Impact of Poly (Styrene–Acrylic Acid) Latex Nanoparticles on Colorectal and Cervical Cancer Cells
Abstract
:1. Introduction
2. Materials and Method
2.1. Preparation of Polystyrene Particles
2.2. Particle Size, Distribution, and Morphology
2.3. Electrokinetic Study
2.4. Cell Culture and Nanoparticle Treatments
2.5. MTT Assay
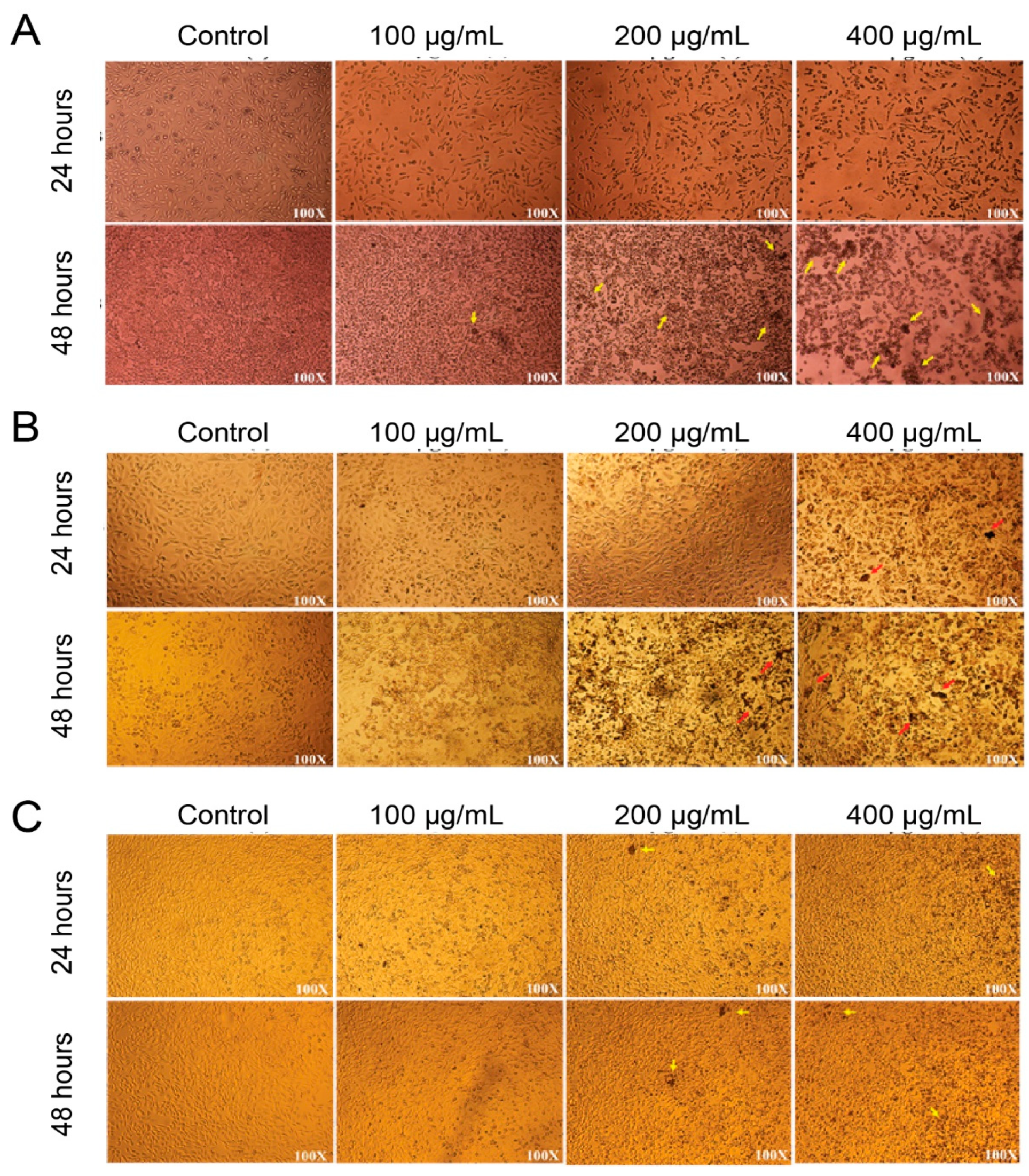
2.6. Morphological Characterization of Nanoparticle-treated HCT-116 and HELA Cells
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Synthesized Poly (Styrene–Acrylic Acid) Latex Particles
3.2. In Vitro MTT Assay of Synthesized Poly (Styrene–Acrylic Acid) Latex Particles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.; DeSantis, C.; Virgo, K.; Stein, K.; Mariotto, A.; Smith, T.; Cooper, D.; Gansler, T.; Lerro, C.; Fedewa, S.; et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J. Clin. 2012, 62, 220–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafiei, P.; Haddadi, A. Docetaxel-loaded PLGA and PLGA-PEG nanoparticles for intravenous application: Pharmacokinetics and biodistribution profile. Int. J. Nanomed. 2017, 12, 935–947. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Kim, Y.H. Cancer prevention from the perspective of global cancer burden patterns. J. Thorac. Dis. 2017, 9, 448–451. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.; Barzi, A.; Jemal, A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 177–193. [Google Scholar] [CrossRef]
- Hacker, N.; Gambone, J.; Hobel, C. Hacker & Moore’s Essentials of Obstetrics and Gynecology—6th Edition n.d. Available online: https://www.elsevier.com/books/hacker-and-moores-essentials-of-obstetrics-and-gynecology/hacker/978-1-4557-7558-3 (accessed on 30 March 2021).
