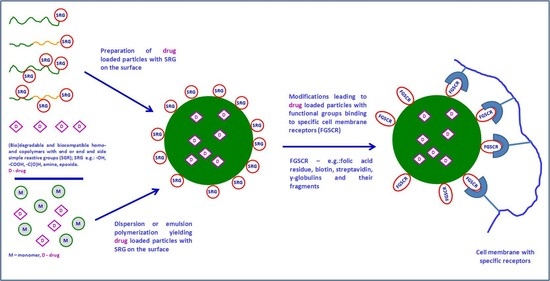
Functionalized Particles Designed for Targeted Delivery
Abstract
1. Introduction
2. Polymers for Preparation of Drug Delivery Carriers
2.1. Polyanhydrides
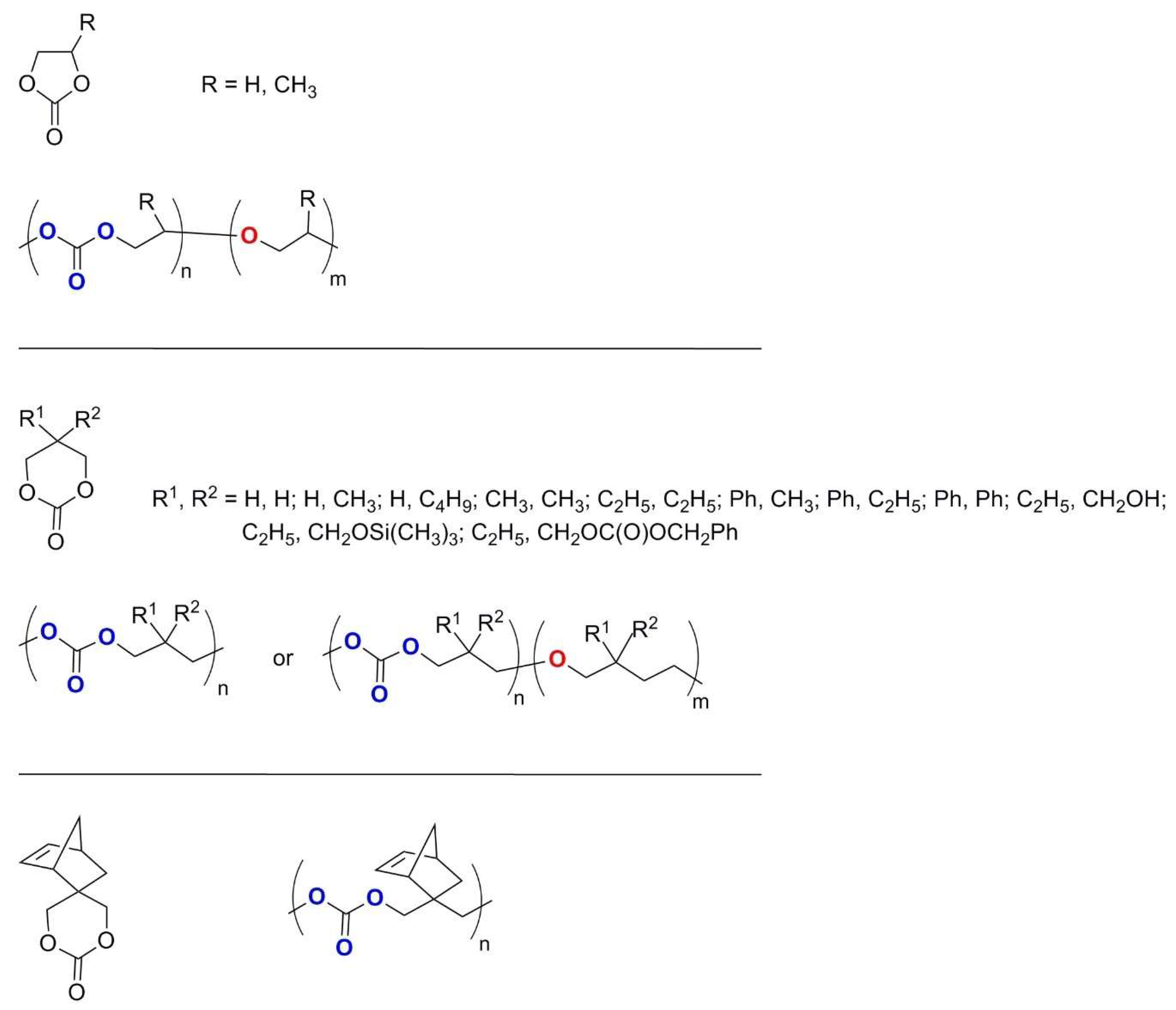
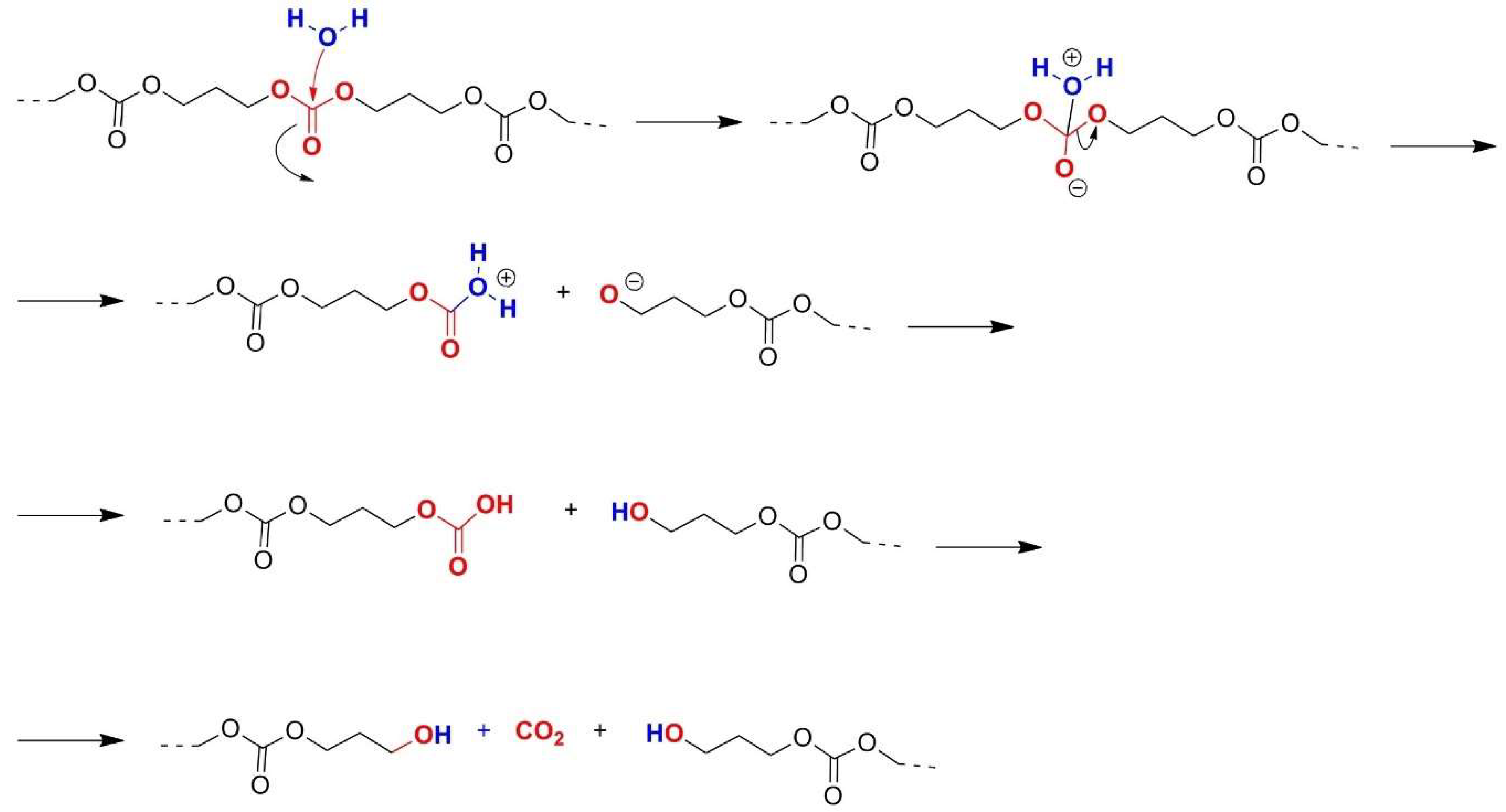
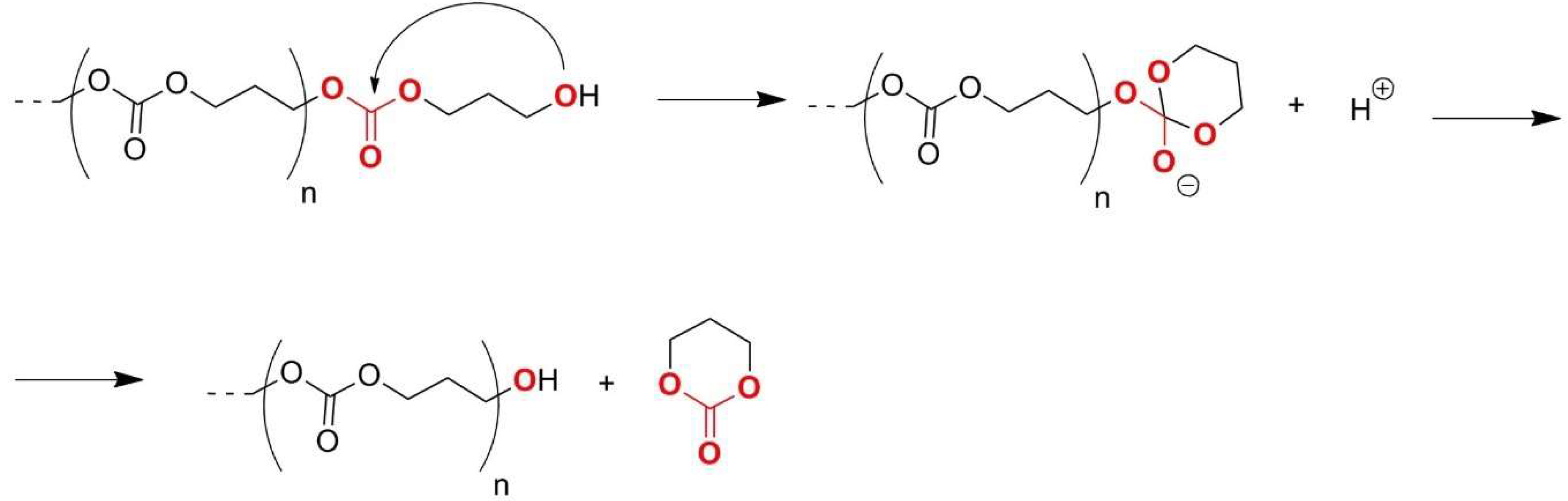
2.2. Polycarbonates
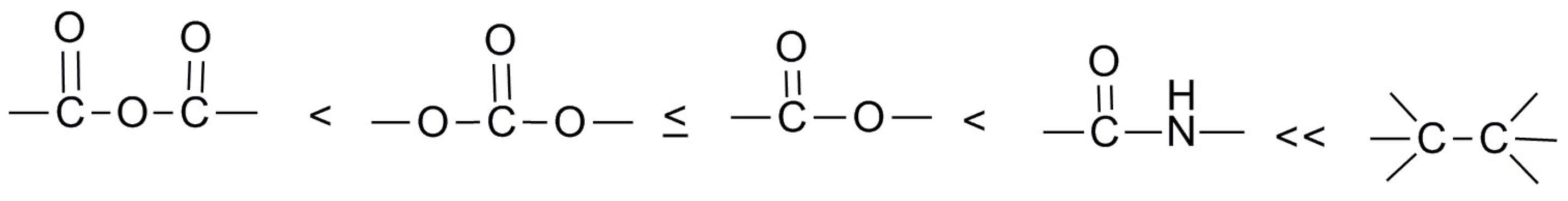
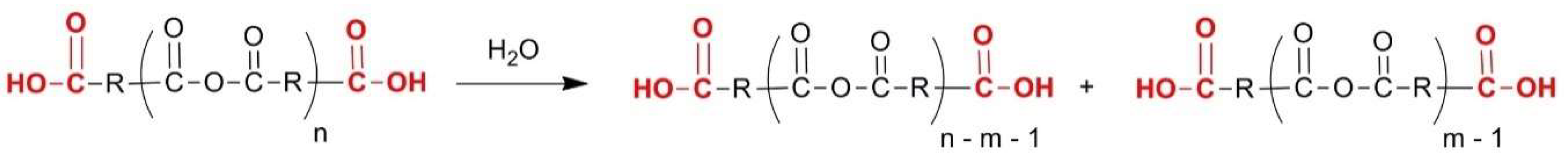
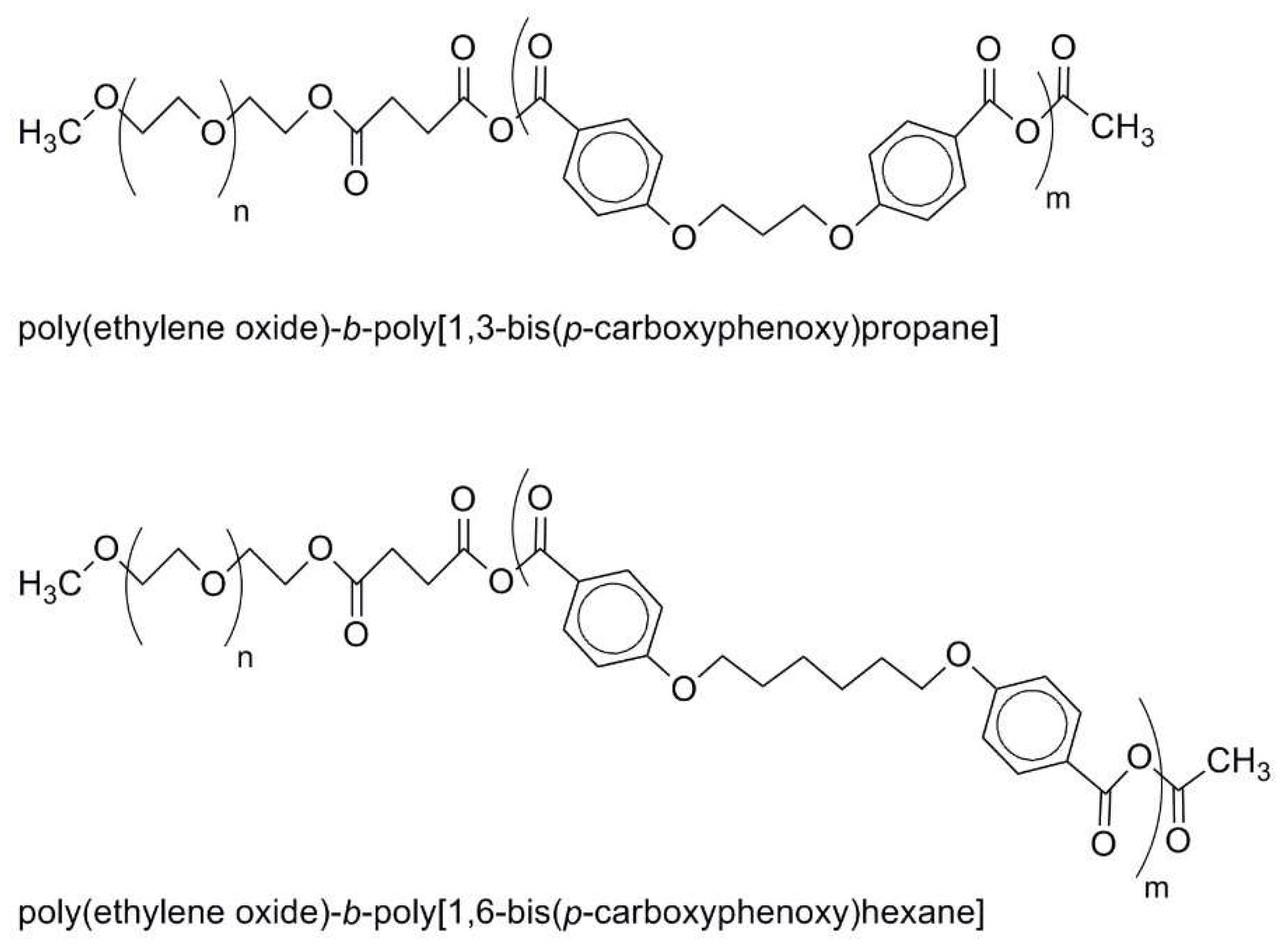
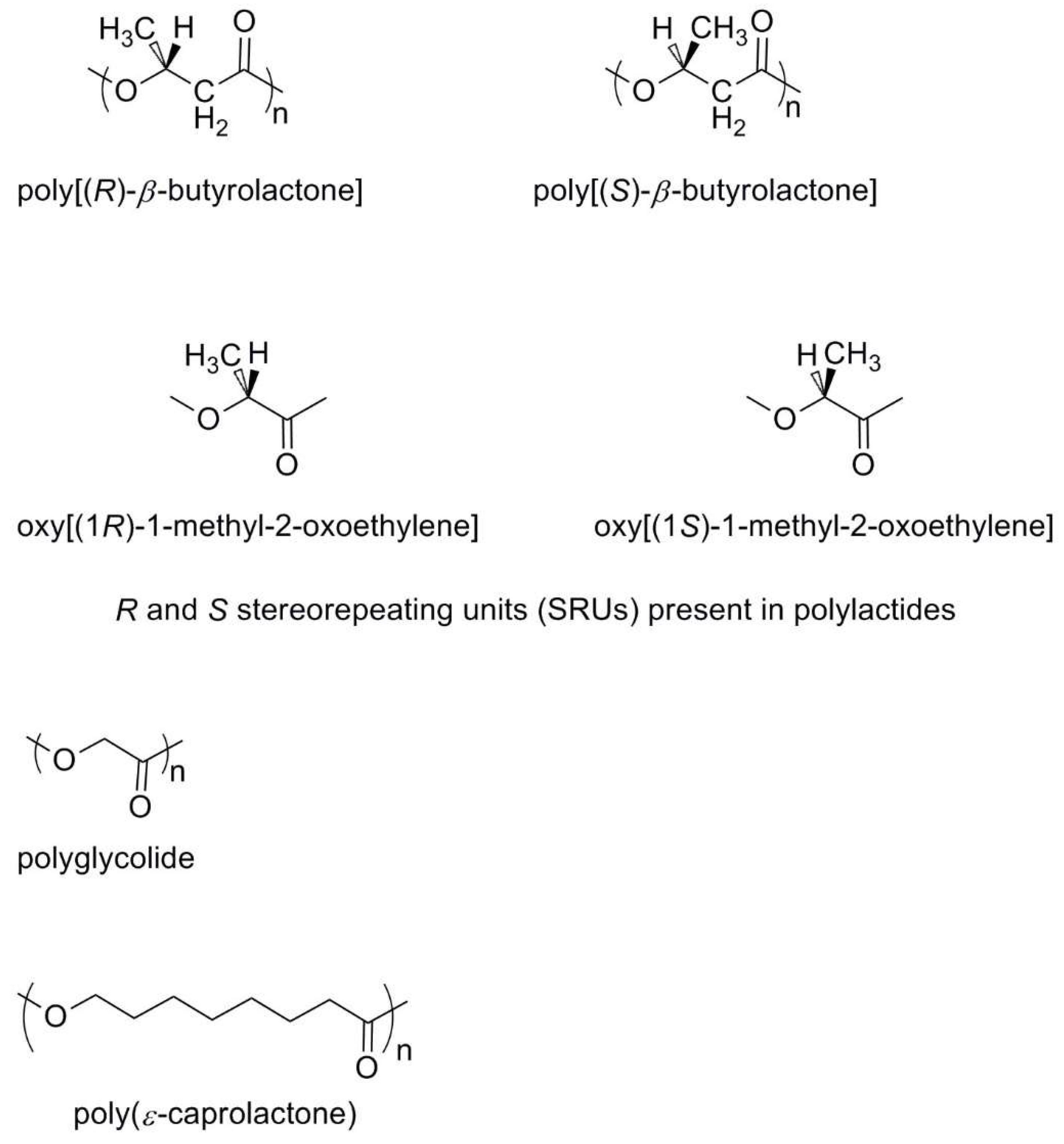
2.3. Aliphatic Polyesters
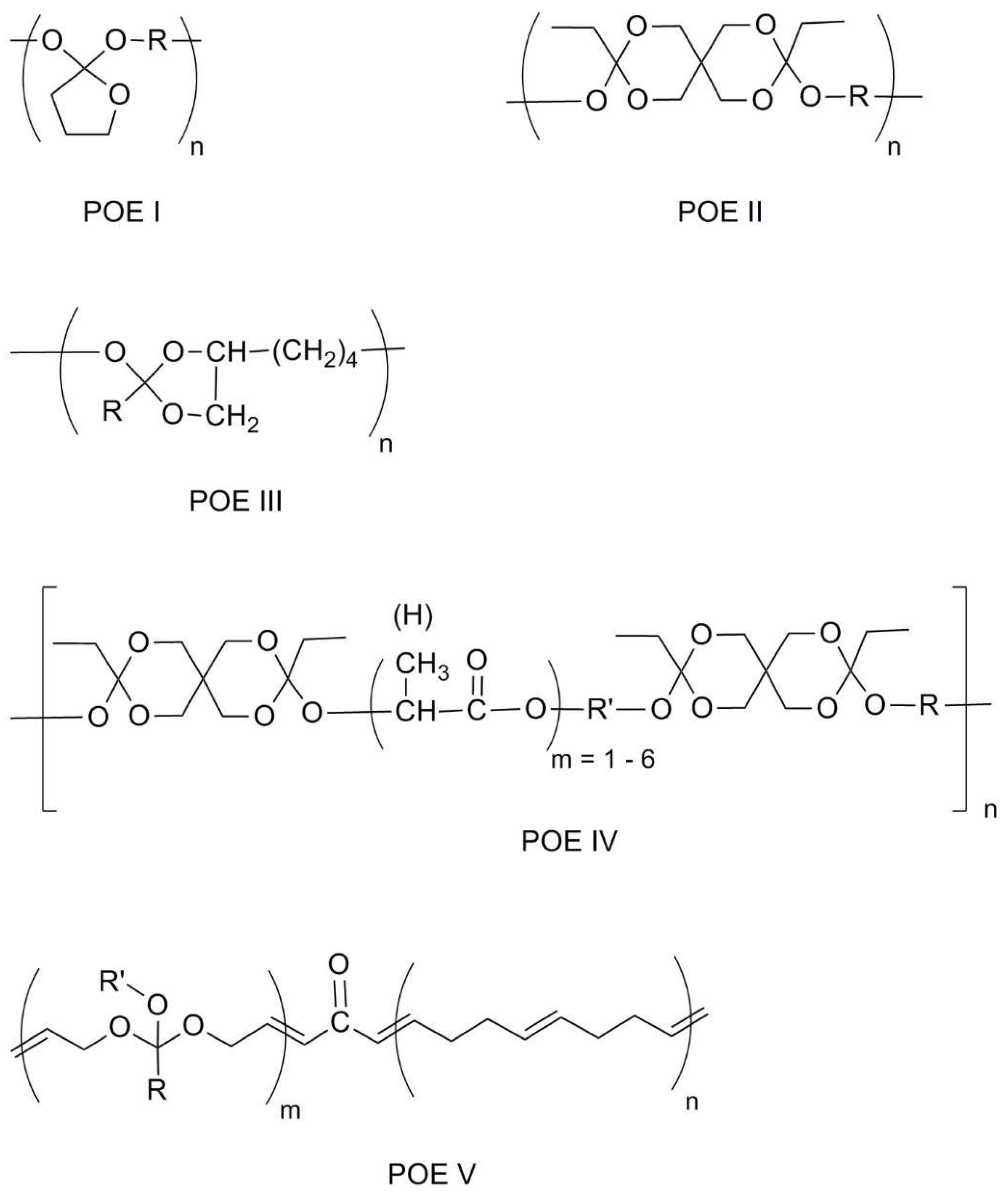
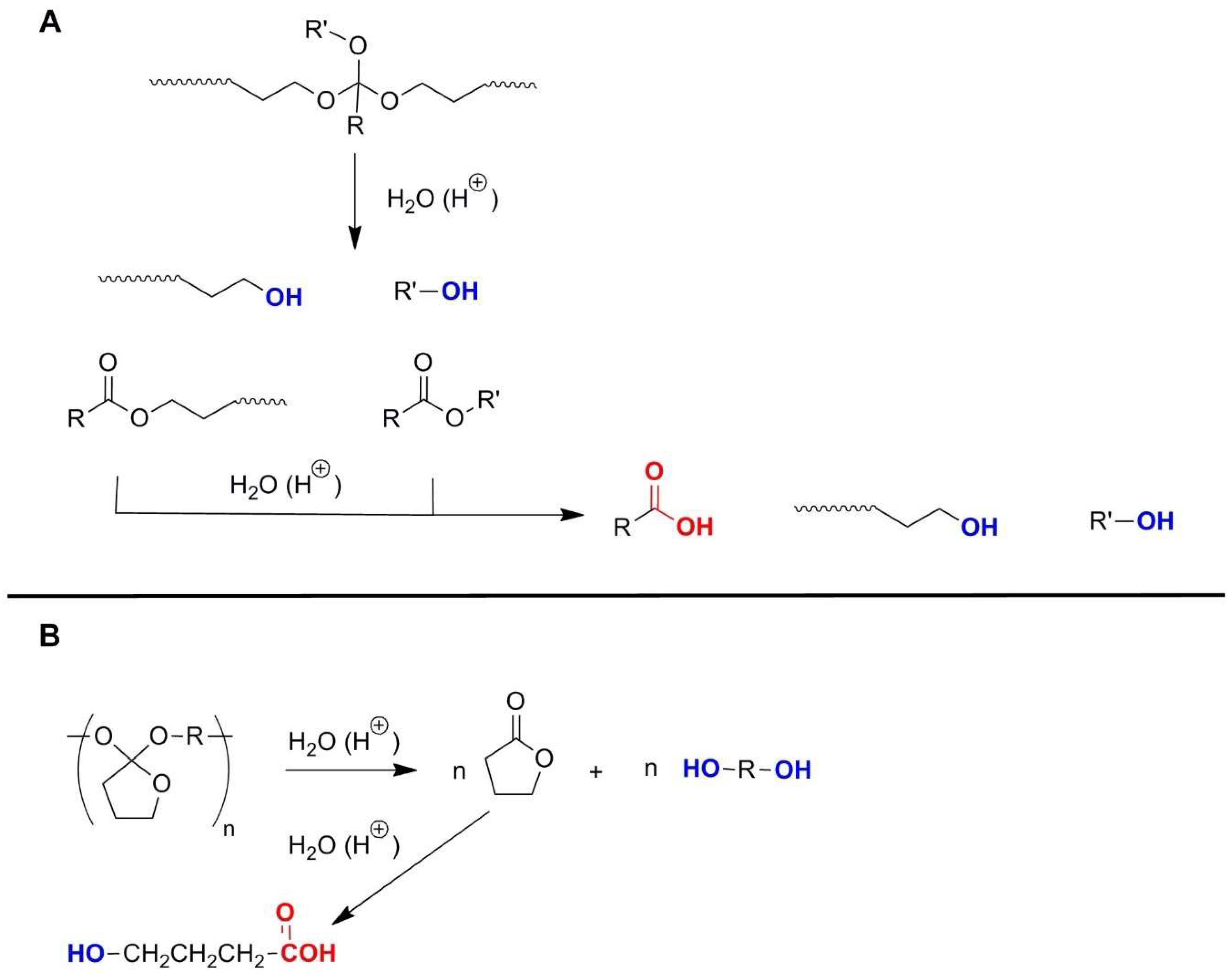
2.4. Polyorthoesters
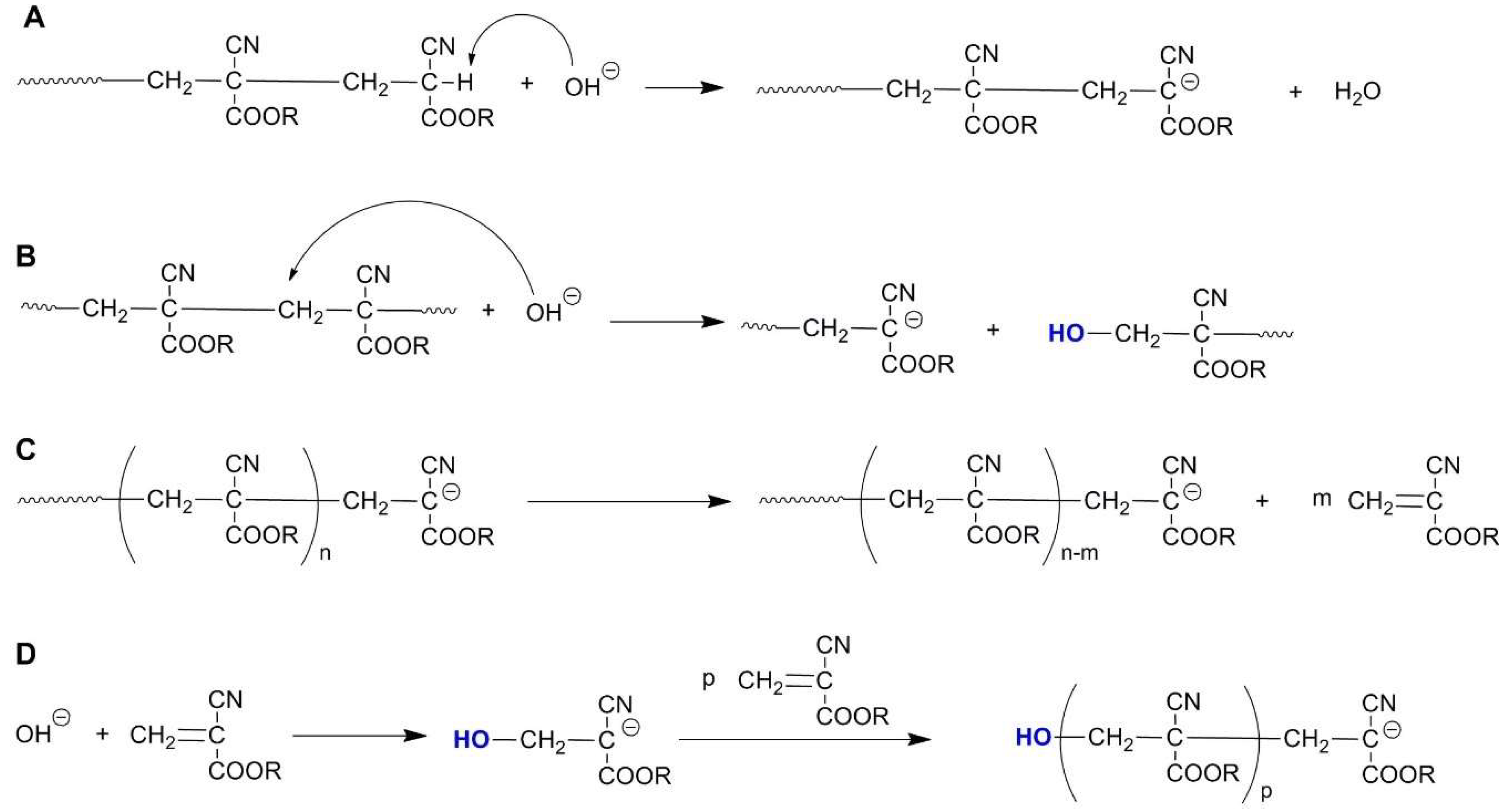
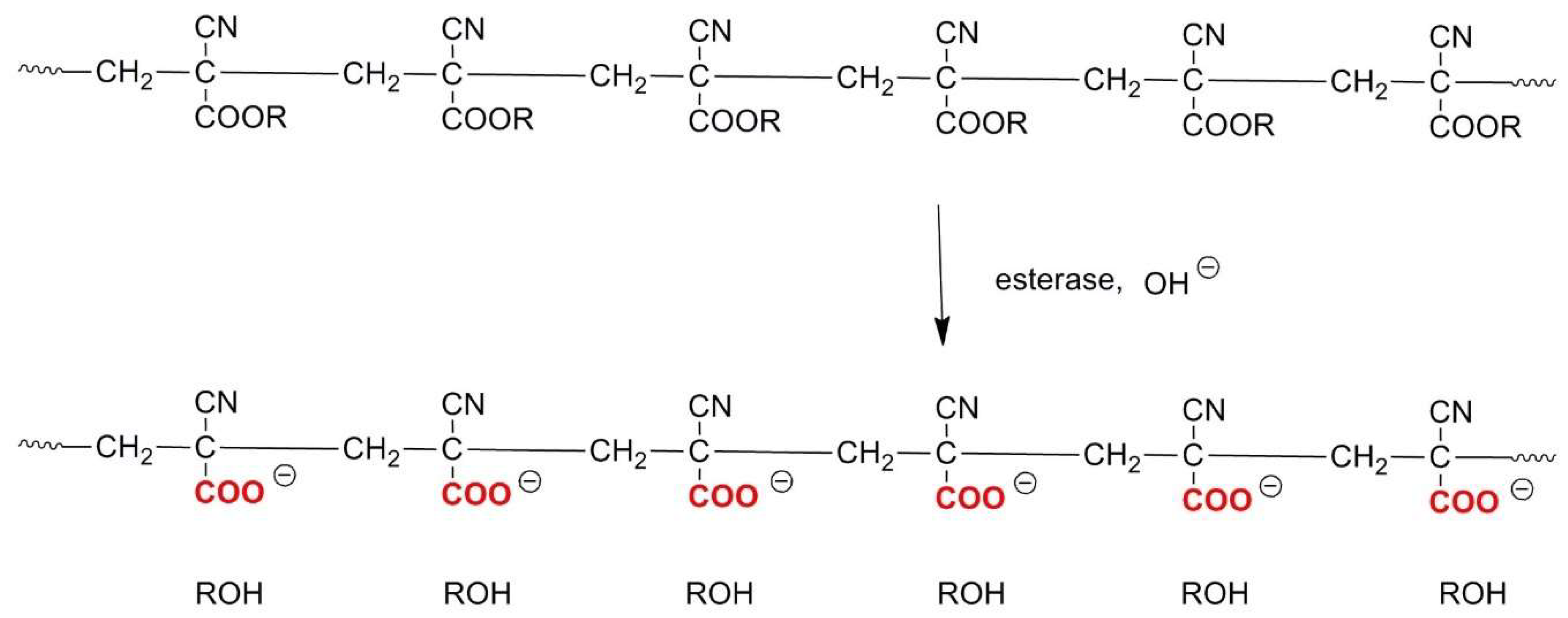
2.5. Polyalkylcyanoacrylates
2.6. Biopolymers
3. Preparation of Functionalized Nano- and Microparticles
3.1. Functional Nano- and Microparticles Prepared by Polymerization
3.2. Nano- and Microparticles by Self-Assembly of Functional (Co)Polymers
- -
- Nanoprecipitation covering “classical” nanoprecipitation and “reverse” nanoprecipitation;
- -
- Flash nanoprecipitation;
- -
- Solvent evaporation/dialysis;
- -
- Salting out;
- -
- Miscellaneous methods including spray-drying.
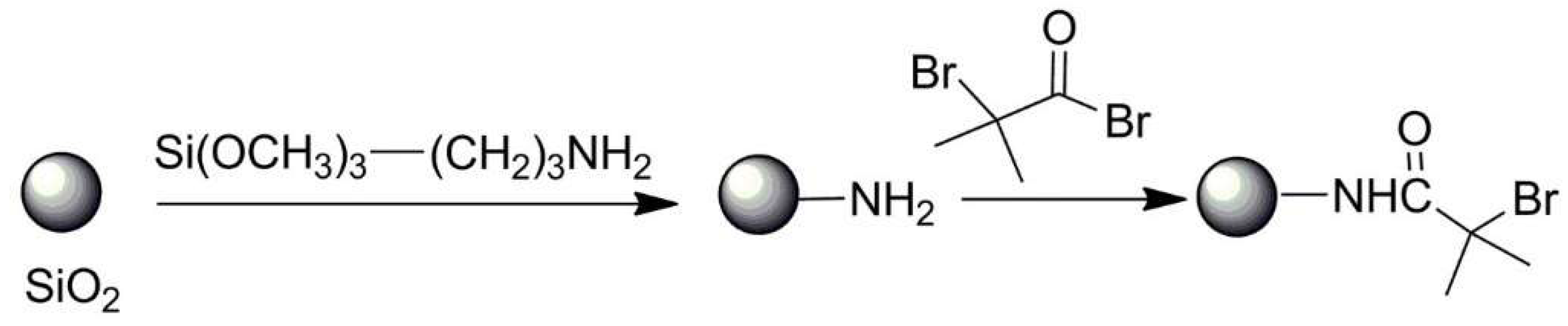
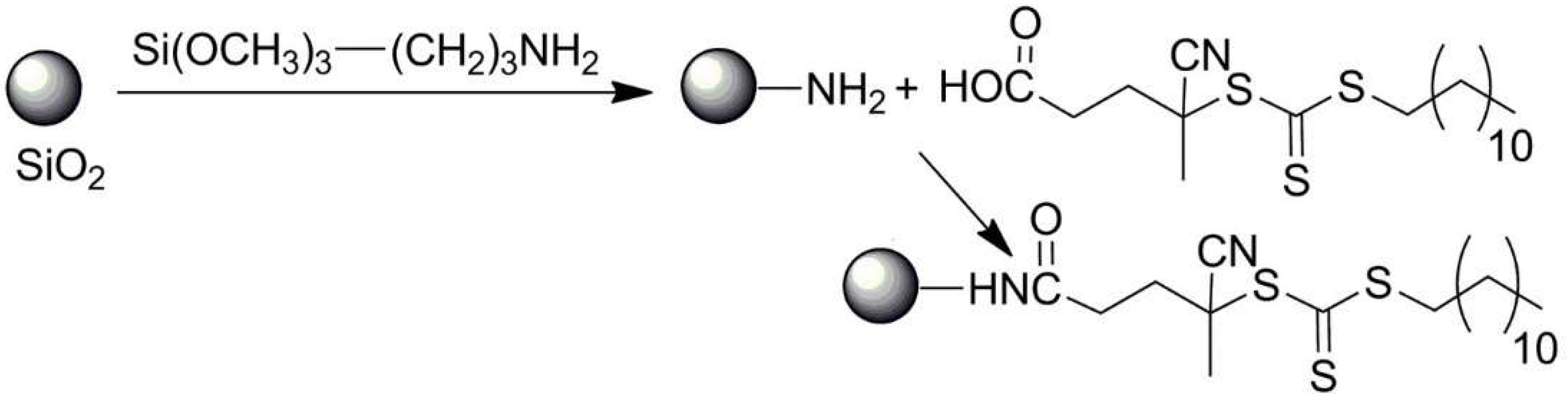
3.3. Hybrid Inorganic and Organic Nano- and Microparticles by Multistep Functionalization of Parent Particles
3.4. Nano- and Microparticles with Immobilized Ligands Specific for Nanoparticle-Selected Cell Interactions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, D.F.; Pye, G.; Bramley, R.; Clark, A.G.; Dyson, T.J.; Hardcastle, J.D. Measurement of Gastrointestinal pH Profiles in Normal Ambulant Human Subjects. Gut 1988, 29, 1035–1041. [Google Scholar] [CrossRef]
- Newby, J.M.; Seim, I.; Lysy, M.; Ling, Y.; Huckaby, J.; Lai, S.K.; Forest, M.G. Technological Strategies to Estimate and Control Diffusive Passage Times Through the Mucus Barrier in Mucosal Drug Delivery. Adv. Drug Deliv. Rev. 2018, 124, 64–81. [Google Scholar] [CrossRef] [PubMed]
- Slomkowski, S.; Alemán, J.V.; Gilbert, R.G.; Hess, M.; Horie, K.; Jones, R.G.; Kubisa, P.; Meisel, I.; Mormann, W.; Penczek, S.; et al. Terminology of Polymers and Polymerization Processes in Dispersed Systems (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 2229–2259. [Google Scholar] [CrossRef]
- Münch, S.; Wohlrab, J.; Neubert, R.H.H. Dermal and Transdermal Delivery of Pharmaceutically Relevant Macromolecules. Eur. J. Pharm. Biopharm. 2017, 119, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.C.; Shukla, S.; Skoog, S.A.; Boehm, R.D.; Narayan, R.J. Current Advancements in Transdermal Biosensing and Targeted Drug Delivery. Sensors 2019, 19, 1028. [Google Scholar] [CrossRef]
- Osman, N.; Kaneko, K.; Carini, V.; Saleem, I. Carriers for the Targeted Delivery of Aerosolized Macromolecules for Pulmonary Pathologies. Expert Opin. Drug Deliv. 2018, 15, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Kuzmov, A.; Minko, T. Nanotechnology Approaches for Inhalation Treatment for Lung Diseases. J. Control. Release 2015, 219, 500–518. [Google Scholar] [CrossRef]
- Gizurarson, S. Anatomical and Histological Factors Affecting Intranasal Drug and Vaccine Delivery. Curr. Drug Deliv. 2012, 9, 566–582. [Google Scholar] [CrossRef] [PubMed]
- Ghadiri, M.; Young, P.M.; Traini, D. Strategies to Enhance Drug Absorption via Nasal and Pulmonary Routes. Pharmaceutics 2019, 11, 113. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, H.M.; Jiang, H.; Yu, J.; Wang, Y.; Ke, H.; Gong, T.; Zhang, Z.R.; Sun, X. Tailoring Polymeric Hybrid Micelles with Lymph Node Targeting Ability to Improve the Potency of Cancer Vaccines. Biomaterials 2017, 122, 105–113. [Google Scholar] [CrossRef]
- Jiang, D.; Mu, W.; Pang, X.; Liu, Y.; Zhang, N.; Song, Y.; Garg, S. Cascade Cytosol Delivery of Dual-sensitive Micelle-tailored Vaccine for Enhancing Cancer Immunotherapy. ACS Appl. Mater. Interfaces 2018, 10, 37797–37811. [Google Scholar] [CrossRef]
- Kuai, R.; Sun, X.; Yuan, W.; Xu, Y.; Schwendeman, A.; Moon, J. Subcutaneous Nanodisc Vaccination with Neoantigens for Combination Cancer Immunotherapy. Bioconjug. Chem. 2018, 29, 771–775. [Google Scholar] [CrossRef]
- Lucke, M.; Mottas, I.; Herbst, T.; Hotz, C.; Römer, L.; Schierling, M.; Herold, H.M.; Slotta, U.; Spinetti, T.; Scheibel, T.; et al. Engineered Hybrid Spider Silk Particles as Delivery System for Peptide Vaccines. Biomaterials 2018, 172, 105–115. [Google Scholar] [CrossRef]
- Howard, G.P.; Verma, G.; Ke, X.; Thayer, W.M.; Hamerly, T.; Baxter, V.K.; Lee, J.E.; Dinglasan, R.R.; Mao, H.-Q. Critical Size Limit of Biodegradable Nanoparticles for Enhanced Lymph Node Trafficking and Paracortex Penetration. Nano Res. 2019, 12, 837–844. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, S.; Qin, Y.; Fan, F.; Zhang, Z.; Huang, C.; Ji, W.; Lu, L.; Wang, C.; Sun, H.; et al. Targeted Codelivery of an Antigen and Dual Agonists by Hybrid Nanoparticles for Enhanced Cancer Immunotherapy. Nano Lett. 2019, 19, 4237–4249. [Google Scholar] [CrossRef]
- Gea, Y.; Chen, D.; Xie, L.; Zhang, R. Optimized Preparation of Daidzein-Loaded Chitosan Microspheres and In Vivo Evaluation After Intramuscular Injection in Rats. Int. J. Pharm. 2007, 338, 142–151. [Google Scholar] [CrossRef]
- Sang, L.; Luo, D.; Wei, Z.; Qi, M. X-Ray Visible and Doxorubicin-loaded Beads Based on Inherently Radiopaque Poly(Lactic Acid)-Polyurethane for Chemoembolization Therapy. Mater. Sci. Eng. C 2017, 75, 1389–1398. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, Y.; He, L.; Sun, R.; Pu, C.; Xie, B.; He, H.; Zhang, Y.; Yin, T.; Wang, Y.; et al. Injectable Sustained-Release Depots of PLGA Microspheres for Insoluble Drugs Prepared by Hot-Melt Extrusion. Pharm. Res. 2017, 34, 2211–2222. [Google Scholar] [CrossRef]
- Tomic, I.; Mueller-Zsigmondy, M.; Vidis-Millward, A.; Cardot, J.-M. In Vivo Release of Peptide-loaded PLGA Microspheres Assessed Through Deconvolution Coupled with Mechanistic Approach. Eur. J. Pharm. Biopharm. 2018, 125, 21–27. [Google Scholar] [CrossRef]
- Andhariya, J.V.; Jog, R.; Shen, J.; Choi, S.; Wang, Y.; Zou, Y. Development of Level A In Vitro-In Vivo Correlations for Peptide Loaded PLGA Microspheres. J. Control. Release 2019, 308, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Bu, R.; Zhang, H.; Yin, J.; Chen, J.; Zhang, A.; Gou, J.; Yin, T.; Zhang, Y.; He, H.; et al. Goserelin Acetate Loaded Poloxamer Hydrogel in PLGA Microspheres: Core-Shell Di-Depot Intramuscular Sustained Release Delivery System. Mol. Pharm. 2019, 16, 3502–3513. [Google Scholar] [CrossRef]
- Ahmed, O.A.A.; Zidan, A.S.; Khayat, M. Mechanistic Analysis of Zein Nanoparticles/PLGA Triblock In Situ Forming Implants for Glimepiride. Int. J. Nanomed. 2016, 11, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Duchi, S.; Ramos-Romero, S.; Dozza, B.; Guerra-Rebollo, M.; Cattini, L.; Ballestri, M.; Dambruoso, P.; Guerrini, A.; Sotgiu, G.; Varchi, G.; et al. Development of Near-infrared Photoactivable Phthalocyanine-loaded Nanoparticles to Kill Tumor Cells: An Improved Tool for Photodynamic Therapy of Solid Cancers. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1885–1897. [Google Scholar] [CrossRef] [PubMed]
- Domb, A.J. Polymeric Site-Specific Pharmacotherapy; John Wiley & Sons: Chichester, UK, 1994. [Google Scholar]
- Zhang, Y.-N.; Poon, W.; Tavares, A.J.; McGilvray, J.D.; Warren, C.W.; Chana, W.C.W. Nanoparticle–Liver Interactions: Cellular Uptake and Hepatobiliary Elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef] [PubMed]
- Karabasz, A.; Szczepanowicz, K.; Cierniak, A.; Mezyk-Kopec, A.; Dyduch, G.; Szczęch, M.; Bereta, J.; Bzowska, M. In Vivo Studies on Pharmacokinetics, Toxicity and Immunogenicity of Polyelectrolyte Nanocapsules Functionalized with Two Different Polymers: Poly-L-Glutamic Acid or PEG. Int. J. Nanomed. 2019, 14, 9587–9602. [Google Scholar] [CrossRef]
