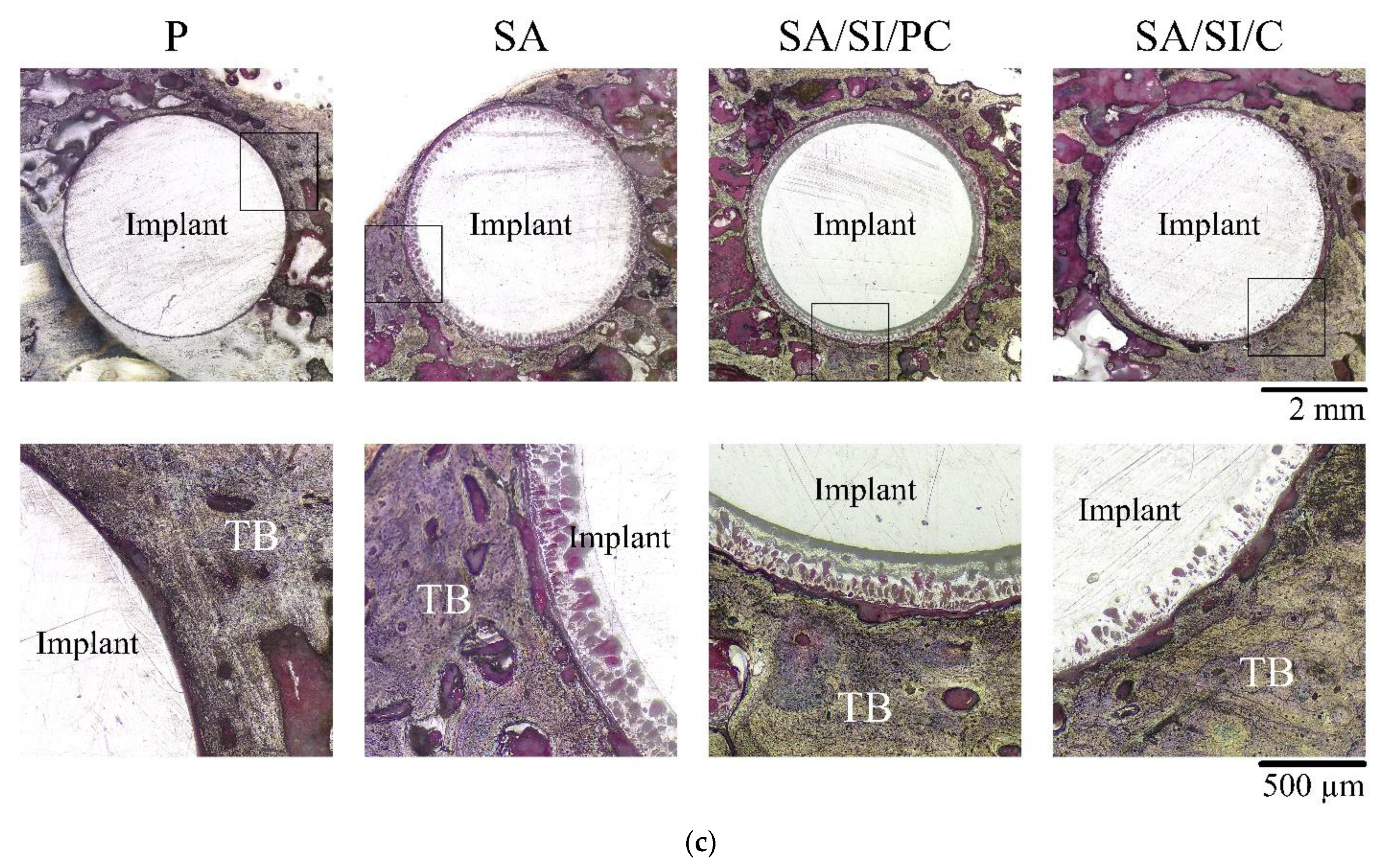
3.3. Observations in In Vivo Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [Green Version]
- Kasliwal, M.K.; O’Toole, J.E. Clinical experience using polyetheretherketone (PEEK) intervertebral structural cage for anterior cervical corpectomy and fusion. J. Clin. Neurosci. 2014, 21, 217–220. [Google Scholar] [CrossRef]
- Sahoo, P. Polyetheretherketone (PEEK) cages for cervical interbody replacement. Apollo Med. 2013, 10, 233–236. [Google Scholar] [CrossRef]
- Boissière, L.; Perrin, G.; Rigal, J.; Michel, F.; Barrey, C. Lumbar-sacral fusion by a combined approach using interbody PEEK cage and posterior pedicle-screw fixation: Clinical and radiological results from a prospective study. Orthop. Traumatol. Surg. Res. 2013, 99, 945–951. [Google Scholar] [CrossRef] [Green Version]
- Jalbert, F.; Boetto, S.; Nadon, F.; Lauwers, F.; Schmidt, E.; Lopez, R. One-step primary reconstruction for complex craniofacial resection with PEEK custom-made implants. J. Cranio-Maxillofac. Surg. 2014, 42, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Hahnel, S.; Scherl, C.; Rosentritt, M. Interim rehabilitation of occlusal vertical dimension using a double-crown-retained removable dental prosthesis with polyetheretherketone framework. J. Prosthet. Dent. 2018, 119, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Zoidis, P.; Bakiri, E.; Polyzois, G. Using modified polyetheretherketone (PEEK) as an alternative material for endocrown restorations: A short-term clinical report. J. Prosthet. Dent. 2017, 117, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Costa-Palau, S.; Torrents-Nicolas, J.; Barberà, M.B.-D.; Cabratosa-Termes, J. Use of polyetheretherketone in the fabrication of a maxillary obturator prosthesis: A clinical report. J. Prosthet. Dent. 2014, 112, 680–682. [Google Scholar] [CrossRef] [PubMed]
- Toth, J.M. Biocompatibility of Polyaryletheretherketone Polymers. In PEEK Biomaterials Handbook; Elsevier: Amsterdam, The Netherlands, 2012; pp. 81–92. [Google Scholar]
- Deng, F.; Wu, X.; Liu, X.; Guo; Wei, J. Nano-TiO2/PEEK bioactive composite as a bone substitute material: In vitro and in vivo studies. Int. J. Nanomed. 2012, 7, 1215–1225. [Google Scholar]
- Johansson, P.; Barkarmo, S.; Hawthan, M.; Peruzzi, N.; Kjellin, P.; Wennerberg, A. Biomechanical, histological, and computed X-ray tomographic analyses of hydroxyapatite coated PEEK implants in an extended healing model in rabbit. J. Biomed. Mater. Res. Part A 2018, 106, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Ourahmoune, R.; Salvia, M.; Mathia, T.G.; Mesrati, N. Surface morphology and wettability of sandblasted PEEK and its composites. Scanning 2014, 36, 64–75. [Google Scholar] [CrossRef]
