Effect of Ultrasonic Vibration on Structural and Physical Properties of Resin-Based Dental Composites
Abstract
:1. Introduction
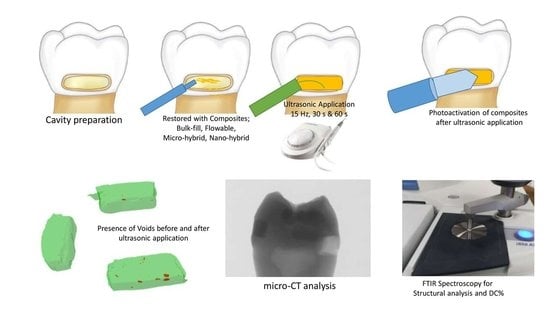
2. Materials and Methods
2.1. Sample Preparation
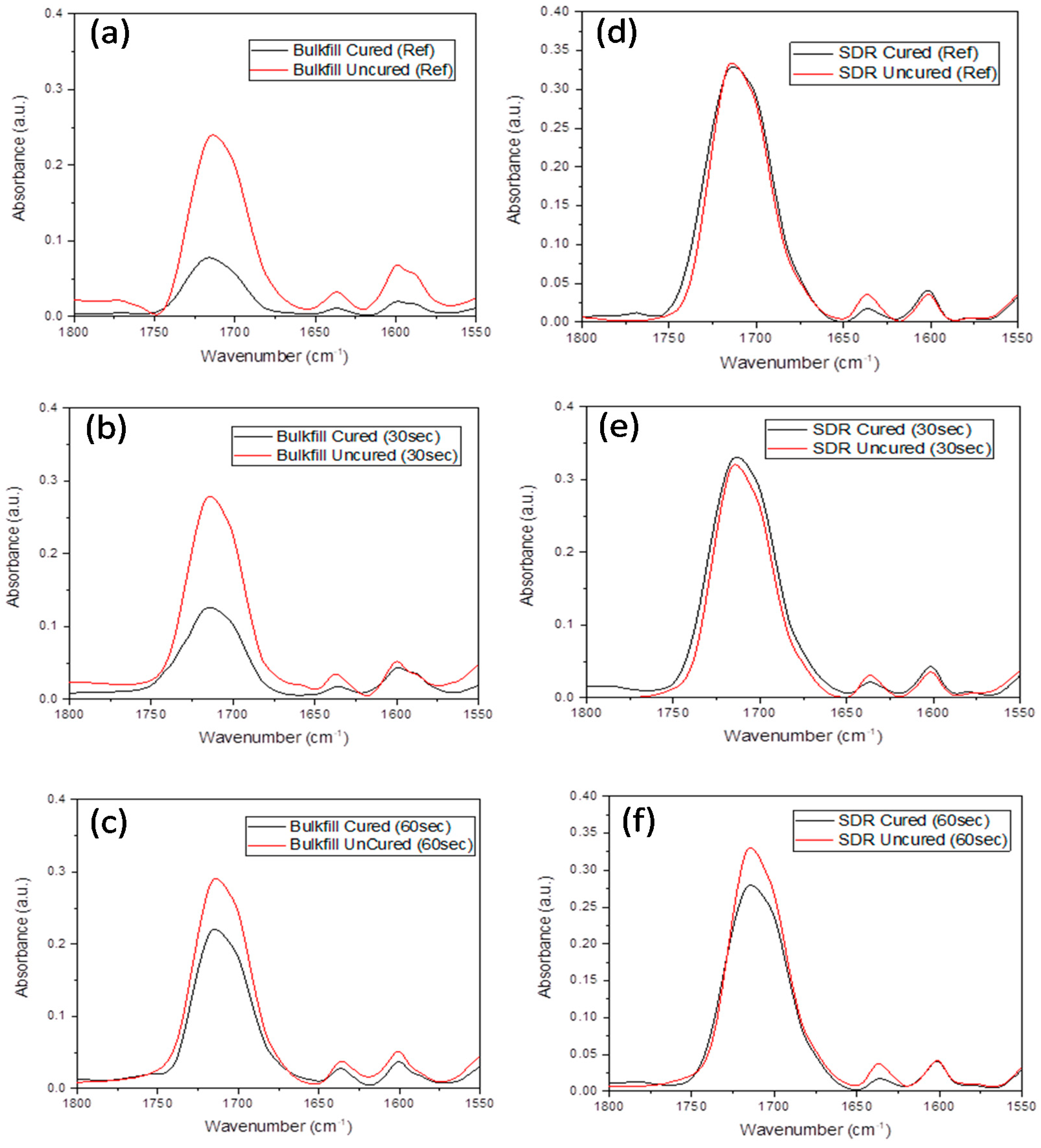
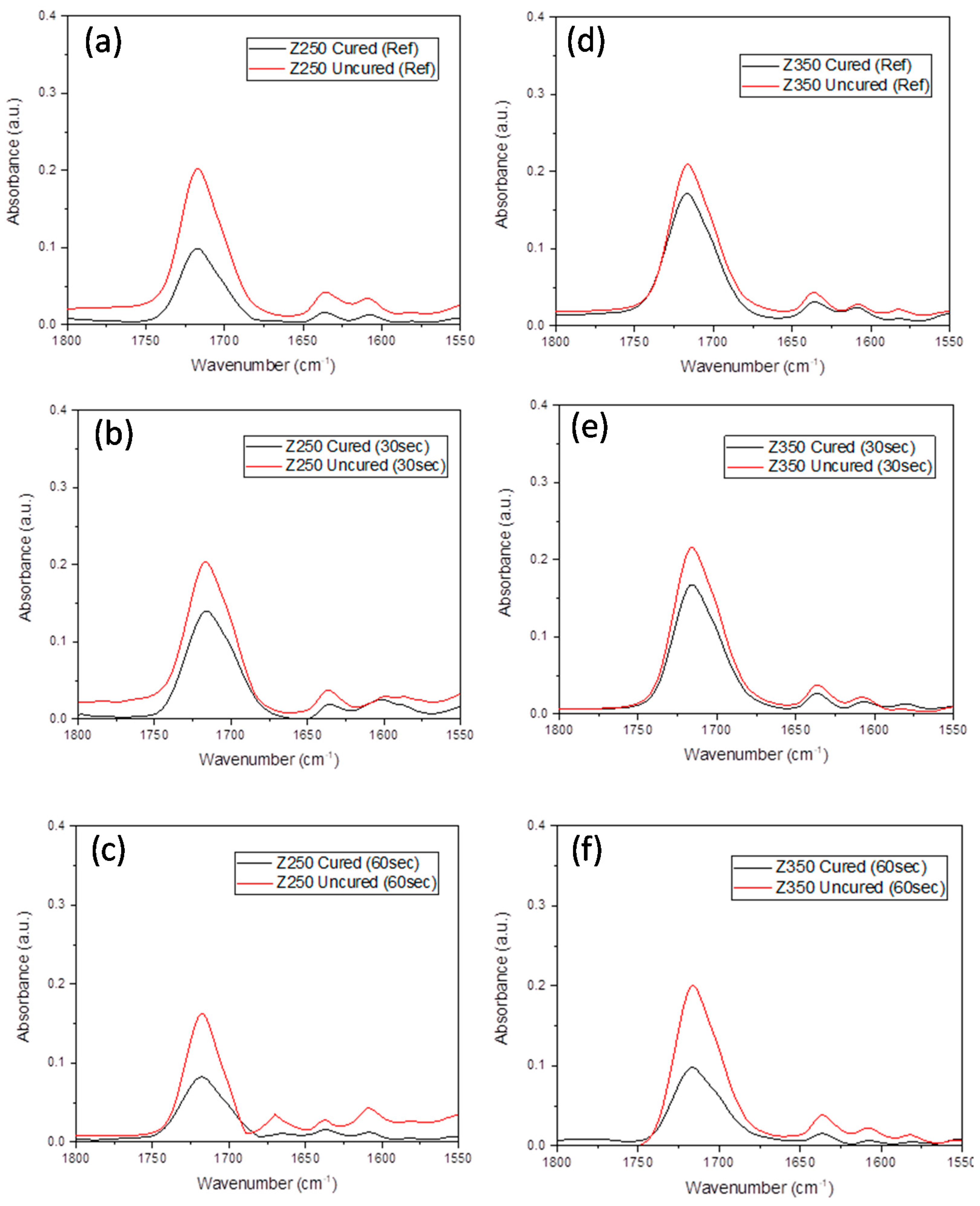
2.2. Degree Of Conversion Analysis
2.3. Methodology For Spectral Data Processing
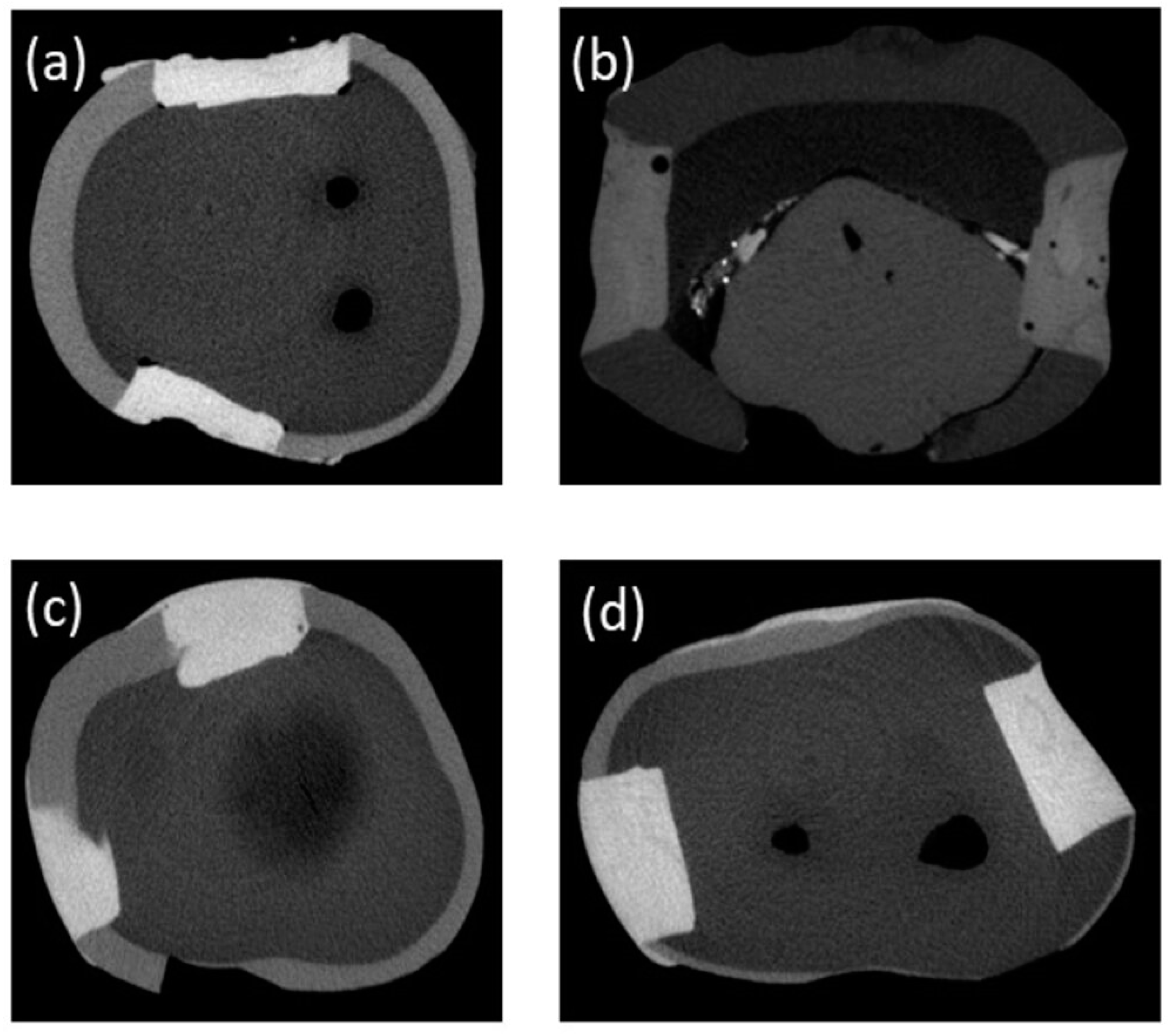
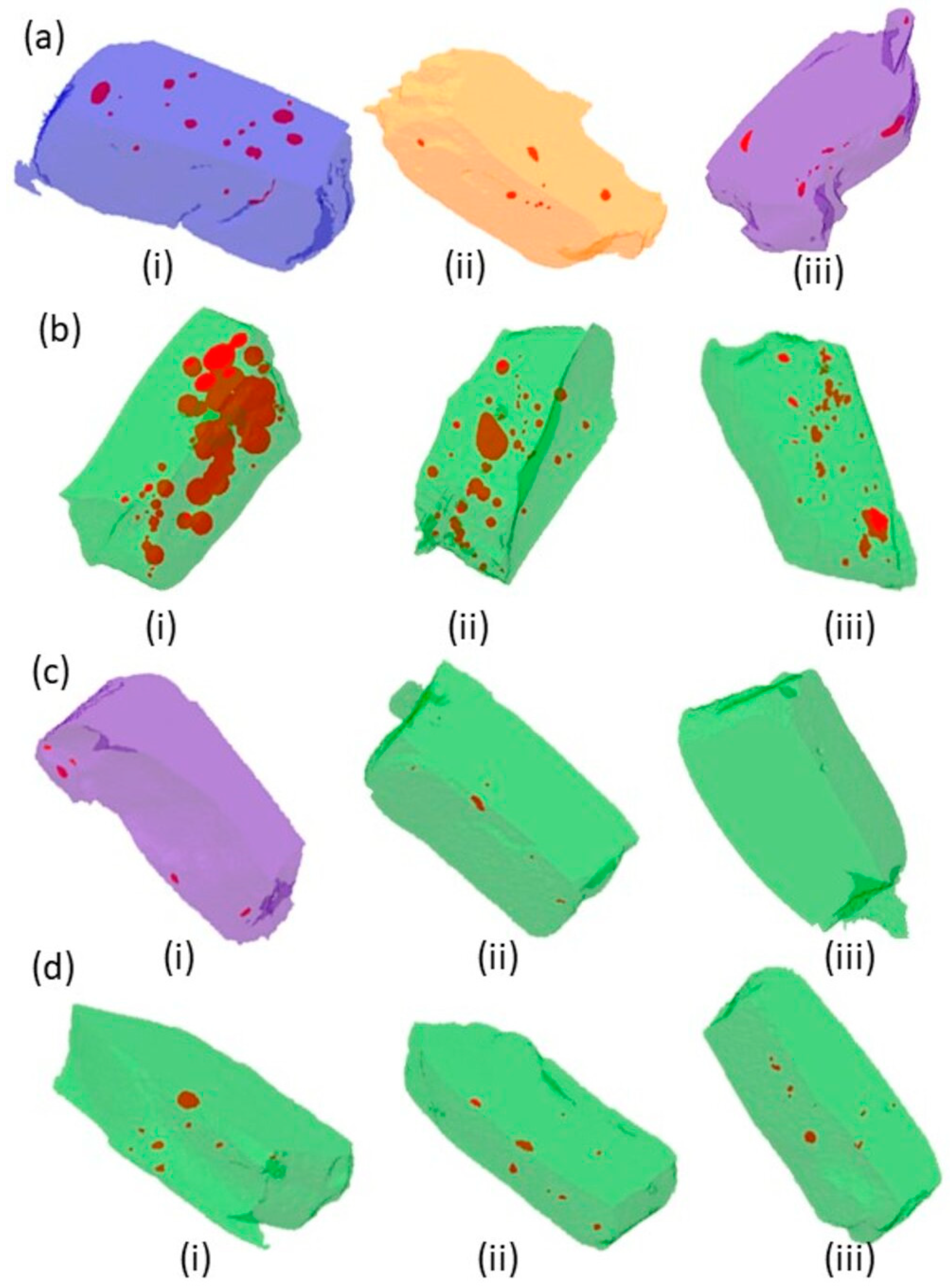
2.4. Micro-Computed Tomography (µ-CT) Analysis
2.5. Statistical Analysis
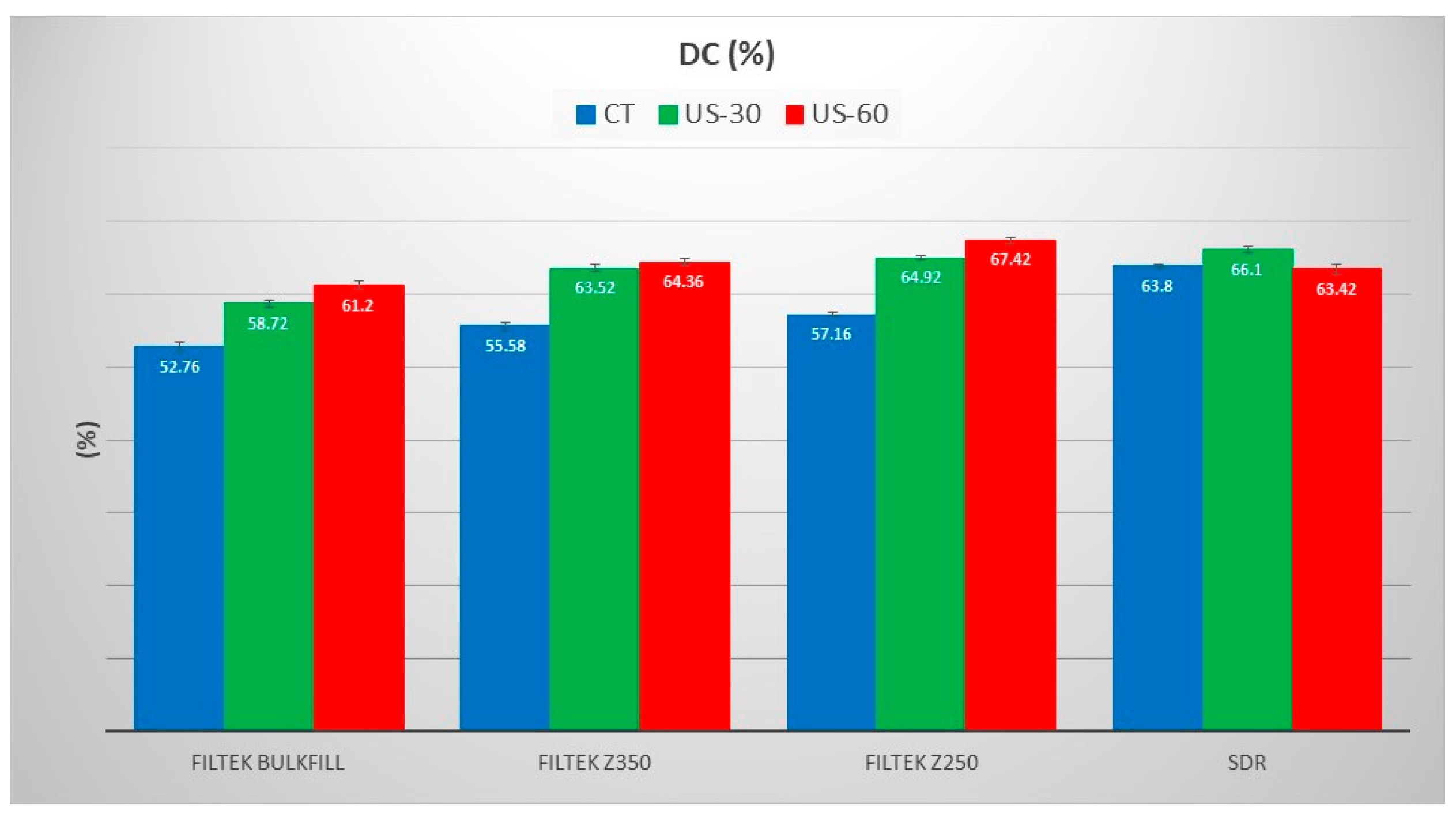
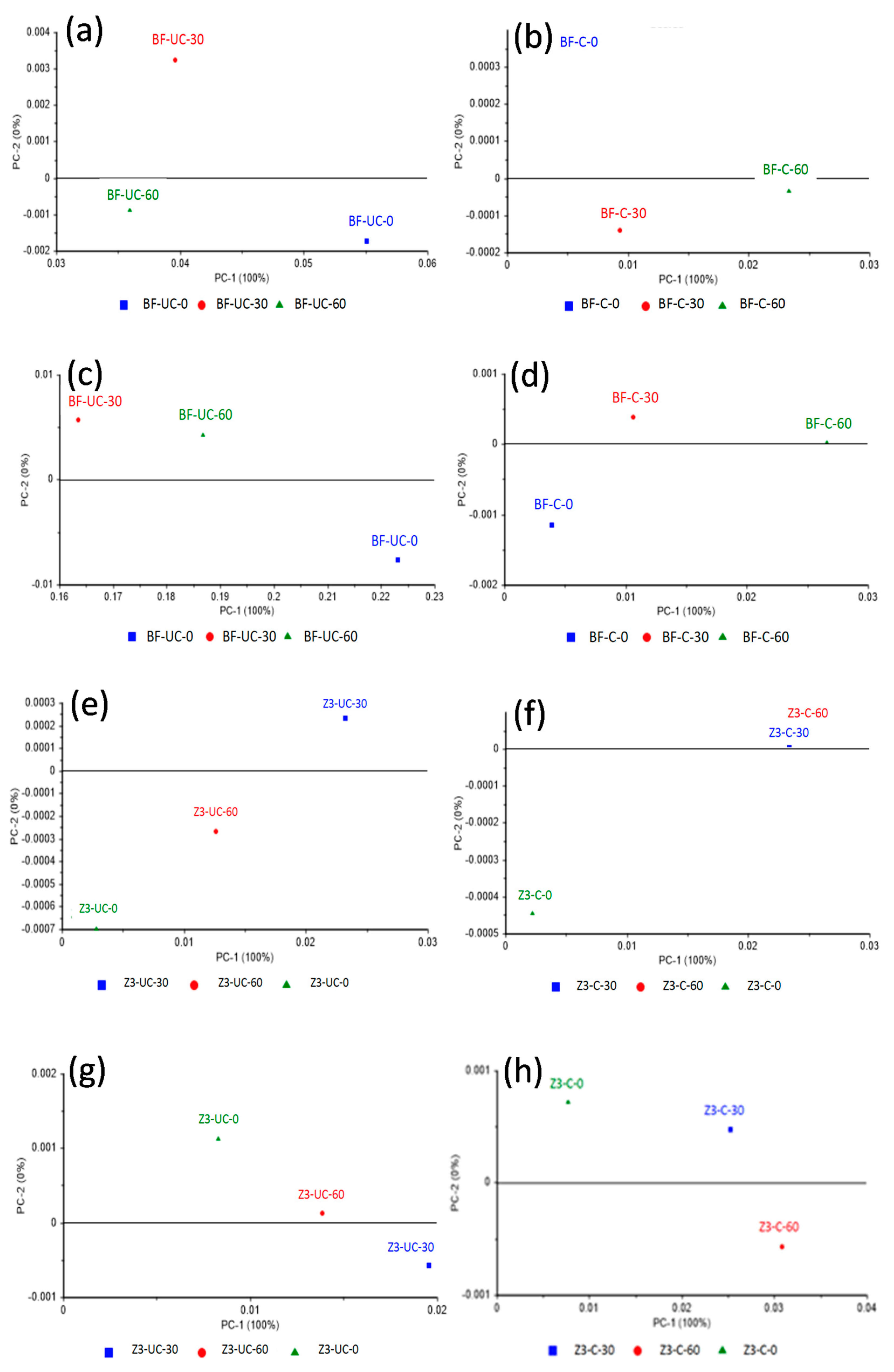
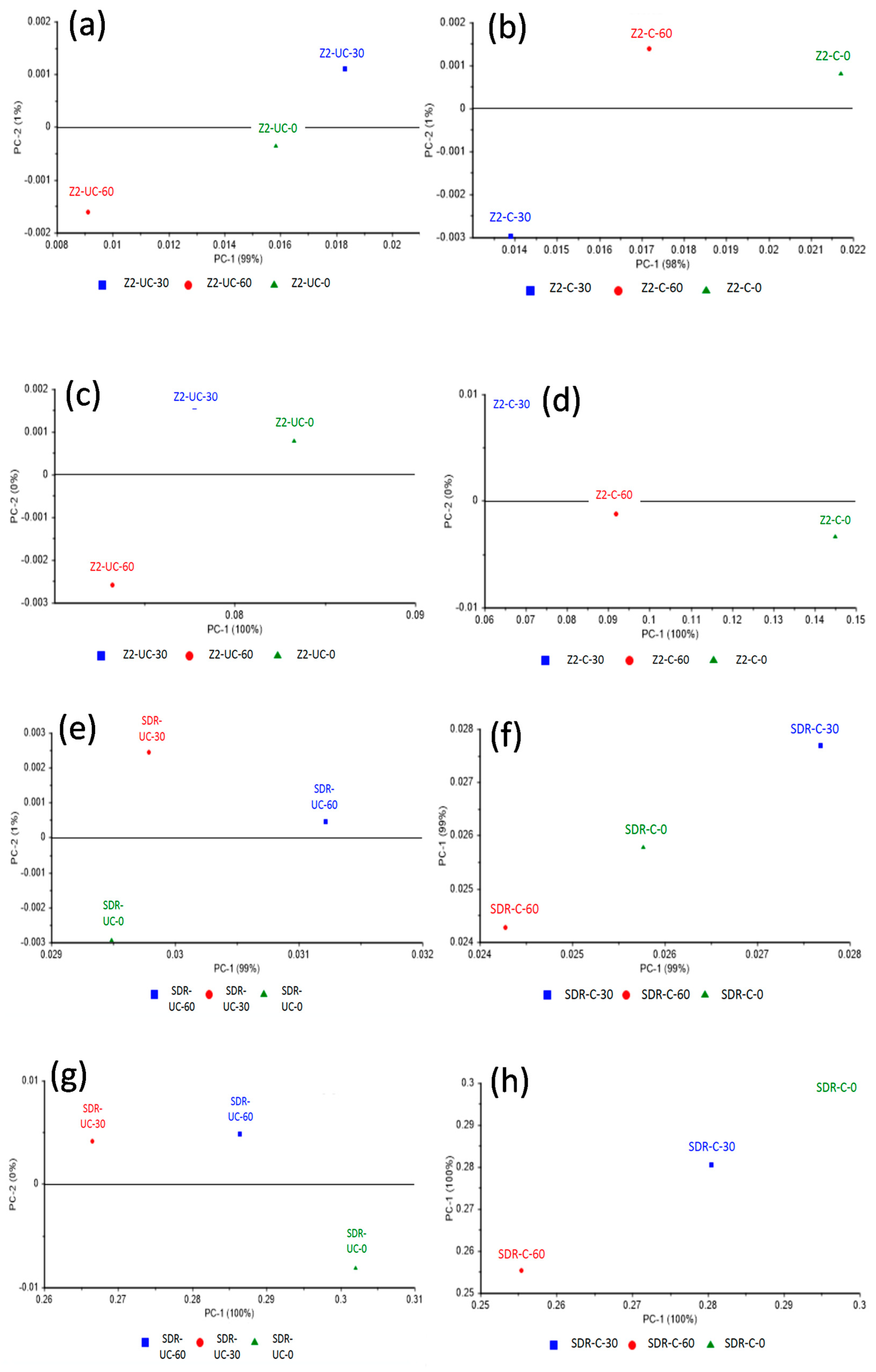
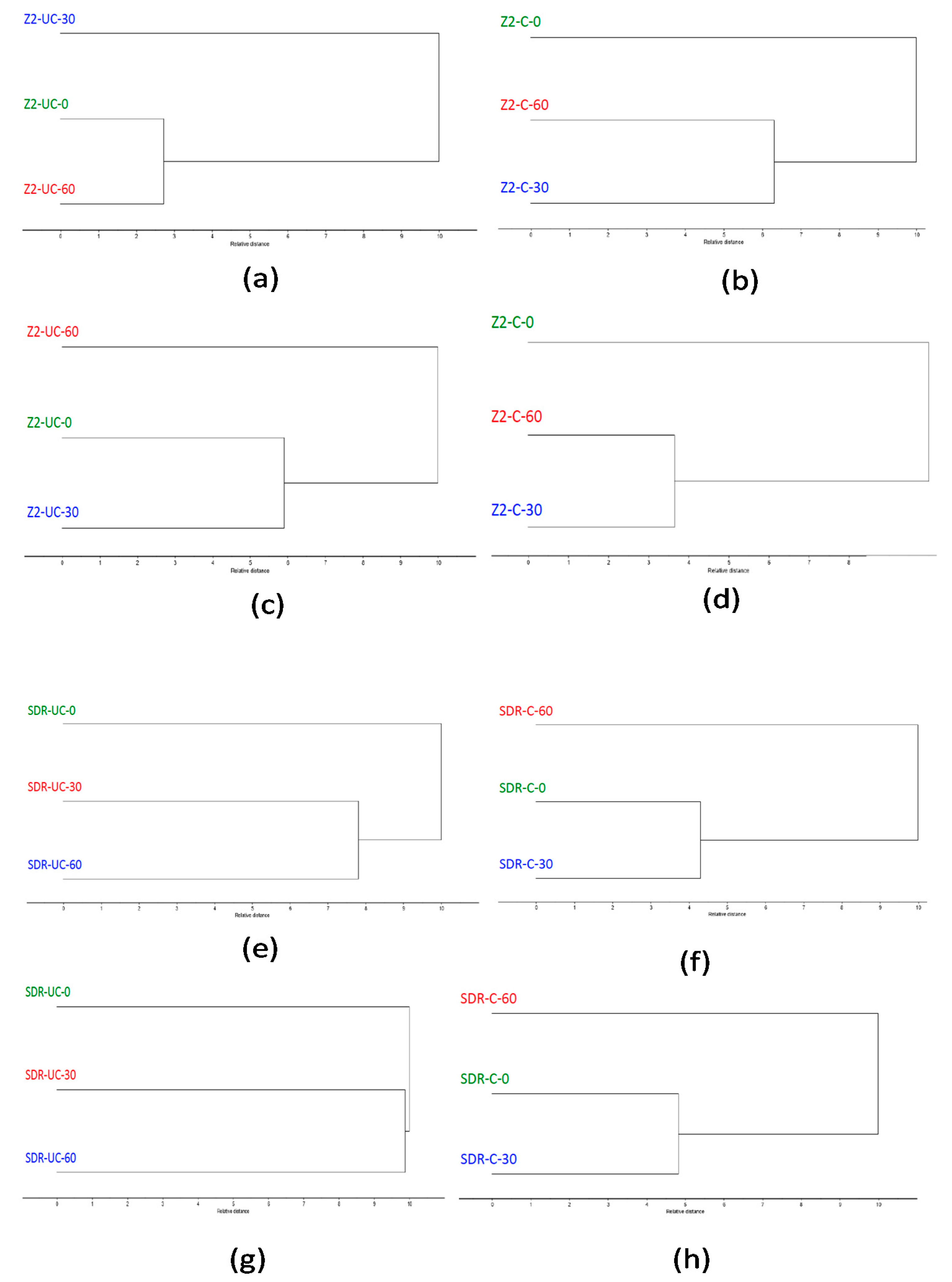
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Nahedh, H.N.; El-Hejazi, A.A.; Habib, S.R. Knowledge and Attitude of Dentists and Patients Toward Use and Health Safety of Dental Amalgam in Saudi Arabia. Eur. J. Dent. 2020, 14, 233–238. [Google Scholar] [CrossRef]
- Mackey, T.K.; Contreras, J.T.; Liang, B.A. The Minamata Convention on Mercury: Attempting to address the global controversy of dental amalgam use and mercury waste disposal. Sci. Total Environ. 2014, 472, 125–129. [Google Scholar] [CrossRef]
- Quek Wei QIAN, C.; Amir Wan Ahmad, W.M.; Yusop, N.; Ghazalli, N.F.; Samsudin, N.A. Anterior Composite Resin Restoration Preference Among General Dental Practitioners in Penang Island, Malaysia. Int. J. Med. Dent. 2020, 24, 506–516. [Google Scholar]
- Khan, A.S.; Ur Rehman, S.; AlMaimouni, Y.K.; Ahmad, S.; Khan, M.; Ashiq, M. Bibliometric Analysis of Literature Published on Antibacterial Dental Adhesive from 1996–2020. Polymers 2020, 12, 2848. [Google Scholar] [CrossRef]
- Rickman, L.J.; Padipatvuthikul, P.; Chee, B. Clinical applications of preheated hybrid resin composite. Br. Dent. J. 2011, 211, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.J.; Faria-E-Silva, A.L.; Rodrigues, M.d.P.; Vilela, A.B.F.; Pfeifer, C.S.; Tantbirojn, D.; Versluis, A. Polymerization shrinkage stress of composite resins and resin cements—What do we need to know? Braz. Oral Res. 2017, 31, e62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celik, C.; Cehreli, S.B.; Arhun, N. Resin composite repair: Quantitative microleakage evaluation of resin-resin and resin-tooth interfaces with different surface treatments. Eur. J. Dent. 2015, 9, 92–99. [Google Scholar] [CrossRef]
