Ultra-Robust Thermoconductive Films Made from Aramid Nanofiber and Boron Nitride Nanosheet for Thermal Management Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of BNNS, BNNS@Ag, and ANF
2.3. Preparation of Thermal Conductive Composite Films
2.4. Characterization
3. Results and Discussion
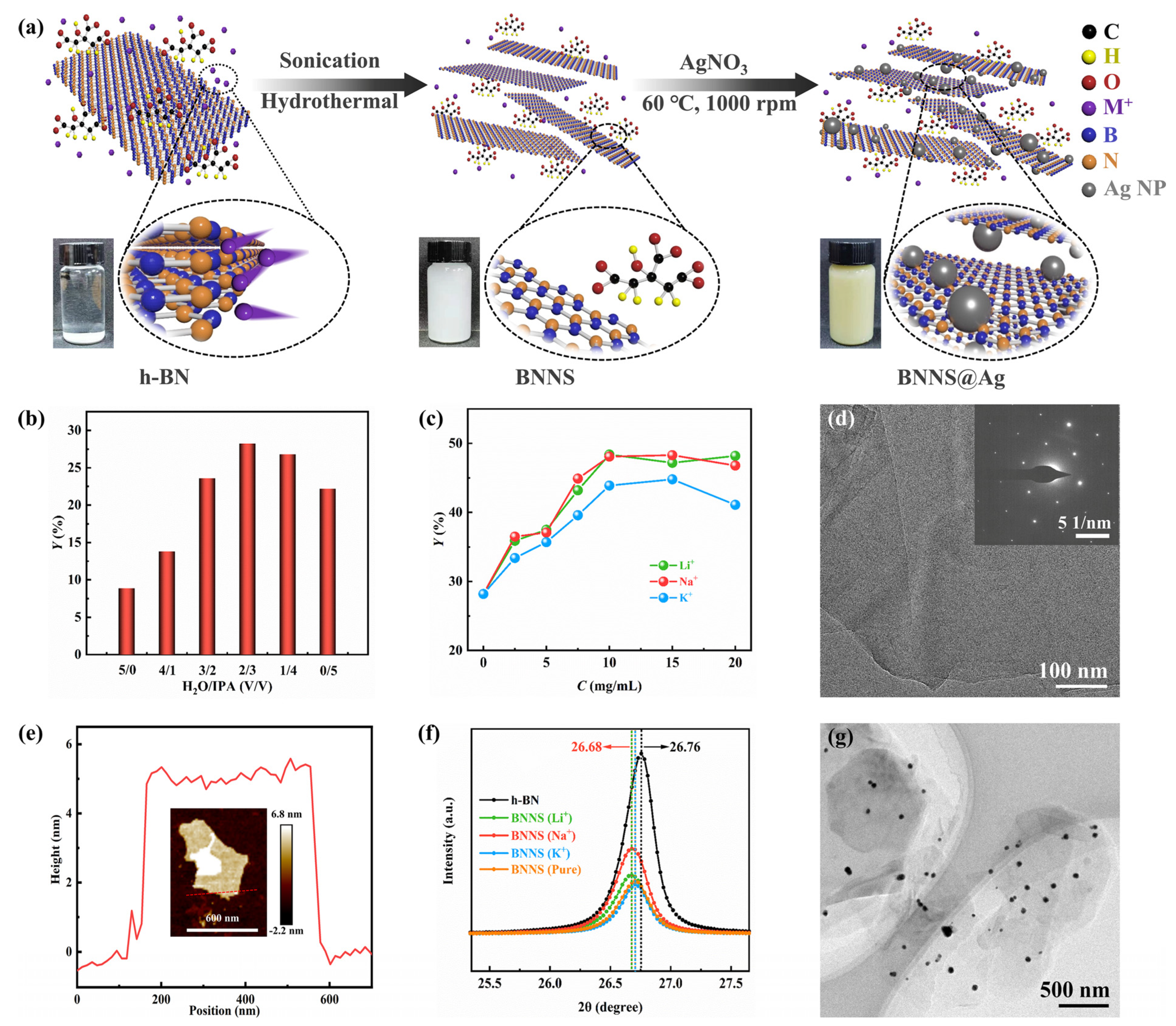
3.1. The Efficient Exfoliation of BNNS@Ag
3.2. Deprotonation Process of ANF and Preparation of Composite Films
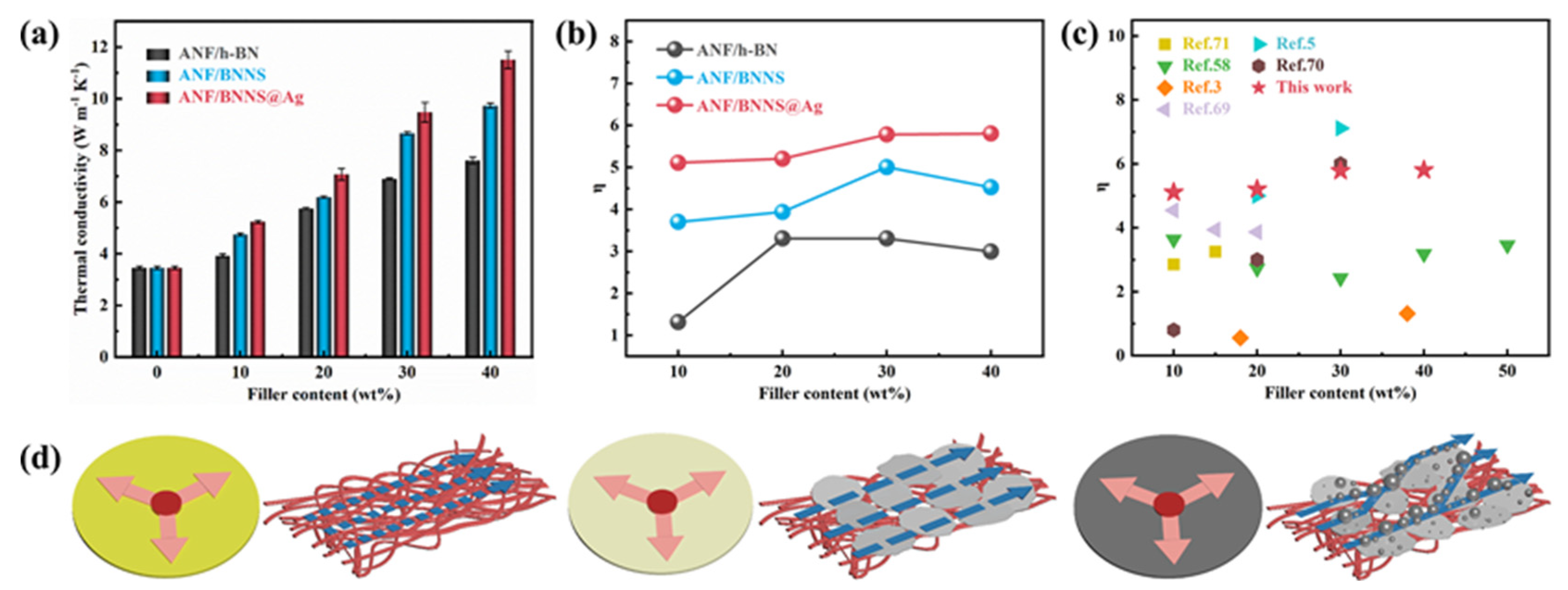
3.3. Thermal Conductivity of Composite Films
3.4. Other Properties of Composite Films
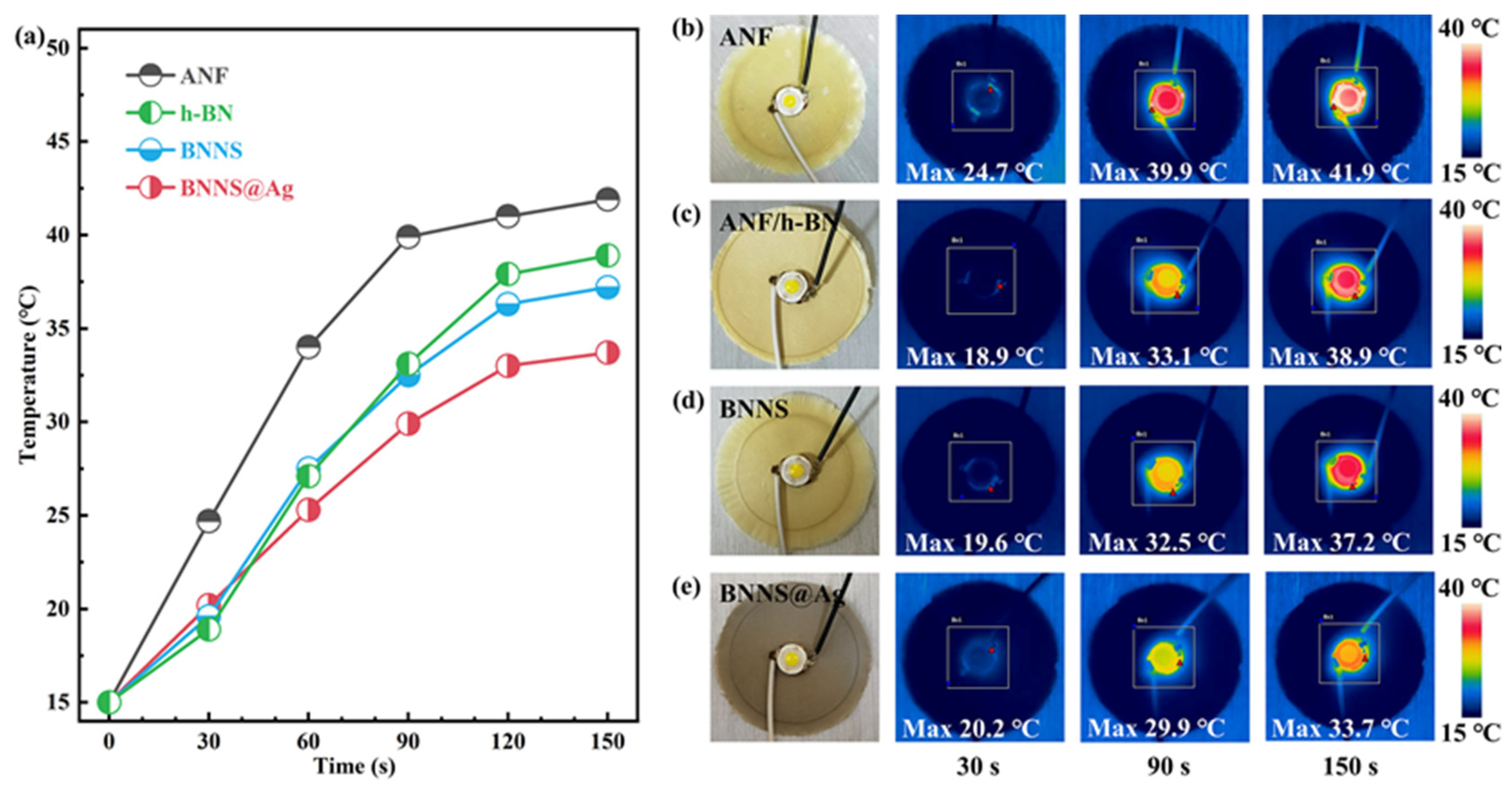
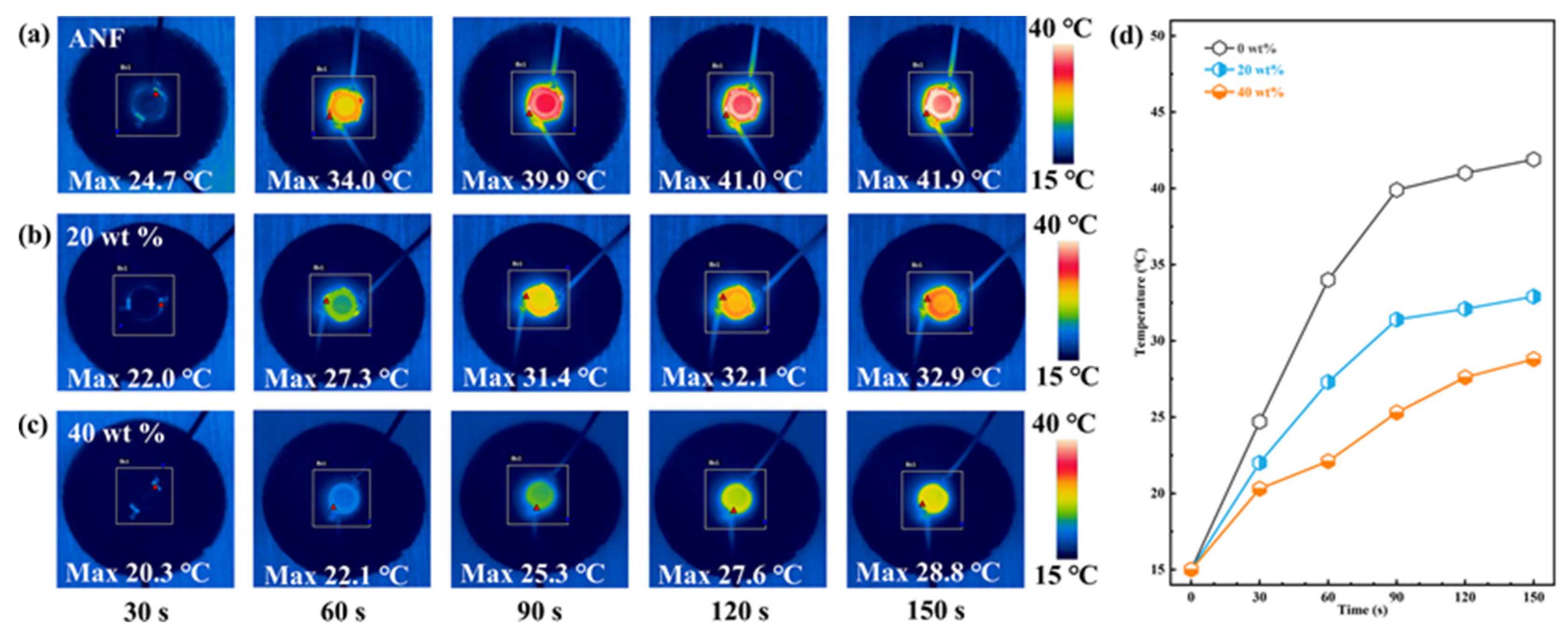
3.5. Simple Applications of Composites
3.6. Finite Element Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, A.; Li, Y.; Liu, W.; Xu, J.-Z.; Yan, D.-X.; Lei, J.; Li, Z.-M. Highly thermally conductive and mechanically robust composite of linear ultrahigh molecular weight polyethylene and boron nitride via constructing nacre-like structure. Compos. Sci. Technol. 2019, 184, 107858. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, J.; Ren, L.; Yao, Y.; Wang, M.; Zeng, X.; Sun, R.; Xu, J.-B.; Wong, C.-P. Nacre-inspired polymer composites with high thermal conductivity and enhanced mechanical strength. Compos. Part A Appl. Sci. Manuf. 2019, 121, 92–99. [Google Scholar] [CrossRef]
- Oh, H.; Kim, J. Fabrication of polymethyl methacrylate composites with silanized boron nitride by in-situ polymerization for high thermal conductivity. Compos. Sci. Technol. 2019, 172, 153–162. [Google Scholar] [CrossRef]
- Jia, L.-C.; Jin, Y.-F.; Ren, J.-W.; Zhao, L.-H.; Yan, D.-X.; Li, Z.-M. Highly thermally conductive liquid metal-based composites with superior thermostability for thermal management. J. Mater. Chem. C 2021, 9, 2904–2911. [Google Scholar] [CrossRef]
- Shen, Z.; Feng, J. Achieving vertically aligned SiC microwires networks in a uniform cold environment for polymer composites with high through-plane thermal conductivity enhancement. Compos. Sci. Technol. 2019, 170, 135–140. [Google Scholar] [CrossRef]
- Yang, D.; Yu, L.; Ai, J.; Wei, Q.; Ni, Y.; Zhang, L. Enhanced thermal conductivity of carboxyl nitrile butadiene rubber composites with low-cost poly(catechol/polyamine) modified Al2O3 via biomimetic method. Compos. Commun. 2021, 23, 100565. [Google Scholar] [CrossRef]
- Wei, Z.; Xie, W.; Ge, B.; Zhang, Z.; Yang, W.; Xia, H.; Wang, B.; Jin, H.; Gao, N.; Shi, Z. Enhanced thermal conductivity of epoxy composites by constructing aluminum nitride honeycomb reinforcements. Compos. Sci. Technol. 2020, 199, 108304. [Google Scholar] [CrossRef]