- Yessine, M.A.; Lafleur, M.; Meier, G.; Petereit, H.-U.; Leroux, J.-C. Characterization of the Membrane-Destabilizing Properties of Different pH-sensitive Methacrylic Acid Copolymers. Biochim. Biophys. Acta 2003, 1613, 28–38. [Google Scholar] [CrossRef]
- Duncan, R. Polymer Conjugates as Anticancer Nanomedicines. Nat. Rev. Cancer 2006, 6, 688–701. [Google Scholar] [CrossRef]
- Nam, J.; Won, N.; Bang, J.; Jin, H.; Park, J.; Jung, S.; Jung, S.; Park, Y.; Kim, S. Surface Engineering of Inorganic Nanoparticles for Imaging and Therapy. Adv. Drug Deliv. Rev. 2013, 65, 622–648. [Google Scholar] [CrossRef]
- Shen, S.; Jiang, D.; Cheng, L.; Chao, Y.; Nie, K.; Dong, Z.; Kutyreff, C.J.; Engle, J.W.; Huang, P.; Cai, W.; et al. Renal-Clearable Ultrasmall Coordination Polymer Nanodots for Chelator-free 64Cu-labeling and Imaging-guided Enhanced Radiotherapy of Cancer. ACS Nano 2017, 11, 9103–9111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Wang, Z.; Wang, F.; Kang, L.; Cao, F.; Dong, K.; Ren, J.; Qu, X. Renal-clearable Ultrasmall Covalent Organic Framework Nanodots as Photodynamic Agents for Effective Cancer Therapy. Biomaterials 2019, 223, 119462. [Google Scholar] [CrossRef] [PubMed]
- Rosen, H.B.; Chang, J.; Wnek, G.E.; Linhard, R.J.; Langer, R. Bioerodible Polyanhydrides for Controlled Drug Delivery. Biomaterials 1983, 4, 131–133. [Google Scholar] [CrossRef]
- Leong, K.W.; Brott, B.C.; Langer, R. Bioerodible Polyanhydrides as Drug-carrier Matrices. I: Characterization, Degradation, and Release Characteristics. J. Biomed. Mater. Res. 1985, 19, 941–955. [Google Scholar] [CrossRef]
- Leong, K.W.; D’Amore, P.; Marletta, M.; Langer, R. Bioerodible Polyanhydrides as Drug-carrier Matrices. II. Biocompatibility and Chemical Reactivity. J. Biomed. Mater. Res. 1986, 20, 51–64. [Google Scholar] [CrossRef]
- Mathiowitz, E.; Saltzman, W.M.; Domb, A.; Dor, P.; Langer, R. Polyanhydride Microspheres as Drug Carriers. 2. Microencapsulation by Solvent Removal. J. Appl. Polym. Sci. 1988, 35, 755–774. [Google Scholar] [CrossRef]
- Bindschaedler, C.; Leong, K.L.; Mathiowitz, E.; Langer, R. Polyanhydride Microsphere Formulation by Solvent Extraction. J. Pharm. Sci. 1988, 77, 696–698. [Google Scholar] [CrossRef]
- Mathiowitz, E.; Amato, C.; Dor, P.; Langer, R. Polyanhydride Microspheres: 3. Morphology and Characterization of Systems Made by Solvent Removal. Polymer 1990, 31, 547–555. [Google Scholar] [CrossRef]
- Mathiowitz, E.; Bernstein, H.; Giannos, S.; Dor, P.; Turek, T.; Langer, R. Polyanhydride Microspheres. IV. Morphology and Characterization of Systems Made by Spray Drying. J. Appl. Polym. Sci. 1992, 45, 125–134. [Google Scholar] [CrossRef]
- Berkland, C.; Kipper, M.J.; Narasimhan, B.; Kim, K.; Pack, D.W. Microsphere Size, Precipitation Kinetics and Drug Distribution Control Drug Release from Biodegradable Polyanhydride Microspheres. J. Control. Release 2004, 94, 129–141. [Google Scholar] [CrossRef]
- Determan, A.S.; Trewyn, B.G.; Lin, V.S.-Y.; Nilsen-Hamilton, M.; Narasimhan, B. Encapsulation, Stabilization, and Release of BSA-FITC from Polyanhydride Microspheres. J. Control. Release 2004, 100, 97–109. [Google Scholar] [CrossRef]
- Hong, D.-W.; Liu, T.-H.; Chu, I.-M. Encapsulation of Curcumin by Methoxy Poly(ethylene glycol-b-aromatic anhydride) Micelles. J. Appl. Polym. Sci. 2011, 122, 898–907. [Google Scholar] [CrossRef]
- Hiremath, J.G.; Rudani, C.G.; Domb, A.J.; Suthar, R.V.; Khamar, N.S. Preparation and In Vitro Characterization of Poly(Sebacic acid-co-Ricinoleic acid)-based Tamoxifen Citrate-loaded Microparticles for Breast Cancer. J. Appl. Polym. Sci. 2012, 124, 4747–4754. [Google Scholar] [CrossRef]
- Vilar, G.; Tulla-Puche, J.; Albericio, F. Polymers and Drug Delivery Systems. Curr. Drug Deliv. 2012, 9, 367–394. [Google Scholar] [CrossRef]
- Bagherifam, S.; Griffiths, G.W.; Maelandsmo, G.M.; Nystrom, B.; Hasirci, V.; Hasirci, N. Poly(Sebacic anhydride) Nanocapsules as Carriers: Effects of Preparation Parameters on Properties and Release of Doxorubicin. J. Microencapsul. 2015, 32, 166–174. [Google Scholar] [CrossRef]
- Shiehzadeh, F.; Tafaghodi, M. Dry Powder Form of Polymeric Nanoparticles for Pulmonary Drug Delivery. Curr. Pharm. Des. 2016, 22, 2549–2560. [Google Scholar] [CrossRef]
- Carothers, W.H.; Van Natta, F.J. Studies on Polymerization and Ring Formation. III. Glycol Esters of Carbonic Acid. J. Am. Chem. Soc. 1930, 52, 314–326. [Google Scholar] [CrossRef]
- Carothers, W.H.; Dorough, G.L.; Van Natta, F.J. Studies of Polymerization and Ring Formation. X. the Reversible Polymerization of Six-membered Cyclic Esters. J. Am. Chem. Soc. 1932, 54, 761–772. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Weegen-Schulz, B. Polymers of Carbonic Acid. 13. Polymerization of Cyclotrimethylenecarbonate with Tin Tetrahalides. Polymer 1995, 36, 4997–5003. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Weegen-Schulz, B. Polymers of Carbonic-Acid. 15. Polymerization of Cyclotrimethylene Carbonate with TiCl4 or SbCl5 as Initiator. J. Macromol. Sci. Pure Appl. Chem. 1995, A32, 1847–1862. [Google Scholar] [CrossRef]
- Albertsson, A.-C.; Eklund, M. Influence of Molecular-Structure on the Degradation Mechanism of Degradable Polymers—In-Vitro Degradation of Poly(Trimethylene carbonate), Poly(Trimethylene carbonate-co-caprolactone), and Poly(Adipic anhydride). J. Appl. Polym. Sci. 1995, 57, 87–103. [Google Scholar] [CrossRef]
- Murayama, M.; Sanda, E.; Endo, T. Anionic Ring-Opening Polymerization of a Cyclic Carbonate Having a Norbornene Structure with Amine Initiators. Macromolecules 1998, 31, 919–923. [Google Scholar] [CrossRef]
- Al-Azemi, T.F.; Bisht, K.S. Novel Functional Polycarbonate by Lipase-catalyzed Ring-opening Polymerization of 5-Methyl-5-benzyloxycarbonyl-1,3-dioxan-2-one. Macromolecules 1999, 32, 6536–6540. [Google Scholar] [CrossRef]
- Keul, H.; Höcker, H. Expected and Unexpected Reactions in Ring-opening (Co)polymerization. Macromol. Rapid Commun. 2000, 21, 869–883. [Google Scholar] [CrossRef]
- Acemoglu, M. Chemistry of Polymer Biodegradation and Implications on Parenteral Drug Delivery. Int. J. Pharm. 2004, 277, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Ree, M.; Kim, H. Acid- and Base-catalyzed Hydrolyses of Aliphatic Polycarbonates and Polyesters. Catal. Today 2006, 115, 283–287. [Google Scholar] [CrossRef]
- Rokicki, G. Aliphatic Cyclic Carbonates and Spiroorthocarbonates as Monomers. Prog. Polym. Sci. 2000, 25, 259–342. [Google Scholar] [CrossRef]
- Soga, K.; Hosoda, S.; Tazuke, Y.; Ikeda, S. Polymerization of Propylene Carbonate. Polym. Sci. Part A Polym. Chem. 1977, 15, 219–229. [Google Scholar] [CrossRef]
- Vogdanis, L.; Heitz, W. Carbon Dioxide as a Monomer, 3. The Polymerization of Ethylene Carbonate. Makromol. Chem. Rapid Commun. 1986, 7, 543–547. [Google Scholar] [CrossRef]
- Feng, J.; Su, W.; Wang, H.-F.; Huang, F.-W.; Zhang, X.-Z.; Zhuo, R.-X. Facile Fabrication of Diblock Methoxy Poly(ethylene glycol)-Poly(tetramethylene carbonate) and its Self-assembled Micelles as Drug Carriers. ACS Appl. Mater. Interfaces 2009, 1, 2729–2737. [Google Scholar] [CrossRef]
- Suriano, F.; Pratt, R.; Tan, J.P.K.; Wiradharma, N.; Nelson, A.; Yang, Y.-Y.; Dubois, P.; Hedrick, J.L. Synthesis of a Family of Amphiphilic Glycopolymers via Controlled Ring-Opening Polymerization of Functionalized Cyclic Carbonates and their Application in Drug Delivery. Biomaterials 2010, 31, 2637–2645. [Google Scholar] [CrossRef]
- Yan, G.-P.; Zong, R.-F.; Li, L.; Fu, T.; Liu, F.; Yu, X.-H. Anticancer Drug-Loaded Nanospheres Based on Biodegradable Amphiphilic Ε-Caprolactone and Carbonate Copolymers. Pharm. Res. 2010, 27, 2743–2752. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, D.; Huang, K.; Liu, S.; Liu, Z. Preparation and Properties of Poly(Propylene Carbonate Maleate) Microcapsules for Controlled Release of Pazufloxacin Mesilate. J. Appl. Polym. Sci. 2011, 122, 3248–3254. [Google Scholar] [CrossRef]