- Ralston, S.; Penman, I.; Strachan, M.; Hobson, R. Davidson’s Principles and Practice of Medicine—23rd Edition n.d. Available online: https://www.elsevier.com/books/davidsons-principles-and-practice-of-medicine/ralston/978-0-7020-7028-0 (accessed on 30 March 2021).
- Ghosn, M.; Kourie, H.R.; Tabchi, S. Gastrointestinal cancers in the era of theranostics: Updates and future perspectives. World J. Gastroenterol. 2015, 21, 8473–8477. [Google Scholar] [CrossRef]
- Wang, L.; Dai, G.; Yang, J.; Wu, W.; Zhang, W. Cervical Cancer Cell Growth, Drug Resistance, and Epithelial-Mesenchymal Transition Are Suppressed by γ-Secretase Inhibitor RO4929097. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 4046–4053. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP–dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Sun, J.; Chen, Q.; Gao, Y.; Li, L.; Li, H.; Leng, D.; Wang, Y.; Sun, Y.; Jing, Y.; et al. Star-shape copolymer of lysine-linked di-tocopherol polyethylene glycol 2000 succinate for doxorubicin delivery with reversal of multidrug resistance. Biomaterials 2012, 33, 6877–6888. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Tao, M.; Jiang, B.; Yao, M.; Jun, Y.; Dai, W.; Tang, Z.; Gao, Y.; Zhang, L.; Chen, X.; et al. Overcoming Drug Resistance in Colon Cancer by Aptamer-Mediated Targeted Co-Delivery of Drug and siRNA Using Grapefruit-Derived Nanovectors. Cell Physiol. Biochem. 2018, 50, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Nabavizadeh, F.; Fanaei, H.; Imani, A.; Vahedian, J.; Amoli, F.A.; Ghorbi, J.; Sohanaki, H.; Mohammadi, S.M.; Golchoobian, R. Evaluation of Nanocarrier Targeted Drug Delivery of Capecitabine-PAMAM Dendrimer Complex in a Mice Colorectal Cancer Model. Acta Med. Iran. 2016, 54, 485–493. [Google Scholar] [PubMed]
- Chen, J.; Solomides, C.; Parekh, H.; Simpkins, F.; Simpkins, H. Cisplatin resistance in human cervical, ovarian and lung cancer cells. Cancer Chemother. Pharmacol. 2015, 75, 1217–1227. [Google Scholar] [CrossRef]
- Gupta, S.; Moulik, S.P. Biocompatible microemulsions and their prospective uses in drug delivery. J. Pharm. Sci. 2008, 97, 22–45. [Google Scholar] [CrossRef]
- Lu, Y.; Park, K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int. J. Pharm. 2013, 453, 198–214. [Google Scholar] [CrossRef] [Green Version]
- Grinberg, S.; Linder, C.; Heldman, E. Progress in lipid-based nanoparticles for cancer therapy. Crit. Rev. Oncog. 2014, 19, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Olenyuk, B.Z.; Okamoto, C.T.; Hamm-Alvarez, S.F. Targeting receptor-mediated endocytotic pathways with nanoparticles: Rationale and advances. Adv. Drug Deliv. Rev. 2013, 65, 121–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Van Der Meel, R.; Theek, B.; Oude Blenke, E.; Pieters, E.H.; Fens, M.H.; Ehling, J.; Schiffelers, R.M.; Storm, G.; Van Nostrum, C.F.; et al. Complete Regression of Xenograft Tumors upon Targeted Delivery of Paclitaxel via Π-Π Stacking Stabilized Polymeric Micelles. ACS Nano 2015, 9, 3740–3752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alomari, M.; Balasamy, R.J.; Almohazey, D.; Ravinayagam, V.; Al Hamad, M.; Ababneh, D.; Bahmdan, H.; Alomari, A.H.; Mokadem, Z.; Elaissari, A. Nile Red-Poly(Methyl Methacrylate)/Silica Nanocomposite Particles Increase the Sensitivity of Cervical Cancer Cells to Tamoxifen. Polymers 2020, 12, 1516. [Google Scholar] [CrossRef]