- Radhika, M.; Sajjan, G.S.; Kumaraswamy, B.N.; Mittal, N. Effect of different placement techniques on marginal microleakage of deep class-II cavities restored with two composite resin formulations. J. Conserv. Dent. 2010, 13, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Tauböck, T.T.; Jäger, F.; Attin, T. Polymerization shrinkage and shrinkage force kinetics of high-and low-viscosity dimethacrylate-and ormocer-based bulk-fill resin composites. Odontology 2019, 107, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Bayne, S.C.; Thompson, J.Y.; Swift, E.J., Jr.; Stamatiades, P.; Wilkerson, M. A characterization of first-generation flowable composites. J. Am. Dent. Assoc. 1998, 129, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Kaisarly, D.; El Gezawi, M.; Keßler, A.; Rösch, P.; Kunzelmann, K.-H. Shrinkage vectors in flowable bulk-fill and conventional composites: Bulk versus incremental application. Clin. Oral Investig. 2021, 25, 1127–1139. [Google Scholar] [CrossRef]
- Rizzante, F.A.P.; Mondelli, R.F.L.; Furuse, A.Y.; Borges, A.F.S.; Mendonça, G.; Ishikiriama, S.K. Shrinkage stress and elastic modulus assessment of bulk-fill composites. J. Appl. Oral Sci. 2019, 27, e20180132. [Google Scholar] [CrossRef] [PubMed]
- Khalid, H.; Syed, M.R.; Rahbar, M.I.; Iqbal, H.; Ahmad, S.; Kaleem, M.; Matinlinna, J.P.; Khan, A.S. Effect of nano-bioceramics on monomer leaching and degree of conversion of resin-based composites. Dent. Mater. J. 2018, 37, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Fronza, B.M.; Rueggeberg, F.A.; Braga, R.R.; Mogilevych, B.; Soares, L.E.S.; Martin, A.A.; Ambrosano, G.; Giannini, M. Monomer conversion, microhardness, internal marginal adaptation, and shrinkage stress of bulk-fill resin composites. Dent. Mater. 2015, 31, 1542–1551. [Google Scholar] [CrossRef]
- Bakhsh, T.A.; Sadr, A.; Shimada, Y.; Turkistani, A.; Abuljadayel, R.; Tagami, J. Does lining class-II cavities with flowable composite improve the interfacial adaptation? J. Adhes. Sci. Technol. 2020, 34, 400–416. [Google Scholar] [CrossRef]
- Sarapultseva, M.; Sarapultsev, A. Flowable Bulk-Fill Materials Compared to Nano Ceramic Composites for Class I Cavities Restorations in Primary Molars: A Two-Year Prospective Case-Control Study. Dent. J. 2019, 7, 94. [Google Scholar] [CrossRef] [Green Version]
- Nocca, G.; De Palma, F.; Minucci, A.; De Sole, P.; Martorana, G.; Callà, C.; Morlacchi, C.; Gozzo, M.; Gambarini, G.; Chimenti, C. Alterations of energy metabolism and glutathione levels of HL-60 cells induced by methacrylates present in composite resins. J. Dent. 2007, 35, 187–194. [Google Scholar] [CrossRef]