- Lee, A.L.Z.; Venkataraman, S.; Sirat, S.B.M.; Gao, S.; Hedrick, J.L.; Yang, Y.Y. The Use of Cholesterol-Containing Biodegradable Block Copolymers to Exploit Hydrophobic Interactions for the Delivery of Anticancer Drugs. Biomaterials 2012, 33, 1921–1928. [Google Scholar] [CrossRef]
- Yan, L.; Wu, W.; Zhao, W.; Qi, R.; Cui, D.; Xie, Z.; Huang, Y.; Tong, T.; Jing, X. Reduction-Sensitive Core-Cross-Linked mPEG–Poly(ester-carbonate) Micelles for Glutathione-Triggered Intracellular Drug Release. Polym. Chem. 2012, 3, 2403–2412. [Google Scholar] [CrossRef]
- Jia, H.-Z.; Wang, H.-f.; Liu, C.-w.; Li, C.; Yang, J.; Xu, X.-d.; Feng, J.; Zhang, X.-Z.; Zhuo, R.-X. A pH-Sensitive Macro- and Nanohydrogel Constructed from Cationic Hydroxyl-containing Hyperbranched Polycarbonate. Soft Matter 2012, 8, 6906–6912. [Google Scholar] [CrossRef]
- Attia, A.B.E.; Yang, C.; Tan, J.P.K.; Gao, S.; Williams, D.F.; Hedrick, J.L.; Yang, Y.-Y. The Effect of Kinetic Stability on Biodistribution and Anti-Tumor Efficacy of Drug-Loaded Biodegradable Polymeric Micelles. Biomaterials 2013, 34, 3132–3140. [Google Scholar] [CrossRef]
- Hu, B.; Ke, X.-J.; Yan, G.-P.; Zhuo, R.-X.; Wu, Y.; Fan, C.-L.; Liu, Y.-J. Preparation and Properties of Polycarbonate Microspheres Containing Tetanus Toxoid Vaccine. J. Appl. Polym. Sci. 2014, 131, 40048. [Google Scholar] [CrossRef]
- Hu, B.; Du, H.-J.; Yan, G.-P.; Zhuo, R.-X.; Wu, Y.; Fan, C.-L. Magnetic Polycarbonate Microspheres for Tumor Targeted Delivery of Tumor Necrosis Factor. Drug Deliv. 2014, 21, 204–212. [Google Scholar] [CrossRef]
- Wang, H.-F.; Jia, H.-Z.; Chu, Y.-F.; Feng, J.; Zhang, X.-Z.; Zhuo, R.-X. Acidity-Promoted Cellular Uptake and Drug Release Mediated by Amine-functionalized Block Polycarbonates Prepared via One-shot Ring-opening Copolymerization. Macromol. Biosci. 2014, 14, 526–536. [Google Scholar] [CrossRef]
- Wang, M.; Sun, J.; Zhai, Y.; Lian, H.; Luo, C.; Li, L.; Du, Y.; Zhang, D.; Ding, W.; Qiu, S.; et al. Enteric Polymer Based on pH-Responsive Aliphatic Polycarbonate Functionalized with Vitamin E to Facilitate Oral Delivery of Tacrolimus. Biomacromolecules 2015, 16, 1179–1190. [Google Scholar] [CrossRef]
- Yang, C.; Liu, S.Q.; Venkataraman, S.; Gao, S.J.; Ke, X.; Chia, X.T.; Hedrick, J.L.; Yang, Y.Y. Structure-Directing Star-Shaped Block Copolymers: Supramolecular Vesicles for the Delivery of Anticancer Drugs. J. Control. Release 2015, 208, 93–105. [Google Scholar] [CrossRef]
- Yu, L.; Xie, M.; Li, Z.; Lin, C.; Zheng, Z.; Zhou, L.; Su, Y.; Wang, X. Facile Construction of Near-monodisperse and Dual Responsive Polycarbonate Mixed Micelles with the Ability of pH-Induced Charge Reversal for Intracellular Delivery of Antitumor Drugs. J. Mater. Chem. B 2016, 4, 6081–6093. [Google Scholar] [CrossRef]
- Xie, M.; Yu, L.; Li, Z.; Zheng, Z.; Wang, X. Synthesis and Character of Novel Polycarbonate for Constructing Biodegradable Multi-Stimuli Responsive Delivery System. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3583–3592. [Google Scholar] [CrossRef]
- Teo, J.Y.; Chin, W.; Ke, X.; Gao, S.; Liu, S.; Cheng, W.; Hedrick, J.L.; Yang, Y.Y. pH and Redox Dual-Responsive Biodegradable Polymeric Micelles with High Drug Loading for Effective Anticancer Drug Delivery. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ono, R.J.; Yang, C.; Gao, S.; Tan, J.Y.M.; Hedrick, J.L.; Yang, Y.Y. Dual pH-responsive Shell-Cleavable Polycarbonate Micellar Nanoparticles for In Vivo Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 19355–19364. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Birnbaum, W.; Anderski, J.; Picker, M.-T.; Mulac, D.; Langer, K.; Kuckling, K. Use of Light-Degradable Aliphatic Polycarbonate Nanoparticles as Drug Carrier for Photosensitizer. Biomacromolecules 2018, 19, 4677–4690. [Google Scholar] [CrossRef]
- Li, H.; Niu, Y. Preparation of Poly(Propylene carbonate-co-ε-caprolactone) and their Applications in Drug Delivery. J. Polym. Mater. Polym. Biomater. 2018, 67, 192–198. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, N.; Qin, Z.; Wu, J.; Wang, F.; Zhang, L.; Xia, X.; Li, J.; Lu, Y. Polycarbonate-Based Core-Crosslinked Redox-Responsive Nanoparticles for Targeted Delivery of Anticancer Drug. J. Mater. Chem. B 2018, 6, 3348–3357. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, X.; Hu, Z.; Hou, Z.; Guo, Z.; Chen, Z.; Hu, J.; Yang, L. Synthesis, Self-Assembly, and Drug-Release Properties of New Amphipathic Liquid Crystal Polycarbonates. Nanomaterials 2018, 8, 195. [Google Scholar] [CrossRef]
- Wang, M.; Zhai, Y.; Ye, H.; Lv, Q.; Sun, B.; Luo, C.; Jiang, O.; Zhang, H.; Xu, Y.; Jing, Y.; et al. High Co-Loading Capacity and Stimuli-Responsive Release Based on Cascade Reaction of Self-Destructive Polymer for Improved Chemo-Photodynamic Therapy. ACS Nano 2019, 13, 7010–7023. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, X.; Chen, Z.; Hu, J.; Yang, L. New Liquid Crystal Polycarbonate Micelles for Intracellular Delivery of Anticancer Drugs. Colloids Surf. B Biointerfaces 2019, 178, 395–403. [Google Scholar] [CrossRef]
- Muller, H.-M.; Seebach, D. Poly(Hydroxyalkanoates)—A 5th Class of Physiologically Important Organic Biopolymers. Angew. Chem. Int. Ed. Eng. 1993, 32, 477–502. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, Structure and Properties of Polyhydroxyalkanoates: Biological Polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Steinbüchel, A.; Doi, Y. Polyesters I: Biological Systems and Biotechnological Production. In Biopolymers; Wiley-VCH: Weinheim, Germany, 2002; Volume 3a, pp. 1–472. [Google Scholar]
- Allmendinger, M.; Eberhardt, R.; Luinstra, G.A.; Rieger, B. The Cobalt-catalyzed Alternating Copolymerization of Epoxides and Carbon Monoxide: A Novel Approach to Polyesters. J. Am. Chem. Soc. 2002, 124, 5646–5647. [Google Scholar] [CrossRef]
- Allmendinger, M.; Eberhardt, R.; Luinstra, G.A.; Rieger, B. Alternating Copolymerization Reaction of Propylene Oxide and CO: Variation of Polymer Stereoregularity and Investigation into Chain Termination. Macromol. Chem. Phys. 2003, 204, 564–569. [Google Scholar] [CrossRef]
- Reichardt, R.; Rieger, B. Poly(3-hydroxybutyrate) from Carbon Monoxide. Adv. Polym. Sci. 2012, 245, 49–90. [Google Scholar] [CrossRef]
- Vert, M.; Chen, J.; Hellwich, K.-H.; Hodge, P.; Nakano, T.; Scholz, C.; Slomkowski, S.; Vohlidal, J. Nomenclature and Terminology for Linear Lactic Acid-based Polymers (IUPAC Recommendations 2019). Pure Appl. Chem. 2020, 92, 193–211. [Google Scholar] [CrossRef]
- Nagy, N.; Varga, Z.; Mihály, J.; Kasza, G.; Iván, B.; Kiss, É. Highly Efficient Encapsulation of Curcumin into and pH-controlled Drug Release from Poly(ε-Caprolactone) Nanoparticles Stabilized with a Novel Amphiphilic Hyperbranched Polyglycerol. eXPRESS Polym. Lett. 2020, 14, 90–101. [Google Scholar] [CrossRef]
- Mohamadpour, H.; Azadi, A.; Rostamizadeh, K.; Andalib, S.; Zanjani, M.R.S.; Hamidi, M. Preparation, Optimization, and Evaluation of Methoxy Poly(ethylene glycol)-co-poly(ε-caprolactone) Nanoparticles Loaded by Rivastigmine for Brain Delivery. ACS Chem. Neurosci. 2020, 11, 783–795. [Google Scholar] [CrossRef]
- Ragusa, J.; Gonzalez, D.; Li, S.; Noriega, S.; Skotak, M.; Larsen, G. Glucosamine/L-lactide Copolymers as Potential Carriers for the Development of a Sustained Rifampicin Release System Using Mycobacterium smegmatis as a Tuberculosis Model. Heliyon 2019, 5, e01539. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, A.E.; Pandey, N.; Shan, D.; Banerjee, S.; Yang, J.; Nguyen, K.T. Characterization of Photoluminescent Polylactone-based Nanoparticles for their Applications in Cardiovascular Disease. Front. Bioeng. Biotechnol. 2019, 7, 353. [Google Scholar] [CrossRef]