- Wu, K.; Wang, J.; Liu, D.; Lei, C.; Liu, D.; Lei, W.; Fu, Q. Highly Thermoconductive, Thermostable, and Super-Flexible Film by Engineering 1D Rigid Rod-Like Aramid Nanofiber/2D Boron Nitride Nanosheets. Adv. Mater. 2020, 32, 1906939. [Google Scholar] [CrossRef]
- Zhang, P.; Ding, X.; Wang, Y.; Gong, Y.; Zheng, K.; Chen, L.; Tian, X.; Zhang, X. Segregated double network enabled effective electromagnetic shielding composites with extraordinary electrical insulation and thermal conductivity. Compos. Part A Appl. Sci. Manuf. 2019, 117, 56–64. [Google Scholar] [CrossRef]
- Luo, F.; Yan, P.; Li, H.; Qian, Q.; Huang, B.; Chen, Q.; Wu, K.; Lu, M. Ultrahigh thermally conductive graphene filled liquid crystalline epoxy composites: Preparation assisted by polyethylene glycol. Compos. Sci. Technol. 2020, 200, 108473. [Google Scholar] [CrossRef]
- Yang, D.; Ni, Y.; Kong, X.; Gao, D.; Wang, Y.; Hu, T.; Zhang, L. Mussel-inspired modification of boron nitride for natural rubber composites with high thermal conductivity and low dielectric constant. Compos. Sci. Technol. 2019, 177, 18–25. [Google Scholar] [CrossRef]
- Chen, J.; Wei, H.; Bao, H.; Jiang, P.; Huang, X. Millefeuille-Inspired Thermally Conductive Polymer Nanocomposites with Overlapping BN Nanosheets for Thermal Management Applications. ACS Appl. Mater. Interfaces 2019, 11, 31402–31410. [Google Scholar] [CrossRef]
- Xie, B.; Liu, H.; Hu, R.; Wang, C.; Hao, J.; Wang, K.; Luo, X. Targeting Cooling for Quantum Dots in White QDs-LEDs by Hexagonal Boron Nitride Platelets with Electrostatic Bonding. Adv. Funct. Mater. 2018, 28, 1801407. [Google Scholar] [CrossRef]
- Yuan, J.; Liew, K.-M. Structure stability and high-temperature distortion resistance of trilayer complexes formed from graphenes and boron nitride nanosheets. Phys. Chem. Chem. Phys. 2014, 16, 88–94. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, J.; Zhang, D.; Guo, H.; Wang, X.; Hu, H. Covalent functionalization of boron nitride nanosheets via reductive activation. Nanoscale 2020, 12, 18379–18389. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, C.; Songfeng, E.; Xie, L.; Geng, R.; Lin, C.-T.; Li, L.; Yao, Y. Enhanced thermal conductivity of polyurethane composites via engineering small/large sizes interconnected boron nitride nanosheets. Compos. Sci. Technol. 2019, 170, 93–100. [Google Scholar] [CrossRef]
- Wang, T.; Wang, M.; Fu, L.; Duan, Z.; Chen, Y.; Hou, X.; Wu, Y.; Li, S.; Guo, L.; Kang, R.; et al. Enhanced Thermal Conductivity of Polyimide Composites with Boron Nitride Nanosheets. Sci. Rep. 2018, 8, 1557. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Li, C.; Cai, Y.; Lin, J.; Long, X.; Wang, L.; Xu, Y.; Sun, J.; Tang, L.; Zhang, Y.-W.; et al. Highly Efficient Mass Production of Boron Nitride Nanosheets via a Borate Nitridation Method. J. Phys. Chem. C 2018, 122, 17370–17377. [Google Scholar] [CrossRef]