- Reichl, F.-X.; Esters, M.; Simon, S.; Seiss, M.; Kehe, K.; Kleinsasser, N.; Folwaczny, M.; Glas, J.; Hickel, R. Cell death effects of resin-based dental material compounds and mercurials in human gingival fibroblasts. Arch. Toxicol. 2006, 80, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Michelsen, V.B.; Kopperud, H.B.; Lygre, G.B.; Björkman, L.; Jensen, E.; Kleven, I.S.; Svahn, J.; Lygre, H. Detection and quantification of monomers in unstimulated whole saliva after treatment with resin-based composite fillings in vivo. Eur. J. Oral Sci. 2012, 120, 89–95. [Google Scholar] [CrossRef]
- Moldovan, M.; Balazsi, R.; Soanca, A.; Roman, A.; Sarosi, C.; Prodan, D.; Vlassa, M.; Cojocaru, I.; Saceleanu, V.; Cristescu, I. Evaluation of the Degree of Conversion, Residual Monomers and Mechanical Properties of Some Light-Cured Dental Resin Composites. Materials 2019, 12, 2109. [Google Scholar] [CrossRef] [Green Version]
- Halvorson, R.H.; Erickson, R.L.; Davidson, C.L. The effect of filler and silane content on conversion of resin-based composite. Dent. Mater. 2003, 19, 327–333. [Google Scholar] [CrossRef]
- Tuna, E.; Aktoren, O.; Oshida, Y.; Gencay, K. Elution of residual monomers from dental composite materials. Eur. J. Paediatr Dent. 2010, 11, 110–114. [Google Scholar] [PubMed]
- Marcondes, R.L.; Lima, V.P.; Barbon, F.J.; Isolan, C.P.; Carvalho, M.A.; Salvador, M.V.; Lima, A.F.; Moraes, R.R. Viscosity and thermal kinetics of 10 preheated restorative resin composites and effect of ultrasound energy on film thickness. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2020, 36, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.H.; Lim, S.; Kum, K.Y.; Chang, S.W. Effect of preheating on the viscoelastic properties of dental composite under different deformation conditions. Dent. Mater. J. 2015, 34, 702–706. [Google Scholar] [CrossRef] [Green Version]
- Prasanna, N.; Pallavi Reddy, Y.; Kavitha, S.; Lakshmi Narayanan, L. Degree of conversion and residual stress of preheated and room-temperature composites. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2007, 18, 173–176. [Google Scholar] [CrossRef]
- Elkaffass, A.-A.; Eltoukhy, R.-I.; Elnegoly, S.-A.-E.; Mahmoud, S.-H. Influence of preheating on mechanical and surface properties of nanofilled resin composites. J. Clin. Exp. Dent. 2020, 12, e494–e500. [Google Scholar] [CrossRef] [PubMed]