- Shao, L.; Li, Q.; Zhao, C.; Lu, J.; Li, X.; Chen, L.; Deng, X.; Ge, G.; Wu, Y. Auto-Fluorescent Polymer Nanotheranostics for Self-monitoring of Cancer Therapy via Triple-collaborative Strategy. Biomaterials 2019, 194, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Pieper, S.; Onafuye, H.; Mulac, D.; Cinatl, J., Jr.; Wass, M.N.; Michaelis, M.; Langer, K. Incorporation of Doxorubicin in Different Polymer Nanoparticles and their Anticancer Activity. Beilstein J. Nanotechnol. 2019, 10, 2062–2072. [Google Scholar] [CrossRef] [PubMed]
- Owiti, A.O.; Pal, D.; Mitra, A. PSMA Antibody-conjugated Pentablock Copolymer Nanomicellar Formulation for Targeted Delivery to Prostate Cancer. AAPS PharmSciTech 2018, 19, 3535–3549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hou, Y.; Chen, H.; Liao, Z.; Chen, J.; Xu, B.B.; Kong, J. Reduction-responsive Amphiphilic Star Copolymers with Long-chain Hyperbranched Poly(ε-caprolactone) Core and Disulfide Bonds for Trigger Release of Anticancer Drugs. Eur. Polym. J. 2018, 108, 364–372. [Google Scholar] [CrossRef]
- Abyaneh, H.S.; Soleimani, A.H.; Reza Vakili, M.R.; Soudy, R.; Kaur, K.; Cuda, F.; Tavassoli, A.; Lavasanifar, A. Modulation of Hypoxia-induced Chemoresistance to Polymeric Micellar Cisplatin: The Effect of Ligand Modification of Micellar Carrier Versus Inhibition of the Mediators of Drug Resistance. Pharmaceutics 2018, 10, 196. [Google Scholar] [CrossRef]
- Chen, F.; Li, Y.; Fu, Y.; Hou, Y.; Chen, Y.; Luo, X. The Synthesis and Co-micellization of PCL-P(HEMA/HEMA-LA) and PCL-P(HEMA/HEMA-FA) as Shell Cross-linked Drug Carriers with Target/Redox Properties. J. Biomater. Sci. Polym. Ed. 2019, 30, 276–294. [Google Scholar] [CrossRef]
- Kost, B.; Brzeziński, M.; Cieślak, M.; Królewska-Golińska, K.; Makowski, T.; Socka, M.; Biela, T. Stereocomplexed Micelles Based on Polylactides with β-Cyclodextrin Core as Anti-cancer Drug Carriers. Eur. Polym. J. 2019, 120, 109271. [Google Scholar] [CrossRef]
- Piao, L.; Li, Y.; Zhang, H.; Jiang, J. Stereocomplex Micelle Loaded with Paclitaxel for Enhanced Therapy of Breast Cancer in an Orthotopic Mouse Model. J. Biomater. Sci. Polym. Ed. 2019, 30, 233–246. [Google Scholar] [CrossRef]
- Lu, M.; Chen, F.; Cao, C.; Garvey, C.J.; Fletcher, N.L.; Houston, Z.H.; Lu, H.; Lord, M.S.; Thurecht, K.J.; Stenzel, M.H. Importance of Polymer Length in Fructose-based Polymeric Micelles for an Enhanced Biological Activity. Macromolecules 2019, 52, 477–486. [Google Scholar] [CrossRef]
- Jiang, J.; Li, J.; Zhou, B.; Niu, C.; Wang, W.; Wu, W.; Liang, J. Fabrication of Polymer Micelles with Zwitterionic Shell and Biodegradable Core for Reductively Responsive Release of Doxorubicin. Polymers 2019, 11, 1019. [Google Scholar] [CrossRef]
- Wang, P.; Liu, W.; Liu, S.; Yang, R.; Pu, Y.; Zhang, W.; Wang, X.; Liu, X.; Ren, Y.; Chi, B. pH-responsive Nanomicelles of Poly(ethylene glycol)-Poly(ε-caprolactone)-Poly(L-histidine) for Targeted Drug Delivery. J. Biomater. Sci. Polym. Ed. 2020, 31, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Kocabay, O.G.; Ismail, O. Preparation and Optimization of Biodegradable Self-assembled PCL-PEG-PCL Nano-sized Micelles for Drug Delivery Systems. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 328–337. [Google Scholar] [CrossRef]
- Gomes, M.L.S.; da Silva Nascimento, N.; Borsato, D.M.; Pretes, A.P.; Nadal, J.M.; Novatski, A.; Gomes, R.Z.; Fernandes, D.; Farago, P.D.; Warumby Zanin, S.M. Long-lasting Anti-platelet Activity of Cilostazol from Poly(ε-caprolactone)-Poly(ethylene glycol) Blend Nanocapsules. Mater. Sci. Eng. C 2019, 94, 694–702. [Google Scholar] [CrossRef]
- Zignani, M.; Merkli, A.; Sintzel, M.B.; Bernatchez, S.F.; Kloeti, W.; Heller, J.; Tabatabay, C.; Gurny, R. New Generation of Poly(ortho esters): Synthesis, Characterization, Kinetics, Sterilization and Biocompatibility. J. Control. Release 1997, 48, 115–129. [Google Scholar] [CrossRef]
- Heller, J.; Barr, J.; Ng, S.Y.; Abdellauoi, K.S.; Gurny, R. Poly(ortho esters): Synthesis, Characterization, Properties and Uses. Adv. Drug Deliv. Rev. 2002, 54, 1015–1039. [Google Scholar] [CrossRef]
- Haider, T.; Shyshov, O.; Suraeva, O.; Lieberwirth, I.; von Delius, M.; Wurm, F.R. Long-chain Polyorthoesters as Degradable Polyethylene Mimics. Macromolecules 2019, 52, 2411–2420. [Google Scholar] [CrossRef] [PubMed]
- Heller, J.; Barr, J. Poly(ortho esters) from Concept to Reality. Biomacromolecules 2004, 5, 1625–1632. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Raghavan, S.S.; Tashima, L.M.; Lin, E.C.; Fredette, S.J.; Langer, R.S.; Wang, C. Enhancement of Poly(orthoester) Microspheres for DNA Vaccine Delivery by Blending with Poly(ethylenimine). Biomaterials 2008, 29, 2783–2793. [Google Scholar] [CrossRef]
- Ryan, B.; McCann, G. Novel Sub-ceiling Temperature Depolymerization–Repolymerization Reactions of Cyanoacrylate Polymers. Macromol. Rapid Commun. 1996, 17, 217–227. [Google Scholar] [CrossRef]
- Lenaerts, V.; Couvreur, P.; Christiaens-Leyh, D.; Joiris, E.; Roland, M.; Rollman, B.; Speiser, P. Degradation of Poly(isobutyl cyanoacrylate) Nanoparticles. Biomaterials 1984, 5, 65–68. [Google Scholar] [CrossRef]
- Vansnick, L.; Couvreur, P.; Christiaens-Ley, D.; Roland, M. Molecular Weights of Free and Drug-loaded Nanoparticles. Pharm. Res. 1985, 2, 36–41. [Google Scholar] [CrossRef]
- Langer, K.; Seegmuller, E.; Zimmer, A.; Kreuter, J. Characterization of Polybutylcyanoacrylate Nanoparticles: Quantification of PBCA Polymer and Dextran. Int. J. Pharm. 1994, 110, 21–27. [Google Scholar] [CrossRef]
- Vauthier, C.; Dubernet, C.; Fattal, E.; Pinto-Alphandary, H.; Couvreur, P. Poly(alkylcyanoacrylates) as Biodegradable Materials for Biomedical Applications. Adv. Drug Deliv. Rev. 2003, 55, 519–548. [Google Scholar] [CrossRef]
- Ren, F.; Chen, R.; Wang, Y.; Sun, Y.; Jiang, Y.; Li, G. Paclitaxel-Loaded Poly(N-butylcyanoacrylate) Nanoparticle Delivery System to Overcome Multidrug Resistance in Ovarian Cancer. Pharm. Res. 2011, 28, 897–906. [Google Scholar] [CrossRef]
- Alhareth, K.; Vauthier, C.; Gueutin, C.; Ponchel, G.; Moussa, F. Doxorubicin Loading and in Vitro Release from Poly(alkylcyanoacrylate) Nanoparticles Produced by Redox Radical Emulsion Polymerization. J. Appl. Polym. Sci. 2011, 119, 816–822. [Google Scholar] [CrossRef]
- Zhao, X.; Li, W.; Qiuhua Luo, Q.; Zhang, X. Enhanced Bioavailability of Orally Administered Flurbiprofen by Combined Use of Hydroxypropyl-Cyclodextrin and Poly(alkyl-cyanoacrylate) Nanoparticles. Eur. J. Drug Metab. Pharmacokinet. 2014, 39, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Sulheim, F.; Baghirov, H.; von Haartman, E.; Bøe, A.; Åslund, A.K.O.; Mørch, Y.; de Lange Davies, C. Cellular Uptake and Intracellular Degradation of Poly(alkyl cyanoacrylate) Nanoparticles. J. Nanobiotechnol. 2016, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.S.R.; Medeiros, M.; Yamashiro-Kanashiro, E.H.; Rocha, M.C.; Cotrim, P.C.; Stephano, M.A.; Lancellotti, M.; Tavares, G.D.; Oliveira-Nascimento, L. Biodegradable Nanocarriers Coated with Polymyxin B: Evaluation of Leishmanicidal and Antibacterial Potential. PLoS Negl. Trop. Dis. 2019, 13, e0007388. [Google Scholar] [CrossRef]
- Vrignaud, S.; Benoit, J.-P.; Saulnier, P. Strategies for the Nanoencapsulation of Hydrophilic Molecules in Polymer-Based Nanoparticles. Biomaterials 2011, 32, 8593–8604. [Google Scholar] [CrossRef]
- Lai, P.; Daear, W.; Raimar Löbenberg, R.; Prenner, E.J. Overview of the Preparation of Organic Polymeric Nanoparticles for Drug Delivery Based on Gelatine, Chitosan, Poly(D,L-lactide-co-glycolic acid) and Polyalkylcyanoacrylate. Colloids Surf. B Biointerfaces 2014, 118, 154–163. [Google Scholar] [CrossRef]