- Han, C.M.; Lee, E.J.; Kim, H.E.; Koh, Y.H.; Kim, K.N.; Ha, Y.; Kuh, S.U. The electron beam deposition of titanium on polyetheretherketone (PEEK) and the resulting enhanced biological properties. Biomaterials 2010, 31, 3465–3470. [Google Scholar] [CrossRef]
- Poulsson, A.H.; Eglin, D.; Zeiter, S.; Camenisch, K.; Sprecher, C.; Agarwal, Y.; Nehrbass, D.; Wilson, J.; Richards, R.G. Osseointegration of machined, injection moulded and oxygen plasma modified PEEK implants in a sheep model. Biomaterials 2014, 35, 3717–3728. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.; Selezneva, I.; Pestov, S.; Tarassov, V.; Ermakov, A.; Mikheev, A.; Lazov, M.; Kirkpatrick, S.R.; Shashkov, D.; Smolkov, A. Surface bioactivation of PEEK by neutral atom beam technology. Bioact. Mater. 2019, 4, 132–141. [Google Scholar] [CrossRef]
- Terpiłowski, K.; Wiącek, A.E.; Jurak, M. Influence of nitrogen plasma treatment on the wettability of polyetheretherketone and deposited chitosan layers. Adv. Polym. Technol. 2018, 37, 1557–1569. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wong, H.M.; Wang, W.; Li, P.; Xu, Z.; Chong, E.Y.; Yan, C.H.; Yeung, K.W.; Chu, P.K. Cytocompatibility, osseointegration, and bioactivity of three-dimensional porous and nanostructured network on polyetheretherketone. Biomaterials 2013, 34, 9264–9277. [Google Scholar] [CrossRef]
- Alghamdi, H.S.; Bosco, R.; Beucken, J.J.V.D.; Walboomers, X.F.; Jansen, J.A. Osteogenicity of titanium implants coated with calcium phosphate or collagen type-I in osteoporotic rats. Biomaterials 2013, 34, 3747–3757. [Google Scholar] [CrossRef] [PubMed]
- Lutz, R.; Srour, S.; Nonhoff, J.; Weisel, T.; Damien, C.J.; Schlegel, K.A. Biofunctionalization of titanium implants with a biomimetic active peptide (P-15) promotes early osseointegration. Clin. Oral Implant. Res. 2010, 21, 726–734. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Cheng, B. Effect of minTBP-1-RGD/titanium implant on osseointegration in rats. Mater. Lett. 2018, 228, 424–426. [Google Scholar] [CrossRef]
- Singh, S.; Prakash, C.; Wang, H.; Yu, X.-F.; Ramakrishna, S. Plasma treatment of polyether-ether-ketone: A means of obtaining desirable biomedical characteristics. Eur. Polym. J. 2019, 118, 561–577. [Google Scholar] [CrossRef]