- Daronch, M.; Rueggeberg, F.A.; De Goes, M.F. Monomer Conversion of Pre-heated Composite. J. Dent. Res. 2005, 84, 663–667. [Google Scholar] [CrossRef]
- Daronch, M.; Rueggeberg, F.A.; De Goes, M.F.; Giudici, R. Polymerization kinetics of pre-heated composite. J. Dent. Res. 2006, 85, 38–43. [Google Scholar] [CrossRef]
- Yao, Y.; Pan, Y.; Liu, S. Power ultrasound and its applications: A state-of-the-art review. Ultrason. Sonochemistry 2020, 62, 104722. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Chang, H.-H.; Chiang, Y.-C.; Lin, C.-P. Application and development of ultrasonics in dentistry. J. Formos. Med. Assoc. 2013, 112, 659–665. [Google Scholar] [CrossRef] [Green Version]
- Cantoro, A.; Goracci, C.; Coniglio, I.; Magni, E.; Polimeni, A.; Ferrari, M. Influence of ultrasound application on inlays luting with self-adhesive resin cements. Clin. Oral Investig. 2011, 15, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Sato, S.; Kishida, M.; Ito, K. A comparison of root surface instrumentation using two piezoelectric ultrasonic scalers and a hand scaler in vivo. J. Periodontal Res. 2007, 42, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Aydin, C.; Inan, U.; Gultekin, M. Comparison of the shaping ability of Twisted Files with ProTaper and RevoS nickel-titanium instruments in simulated canals. J. Dental Sci. 2012, 7, 283–288. [Google Scholar] [CrossRef] [Green Version]
- Coldebella, C.R.; Santos-Pinto, L.; Zuanon, A.C.C. Effect of ultrasonic excitation on the porosity of glass ionomer cement: A scanning electron microscope evaluation. Microsc. Res. Tech. 2011, 74, 54–57. [Google Scholar] [CrossRef]
- Barata, T.J.E.; Bresciani, E.; Adachi, A.; Fagundes, T.C.; Carvalho, C.A.R.; Navarro, M.F.L. Influence of ultrasonic setting on compressive and diametral tensile strengths of glass ionomer cements. Mater. Res. 2008, 11, 57–61. [Google Scholar] [CrossRef]
- Vanderlei, A.D.; Borges, A.L.S.; Cavalcanti, B.N.; Rode, S.M. Ultrasonic versus high-speed cavity preparation: Analysis of increases in pulpal temperature and time to complete preparation. J. Prosthet. Dent. 2008, 100, 107–109. [Google Scholar] [CrossRef]
- Monteiro, G.Q.d.M.; Montes, M.A.J.R. Evaluation of linear polymerization shrinkage, flexural strength and modulus of elasticity of dental composites. Mater. Res. 2010, 13, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Camassari, J.R.; Correr-Sobrinho, L.; Correr, A.B.; Puppin-Rontani, J.; Stipp, R.N.; Puppin-Rontani, R.M.; Paula, A.B. Physical-Mechanical Properties of Bulk Fill Composites Submitted to Biodegradation by Streptococcus mutans. Braz. Dent. J. 2020, 31, 431–439. [Google Scholar] [CrossRef]