- Arpicco, S.; Battaglia, L.; Brus, P.; Cavalli, R.; Chirio, D.; Dosio, F.; Gallarate, M.; Milla, P.; Peira, E.; Rocco, F.; et al. Recent Studies on the Delivery of Hydrophilic Drugs in Nanoparticulate Systems. J. Drug Deliv. Sci. Technol. 2016, 32, 298–312. [Google Scholar] [CrossRef]
- Lopes, M.A.; Abrahim, B.A.; Cabral, L.M.; Rodrigues, C.R.; Seica, R.M.F.; de Baptista Veiga, F.J.; Ribeiro, A.J. Intestinal Absorption of Insulin Nanoparticles: Contribution of M Cells. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Fattal, E.; Nicolas, J. From Poly(alkyl cyanoacrylate) to Squalene as Core Material for the Design of Nanomedicines. J. Drug Target. 2019, 27, 470–501. [Google Scholar] [CrossRef] [PubMed]
- Vauthier, C. A Journey Through the Emergence of Nanomedicines with Poly(alkylcyanoacrylate) Based Nanoparticles. J. Drug Target. 2019, 27, 502–524. [Google Scholar] [CrossRef]
- Jones, R.G.; Kahovec, J.; Stepto, R.; Wilks, E.S.; Hess, M.; Kitayama, T.; Metanomski, W.V. Compendium of Polymer Terminology and Nomenclature: IUPAC Recommendations 2008; The Royal Society of Chemistry: Cambridge, UK, 2008. [Google Scholar]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef]
- Singh, I.; Luxami, V.; Paul, K. Spectroscopy and Molecular Docking Approach for Investigation on the Binding of Nocodazole to Human Serum Albumin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 235, 118289. [Google Scholar] [CrossRef] [PubMed]
- Claire, A.; Lethier, L.; Guillaume, Y.C. An Organic Monolithic Capillary Column Functionalized with Human Serum Albumin and its Application for the Nano-Chromatography Study of its Binding with Universal Cancer Peptides and its Impact on Immunogenicity. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 777–783. [Google Scholar] [CrossRef]
- Callmann, C.E.; LeGuyader, C.L.M.; Burton, S.T.; Thompson, M.P.; Hennis, R.; Barback, C.; Henriksen, N.M.; Chan, W.C.; Jaremko, M.J.; Yang, J.; et al. Antitumor Activity of 1,18-Octadecanedioic Acid-Paclitaxel Complexed with Human Serum Albumin. J. Am. Chem. Soc. 2019, 141, 11765–11769. [Google Scholar] [CrossRef]
- Yokoyama, M.; Inoue, S.; Kataoka, K.; Yui, N.; Sakurai, Y. Preparation of Adriamycin-Conjugated Poly(ethylene glycol)-Poly(aspartic acid) Block Copolymer—A New Type of Polymeric Anticancer Agent. Makromol. Chem. Rapid Commun. 1987, 8, 431–435. [Google Scholar] [CrossRef]
- Yokoyama, M.; Miyauchi, M.; Yamada, N.; Okano, T.; Sakurai, Y.; Kataoka, K.; Inoue, S. Characterization and Anticancer Activity of the Micelle-Forming Polymeric Anticancer Drug Adriamycin-Conjugated Poly(ethylene glycol)-Poly(aspartic acid) Block Copolymer. Cancer Res. 1990, 50, 1693–1700. [Google Scholar]
- Horise, Y.; Maeda, M.; Konishi, Y.; Okamoto, J.; Ikuta, S.; Okamoto, Y.; Ishii, H.; Yoshizawa, S.; Umemura, S.; Ueyama, T.; et al. Sonodynamic Therapy With Anticancer Micelles and High-Intensity Focused Ultrasound in Treatment of Canine Cancer. Front. Pharmacol. 2019, 10, 545. [Google Scholar] [CrossRef]
- Takemae, K.; Okamoto, J.; Horise, Y.; Masamune, K.; Muragaki, Y. Function of Epirubicin-Conjugated Polymeric Micelles in Sonodynamic Therapy. Front. Pharmacol. 2019, 10, 546. [Google Scholar] [CrossRef]
- Florinas, S.; Liu, M.; Fleming, R.; Van Vlerken-Ysla, L.; Ayriss, J.; Gilbreth, R.; Dimasi, N.; Gao, C.; Wu, H.; Xu, Z.-Q.; et al. A Nanoparticle Platform To Evaluate Bioconjugation and Receptor-Mediated Cell Uptake Using Cross-Linked Polyion Complex Micelles Bearing Antibody Fragments. Biomacromolecules 2016, 17, 1818–1833. [Google Scholar] [CrossRef]
- Takashima, H.; Koga, Y.; Tsumura, R.; Yasunaga, M.; Tsuchiya, M.; Inoue, T.; Negishi, E.; Harada, M.; Yoshida, S.; Matsumura, Y. Reinforcement of Antitumor Effect of Micelles Containing Anticancer Drugs by Binding of an Anti-Tissue Factor Antibody Without Direct Cytocidal Effects. J. Control. Release 2020, 323, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.L.; Dhanya, B.S.; Rani, V.; Thakur, M.; Jeslin, J.; Kushwaha, R. Carbohydrate and Protein Based Biopolymeric Nanoparticles: Current Status and Biotechnological Applications. Int. J. Biol. Macromol. 2020, 154, 390–412. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I.S. Hyaluronic Acid and Controlled Release: A Review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Maji, B.; Hari Narayana Moorthy, N.S.; Maiti, S. Xanthan Gum Derivatives: Review of Synthesis, Properties and Diverse Applications. RSC Adv. 2020, 10, 27103–27136. [Google Scholar] [CrossRef]
- Heidarian, P.; Kouzani, A.Z.; Kaynak, A.; Paulino, M.; Nasri-Nasrabadi, B.; Zolfagharian, A.; Varley, R. Dynamic Plant-Derived Polysaccharide-Based Hydrogels. Carbohydr. Polym. 2020, 231, 115743. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, X.; Wang, G. Review on Marine Carbohydrate-based Gold Nanoparticles Represented by Alginate and Chitosan for Biomedical Application. Carbohydr. Polym. 2020, 244, 116311. [Google Scholar] [CrossRef]
- Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fischer, A.-M.; Helley, D.; Colliec-Jouault, S. Marine Polysaccharides: A Source of Bioactive Molecules for Cell Therapy and Tissue Engineering. Mar. Drugs 2011, 9, 1664–1681. [Google Scholar] [CrossRef] [PubMed]
- Gericke, M.; Schulze, P.; Heinze, T. Nanoparticles Based on Hydrophobic Polysaccharide Derivatives—Formation, Principles, Characterization Techniques, and Biomedical Applications. Macromol. Biosci. 2020, 20, 1900415. [Google Scholar] [CrossRef]
- Almalik, A.; Alradwan, I.; Kalam, M.A.; Alshamsan, A. Effect of Cryoprotection on Particle Size Stability and Preservation of Chitosan Nanoparticles with and without Hyaluronate or Alginate Coating. Saudi Pharm. J. 2017, 25, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Eliyahu, S.; Almeida, A.; Macedo, M.H.; das Neves, J.; Sarmento, B.; Bianco-Peled, H. The Effect of Freeze-drying on Mucoadhesion and Transport of Acrylated Chitosan Nanoparticles. Int. J. Pharm. 2020, 573, 118739. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Marchetti, R.; Aman, A.; Silipo, A.; Ul Qader, S.A.; Molinaro, A. Enzymatic and Acidic Degradation of High Molecular Weight Dextran into Low Molecular Weight and its Characterizations Using Novel Diffusion-ordered NMR Spectroscopy. Int. J. Biol. Macromol. 2017, 103, 744–750. [Google Scholar] [CrossRef]
- Beaubier, S.; Framboisier, X.; Ioannou, I.; Galet, O.; Kapel, R. Simultaneous Quantification of the Degree of Hydrolysis, Protein Conversion Rate and Mean Molar Weight of Peptides Released in the Course of Enzymatic Proteolysis. J. Chromatogr. B 2019, 1105, 1–9. [Google Scholar] [CrossRef]
- Del Castillo-Santaella, T.; Maldonado-Valderrama, J.; Molina-Bolivar, J.A.; Galisteo-Gonzalez, F. Effect of Cross-Linker Glutaraldehyde on Gastric Digestion of Emulsified Albumin. Colloids Surf. B Biointerfaces 2016, 145, 899–905. [Google Scholar] [CrossRef]
- Varkhede, N.; Bommana, R.; Schöneich, C.; Forrest, M.L. Proteolysis and Oxidation of Therapeutic Proteins After Intradermal or Subcutaneous Administration. J. Pharm. Sci. 2020, 109, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Barakat, I.; Dubois, P.; Jérôme, R.; Teyssié, P.; Mazurek, M. Polymerization of Glycolide Promoted by ω-Al-alkoxide Poly(ε-caprolactone) Macro-Initiators and Formation of Stable Colloidal Dispersions. Macromol. Symp. 1994, 88, 227–244. [Google Scholar] [CrossRef]
- Sosnowski, S.; Gadzinowski, M.; Slomkowski, S.; Penczek, S. Synthesis of Bioerodible Poly(ε-caprolactone) Latexes and Poly(D, L-lactide) Microspheres by Ring-Opening Polymerization. J. Bioact. Compat. Polym. 1994, 9, 345–366. [Google Scholar] [CrossRef]
- Slomkowski, S.; Sosnowski, S.; Gadzinowski, M. Synthesis and Properties of Bioerodible Latexes and Microspheres. Polym. Prepr. 1996, 37, 135. [Google Scholar]