- Xian, P.; Chen, Y.; Gao, S.; Qian, J.; Zhang, W.; Udduttula, A.; Huang, N.; Wan, G. Polydopamine (PDA) mediated nanogranular-structured titanium dioxide (TiO2) coating on polyetheretherketone (PEEK) for oral and maxillofacial implants application. Surf. Coat. Technol. 2020, 401, 126282. [Google Scholar] [CrossRef]
- Xue, Z.; Wang, Z.; Huang, J.; Wu, W.; Chen, M.; Hao, X.; Huang, Z.; Lin, X.; Weng, S.J.M.S. Rapid construction of polyetheretherketone (PEEK) biological implants incorporated with brushite (CaHPO4· 2H2O) and antibiotics for anti-infection and enhanced osseointegration. Mater. Sci. Eng. C 2020, 111, 110782. [Google Scholar] [CrossRef]
- Wei, R.; Wu, J.; Li, Y. Macrophage polarization following three-dimensional porous PEEK. Mater. Sci. Eng. C 2019, 104, 109948. [Google Scholar] [CrossRef]
- Torstrick, F.B.; Lin, A.S.; Potter, D.; Safranski, D.L.; Sulchek, T.A.; Gall, K.; Guldberg, R.E. Porous PEEK improves the bone-implant interface compared to plasma-sprayed titanium coating on PEEK. Biomaterials 2018, 185, 106–116. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lin, J.H.C.; Wu, Y.R.; Chang, C.W.; Chang, K.C.; Chen, C.C.; Chen, C.H.; Chen, W.C. Characterizing the differentiation of osteoprogenitor cells on surface modified polyether-ether-ketone. Surf. Coat. Technol. 2018, 350, 904–912. [Google Scholar] [CrossRef]
- Chen, W.-C.; Ju, C.-P.; Tien, Y.-C.; Lin, J.-H.C. In vivo testing of nanoparticle-treated TTCP/DCPA-based ceramic surfaces. Acta Biomater. 2009, 5, 1767–1774. [Google Scholar] [CrossRef]
- Chen, J.-C.; Ko, C.-L.; Shih, C.-J.; Tien, Y.-C.; Chen, W.-C. Calcium phosphate bone cement with 10 wt% platelet-rich plasma in vitro and in vivo. J. Dent. 2012, 40, 114–122. [Google Scholar] [CrossRef]
- Tukey, J.W. Comparing Individual Means in the Analysis of Variance. Biometrics 1949, 5, 99. [Google Scholar] [CrossRef]
- Nanci, A.; Wuest, J.D.; Peru, L.; Brunet, P.; Sharma, V.; Zalzal, S.; McKee, M.D. Chemical modification of titanium surfaces for covalent attachment of biological molecules. J. Biomed. Mater. Res. 1998, 40, 324–335. [Google Scholar] [CrossRef]
- Desmet, T.; Morent, R.; De Geyter, N.; Leys, C.; Schacht, E.; Dubruel, P. Nonthermal Plasma Technology as a Versatile Strategy for Polymeric Biomaterials Surface Modification: A Review. Biomacromolecules 2009, 10, 2351–2378. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.R.; Vinothkannan, M.; Yoo, D.J. Sulfonated-fluorinated copolymer blending membranes containing SPEEK for use as the electrolyte in polymer electrolyte fuel cells (PEFC). Int. J. Hydrogen Energy 2017, 42, 4349–4365. [Google Scholar] [CrossRef]