- Lassila, L.; Säilynoja, E.; Prinssi, R.; Vallittu, P.; Garoushi, S. Characterization of a new fiber-reinforced flowable composite. Odontology 2019, 107, 342–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Secilmis, A.; Dilber, E.; Gokmen, F.; Ozturk, N.; Telatar, T. Effects of storage solutions on mineral contents of dentin. J. Dent. Sci. 2011, 6, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Firzok, H.; Zahid, S.; Asad, S.; Manzoor, F.; Khan, A.S.; Shah, A.T. Sol-gel derived fluoridated and non-fluoridated bioactive glass ceramics-based dental adhesives: Compositional effect on re-mineralization around orthodontic brackets. J. Non Cryst. Solids 2019, 521, 119469. [Google Scholar] [CrossRef]
- Luong, E.; Shayegan, A. Assessment of microleakage of class V restored by resin composite and resin-modified glass ionomer and pit and fissure resin-based sealants following Er:YAG laser conditioning and acid etching: In vitro study. Clin. Cosmet Investig Dent. 2018, 10, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemeshow, S.; Lwanga, S. Sample Size Determination in Health Studies; Version 2.0.21; World Health Organization: Geneva, Switzerland, 1991. [Google Scholar]
- Sakaguchi, R.; Ferracane, J.; Powers, J. (Eds.) Chapter 9—Restorative Materials: Resin Composites and Polymers. In Craig’s Restorative Dental Materials, 4th ed.; Elsevier: Philadelphia, PA, USA, 2019; pp. 135–170. Available online: https://doi.org/10.1016/B978-0-323-47821-2.00009-3 (accessed on 19 June 2021).
- Nayyer, M.; Zahid, S.; Hassan, S.H.; Mian, S.A.; Mehmood, S.; Khan, H.A.; Kaleem, M.; Zafar, M.S.; Khan, A.S. Comparative abrasive wear resistance and surface analysis of dental resin-based materials. Eur. J. Dent. 2018, 12, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Bayne, S.C.; Ferracane, J.L.; Marshall, G.W.; Marshall, S.J.; van Noort, R. The Evolution of Dental Materials over the Past Century: Silver and Gold to Tooth Color and Beyond. J. Dent. Res. 2019, 98, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Arbildo-Vega, H.I.; Lapinska, B.; Panda, S.; Lamas-Lara, C.; Khan, A.S.; Lukomska-Szymanska, M. Clinical Effectiveness of Bulk-Fill and Conventional Resin Composite Restorations: Systematic Review and Meta-Analysis. Polymers 2020, 12, 1786. [Google Scholar] [CrossRef] [PubMed]