- Gadzinowski, M.; Slomkowski, S.; Elaïssari, A.; Pichot, C. Phase Transfer and Characterization of Poly(ε-caprolactone) and Poly(L-lactide) Microspheres. J. Biomater. Sci. Polym. Ed. 2000, 11, 459–480. [Google Scholar] [CrossRef]
- Lassalle, V.; Ferreira, M.L. PLA Nano- and Microparticles for Drug Delivery: An Overview of the Methods of Preparation. Macromol. Biosci. 2007, 7, 767–783. [Google Scholar] [CrossRef]
- Allen, S.D.; Bobbala, S.; Karabin, N.B.; Scott, E.A. on the Advancement of Polymeric Bicontinuous Nanospheres Toward Biomedical Applications. Nanoscale Horiz. 2019, 4, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Bobbala, S.; Allen, S.D.; Scott, E.A. Flash Nanoprecipitation Permits Versatile Assembly and Loading of Polymeric Bicontinuous Cubic Nanospheres. Nanoscale 2018, 10, 5078–5088. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, B.E.; De Visser, J.F.; Portale, G.; Hermida-Merino, D.; Friedrich, H.; Bomans, P.H.H.; Bras, W.; Monaghan, O.R.; Holder, S.J.; Sommerdijk, N.A.J.M. The Evolution of Bicontinuous Polymeric Nanospheres in Aqueous Solution. Soft Matter 2016, 12, 4113–4122. [Google Scholar] [CrossRef]
- Jain, R.A. The Manufacturing Techniques of Various Drug Loaded Biodegradable Poly(lactide-co-glycolide) (PLGA) Devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef]
- Prosapio, V.; De Marco, J.; Reverchon, E. Supercritical Antisolvent Coprecipitation Mechanisms. J. Supercrit. Fluids 2018, 138, 247–258. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Scognamiglio, M.; Reverchon, E. Control of Liposomes Diameter at Micrometric and Nanometric Level Using a Supercritical Assisted Technique. J. CO2 Util. 2019, 32, 119–127. [Google Scholar] [CrossRef]
- La, Y.; An, T.H.; Shin, T.J.; Park, C.; Kim, K.T. A Morphological Transition of Inverse Mesophases of a Branched-Linear Block Copolymer Guided by Using Cosolvents. Angew. Chem. Int. Ed. 2015, 54, 10483–10487. [Google Scholar] [CrossRef]
- Cho, A.; La, Y.; Shin, T.J.; Park, C.; Kim, K.T. Structural Requirements of Block Copolymers for Self-Assembly into Inverse Bicontinuous Cubic Mesophases in Solution. Macromolecules 2016, 49, 4510–4519. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, S.; Mao, W.; Tian, H.; Wang, N.; Zhang, N.; Tian, F.; Han, L.; Feng, X.; Mai, Y. Tunable Self-assembly of Diblock Copolymers into Colloidal Particles with Triply Periodic Minimal Surfaces. Angew. Chem. Int. Ed. 2017, 56, 7135–7140. [Google Scholar] [CrossRef]
- Ku, K.H.; Shin, J.M.; Yun, H.; Yi, G.-R.; Jang, S.G.; Kim, B.J. Multidimensional Design of Anisotropic Polymer Particles from Solvent-evaporative Emulsion. Adv. Funct. Mater. 2018, 28, 1802961. [Google Scholar] [CrossRef]
- Pichot, C. Surface-Functionalized Latexes for Biotechnological Applications. Curr. Opin. Colloid Interface Sci. 2004, 9, 213–221. [Google Scholar] [CrossRef]
- Kang, Y.; Pitto-Barry, A.; Rolph, M.S.; Hua, Z.; Hands-Portman, I.; Kirby, N.; O’Reilly, R.K. Use of Complementary Nucleobase-containing Synthetic Polymers to Prepare Complex Self-assembled Morphologies in Water. Polym. Chem. 2016, 7, 2836–2846. [Google Scholar] [CrossRef]
- Gaitzsch, J.; Chudasama, V.; Morecroft, E.; Messager, L.; Battaglia, G. Synthesis of an Amphiphilic Miktoarm Star Terpolymer for Self-assembly into Patchy Polymersomes. ACS Macro Lett. 2016, 5, 351–354. [Google Scholar] [CrossRef]
- Socka, M.; Brzezinski, M.; Michalski, A.; Kacprzak, A.; Makowski, T.; Duda, A. Self-assembly of Triblock Copolymers from Cyclic Esters as a Tool for Tuning their Particle Morphology. Langmuir 2018, 34, 3701–3710. [Google Scholar] [CrossRef]
- Nagasaki, Y.; Okada, T.; Scholz, C.; Iijima, M.; Kato, M.; Kataoka, K. The Reactive Polymeric Micelle Based on an Aldehyde-Ended Poly(ethylene glycol)/Poly(lactide) Block Copolymer. Macromolecules 1998, 31, 1473–1479. [Google Scholar] [CrossRef]
- Le Fer, G.L.; Le Coeur, C.; Guigner, J.-M.; Amiel, C.; Volet, G. Amphiphilic Diblock and Triblock Copolymers Based on Poly(2-Methyl-2-oxazoline) and Poly(D,L-lactide): Synthesis, Physicochemical Characterizations and Self-assembly Properties. Polymer 2019, 171, 149–160. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, C.Y.; Wu, J.; Zhou, H.; Bai, R.; Shen, Z.; Deng, F.; Liu, Y.; Liu, J. PEG-detachable Polymeric Micelles Self-assembled from Amphiphilic Copolymers for Tumor-Acidity-Triggered Drug Delivery and Controlled Release. ACS Appl. Mater. Interfaces 2019, 11, 5701–5713. [Google Scholar] [CrossRef]
- Staubli, A.; Mathiowitz, E.; Lucarelli, M.; Langer, R. Characterization of Hydrolytically Degradable Amino Acid Containing Poly(anhydride-co-imides). Macromolecules 1991, 24, 2283–2290. [Google Scholar] [CrossRef]
- Ray, P.; Ferraro, M.; Haag, R.; Quadir, M. Dendritic Polyglycerol-derived Nano-Architectures as Delivery Platforms of Gemcitabine for Pancreatic Cancer. Macromol. Biosci. 2019, 19, 1900073. [Google Scholar] [CrossRef]
- Yi, X.; Zhao, D.; Zhang, Q.; Xu, J.; Yuan, G.; Zhuo, R.; Li, F. A Co-delivery System Based on a Reduction-Sensitive Polymeric Prodrug Capable of Loading Hydrophilic and Hydrophobic Drugs for Combination Chemotherapy. Polym. Chem. 2016, 7, 5966–5977. [Google Scholar] [CrossRef]
- Tsai, F.-T.; Wang, Y.; Darensbourg, D.J. Environmentally Benign CO2-Based Copolymers: Degradable Polycarbonates Derived from Dihydroxybutyric Acid and their Platinum–Polymer Conjugates. J. Am. Chem. Soc. 2016, 138, 4626–4633. [Google Scholar] [CrossRef]
- Stevens, D.M.; Rahalkar, A.; Spears, B.; Gilmore, K.; Douglas, E.; Muthukumar, M.; Harth, E. Semibranched Polyglycidols as “Fillers” in Polycarbonate Hydrogels to Tune Hydrophobic Drug Release. Polym. Chem. 2015, 6, 1096–1102. [Google Scholar] [CrossRef]
- Wang, Y.; Ke, X.; Voo, Z.X.; Yap, S.S.L.; Yang, C.; Gao, S.; Liu, S.; Venkataraman, S.; Obuobi, S.A.O.; Khara, J.S.; et al. Biodegradable Functional Polycarbonate Micelles for Controlled Release of Amphotericin B. Acta Biomater. 2016, 46, 211–220. [Google Scholar] [CrossRef]
- Ke, X.; Coady, D.J.; Yang, C.; Engler, A.C.; Hedrick, J.L.; Yang, Y.Y. pH-Sensitive Polycarbonate Micelles for Enhanced Intracellular Release of Anticancer Drugs: A Strategy to Circumvent Multidrug Resistance. Polym. Chem. 2014, 5, 2621–2628. [Google Scholar] [CrossRef]
- Peng, T.; Su, J.; Cheng, S.-X.; Zhuo, R.-X. Poly-α,β-(N-(2-hydroxyethyl)-L-aspartamide)-g-poly (1,3-trimethylene carbonate) Amphiphilic Graft Co-Polymer as a Potential Drug Carrier. J. Biomater. Sci. Polym. Ed. 2006, 17, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Singh, S.K.; Arya, S.K.; Kundu, S.C.; Kapoor, S. Protein Nanoparticles: Promising Platforms for Drug Delivery Applications. ACS Biomater. Sci. Eng. 2018, 4, 3939–3961. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-Based Biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kundu, S.C. Self-assembled Silk Sericin/Poloxamer Nanoparticles as Nanocarriers of Hydrophobic and Hydrophilic Drugs for Targeted Delivery. Nanotechnology 2009, 20, 355101. [Google Scholar] [CrossRef] [PubMed]
- Lomis, N.; Westfall, S.; Farahdel, L.; Malhotra, M.; Shum-Tim, D.; Prakash, S. Human Serum Albumin Nanoparticles for Use in Cancer Drug Delivery: Process Optimization and In Vitro Characterization. Nanomaterials 2016, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Arangoa, M.A.; Ponchel, G.; Orecchioni, A.M.; Renedo, M.J.; Duchene, D.; Irache, J.M. Bioadhesive Potential of Gliadin Nanoparticulate Systems. Eur. J. Pharm. Sci. 2000, 11, 333–341. [Google Scholar] [CrossRef]
- Bayrak, A.; Pruger, P.; Stock, U.A.; Seifert, M. Absence of Immune Responses with Xenogeneic Collagen and Elastin. Tissue Eng. Part A 2013, 19, 1592–1600. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Hnatuszko-Konka, K.; Gerszberg, A.; Kononowicz, A.K. Elastin-like Polypeptides as a Promising Family of Genetically-engineered Protein Based Polymers. World J. Microbiol. Biotechnol. 2014, 30, 2141–2152. [Google Scholar] [CrossRef]