- Wang, N.; Yang, G.; Wang, H.; Yan, C.; Sun, R.; Wong, C.-P. A universal method for large-yield and high-concentration exfoliation of two-dimensional hexagonal boron nitride nanosheets. Appl. Mater. Today 2019, 27, 33–42. [Google Scholar] [CrossRef]
- Lin, Y.; Connell, J.-W. Advances in 2D boron nitride nanostructures: Nanosheets, nanoribbons, nanomeshes, and hybrids with graphene. Nanoscale 2012, 4, 6908–6939. [Google Scholar] [CrossRef]
- Sajjad, M.; Ahmadi, M.; Guinel, M.-J.-F.; Lin, Y.; Feng, P. Large scale synthesis of single-crystal and polycrystalline boron nitride nanosheets. J. Mater. Sci. 2012, 48, 2543–2549. [Google Scholar] [CrossRef]
- Wang, Z.; Priego, P.; Meziani, M.-J.; Wirth, K.; Bhattacharya, S.; Rao, A.; Wang, P.; Sun, Y.-P. Dispersion of high-quality boron nitride nanosheets in polyethylene for nanocomposites of superior thermal transport properties. Nanoscale Adv. 2020, 2, 2507–2513. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chen, Y.; Wang, X. Two-dimensional covalent carbon nitride nanosheets: Synthesis, functionalization, and applications. Energy Envion. Sci. 2015, 8, 3092–3108. [Google Scholar] [CrossRef]
- An, D.; Cheng, S.; Xi, S.; Zhang, Z.; Duan, X.; Ren, Y.; Li, J.; Sun, Z.; Liu, Y.; Wong, C.-P. Flexible thermal interfacial materials with covalent bond connections for improving high thermal conductivity. Chem. Eng. J. 2020, 383, 123151. [Google Scholar] [CrossRef]
- Fu, C.; Yan, C.; Ren, L.; Zeng, X.; Du, G.; Sun, R.; Xu, J.; Wong, C.-P. Improving thermal conductivity through welding boron nitride nanosheets onto silver nanowires via silver nanoparticles. Compos. Sci. Technol. 2019, 177, 118–126. [Google Scholar] [CrossRef]
- Ren, L.; Zeng, X.; Sun, R.; Xu, J.-B.; Wong, C.-P. Spray-assisted assembled spherical boron nitride as fillers for polymers with enhanced thermally conductivity. Chem. Eng. J. 2019, 370, 166–175. [Google Scholar] [CrossRef]
- Bryning, M.-B.; Milkie, D.-E.; Islam, M.-F.; Kikkawa, J.-M.; Yodh, A.-G. Thermal conductivity and interfacial resistance in single-wall carbon nanotube epoxy composites. Appl. Phys. Lett. 2005, 87, 161909. [Google Scholar] [CrossRef]
- Nan, C.-W.; Birringer, R.; Clarke, D.-R.; Gleiter, H. Effective thermal conductivity of particulate composites with interfacial thermal resistance. J. Appl. Phys. 1997, 81, 6692–6699. [Google Scholar] [CrossRef]
- Kwon, Y.-J.; Park, J.-B.; Jeon, Y.-P.; Hong, J.-Y.; Park, H.-S.; Lee, J.-U. A Review of Polymer Composites Based on Carbon Fillers for Thermal Management Applications: Design, Preparation, and Properties. Polymers 2021, 13, 1312. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Dong, X.; Lv, W.; Ji, C.; Jiang, Z.; Zhang, X.; Gao, T.; Yue, K.; Zhang, X. Synergistic enhancement of thermal conductivity for low dielectric constant boron nitride–polytetrafluoroethylene composites by adding small content of graphene nanosheets. Compos. Commun. 2020, 17, 163–169. [Google Scholar] [CrossRef]