- Ilie, N.; Hickel, R. Resin composite restorative materials. Aust. Dent. J. 2011, 56, 59–66. [Google Scholar] [CrossRef]
- Ghorayeb, S.R.; Xue, T.; Lord, W. A finite element study of ultrasonic wave propagation in a tooth phantom. J. Dent. Res. 1998, 77, 39–49. [Google Scholar] [CrossRef]
- Dionysopoulos, D.; Tolidis, K.; Gerasimou, P.; Koliniotou-Koumpia, E. Effect of preheating on the film thickness of contemporary composite restorative materials. J. Dent. Sci. 2014, 9, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Schmidlin, P.R.; Zehnder, M.; Schlup-Mityko, C.; Göhring, T.N. Interface evaluation after manual and ultrasonic insertion of standardized class I inlays using composite resin materials of different viscosity. Acta Odontol. Scand. 2005, 63, 205–212. [Google Scholar] [CrossRef]
- Kaisarly, D.; Gezawi, M.E. Polymerization shrinkage assessment of dental resin composites: A literature review. Odontology 2016, 104, 257–270. [Google Scholar] [CrossRef]
- Salem, H.N.; Hefnawy, S.M.; Nagi, S.M. Degree of Conversion and Polymerization Shrinkage of Low Shrinkage Bulk-Fill Resin Composites. Contemp Clin. Dent. 2019, 10, 465–470. [Google Scholar] [CrossRef]
- Garoushi, S.; Vallittu, P.K.; Watts, D.C.; Lassila, L.V.J. Polymerization shrinkage of experimental short glass fiber-reinforced composite with semi-inter penetrating polymer network matrix. Dent. Mater. 2008, 24, 211–215. [Google Scholar] [CrossRef]
- Almeida Junior, L.J.d.S.; Penha, K.J.d.S.; Souza, A.F.; Lula, E.C.O.; Magalhães, F.C.; Lima, D.M.; Firoozmand, L.M. Is there correlation between polymerization shrinkage, gap formation, and void in bulk fill composites? A μCT study. Braz. Oral Res. 2017, 31, e100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakaboura, A.; Rahiotis, C.; Watts, D.; Silikas, N.; Eliades, G. 3D-marginal adaptation versus setting shrinkage in light-cured microhybrid resin composites. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2007, 23, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Opdam, N.J.; Roeters, J.J.; Joosten, M.; Veeke, O. Porosities and voids in Class I restorations placed by six operators using a packable or syringable composite. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2002, 18, 58–63. [Google Scholar] [CrossRef]
- Mulder, R.; Mohammed, N.; Du Plessis, A.; Le Roux, S. A pilot study investigating the presence of voids in bulk fill flowable composites. South. Afr. Dent. J. 2017, 72, 462–465. [Google Scholar] [CrossRef] [Green Version]