- Garland, S.M.; Hernandez-Avila, M.; Wheeler, C.M.; Perez, G.; Harper, D.M.; Leodolter, S.; Tang, G.W.K.; Ferris, D.G.; Steben, M.; Bryan, J.; et al. Quadrivalent Vaccine Against Human Papillomavirus to Prevent Anogenital Diseases. N. Engl. J. Med. 2007, 356, 1928–1943. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cui, F.; Cun, D.; Tao, A.; Shi, K.; Lin, W. Preparation, Characterization and Biodistribution of the Lactone Form of 10-Hydroxycamptothecin (HCPT)-loaded Bovine Serum Albumin (BSA) Nanoparticles. Int. J. Pharm. 2007, 340, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Coester, C.; Kreuter, J.; Langer, K. Desolvation Process and Surface Characterisation of Protein Nanoparticles. Int. J. Pharm. 2000, 194, 91–102. [Google Scholar] [CrossRef]
- Fan, Y.F.; Wang, Y.N.; Fan, Y.G.; Ma, J.B. Preparation of Insulin Nanoparticles and their Encapsulation with Biodegradable Polyelectrolytes via the Layer-By-Layer Adsorption. Int. J. Pharm. 2006, 324, 158–167. [Google Scholar] [CrossRef]
- Jaworek, A. Micro- and Nanoparticle Production by Electrospraying. Powder Technol. 2007, 176, 18–35. [Google Scholar] [CrossRef]
- Islam, N.; Ferro, V. Recent Advances in Chitosan-Based Nanoparticulate Pulmonary Drug Delivery. Nanoscale 2016, 8, 14341–14358. [Google Scholar] [CrossRef]
- Muhsin, M.D.A.; George, G.; Beagley, K.; Ferro, V.; Armitage, C.; Islam, N. Synthesis and Toxicological Evaluation of a Chitosan-L-leucine Conjugate for Pulmonary Drug Delivery Applications. Biomacromolecules 2014, 15, 3596–3607. [Google Scholar] [CrossRef]
- Choi, M.; Cho, M.; Han, B.S.; Hong, J.; Jeong, J.; Park, S.; Cho, M.-H.; Kim, K.; Cho, W.-S. Chitosan Nanoparticles Show Rapid Extrapulmonary Tissue Distribution and Excretion with Mild Pulmonary Inflammation to Mice. Toxicol. Lett. 2010, 199, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Merchant, Z.; Taylor, K.M.G.; Stapleton, P.; Razak, S.A.; Kunda, N.; Alfagih, I.; Sheikh, K.; Saleem, I.Y.; Somavarapu, S. Engineering Hydrophobically Modified Chitosan for Enhancing the Dispersion of Respirable Microparticles of Levofloxacin. Eur. J. Pharm. Biopharm. 2014, 88, 816–829. [Google Scholar] [CrossRef]
- Makhlof, A.; Werle, M.; Tozuka, Y.; Takeuchi, H. Nanoparticles of Glycol Chitosan and its Thiolated Derivative Significantly Improved the Pulmonary Delivery of Calcitonin. Int. J. Pharm. 2010, 397, 92–95. [Google Scholar] [CrossRef]
- Jin, H.; Xu, C.X.; Kim, H.W.; Chung, Y.S.; Shin, J.Y.; Chang, S.H.; Park, S.J.; Lee, E.S.; Hwang, S.K.; Kwon, J.T.; et al. Urocanic Acid-modified Chitosan-mediated PTEN Delivery via Aerosol Suppressed Lung Tumorigenesis in K-rasLA1 Mice. Cancer Gene Ther. 2008, 15, 275–283. [Google Scholar] [CrossRef]
- Luo, Y.; Zhai, X.; Ma, C.; Sun, P.; Fu, Z.; Liu, W.; Xu, J. An Inhalable β2-adrenoceptor Ligand-directed Guanidinylated Chitosan Carrier for Targeted Delivery of siRNA to Lung. J. Control. Release 2012, 162, 28–36. [Google Scholar] [CrossRef]
- Fischer, N.O.; Blanchette, C.D.; Segelke, B.W.; Corzett, M.; Chromy, B.A.; Kuhn, E.A.; Bench, G.; Hoeprich, P.D. Isolation, Characterization, and Stability of Discretely-sized Nanolipoprotein Particles Assembled with Apolipophorin-III. PLoS ONE 2010, 5, e11643. [Google Scholar] [CrossRef] [PubMed]
- Nisha, C.K.; Manorama, S.V.; Ganguli, M.; Maiti, S.; Kizhakkedathu, J.N. Complexes of Poly(ethylene glycol)-based Cationic Random Copolymer and Calf Thymus DNA: A Complete Biophysical Characterization. Langmuir 2004, 20, 2386–2396. [Google Scholar] [CrossRef]
- Van de Manakker, F.; Vermonden, T.; van Nostrum, C.F.; Hennink, W.E. Cyclodextrin-Based Polymeric Materials: Synthesis, Properties, and Pharmaceutical/Biomedical Applications. Biomacromolecules 2009, 10, 3157–3175. [Google Scholar] [CrossRef]
- Yao, X.; Huang, P.; Nie, Z. Cyclodextrin-Based Polymer Materials: From Controlled Synthesis to Applications. Prog. Polym. Sci. 2019, 93, 1–35. [Google Scholar] [CrossRef]
- Prochowicz, D.; Kornowicz, A.; Lewinski, J. Interactions of Native Cyclodextrins with Metal Ions and Inorganic Nanoparticles: Fertile Landscape for Chemistry and Materials Science. Chem. Rev. 2017, 117, 13461–13501. [Google Scholar] [CrossRef]
- Loh, X.J. Supramolecular Host-Guest Polymeric Materials for Biomedical Applications. Mater. Horiz. 2014, 1, 185–195. [Google Scholar] [CrossRef]
- Bukchin, A.; Kuplennik, N.; Carcaboso, Á.M.; Sosnik, A. Effect of Growing Glycosylation Extents on the Self-Assembly and Active Targeting in Vitro of Branched Poly(ethylene oxide)-Poly(propylene oxide) Block Copolymers. Appl. Mater. Today 2018, 11, 57–69. [Google Scholar] [CrossRef]
- Willersinn, J.; Schmidt, B.V.K.J. Pure Hydrophilic Block Copolymer Vesicles with Redox- and pH-Cleavable Crosslinks. Polym. Chem. 2018, 9, 1626–1637. [Google Scholar] [CrossRef]
- Kumar, J.N.; Wu, Y.-L.; Loh, X.J.; Ho, N.Y.; Aik, S.X.; Pang, V.Y. the Effective Treatment of Multi-Drug Resistant Tumors with Self-Assembling Alginate Copolymers. Polym. Chem. 2019, 10, 278–286. [Google Scholar] [CrossRef]
- Liu, B.W.; Zhou, H.; Zhou, S.T.; Zhang, H.J.; Feng, A.C.; Jian, C.M.; Hu, J.; Gao, W.P.; Yuan, J.Y. Synthesis and Self-Assembly of CO2-Temperature Dual Stimuli-Responsive Triblock Copolymers. Macromolecules 2014, 47, 2938–2946. [Google Scholar] [CrossRef]
- Feng, A.C.; Yan, Q.; Zhang, H.J.; Peng, L.; Yuan, J.Y. Electrochemical Redox Responsive Polymeric Micelles Formed from Amphiphilic Supramolecular Brushes. Chem. Commun. 2014, 50, 4740–4742. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yan, C.; Chen, Z.; Liu, J.; Song, H.; Wang, W.; Liu, J.; Yang, N.; Zhao, Y.; Chen, L. Dual-targeting Nanoparticles with Core-Crosslinked and pH/Redox Bioresponsive Properties for Enhanced Intracellular Drug Delivery. J. Colloid Interface Sci. 2019, 540, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Canosa, J.B.; Medintz, I.L.; Farrell, D.; Mattoussi, H.; Dawson, P.E. Rapid Covalent Ligation of Fluorescent Peptides to Water Solubilized Quantum Dots. J. Am. Chem. Soc. 2010, 132, 10027–10033. [Google Scholar] [CrossRef] [PubMed]
- Connal, L.A.; Kinnane, C.R.; Zelikin, A.N.; Caruso, F. Stabilization and Functionalization of Polymer Multilayers and Capsules via Thiol-ene Click Chemistry. Chem. Mater. 2009, 21, 576–578. [Google Scholar] [CrossRef]
- Ma, H.; Jiang, L.; Hajizadeh, S.; Gong, H.; Lu, B.; Ye, L. Nanoparticle-supported Polymer Brushes for Temperature-regulated Glycoprotein Separation: Investigation of Structure–Function Relationship. J. Mater. Chem. B 2018, 6, 3770–3781. [Google Scholar] [CrossRef]
- Bagheri, A.; Sadrearhami, Z.; Adnan, N.N.M.; Boyer, C.; Lim, M. Surface Functionalization of Upconversion Nanoparticles Using Visible Light-mediated Polymerization. Polymer 2018, 151, 6–14. [Google Scholar] [CrossRef]
- Finetti, C.; Sola, L.; Pezzullo, M.; Prosperi, D.; Colombo, M.; Riva, B.; Avvakumova, S.; Morasso, C.; Picciolini, S.; Chiari, M. Click Chemistry Immobilization of Antibodies on Polymer Coated Gold Nanoparticles. Langmuir 2016, 32, 7435–7441. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.; Karimian, R.; Mostafidi, E.; Noruzi, E.B.; Taghizadeh, S.; Shokouhid, B.; Kafil, H.S. Highly Branched Amine-Functionalized P-sulfonatocalix[4]arene Decorated with Human Plasma Proteins as a Smart, Targeted, and Stealthy Nano-Vehicle for the Combination Chemotherapy of MCF7 Cells. New J. Chem. 2018, 42, 13010–13024. [Google Scholar] [CrossRef]
- De Moraes Profirio, D.; Pessine, F.B.T. Formulation of Functionalized PLGA Nanoparticles with Folic Acid Conjugated Chitosan for Carboplatin Encapsulation. Eur. Polym. J. 2018, 108, 311–321. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, J.; Han, Q.; Hu, X.; Wang, D.; Zhang, X.; Yang, P. One-step Assembly of a Biomimetic Biopolymer Coating for Particle Surface Engineering. Adv. Mater. 2018, 30, 1802851. [Google Scholar] [CrossRef]