- Sun, D.-x.; Bai, Q.-q.; Jin, X.-z.; Qi, X.-d.; Yang, J.-h.; Wang, Y. Simultaneously enhanced thermal conductivity and fracture toughness in polystyrene/carbon nanofiber composites by adding elastomer. Compos. Sci. Technol. 2019, 184, 107864. [Google Scholar] [CrossRef]
- Moradi, S.; Roman, F.; Calventus, Y.; Hutchinson, J.-M. Remarkable Thermal Conductivity of Epoxy Composites Filled with Boron Nitride and Cured under Pressure. Polymers 2021, 13, 955. [Google Scholar] [CrossRef]
- Wang, F.; Yao, Y.; Zeng, X.; Huang, T.; Sun, R.; Xu, J.; Wong, C.-P. Highly thermally conductive polymer nanocomposites based on boron nitride nanosheets decorated with silver nanoparticles. RSC Adv. 2016, 6, 41630–41636. [Google Scholar] [CrossRef]
- Wang, F.; Zeng, X.; Yao, Y.; Sun, R.; Xu, J.; Wong, C.-P. Silver Nanoparticle-Deposited Boron Nitride Nanosheets as Fillers for Polymeric Composites with High Thermal Conductivity. Sci. Rep. 2016, 6, 19394. [Google Scholar] [CrossRef] [Green Version]
- Fu, C.; Li, Q.; Lu, J.; Mateti, S.; Cai, Q.; Zeng, X.; Du, G.; Sun, R.; Chen, Y.; Xu, J.; et al. Improving thermal conductivity of polymer composites by reducing interfacial thermal resistance between boron nitride nanotubes. Compos. Sci. Technol. 2018, 165, 322–330. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, S.; Liu, Y.; Qu, Z.; Tan, Z.; Wu, K.; Shi, J.; Liang, L.; Lu, M. Constructing a Layer-by-Layer Architecture to Prepare a Transparent, Strong, and Thermally Conductive Boron Nitride Nanosheet/Cellulose Nanofiber Multilayer Film. Ind. Eng. Chem. Res. 2020, 59, 4437–4446. [Google Scholar] [CrossRef]
- Ren, J.; Li, Q.; Yan, L.; Jia, L.; Huang, X.; Zhao, L.; Ran, Q.; Fu, M. Enhanced thermal conductivity of epoxy composites by introducing graphene@boron nitride nanosheets hybrid nanoparticles. Mater. Des. 2020, 191, 108663. [Google Scholar] [CrossRef]
- Gilliam, M.-S.; Yousaf, A.; Guo, Y.; Li, D.-O.; Momenah, A.; Wang, Q.-H.; Green, A.-A. Evaluating the Exfoliation Efficiency of Quasi-2D Metal Diboride Nanosheets Using Hansen Solubility Parameters. Langmuir 2021, 37, 1194–1205. [Google Scholar] [CrossRef]
- Guo, Y.; Gupta, A.; Gilliam, M.-S.; Debnath, A.; Yousaf, A.; Saha, S.; Levin, M.-D.; Green, A.-A.; Singh, A.-K.; Wang, Q.-H. Exfoliation of boron carbide into ultrathin nanosheets. Nanoscale 2021, 13, 1652–1662. [Google Scholar] [CrossRef]
- Wang, S.; Tao, B.; Yu, S.; Wei, C.; Zhou, T.; Chen, X.; Han, C.; Wang, C. Insight into the liquid-phase exfoliation to prepare BN nanosheets. Mater. Lett. 2020, 269, 127644. [Google Scholar] [CrossRef]