- Chuang, S.-F.; Liu, J.-K.; Chao, C.-C.; Liao, F.-P.; Chen, Y.-H.M. Effects of flowable composite lining and operator experience on microleakage and internal voids in class II composite restorations. J. Prosthet. Dent. 2001, 85, 177–183. [Google Scholar] [CrossRef]
- Opdam, N.; Roeters, J.; de Boer, T.; Pesschier, D.; Bronkhorst, E. Voids and porosities in class I micropreparations filled with various resin composites. Oper. Dent. 2003, 28, 9–14. [Google Scholar]
- Mohammadi, N.; Jafari-Navimipour, E.; Kimyai, S.; Ajami, A.-A.; Bahari, M.; Ansarin, M.; Ansarin, M. Effect of pre-heating on the mechanical properties of silorane-based and methacrylate-based composites. J. Clin. Exp. Dent. 2016, 8, e373–e378. [Google Scholar] [CrossRef] [Green Version]
- Jin, M.U. Prepare the pre-heated composite resin. Restor. Dent. Endod. 2013, 38, 103–104. [Google Scholar] [CrossRef] [Green Version]
- Daronch, M.; Rueggeberg, F.A.; Moss, L.; de Goes, M.F. Clinically relevant issues related to preheating composites. J. Esthet. Restor. Dent. Off. Publ. Am. Acad. Esthet. Dent. 2006, 18, 340–350. [Google Scholar] [CrossRef]
- Daronch, M.; Rueggeberg, F.A.; Hall, G.; De Goes, M.F. Effect of composite temperature on in vitro intrapulpal temperature rise. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2007, 23, 1283–1288. [Google Scholar] [CrossRef]
- Walter, R.; Swift, E.J., Jr.; Sheikh, H.; Ferracane, J.L. Effects of temperature on composite resin shrinkage. Quintessence Int. 2009, 40, 843–847. [Google Scholar]
- Tantbirojn, D.; Chongvisal, S.; Augustson, D.G.; Versluis, A. Hardness and postgel shrinkage of preheated composites. Quintessence Int. 2011, 42, e51–e59. [Google Scholar] [PubMed]
- Nada, K.; El-Mowafy, O. Effect of precuring warming on mechanical properties of restorative composites. Int. J. Dent. 2011, 2011, 536212. [Google Scholar] [CrossRef] [PubMed]
- Bijle, M.N.; Ekambaram, M.; Lo, E.; Yiu, C. Physicochemical Characteristics of Arginine Enriched NaF Varnish: An In Vitro Study. Polymers 2020, 12, 2998. [Google Scholar] [CrossRef]
- Scribante, A.; Dermenaki Farahani, M.R.; Marino, G.; Matera, C.; Rodriguez, Y.B.R.; Lanteri, V.; Butera, A. Biomimetic Effect of Nano-Hydroxyapatite in Demineralized Enamel before Orthodontic Bonding of Brackets and Attachments: Visual, Adhesion Strength, and Hardness in In Vitro Tests. Biomed. Res. Int. 2020, 2020, 6747498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darabi, F.; Tayefeh-Davalloo, R.; Tavangar, S.-M.; Naser-Alavi, F.; Boorboo-Shirazi, M. The effect of composite resin preheating on marginal adaptation of class II restorations. J. Clin. Exp. Dent. 2020, 12, e682–e687. [Google Scholar] [CrossRef]