- Teli, S.B.; Benamor, A.; Nasser, M.; Hawari, A.; Zaidi, S.J.; Ba-Abbad, M.; Mohammad, A.W. Effects of amphiphilic pluronic F127 on the performance of PS/SPEEK blend ultrafiltration membrane: Characterization and antifouling study. J. Water Process. Eng. 2017, 18, 176–184. [Google Scholar] [CrossRef]
- Salleh, M.T.; Jaafar, J.; Mohamed, M.A.; Norddin, M.; Ismail, A.; Othman, M.; Rahman, M.A.; Yusof, N.; Aziz, F.; Salleh, W. Stability of SPEEK/Cloisite®/TAP nanocomposite membrane under Fenton reagent condition for direct methanol fuel cell application. Polym. Degrad. Stab. 2017, 137, 83–99. [Google Scholar] [CrossRef]
- Sasikala, S.; Meenakshi, S.; Bhat, S.D.; Sahu, A.K. Functionalized Bentonite clay-sPEEK based composite membranes for direct methanol fuel cells. Electrochim. Acta 2014, 135, 232–241. [Google Scholar] [CrossRef]
- Montero, J.F.; Tajiri, H.A.; Barra, G.M.; Fredel, M.C.; Benfatti, C.A.; Magini, R.S.; Pimenta, A.L.; Souza, J.C. Biofilm behavior on sulfonated poly(ether-ether-ketone) (sPEEK). Mater. Sci. Eng. C 2017, 70, 456–460. [Google Scholar] [CrossRef]
- Mansur, H.S.; Oréfice, R.L.; Vasconcelos, W.L.; Lobato, Z.P.; Machado, L.J.C. Biomaterial with chemically engineered surface for protein immobilization. J. Mater. Sci. 2005, 16, 333–340. [Google Scholar] [CrossRef]
- Wei, J.; Igarashi, T.; Okumori, N.; Igarashi, T.; Maetani, T.; Liu, B.; Yoshinari, M. Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts. Biomed. Mater. 2009, 4, 045002. [Google Scholar] [CrossRef]
- Goddard, J.; Hotchkiss, J. Polymer surface modification for the attachment of bioactive compounds. Prog. Polym. Sci. 2007, 32, 698–725. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 172–184. [Google Scholar] [CrossRef]
- Sengupta, S.; Park, S.-H.; Patel, A.; Carn, J.; Lee, K.; Kaplan, D.L. Hypoxia and amino acid supplementation synergistically promote the osteogenesis of human mesenchymal stem cells on silk protein scaffolds. Tissue Eng. Part A 2010, 16, 3623–3634. [Google Scholar] [CrossRef] [Green Version]
- Washington, J.M.; Rathjen, J.; Felquer, F.; Lonic, A.; Bettess, M.D.; Hamra, N.; Semendric, L.; Tan, B.S.N.; Lake, J.-A.; Keough, R.A.; et al. l-Proline induces differentiation of ES cells: A novel role for an amino acid in the regulation of pluripotent cells in culture. Am. J. Physiol. Physiol. 2010, 298, C982–C992. [Google Scholar] [CrossRef]
- Zhu, N.; Cui, F.; Hu, K.; Zhu, L. Biomedical modification of poly(L-lactide) by blending with lecithin. J. Biomed. Mater. Res. Part A 2007, 82, 455–461. [Google Scholar] [CrossRef]