- Coleman, J.-N.; Lotya, M.; O’Neill, A.; Bergin, S.-D.; King, P.-J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.-J.; et al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Zhang, J.; Tian, W.; Fan, X.; Yao, Y. Polymer composites based on hexagonal boron nitride and their application in thermally conductive composites. RSC Adv. 2018, 8, 21948–21967. [Google Scholar] [CrossRef]
- Cheng, H.; Zhao, K.; Gong, Y.; Wang, X.; Wang, R.; Wang, F.; Hu, R.; Wang, F.; Zhang, X.; He, J.; et al. Covalent coupling regulated thermal conductivity of poly(vinyl alcohol)/boron nitride composite film based on silane molecular structure. Compos. Part A Appl. Sci. Manuf. 2020, 137, 106026. [Google Scholar] [CrossRef]
- Jin, X.; Li, W.; Liu, Y.; Gan, W. Self-constructing thermal conductive filler network via reaction-induced phase separation in BNNSs/epoxy/polyetherimide composites. Compos. Part A Appl. Sci. Manuf. 2020, 130, 105727. [Google Scholar] [CrossRef]
- Zhao, L.; Yan, L.; Wei, C.; Wang, Z.; Jia, L.; Ran, Q.; Huang, X.; Ren, J. Aqueous-Phase Exfoliation and Functionalization of Boron Nitride Nanosheets Using Tannic Acid for Thermal Management Applications. Ind. Eng. Chem. Res. 2020, 59, 16273–16282. [Google Scholar] [CrossRef]
- Zeena, S.-P.; Prashant, V.-K. What Factors Control the Size and Shape of Silver Nanoparticles in the Citrate Ion Reduction Method? J. Phys. Chem. B 2004, 108, 945–951. [Google Scholar]
- Rehan, M.; Mashaly, H.-M.; Mowafi, S.; Abou El-Kheir, A.; Emam, H.-E. Multi-functional textile design using in-situ Ag NPs incorporation into natural fabric matrix. Dye. Pigm. 2015, 118, 9–17. [Google Scholar] [CrossRef]
- Jiang, X.-C.; Chen, C.-Y.; Chen, W.-M.; Yu, A.-B. Role of citric acid in the formation of silver nanoplates through a synergistic reduction approach. Langmuir 2010, 26, 4400–4408. [Google Scholar] [CrossRef]
- Giannis, M.; Dionisios, G.-V. Insights into the Early Stages of Metal Nanoparticle Formation via First-Principle Calculations: The Roles of Citrate and Water. Langmuir 2008, 24, 7465–7473. [Google Scholar]
- Montazer, M.; Keshvari, A.; Kahali, P. Tragacanth gum/nano silver hydrogel on cotton fabric: In-situ synthesis and antibacterial properties. Carbohydr. Polym. 2016, 154, 257–266. [Google Scholar] [CrossRef]
- Ramon, A.-A.; Ricardo, F.-A. Synthesis of Silver Nanoparticles with Controllable Surface Charge and Their Application to Surface-Enhanced Raman Scattering. Anal. Chem. 2009, 81, 2280–2285. [Google Scholar]
- Patra, S.; Pandey, A.-K.; Sen, D.; Ramagiri, S.-V.; Bellare, J.-R.; Mazumder, S.; Goswami, A. Redox decomposition of silver citrate complex in nanoscale confinement: An unusual mechanism of formation and growth of silver nanoparticles. Langmuir 2014, 30, 2460–2469. [Google Scholar] [CrossRef]