- Khan, F.A.; Akhtar, S.; Almohazey, D.; Alomari, M.; Almofty, S.A.; Badr, I.; Elaissari, A. Targeted delivery of poly (methyl methacrylate) particles in colon cancer cells selectively attenuates cancer cell proliferation. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1533–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, K.; Li, D.; Shi, C.; Ma, X.; Rong, G.; Kang, H.; Wang, X.; Sun, B. Biodegradable Polymeric Nanoparticles as the Delivery Carrier for Drug. Curr. Drug Deliv. 2016, 13, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-J.; Chen, J.-W.; Lin, F.-H.; Young, T.-H.; Lou, P.-J.; Shieh, M.-J. Colorectal cancer cell detection by folic acid-conjugated chitosan nanoparticles. Biomed. Eng. Appl. Basis Commun. 2010, 22, 9–17. [Google Scholar] [CrossRef]
- El-Aassar, M.R.; Soliman, E.A.; Hashem, A.I.; Sun, G.; Amaly, N. Preparation and characterization of poly (styrene-co-Methacrylic acid) copolymer nanoparticles via precipitation polymerization. J. Polym. Res. 2017, 24, 1–12. [Google Scholar] [CrossRef]
- Wang, L.; Gao, J.; An, Z.; Zhao, X.; Yao, H.; Zhang, M.; Tian, Q.; Zhai, X.; Liu, Y. Polymer microsphere for water-soluble drug delivery via carbon dot-stabilizing W/O emulsion. J. Mater. Sci. 2019, 54, 5160–5175. [Google Scholar] [CrossRef]
- Silvério Neto, W.; Thyago Jensen, A.; Ribeiro Ferreira, G.; Fonseca Valadares, L.; Gambetta, R.; Belém Gonçalves, S.; Machado, F. A Survey on Synthesis Processes of Structured Materials for Biomedical Applications: Iron-based Magnetic Nanoparticles, Polymeric Materials and Polymerization Processes. Curr. Pharm. Des. 2015, 21, 5336–5358. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, A.; Mahdavian, A.R.; Salehi-Mobarakeh, H. Preparation of Stimuli-Responsive Functionalized Latex Nanoparticles: The Effect of Spiropyran Concentration on Size and Photochromic Properties. Langmuir 2015, 31, 10672–10682. [Google Scholar] [CrossRef] [PubMed]
- Khakzad, F.; Mahdavian, A.R.; Salehi-Mobarakeh, H.; Sharifian, M.H. A step-wise self-assembly approach in preparation of multi-responsive poly(styrene-co-methyl methacrylate) nanoparticles containing spiropyran. J. Colloid Interface Sci. 2018, 515, 58–69. [Google Scholar] [CrossRef]
- Shah, S.; Pal, A.; Gude, R.; Devi, S. Synthesis and characterization of thermo-responsive copolymeric nanoparticles of poly(methyl methacrylate-co-N-vinylcaprolactam). Eur. Polym. J. 2010, 46, 958–967. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Liu, T.Y.; Wang, K.S.; Hardiansyah, A.; Lin, Y.T.; Chen, H.Y.; Chiu, W.Y. Magnetic and Thermal-sensitive Poly(N-isopropylacrylamide)-based Microgels for Magnetically Triggered Controlled Release. J. Vis. Exp. JoVE 2017, 125, 55648. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Duan, L.; Wu, M.; Wang, X.; Sun, Z.; Zhang, Y.; Li, Y.; He, P. Preparation of thermo/redox/pH-stimulative poly(N-isopropylacrylamide-co-N,N’-dimethylaminoethyl methacrylate) nanogels and their DOX release behaviors. J. Biomed. Mater. Res. A 2019, 107, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-F.; Young, T.-H.; Huang, Y.-H.; Chiu, W.-Y. Synthesis and properties of polymer latex with carboxylic acid functional groups for immunological studies. Polymer 2000, 41, 8565–8571. [Google Scholar] [CrossRef]