- Quarles, L.D.; Yohay, D.A.; Lever, L.W.; Caton, R.; Wenstrup, R.J. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: An in vitro model of osteoblast development. J. Bone Miner. Res. 2009, 7, 683–692. [Google Scholar] [CrossRef]
- Schwartz, Z.; Lohmann, C.; Oefinger, J.; Bonewald, L.; Dean, D.; Boyan, B. Implant Surface Characteristics Modulate Differentiation Behavior of Cells in the Osteoblastic Lineage. Adv. Dent. Res. 1999, 13, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Kim, C.-W.; Lim, Y.-J.; Heo, S.J. Microrough titanium surface affects biologic response in MG63 osteoblast-like cells. J. Biomed. Mater. Res. Part A 2006, 79, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. Part A 2005, 74, 49–58. [Google Scholar] [CrossRef]
- Fu, P.-S.; Wang, J.-C.; Lai, P.-L.; Liu, S.-M.; Chen, Y.-S.; Chen, W.-C.; Hung, C.-C. Effects of Gamma Radiation on the Sterility Assurance, Antibacterial Ability, and Biocompatibility of Impregnated Hydrogel Macrosphere Protein and Drug Release. Polymers 2021, 13, 938. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Cheng, I.-T.; Chang, K.-C.; Haung, S.-M.; Chen, J.-C.; Shih, C.-J. Heparin as a biomimetic template on nanoapatite rods with tunable aspect ratio: Synthesis and biocompatibility. J. Aust. Ceram. Soc. 2021, 1–10. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-C.; Chen, C.-H.; Chang, K.-C.; Liu, S.-M.; Ko, C.-L.; Shih, C.-J.; Sun, Y.-S.; Chen, W.-C. Evaluation of the Grafting Efficacy of Active Biomolecules of Phosphatidylcholine and Type I Collagen on Polyether Ether Ketone: In Vitro and In Vivo. Polymers 2021, 13, 2081. https://doi.org/10.3390/polym13132081
Chen J-C, Chen C-H, Chang K-C, Liu S-M, Ko C-L, Shih C-J, Sun Y-S, Chen W-C. Evaluation of the Grafting Efficacy of Active Biomolecules of Phosphatidylcholine and Type I Collagen on Polyether Ether Ketone: In Vitro and In Vivo. Polymers. 2021; 13(13):2081. https://doi.org/10.3390/polym13132081
Chicago/Turabian StyleChen, Jian-Chih, Chih-Hua Chen, Kai-Chi Chang, Shih-Ming Liu, Chia-Ling Ko, Chi-Jen Shih, Ying-Sui Sun, and Wen-Cheng Chen. 2021. "Evaluation of the Grafting Efficacy of Active Biomolecules of Phosphatidylcholine and Type I Collagen on Polyether Ether Ketone: In Vitro and In Vivo" Polymers 13, no. 13: 2081. https://doi.org/10.3390/polym13132081
APA StyleChen, J.-C., Chen, C.-H., Chang, K.-C., Liu, S.-M., Ko, C.-L., Shih, C.-J., Sun, Y.-S., & Chen, W.-C. (2021). Evaluation of the Grafting Efficacy of Active Biomolecules of Phosphatidylcholine and Type I Collagen on Polyether Ether Ketone: In Vitro and In Vivo. Polymers, 13(13), 2081. https://doi.org/10.3390/polym13132081