- Shen, H.; Duan, C.; Guo, J.; Zhao, N.; Xu, J. Facile in situ synthesis of silver nanoparticles on boron nitride nanosheets with enhanced catalytic performance. J. Mater. Chem. A 2015, 3, 16663–16669. [Google Scholar] [CrossRef]
- Liu, K.; Chen, S.; Luo, Y.; Jia, D.; Gao, H.; Hu, G.; Liu, L. Noncovalently functionalized pristine graphene/metal nanoparticle hybrid for conductive composites. Compos. Sci. Technol. 2014, 94, 1–7. [Google Scholar] [CrossRef]
- Pang, J.; Chao, Y.; Chang, H.; Li, H.; Xiong, J.; Zhang, Q.; Chen, G.; Qian, J.; Zhu, W.; Li, H. Silver Nanoparticle-Decorated Boron Nitride with Tunable Electronic Properties for Enhancement of Adsorption Performance. ACS Sustain. Chem. Eng. 2018, 6, 4948–4957. [Google Scholar] [CrossRef]
- Liu, P.; Ding, J.; Su, S.; Yu, H. A universal strategy for high-yield producing water compatible boron nitride nanosheets. New J. Chem. 2020, 44, 19812–19819. [Google Scholar] [CrossRef]
- Guan, Y.; Li, W.; Zhang, Y.; Shi, Z.; Tan, J.; Wang, F.; Wang, Y. Aramid nanofibers and poly (vinyl alcohol) nanocomposites for ideal combination of strength and toughness via hydrogen bonding interactions. Compos. Sci. Technol. 2017, 144, 193–201. [Google Scholar] [CrossRef]
- Li, J.; Fan, J.; Liao, K.; Xie, J.; Chen, Y.; Liu, P.; Min, Y.; Xu, Q. Facile fabrication of a multifunctional aramid nanofiber-based composite paper. RSC Adv. 2016, 6, 90263–90272. [Google Scholar] [CrossRef]
- Yang, M.; Cao, K.-Q.; Sui, L.; Qi, Y.; Zhu, J.; Waas, A.; Arruda, E.-M.; Kieffer, J.; Thouless, M.-D.; Kotov, N.-A. Dispersions of Aramid Nanofibers: A New Nanoscale Building Block. ACS Nano 2011, 5, 6945–6954. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zhu, Y.; Teng, C. Facial fabrication of aramid composite insulating paper with high strength and good thermal conductivity. Compos. Commun. 2020, 21, 100370. [Google Scholar] [CrossRef]
- Ma, T.; Zhao, Y.; Ruan, K.; Liu, X.; Zhang, J.; Guo, Y.; Yang, X.; Kong, J.; Gu, J. Highly Thermal Conductivities, Excellent Mechanical Robustness and Flexibility, and Outstanding Thermal Stabilities of Aramid Nanofiber Composite Papers with Nacre-Mimetic Layered Structures. ACS Appl. Mater. Interfaces 2020, 12, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, L.; Zhang, M.; Luo, J.; Ding, X. Timesaving, High-Efficiency Approaches To Fabricate Aramid Nanofibers. ACS Nano 2019, 13, 7886–7897. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Li, J.; Tian, W.; He, L.; Tuo, X.; Qiu, T. A new approach to the preparation of poly(p-phenylene terephthalamide) nanofibers. RSC Adv. 2016, 6, 26599–26605. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Shen, J.; Gao, C.; Van der Bruggen, B. The potential of Kevlar aramid nanofiber composite membranes. J. Mater. Chem. A 2020, 8, 7548–7568. [Google Scholar] [CrossRef]