- Braconnot, S.; Hoang, C.; Fessi, H.; Elaissari, A. Elaboration of perfect core-shell submicronic magnetic latexes from oil in water ferrofluid droplets for bionanotechnology applications. Mater. Sci. Eng. C 2009, 29, 624–630. [Google Scholar] [CrossRef]
- Polpanich, D.; Tangboriboonrat, P.; Elaïssari, A. The effect of acrylic acid amount on the colloidal properties of polystyrene latex. Colloid Polym. Sci. 2005, 284, 183–191. [Google Scholar] [CrossRef]
- Ríos-Osuna, L.A.; Licea-Claverie, A.; Paraguay-Delgado, F.; Cortez-Lemus, N.A. Synthesis of Poly(styrene-acrylates-acrylic acid) Microspheres and Their Chemical Composition towards Colloidal Crystal Films. Int. J. Polym. Sci. 2016, 2016, 4527526. [Google Scholar] [CrossRef] [Green Version]
- Zein, R.; Sharrouf, W.; Selting, K. Physical Properties of Nanoparticles That Result in Improved Cancer Targeting. J. Oncol. 2020, 2020, e5194780. [Google Scholar] [CrossRef]
- Jiang, B.P.; Zhang, L.; Zhu, Y.; Shen, X.C.; Ji, S.C.; Tan, X.Y.; Cheng, L.; Liang, H. Water-soluble hyaluronic acid–hybridized polyaniline nanoparticles for effectively targeted photothermal therapy. J. Mater. Chem. B 2015, 3, 3767–3776. [Google Scholar] [CrossRef]
- Cho, W.S.; Thielbeer, F.; Duffin, R.; Johansson, E.M.; Megson, I.L.; MacNee, W.; Bradley, M.; Donaldson, K. Surface functionalization affects the zeta potential, coronal stability and membranolytic activity of polymeric nanoparticles. Nanotoxicology 2014, 8, 202–211. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alomari, M.; Almahasheer, A.; Jermy, B.R.; Al-Dossary, A.A.; Bahmdan, H.; Ravinayagam, V.; Ababneh, D.; Tarhini, M.; Elaissari, A. Impact of Poly (Styrene–Acrylic Acid) Latex Nanoparticles on Colorectal and Cervical Cancer Cells. Polymers 2021, 13, 2025. https://doi.org/10.3390/polym13132025
Alomari M, Almahasheer A, Jermy BR, Al-Dossary AA, Bahmdan H, Ravinayagam V, Ababneh D, Tarhini M, Elaissari A. Impact of Poly (Styrene–Acrylic Acid) Latex Nanoparticles on Colorectal and Cervical Cancer Cells. Polymers. 2021; 13(13):2025. https://doi.org/10.3390/polym13132025
Chicago/Turabian StyleAlomari, Munther, Arwa Almahasheer, Balasamy Rabindran Jermy, Amal A. Al-Dossary, Hiba Bahmdan, Vijaya Ravinayagam, Deena Ababneh, Mohamad Tarhini, and Abdelhamid Elaissari. 2021. "Impact of Poly (Styrene–Acrylic Acid) Latex Nanoparticles on Colorectal and Cervical Cancer Cells" Polymers 13, no. 13: 2025. https://doi.org/10.3390/polym13132025
APA StyleAlomari, M., Almahasheer, A., Jermy, B. R., Al-Dossary, A. A., Bahmdan, H., Ravinayagam, V., Ababneh, D., Tarhini, M., & Elaissari, A. (2021). Impact of Poly (Styrene–Acrylic Acid) Latex Nanoparticles on Colorectal and Cervical Cancer Cells. Polymers, 13(13), 2025. https://doi.org/10.3390/polym13132025