- Kaboorani, A.; Englund, K.R. Water sorption and mechanical performance of preheated wood/thermoplastic composites. J. Compos. Mater. 2011, 45, 1423–1433. [Google Scholar] [CrossRef]


| Commercial Name | Type | Composition | References |
|---|---|---|---|
| FiltekTM Z250 (Lot No: NB29540; 3M ESPE, St. Paul, MN, USA) | Micro-hybrid | bis-GMA, UDMA, and bis-EMA resins Zirconia/Silica (82 wt.%) | [37] |
| FiltekTM Bulk-Fill (Lot No: N7060903M ESPE, St. Paul, MN, USA) | Bulk-fill nanocomposite | ERGP-DMA, diurethane-DMA, and 1,12-dodecane-DMA zirconia/silica cluster filler, ytterbium trifluoride (74 wt.%) | [38] |
| FiltekTM Z350 (Lot No: N582322; 3M ESPE, St. Paul, MN, USA) | Nano-hybrid | bis-GMA, UDMA, TEGDMA and bis-EMA resins, PEGDMA Nanoclusters of silica (78.5 wt.%) | [37] |
| SureFil®SDRTM flow (Lot No: 1705000885: DENTSPLY, Konstanz, Germany) | Flowable | UDMA, TEGDMA, Di-methacrylate resin, trimethacrylate resins barium-alumino-fluoro-borosilicate glass; silanated strontium alumino-fluoro-silicate glass; surface treated fume silicas; ytterbium fluoride; synthetic inorganic iron oxide pigments and titanium dioxide (68 wt.%) | [39] |
| Composites | CT | US-30 | US-60 |
|---|---|---|---|
| Bulk-fill | 0.479 ± 0.114 | 0.180 ± 0.036 | 0.077 ± 0.021 |
| SDR | 0.821 ± 0.096 | 0.214 ± 0.089 | 0.053 ± 0.038 |
| Z250 | 0.038 ± 0.007 | 0.047 ± 0.006 | 0.035 ± 0.001 |
| Z350 | 0.015 ± 0.007 | 0.006 ± 0.005 | 0.014 ± 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.S. Effect of Ultrasonic Vibration on Structural and Physical Properties of Resin-Based Dental Composites. Polymers 2021, 13, 2054. https://doi.org/10.3390/polym13132054
Khan AS. Effect of Ultrasonic Vibration on Structural and Physical Properties of Resin-Based Dental Composites. Polymers. 2021; 13(13):2054. https://doi.org/10.3390/polym13132054
Chicago/Turabian StyleKhan, Abdul Samad. 2021. "Effect of Ultrasonic Vibration on Structural and Physical Properties of Resin-Based Dental Composites" Polymers 13, no. 13: 2054. https://doi.org/10.3390/polym13132054
APA StyleKhan, A. S. (2021). Effect of Ultrasonic Vibration on Structural and Physical Properties of Resin-Based Dental Composites. Polymers, 13(13), 2054. https://doi.org/10.3390/polym13132054