- Nie, C.; Yang, Y.; Peng, Z.; Cheng, C.; Ma, L.; Zhao, C. Aramid nanofiber as an emerging nanofibrous modifier to enhance ultrafiltration and biological performances of polymeric membranes. J. Membr. Sci. 2017, 528, 251–263. [Google Scholar] [CrossRef]
- Fan, J.; Wang, J.; Shi, Z.; Yu, S.; Yin, J. Kevlar nanofiber-functionalized multiwalled carbon nanotubes for polymer reinforcement. Mater. Chem. Phys. 2013, 141, 861–868. [Google Scholar] [CrossRef]
- Burch, R.-R.; Sweeny, W.; Schmidt, H.-W. Preparation of aromatic polyamide polyanions: A novel processing strategy for aromatic polyamides. Macromolecules 1990, 23, 1065–1072. [Google Scholar] [CrossRef]
- Tian, W.; Qiu, T.; Shi, Y.; He, L.; Tuo, X. The facile preparation of aramid insulation paper from the bottom-up nanofiber synthesis. Mater. Lett. 2017, 202, 158–161. [Google Scholar] [CrossRef]
- Lee, J.-U.; Park, B.; Kim, B.-S.; Bae, D.-R.; Lee, W. Electrophoretic deposition of aramid nanofibers on carbon fibers for highly enhanced interfacial adhesion at low content. Compos. Part A Appl. Sci. Manuf. 2016, 84, 482–489. [Google Scholar] [CrossRef]
- Zhou, W. Effect of coupling agents on the thermal conductivity of aluminum particle/epoxy resin composites. J. Mater. Sci. 2011, 46, 3883–3889. [Google Scholar] [CrossRef]
- Xu, Y.; Chung, D.-D.-L. Increasing the thermal conductivity of boron nitride and aluminum nitride particle epoxy-matrix composites by particle surface treatments. Compos. Interfaces 2012, 7, 243–256. [Google Scholar] [CrossRef]
- Han, Y.; Shi, X.; Yang, X.; Guo, Y.; Zhang, J.; Kong, J.; Gu, J. Enhanced thermal conductivities of epoxy nanocomposites via incorporating in-situ fabricated hetero-structured SiC-BNNS fillers. Compos. Sci. Technol. 2020, 187, 107944. [Google Scholar] [CrossRef]
- Wang, J.; Mubarak, S.; Dhamodharan, D.; Divakaran, N.; Wu, L.; Zhang, X. Fabrication of thermoplastic functionally gradient composite parts with anisotropic thermal conductive properties based on multicomponent fused deposition modeling 3D printing. Compos. Commun. 2020, 19, 142–146. [Google Scholar] [CrossRef]
- Shang, Y.; Yang, G.; Su, F.; Feng, Y.; Ji, Y.; Liu, D.; Yin, R.; Liu, C.; Shen, C. Multilayer polyethylene/ hexagonal boron nitride composites showing high neutron shielding efficiency and thermal conductivity. Compos. Commun. 2020, 19, 147–153. [Google Scholar] [CrossRef]
- Yang, D.; Ni, Y.; Liang, Y.; Li, B.; Ma, H.; Zhang, L. Improved thermal conductivity and electromechanical properties of natural rubber by constructing Al2O3-PDA-Ag hybrid nanoparticles. Compos. Sci. Technol. 2019, 180, 86–93. [Google Scholar] [CrossRef]
- Wang, H.; Yang, H.; Wang, Q.; Tong, J.; Wen, J.; Zhang, Q. Surface-modified Li3Mg2NbO6 ceramic particles and hexagonal boron nitride sheets filled PTFE composites with high through-plane thermal conductivity and extremely low dielectric loss. Compos. Commun. 2020, 22, 100523. [Google Scholar] [CrossRef]
- Yang, D.; Huang, S.; Ruan, M.; Wu, Y.; Li, S.; Wang, H.; Zhang, J.; Ma, H.; Guo, W.; Zhang, L. Controllable dielectric performance of polymer composites via the Coulomb-blockade effect with core–shell structured nano-particles. J. Mater. Chem. C 2017, 5, 7759–7767. [Google Scholar] [CrossRef]
- Xiao, X.; Xu, N.; Jiang, Y.; Zhang, Q.; Yu, E.; Yang, H. TiO2@Ag/P (VDF-HFP) composite with enhanced dielectric permittivity and rather low dielectric loss. RSC Adv. 2016, 6, 69580–69585. [Google Scholar] [CrossRef]
- Xiao, G.; Di, J.; Li, H.; Wang, J. Highly thermally conductive, ductile biomimetic boron nitride/aramid nanofiber composite film. Compos. Sci. Technol. 2020, 189, 108021. [Google Scholar] [CrossRef]
- Droval, G.; Feller, J.-F.; Salagnac, P.; Glouannec, P. Thermal conductivity enhancement of electrically insulating syndiotactic poly(styrene) matrix for diphasic conductive polymer composites. Polym Adv. Technol. 2006, 17, 732–745. [Google Scholar] [CrossRef]
- Lv, X.; Tang, Y.; Tian, Q.; Wang, Y.; Ding, T. Ultra-stretchable membrane with high electrical and thermal conductivity via electrospinning and in-situ nanosilver deposition. Compos. Sci. Technol. 2020, 200, 108414. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.-H.; Liao, Y.; Jia, L.-C.; Wang, Z.; Huang, X.-L.; Ning, W.-J.; Zhang, Z.-X.; Ren, J.-W. Ultra-Robust Thermoconductive Films Made from Aramid Nanofiber and Boron Nitride Nanosheet for Thermal Management Application. Polymers 2021, 13, 2028. https://doi.org/10.3390/polym13132028
Zhao L-H, Liao Y, Jia L-C, Wang Z, Huang X-L, Ning W-J, Zhang Z-X, Ren J-W. Ultra-Robust Thermoconductive Films Made from Aramid Nanofiber and Boron Nitride Nanosheet for Thermal Management Application. Polymers. 2021; 13(13):2028. https://doi.org/10.3390/polym13132028
Chicago/Turabian StyleZhao, Li-Hua, Yun Liao, Li-Chuan Jia, Zhong Wang, Xiao-Long Huang, Wen-Jun Ning, Zong-Xi Zhang, and Jun-Wen Ren. 2021. "Ultra-Robust Thermoconductive Films Made from Aramid Nanofiber and Boron Nitride Nanosheet for Thermal Management Application" Polymers 13, no. 13: 2028. https://doi.org/10.3390/polym13132028
APA StyleZhao, L.-H., Liao, Y., Jia, L.-C., Wang, Z., Huang, X.-L., Ning, W.-J., Zhang, Z.-X., & Ren, J.-W. (2021). Ultra-Robust Thermoconductive Films Made from Aramid Nanofiber and Boron Nitride Nanosheet for Thermal Management Application. Polymers, 13(13), 2028. https://doi.org/10.3390/polym13132028