- Zlitni, A.; Janzen, N.; Foster, F.S.; Valliant, J.F. Catching Bubbles: Targeting Ultrasound Microbubbles Using Bioorthogonal Inverse-Electron-Demand Diels–Alder Reactions. Angew. Chem. Int. Ed. 2014, 53, 6459–6463. [Google Scholar] [CrossRef]
- Zhang, Q.; Nurumbetov, G.; Simula, A.; Zhu, C.; Li, M.; Wilson, P.; Kempe, K.; Yang, B.; Taob, L.; Haddleton, D.M. Synthesis of Well-Defined Catechol Polymers for Surface Functionalization of Magnetic Nanoparticles. Polym. Chem. 2016, 7, 7002–7010. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Burov, A.M.; Pylaev, T.E.; Khlebtsov, N.G. Polydopamine-coated Au Nanorods for Targeted Fluorescent Cell Imaging and Photothermal Therapy. Beilstein J. Nanotechnol. 2019, 10, 794–803. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Cao, J.; Choi, J.-S.; Oshi, M.A.; Lee, J.; Hasan, N.; Kim, J.; Yoo, J.-W. Development of PLGA Micro- and Nanorods with High Capacity of Surface Ligand Conjugation for Enhanced Targeted Delivery. Asian J. Pharm. Sci. 2019, 14, 86–94. [Google Scholar] [CrossRef]
- Hao, J.; Huang, L.L.; Zhang, R.; Wang, H.Z.; Xie, H.Y. A Mild and Reliable Method to Label Enveloped Virus with Quantum Dots by Copper-Free Click Chemistry. Anal. Chem. 2012, 84, 8364–8370. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, T.; Liu, X.; Ouyang, H.; Ren, L. Recent Trends in Click Chemistry as a Promising Technology for Virus-related Research. Virus Res. 2018, 256, 21–28. [Google Scholar] [CrossRef]
- Basiruddin, S.K.; Maity, A.R.; Jana, N.R. Glucose/Galactose/Dextran-functionalized Quantum Dots, Iron Oxide and Doped Semiconductor Nanoparticles with < 100 nm Hydrodynamic Diameter. RSC Adv. 2012, 2, 11915–11921. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Abdullah, F.; Mukherjee, A. Fabrication and Fluorescent Labeling of Guar Gum Nanoparticles in a Surfactant Free Aqueous Environment. Mater. Sci. Eng. C 2015, 46, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Kadam, R.; Zilli, M.; Maas, M.; Rezwan, K. Nanoscale Janus Particles with Dual Protein Functionalization. Part. Part. Syst. Charact. 2018, 35, 1700332. [Google Scholar] [CrossRef]
- Clarke, K.C.; Lyon, L.A. Microgel Surface Modification with Self-assembling Peptides. Macromolecules 2016, 49, 5366–5373. [Google Scholar] [CrossRef]
- Hong, V.; Presolski, S.I.; Ma, C.; Finn, M.G. Analysis and Optimization of Copper-Catalyzed Azide–Alkyne Cycloaddition for Bioconjugation. Angew. Chem. Int. Ed. 2009, 48, 9879–9883. [Google Scholar] [CrossRef]
- Cao, X.; Horak, D.; An, Z.; Plichta, Z. Raft Polymerization of N,N-dimethylacrylamide from Magnetic Poly(2-hydroxyethyl methacrylate) Microspheres to Suppress Nonspecific Protein Adsorption. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1036–1043. [Google Scholar] [CrossRef]
- Gindy, M.E.; Ji, S.; Hoye, T.R.; Panagiotopoulos, A.Z.; Prud’homme, R.K. Preparation of Poly(ethylene glycol) Protected Nanoparticles with Variable Bioconjugate Ligand Density. Biomacromolecules 2008, 9, 2705–2711. [Google Scholar] [CrossRef]
- Ji, S.; Zhu, Z.; Hoye, T.R.; Macosko, C.W. Maleimide Functionalized Poly(ε-caprolactone)-Block-Poly (ethylene glycol) (PCL-PEG-MAL): Synthesis, Nanoparticle Formation, and Thiol Conjugation. Macromol. Chem. Phys. 2009, 210, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, H.; Yang, L.; Du, G.; Pai-Panandiker, A.S.; Huang, X.; Yan, B. Enhancement of Cell Recognition in Vitro by Dual-Ligand Cancer Targeting Gold Nanoparticles. Biomaterials 2011, 32, 2540–2545. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Choi, J.-S.; Garcia, M.A.; Xing, Y.; Chen, K.-J.; Chen, Y.-M.; Jiang, Z.K.; Ro, T.; Wu, L.; Stout, D.B.; et al. Pretargeted Positron Emission Tomography Imaging That Employs Supramolecular Nanoparticles with in Vivo Bioorthogonal Chemistry. ACS Nano 2016, 10, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Kim, H.L.; Jeong, H.J.; Lim, S.T.; Sohn, M.H.; Kim, D.W. Mesoporous Silica Nanoparticle Pretargeting for PET Imaging Based on a Rapid Bioorthogonal Reaction in a Living Body. Angew. Chem. Int. Ed. 2013, 52, 10549–10552. [Google Scholar] [CrossRef] [PubMed]
- Hooks, M.A.; Wade, C.S.; Millikan, W.J. Muromonab CD-3: A Review of its Pharmacology, Pharmacokinetics, and Clinical Use in Transplantation. Pharmacotherapy 1991, 11, 26–37. [Google Scholar] [CrossRef]
- Tiller, K.E.; Tessier, P.M. Advances in Antibody Design. Annu. Rev. Biomed. Eng. 2015, 17, 191–216. [Google Scholar] [CrossRef]
- Jung, Y.; Jeong, J.Y.; Chung, B.H. Recent Advances in Immobilization Methods of Antibodies on Solid Supports. Analyst 2008, 133, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Norret, M.; House, M.J.; Galabura, Y.; Bradshaw, M.; Ho, D.W.; Woodward, R.C.; Pierre, T.G.S.; Luzinov, I.; Smith, N.M.; et al. Dose-Dependent Therapeutic Distinction Between Active and Passive Targeting Revealed Using Transferrin-Coated PGMA Nanoparticles. Small 2016, 12, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.P.; Prado, A.R.; Keijok, W.J.; Antunes, P.W.P.; Yapuchura, E.R.; Guimarães, M.C.C. Impact of Conjugation Strategies for Targeting of Antibodies in Gold Nanoparticles for Ultrasensitive Detection of 17β-Estradiol. Sci. Rep. 2019, 9, 13859. [Google Scholar] [CrossRef]
- Sun, L.; Wan, J.; Schaefer, C.G.; Zhang, Z.; Tan, J.; Guo, J.; Wu, L.; Wang, C. Specific On-Site Assembly of Multifunctional Magnetic Nanocargos Based on Highly Efficient and Parallelized Bioconjugation: Toward Personalized Cancer Targeting Therapy. ACS Biomater. Sci. Eng. 2017, 3, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Mukai, H.; Kogawa, T.; Matsubara, N.; Naito, Y.; Sasaki, M.; Hosono, A. A First-in-Human Phase 1 Study of Epirubicin-Conjugated Polymer Micelles (K-912/NC-6300) in Patients with Advanced Or Recurrent Solid Tumors. Investig. New Drugs 2017, 35, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.; Kataoka, K. Chemo-physical Strategies to Advance the in Vivo Functionality of Targeted Nanomedicine: The Next Generation. J. Am. Chem. Soc. 2021, 143, 538–559. [Google Scholar] [CrossRef]
- Li, J.; Dirisala, A.; Ge, Z.; Wang, Y.; Yin, W.; Ke, W.; Toh, K.; Xie, J.; Matsumoto, Y.; Anraku, Y.; et al. Therapeutic Vesicular Nanoreactors with Tumor-Specific Activation and Self-Destruction for Synergistic Tumor Ablation. Angew. Chem. Int. Ed. 2017, 56, 14025–14030. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Wang, Y.; Ke, W.; Chen, W.; Wang, W.; Ge, Z. Polymer Prodrug-Based Nanoreactors Activated by Tumor Acidity for Orchestrated Oxidation/Chemotherapy. Nano Lett. 2017, 17, 6983–6990. [Google Scholar] [CrossRef]
- Li, J.; Anraku, Y.; Kataoka, K. Self-Boosting Catalytic Nanoreactors Integrated with Triggerable Crosslinking Membrane Networks for Initiation of Immunogenic Cell Death by Pyroptosis. Angew. Chem. Int. Ed. 2020, 59, 13526–13530. [Google Scholar] [CrossRef] [PubMed]
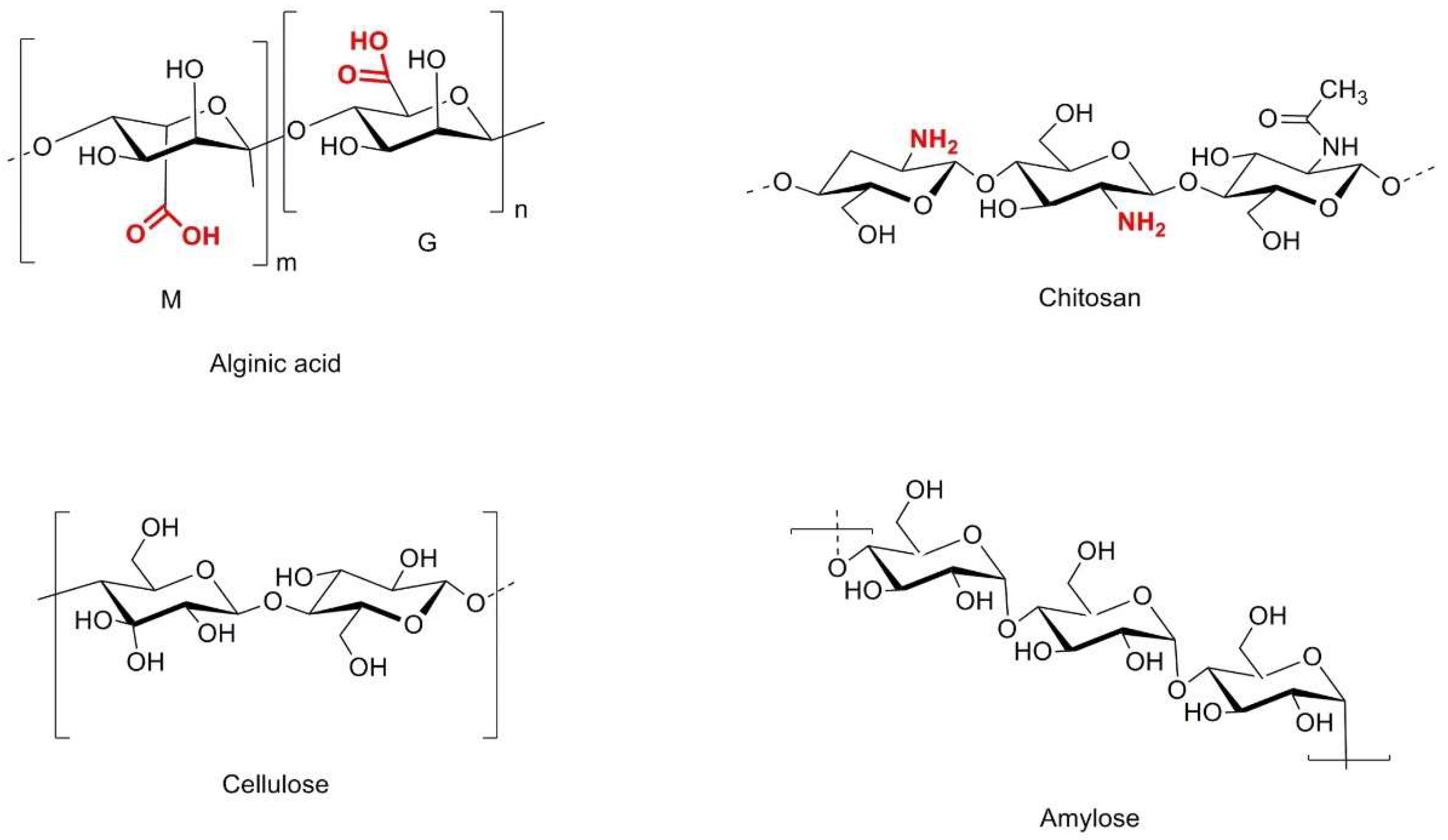
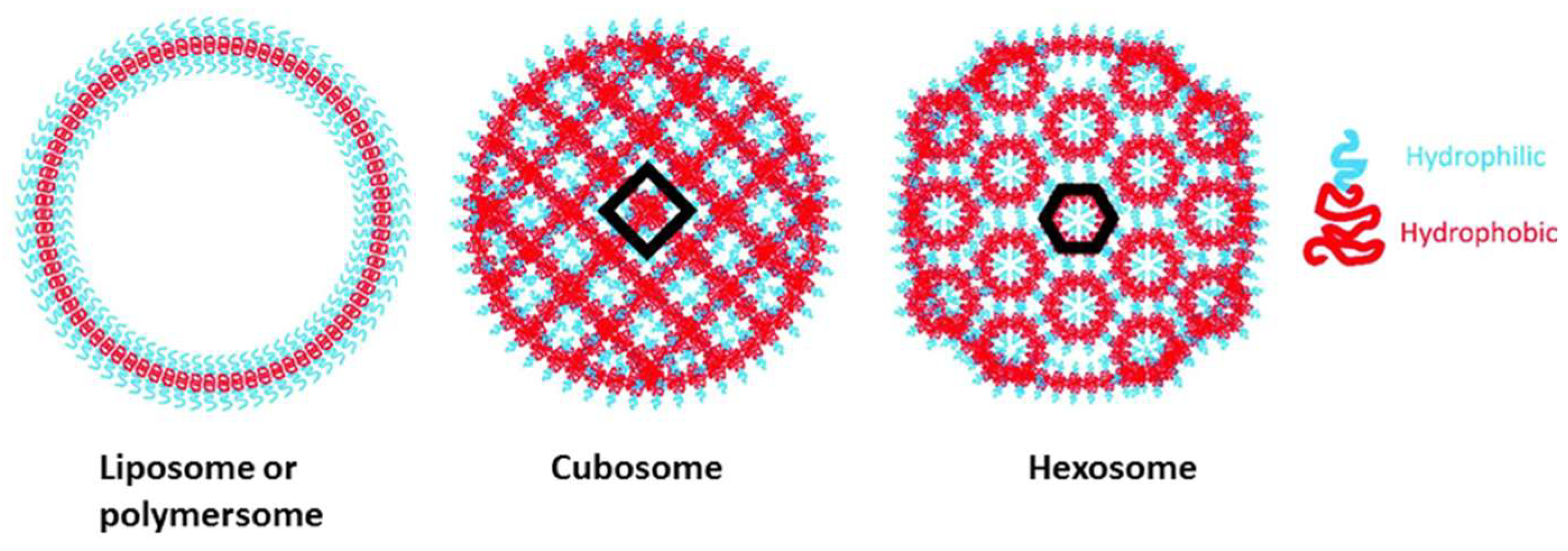
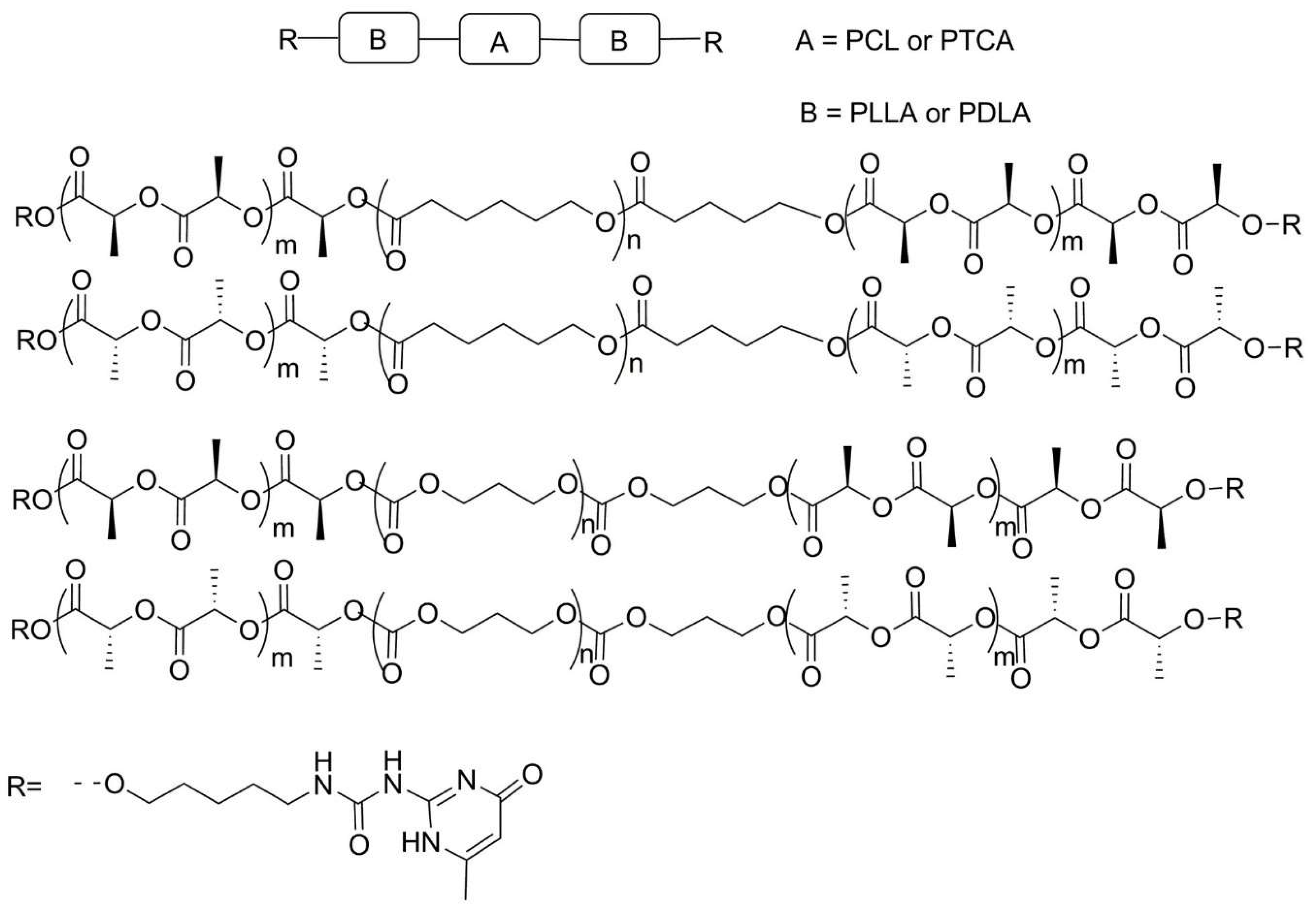
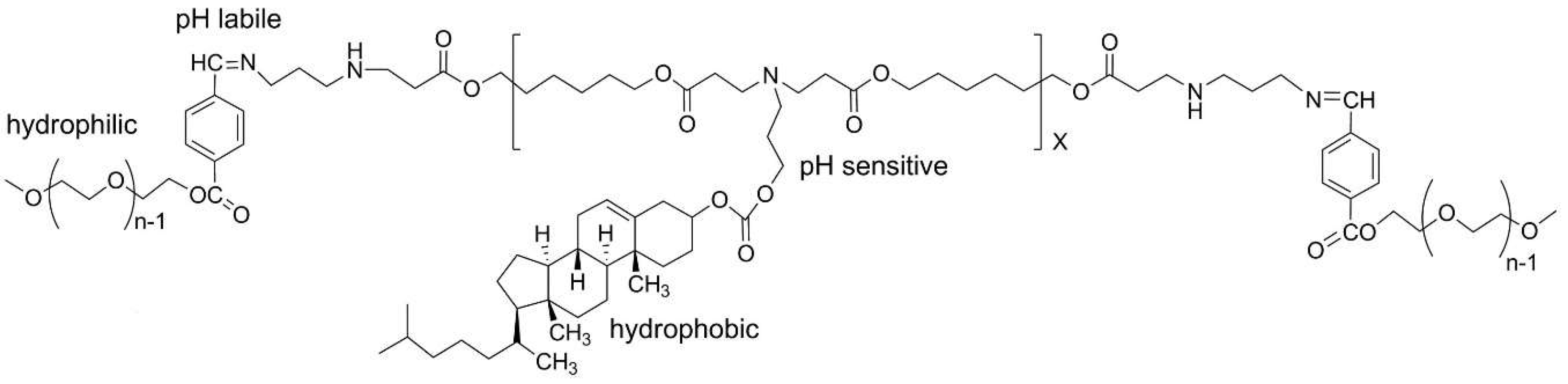
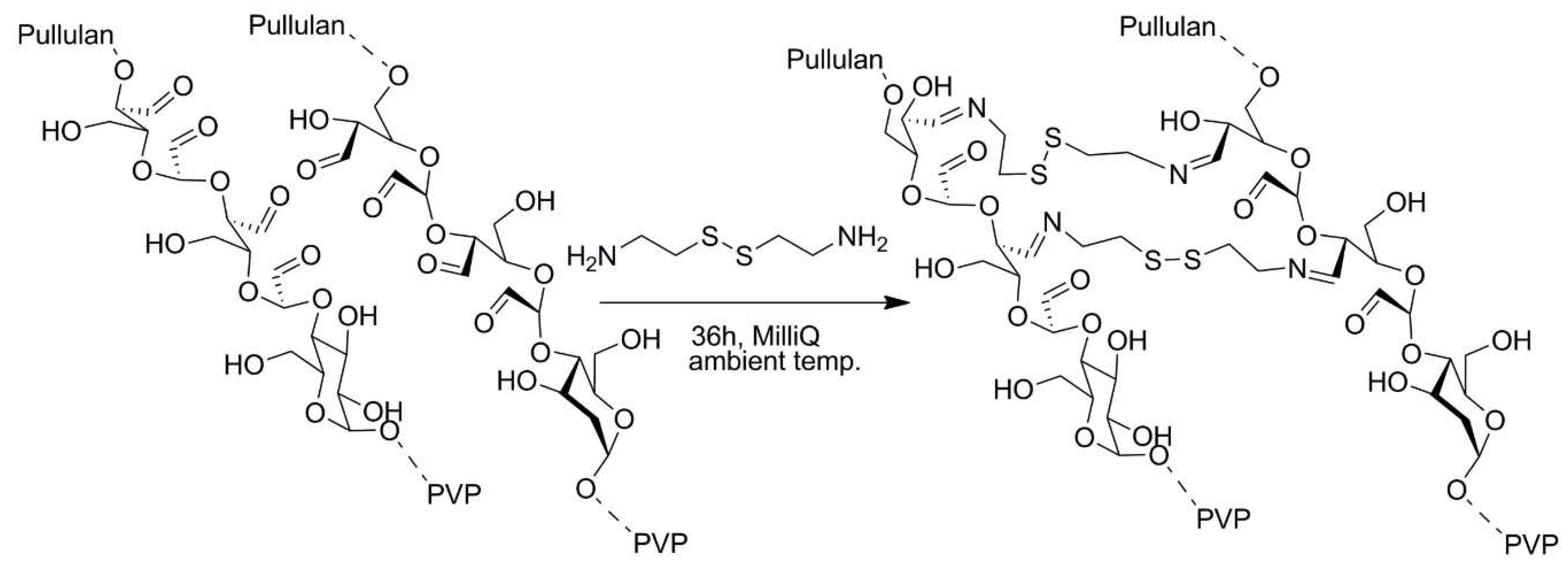
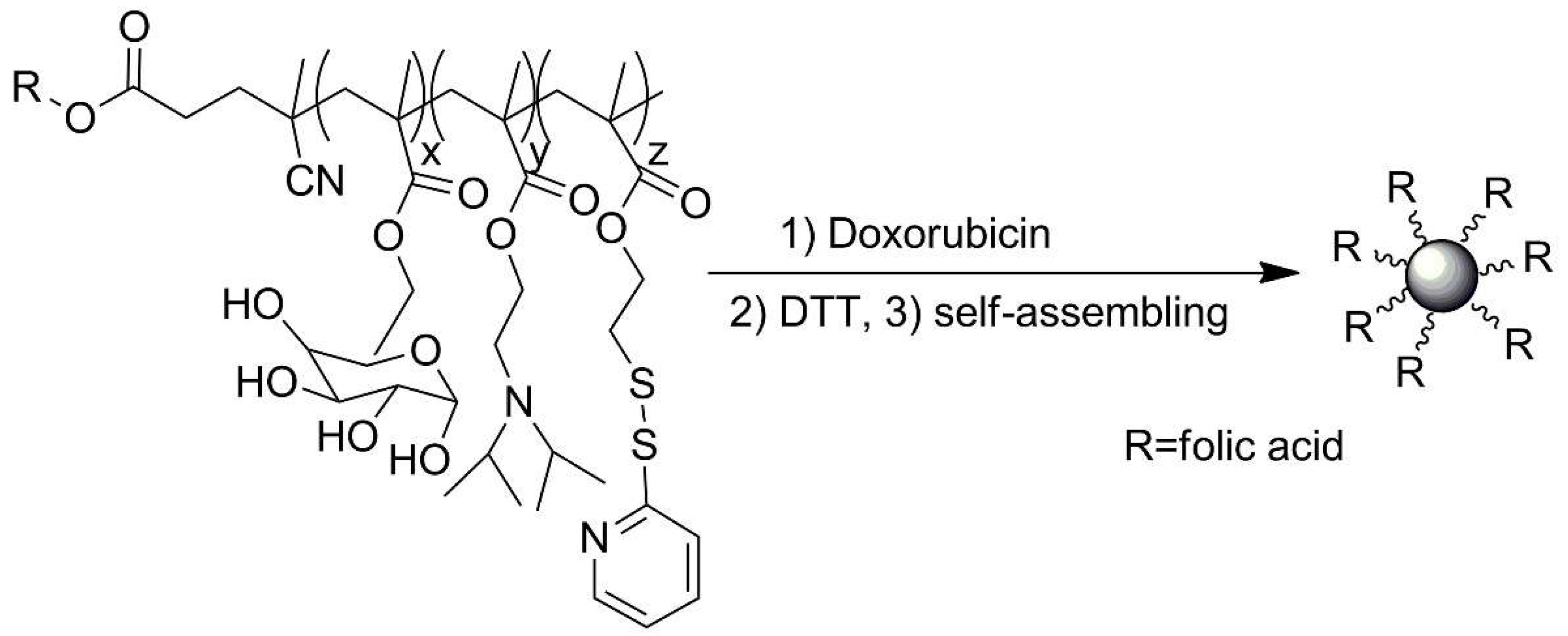
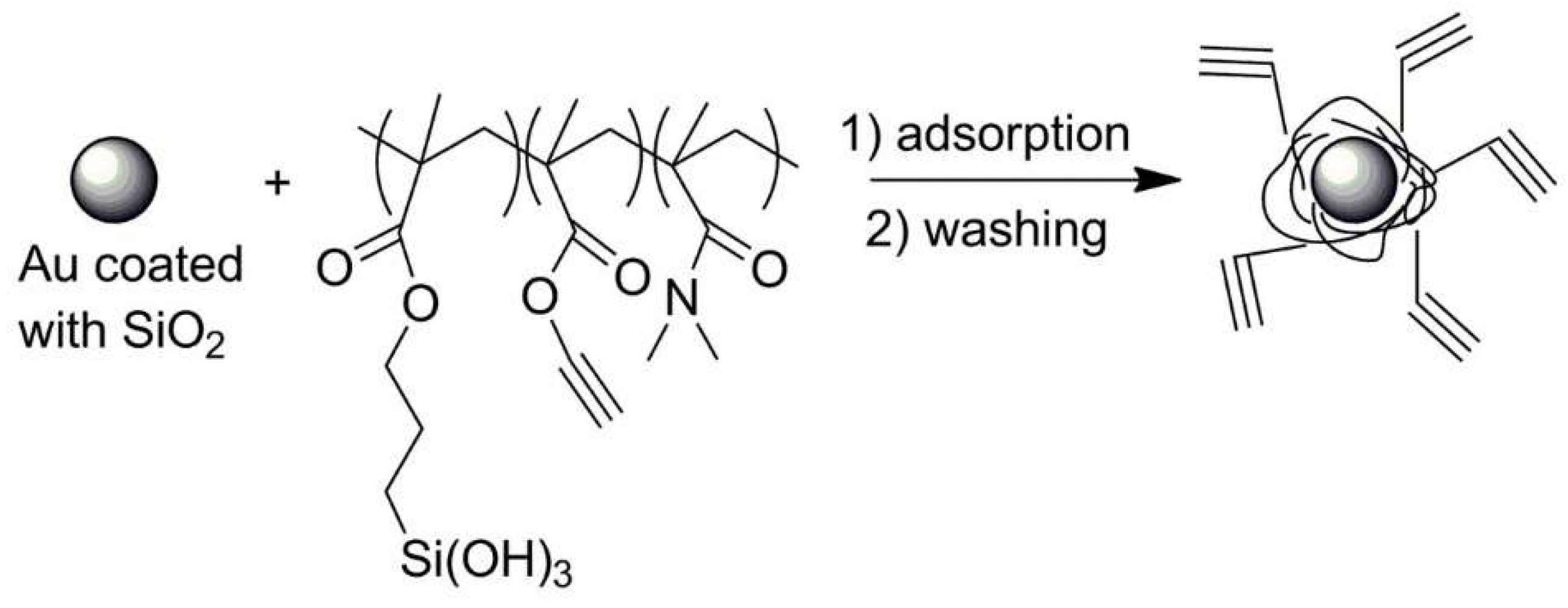
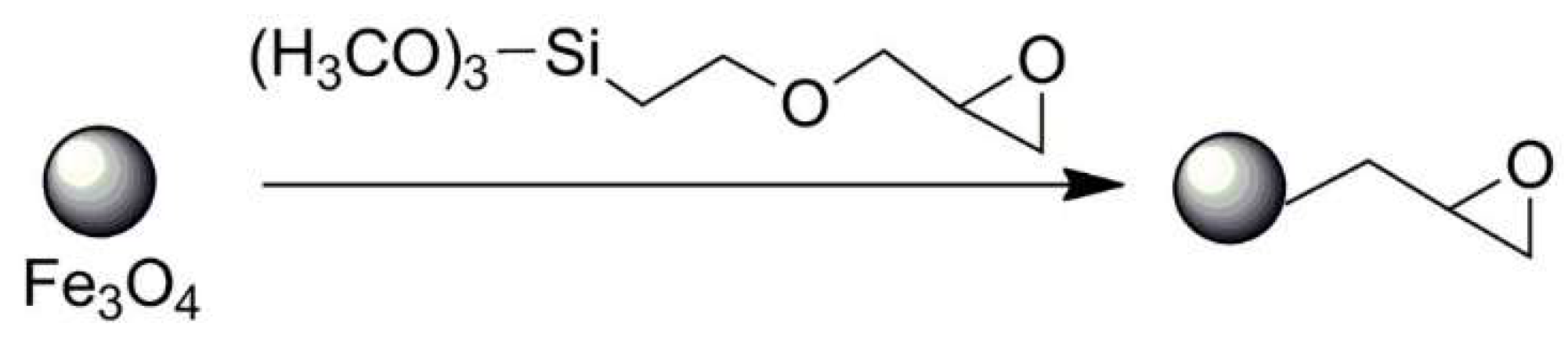
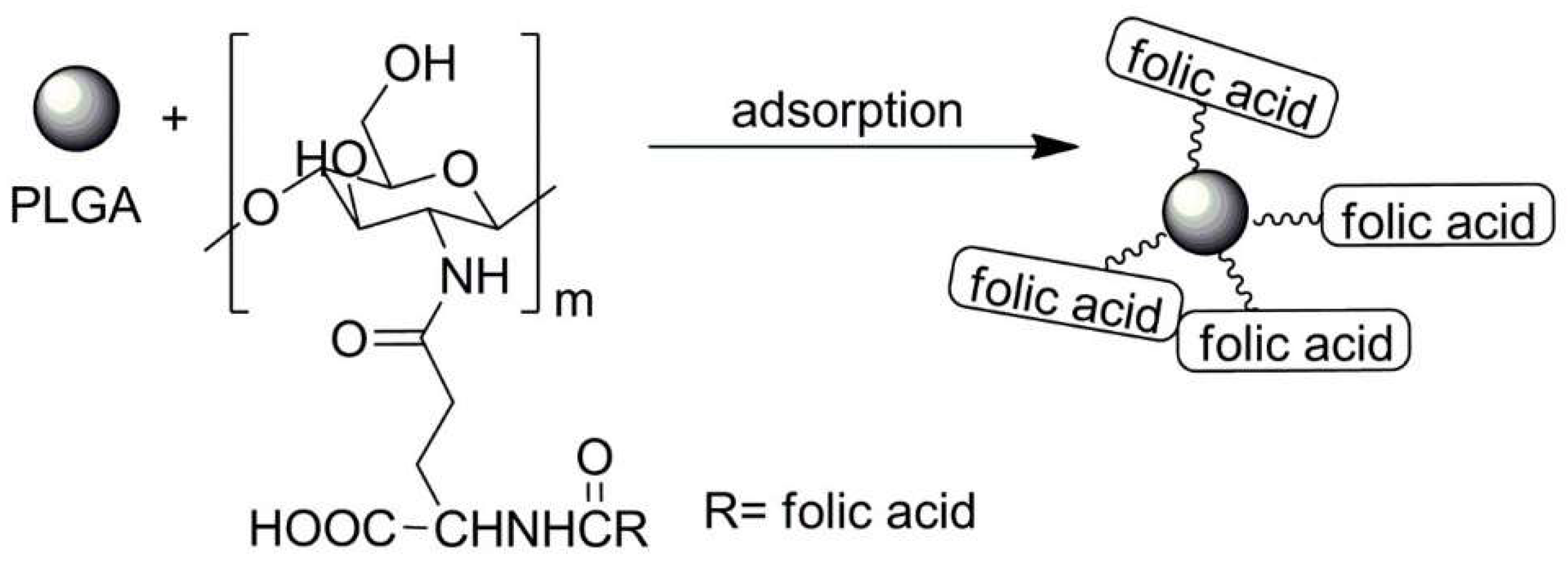
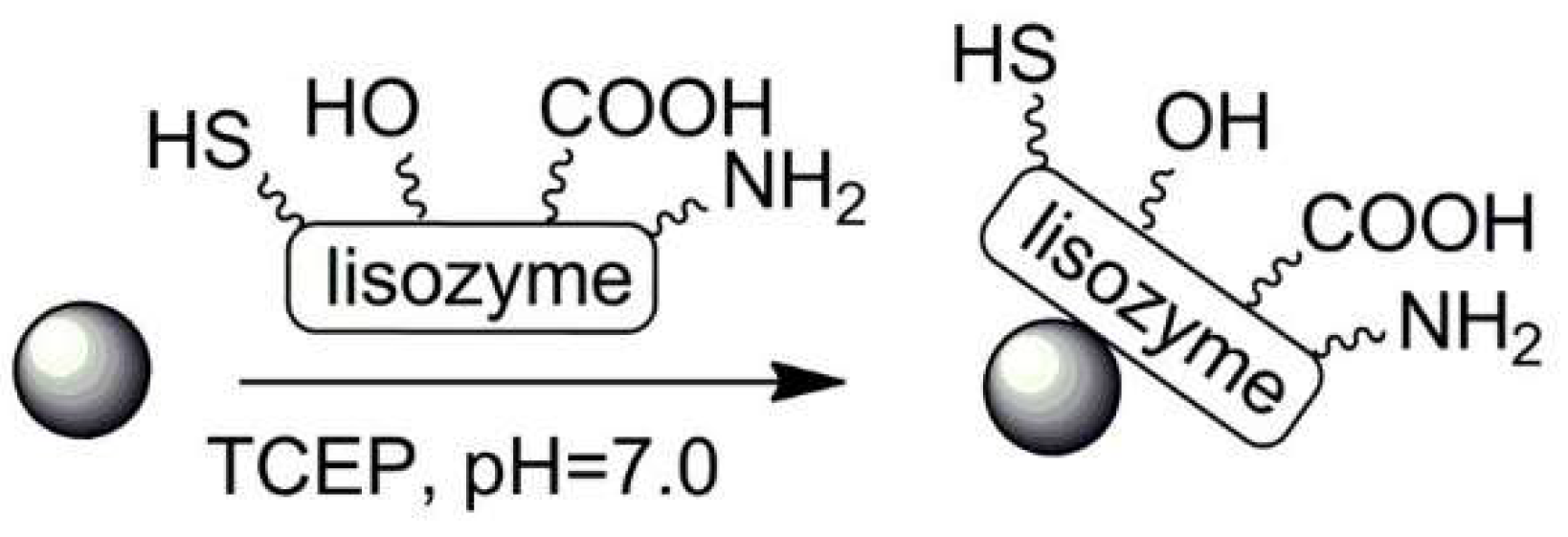
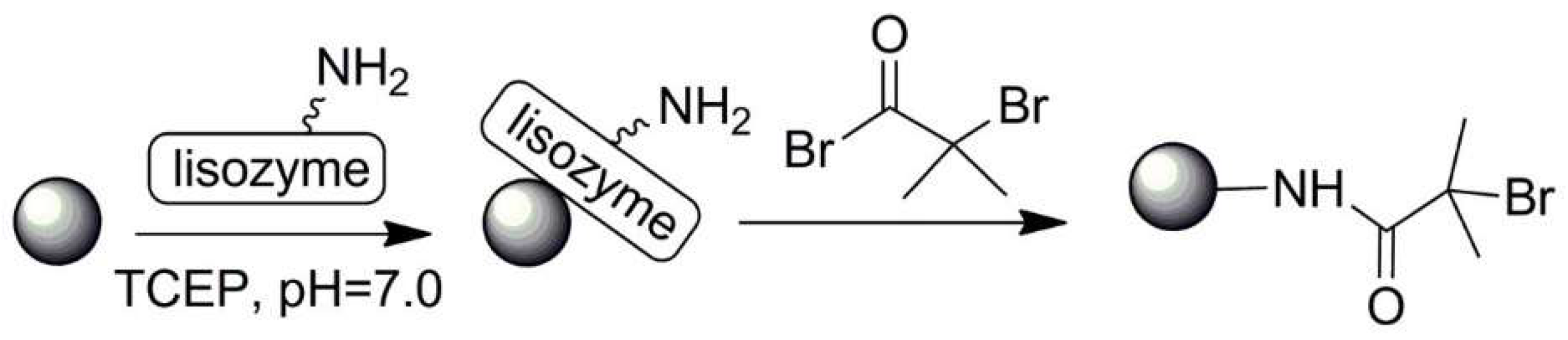
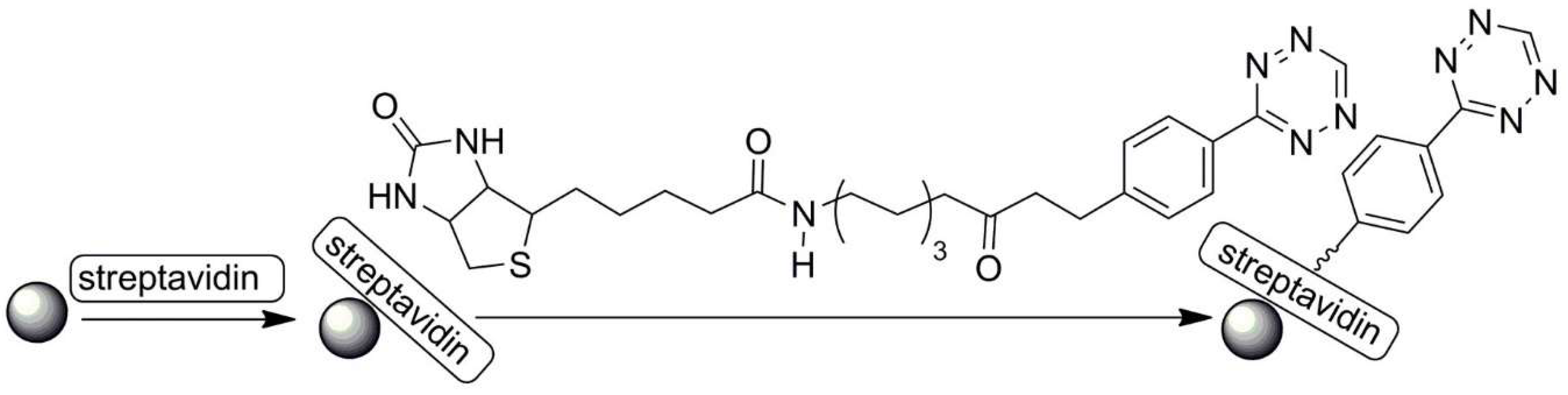
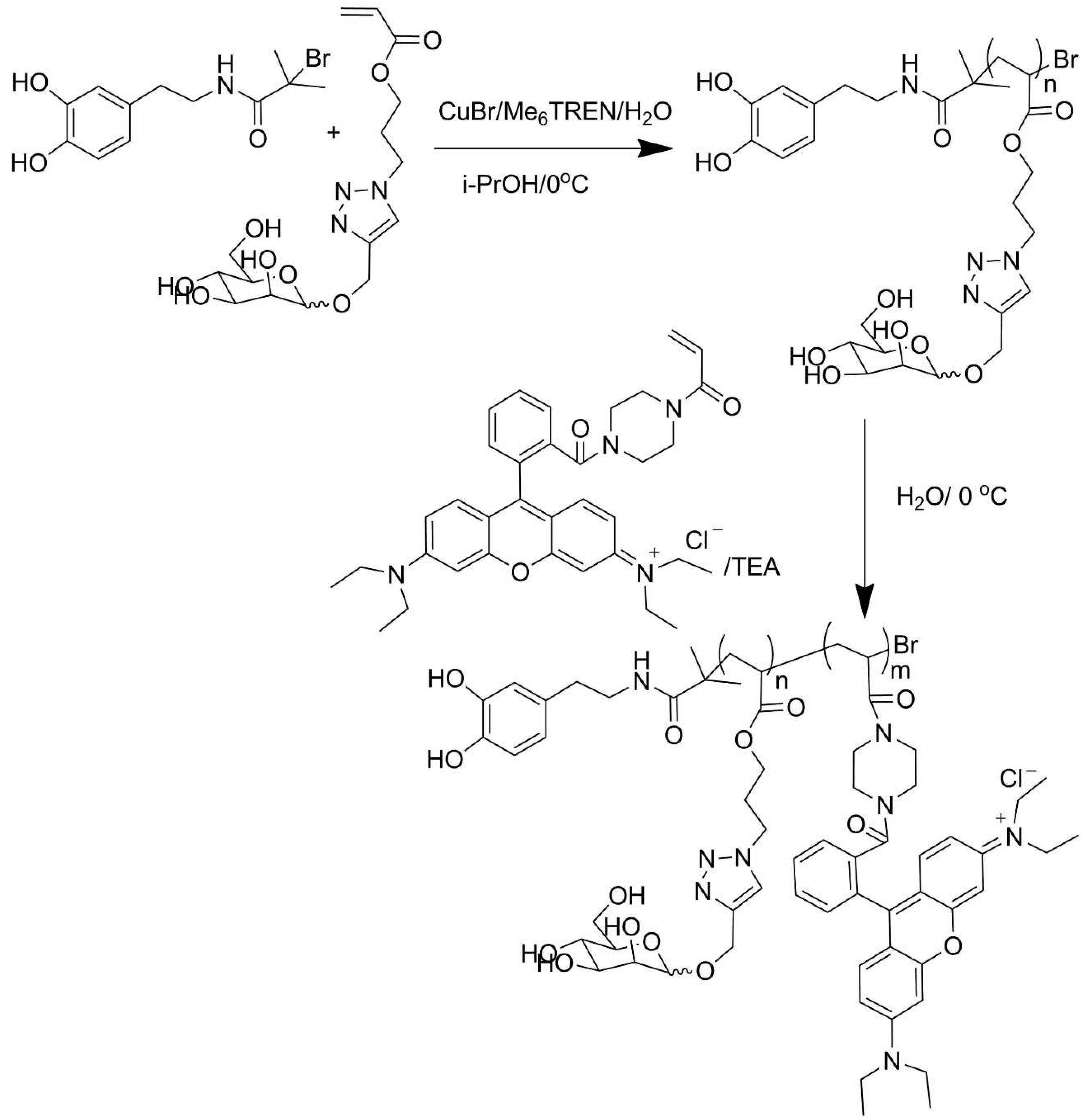
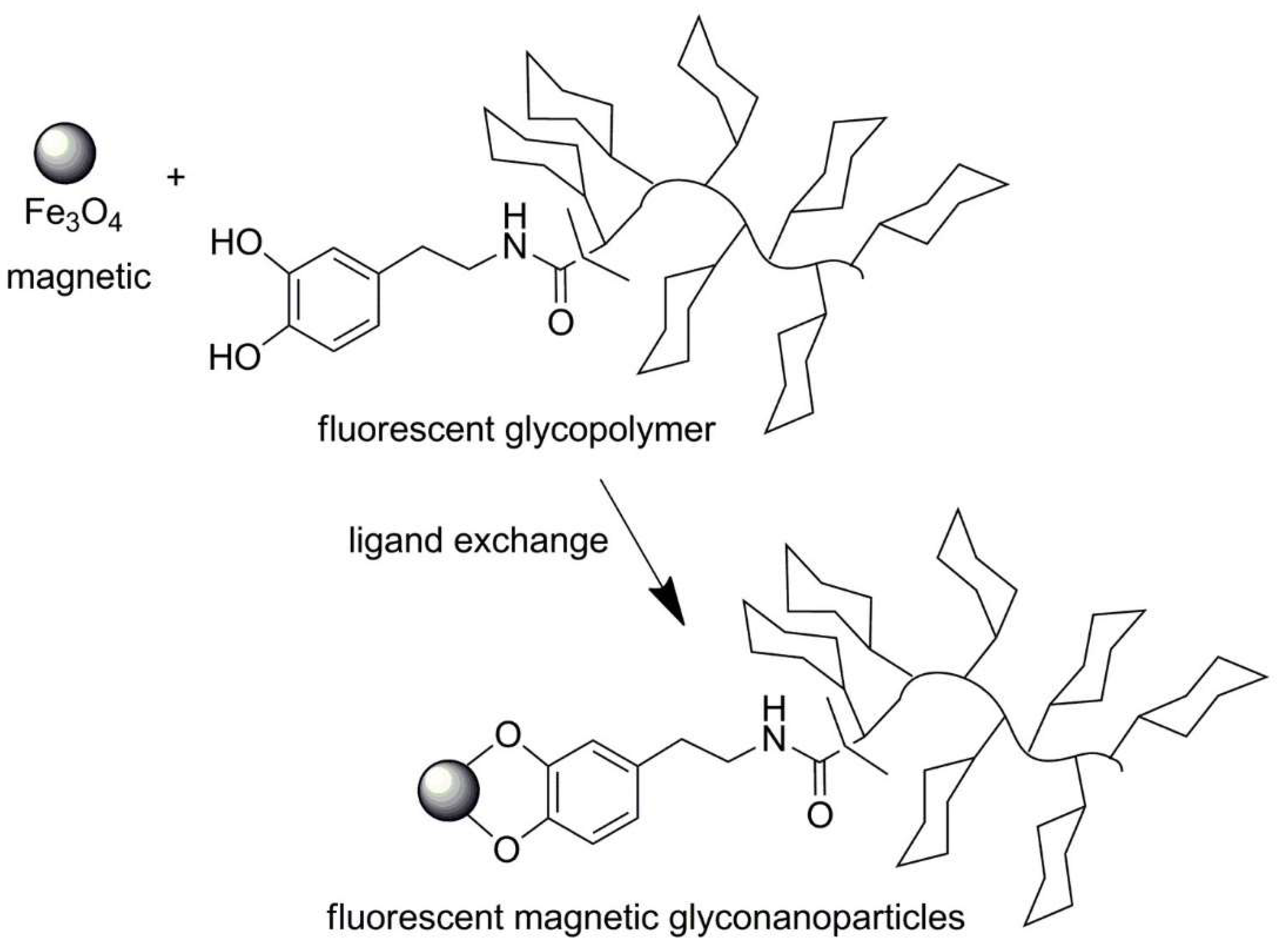
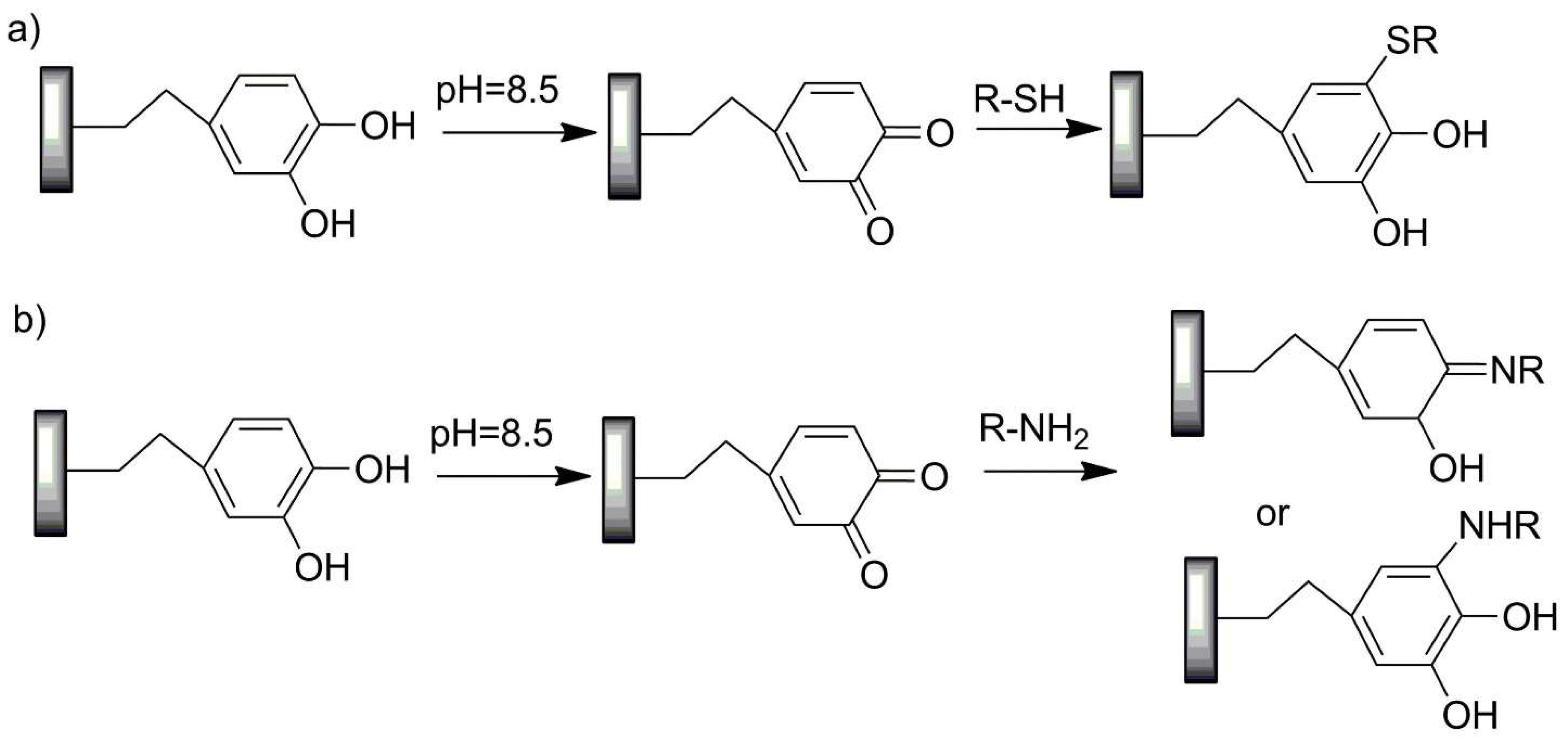
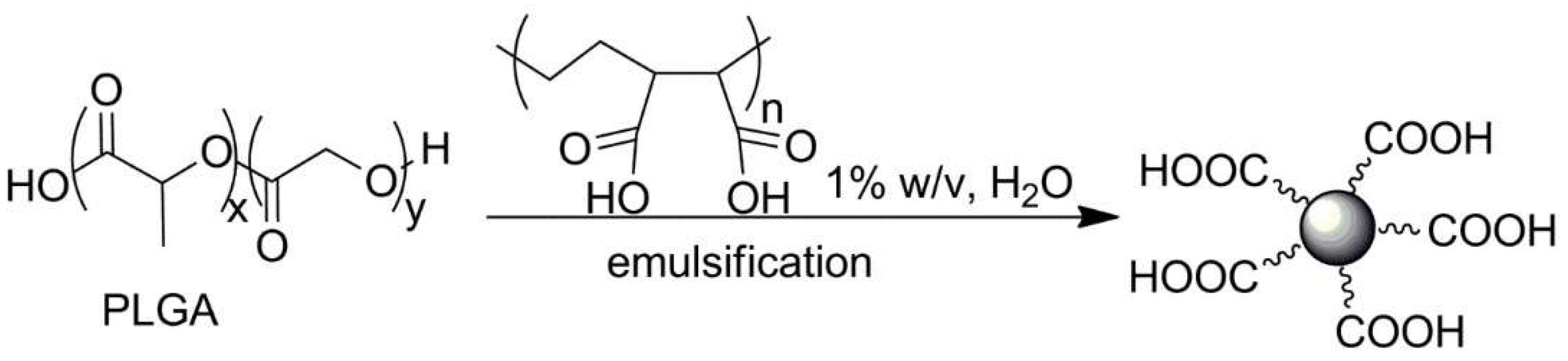
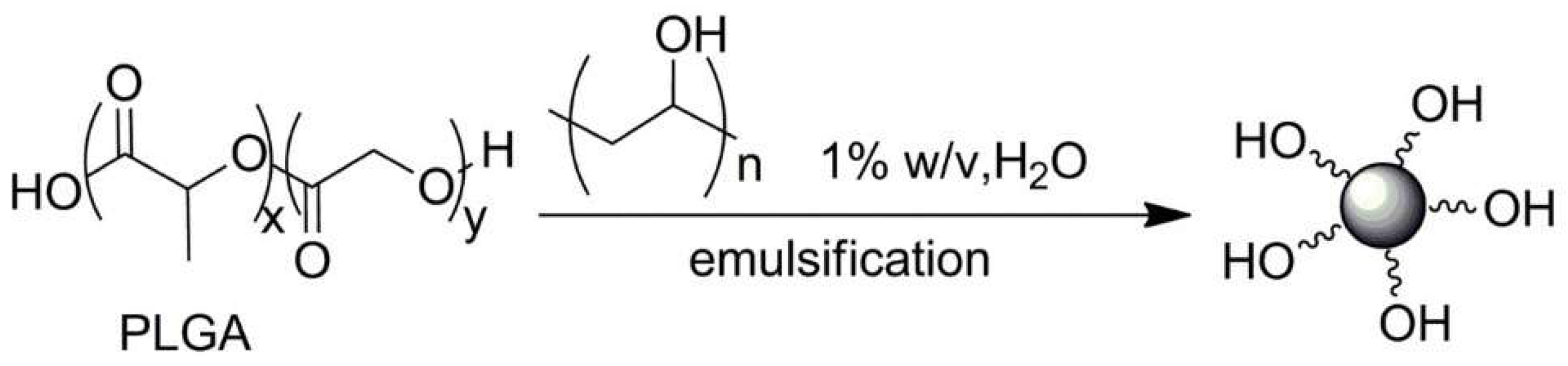
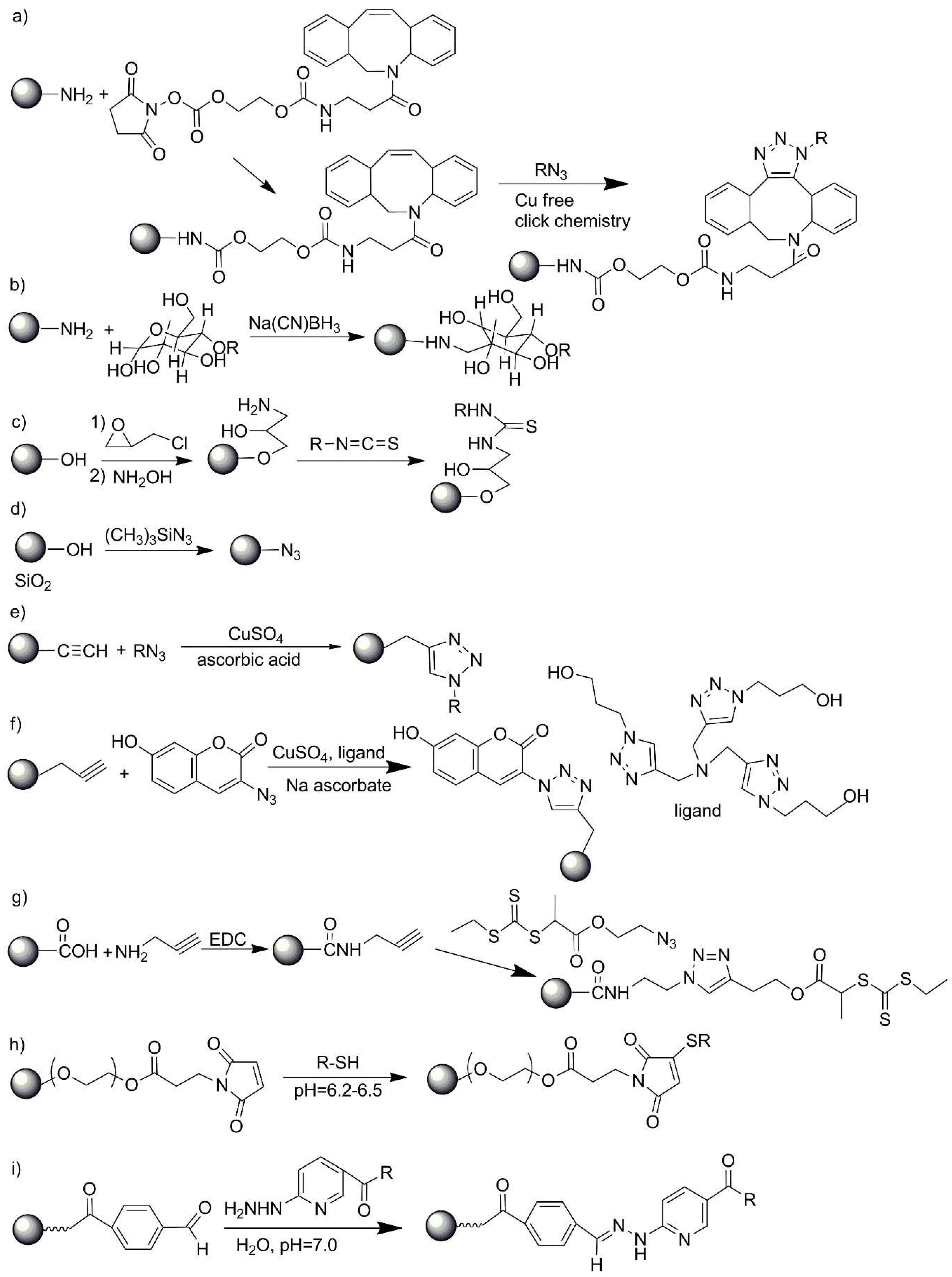
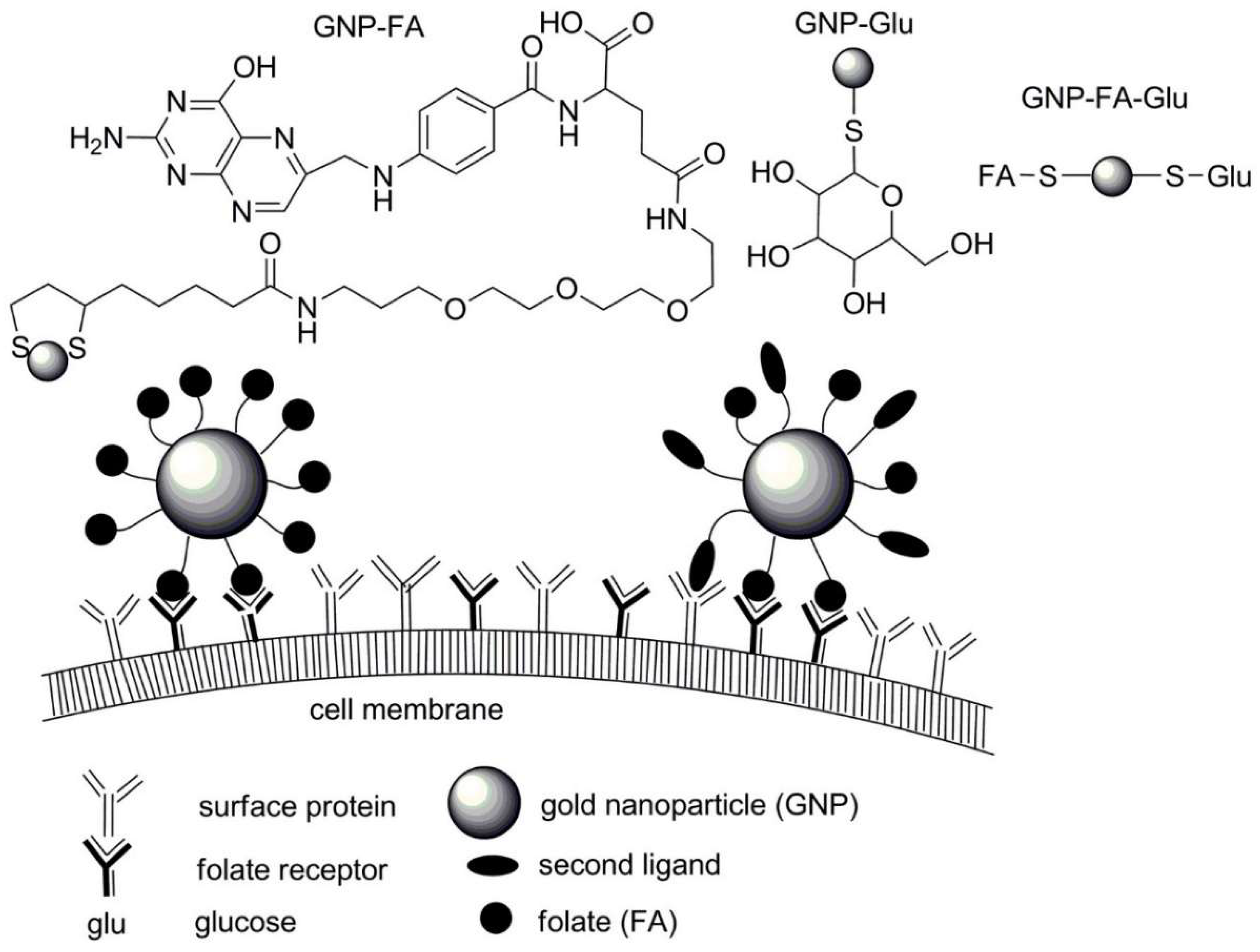
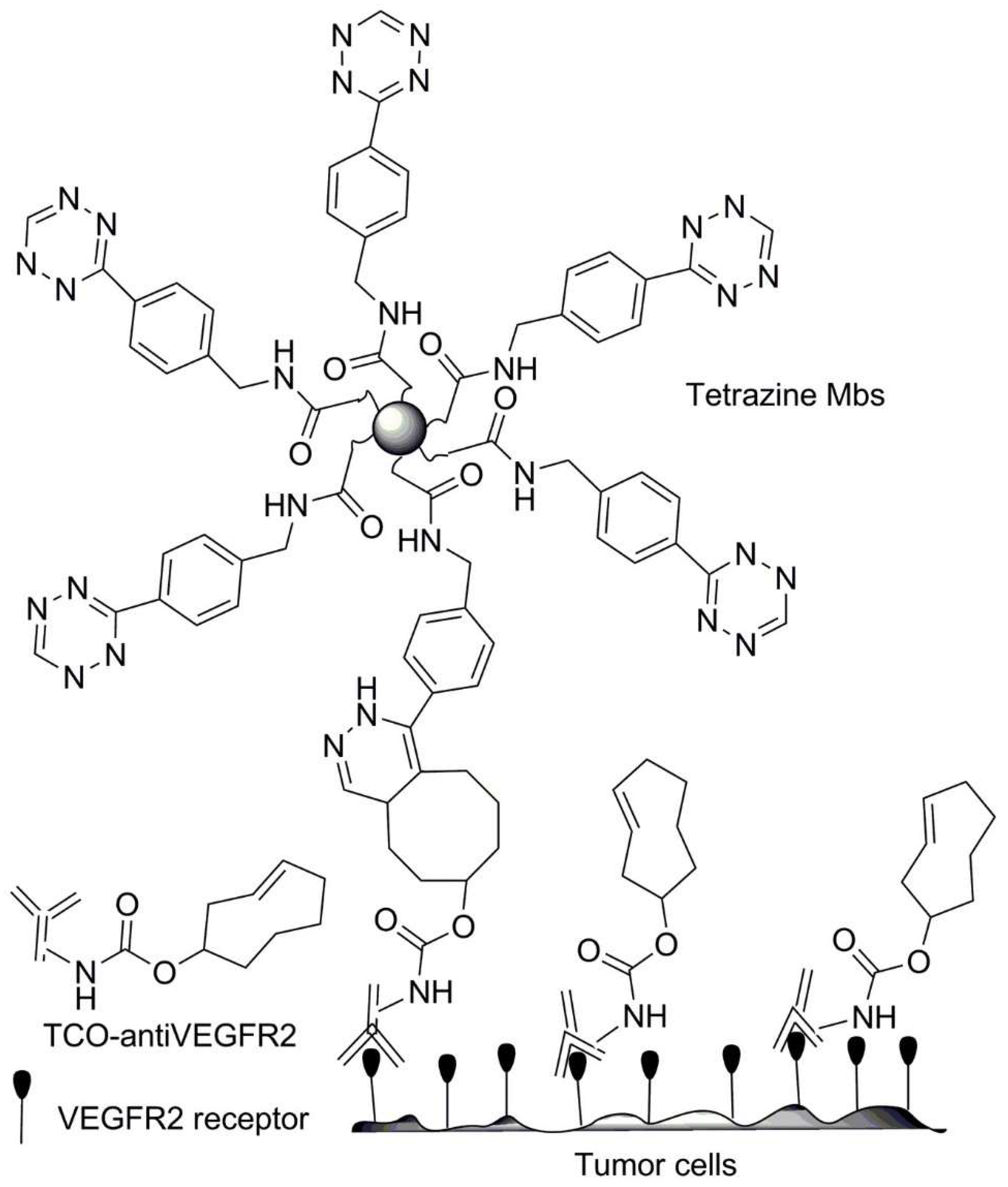
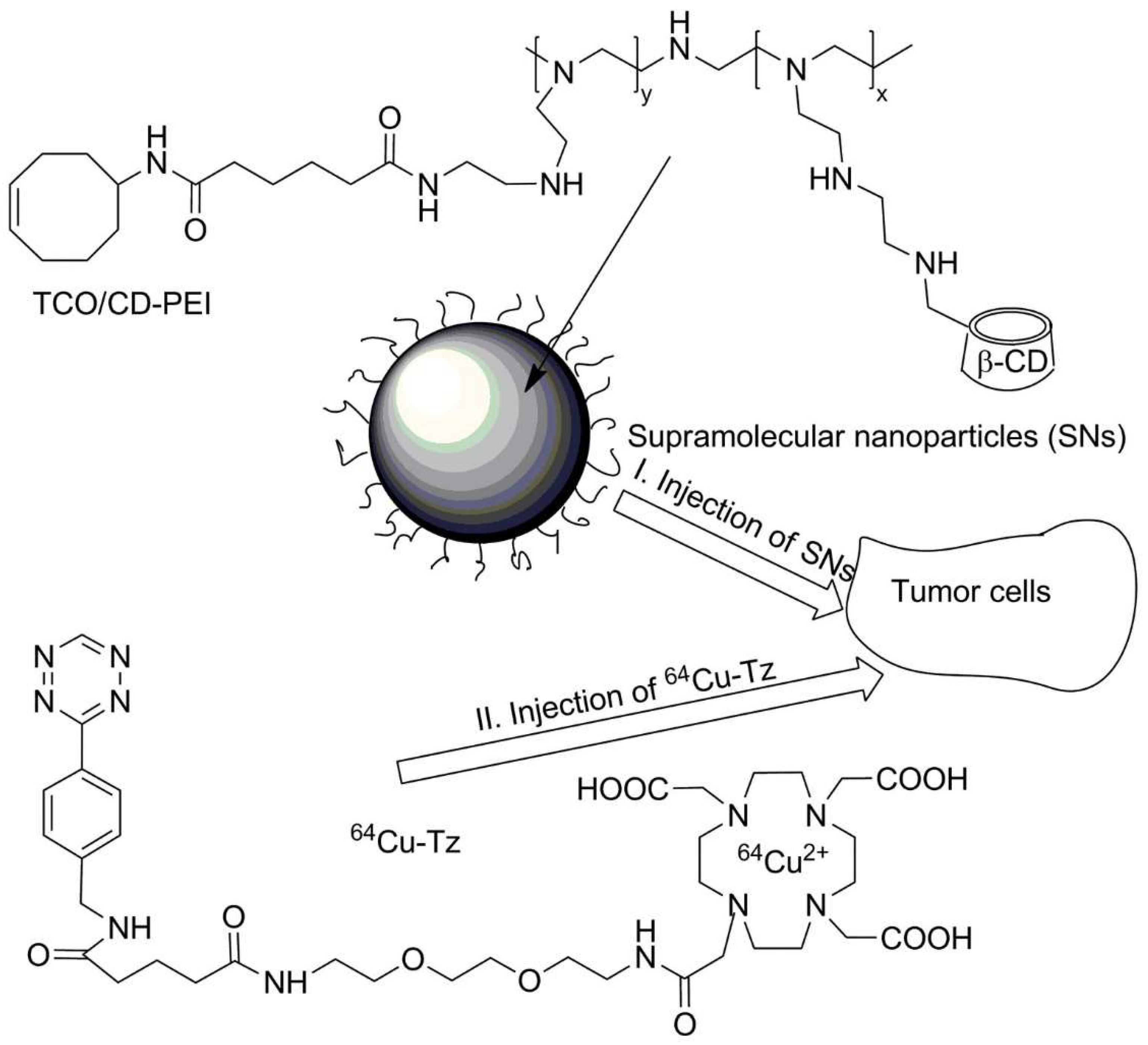
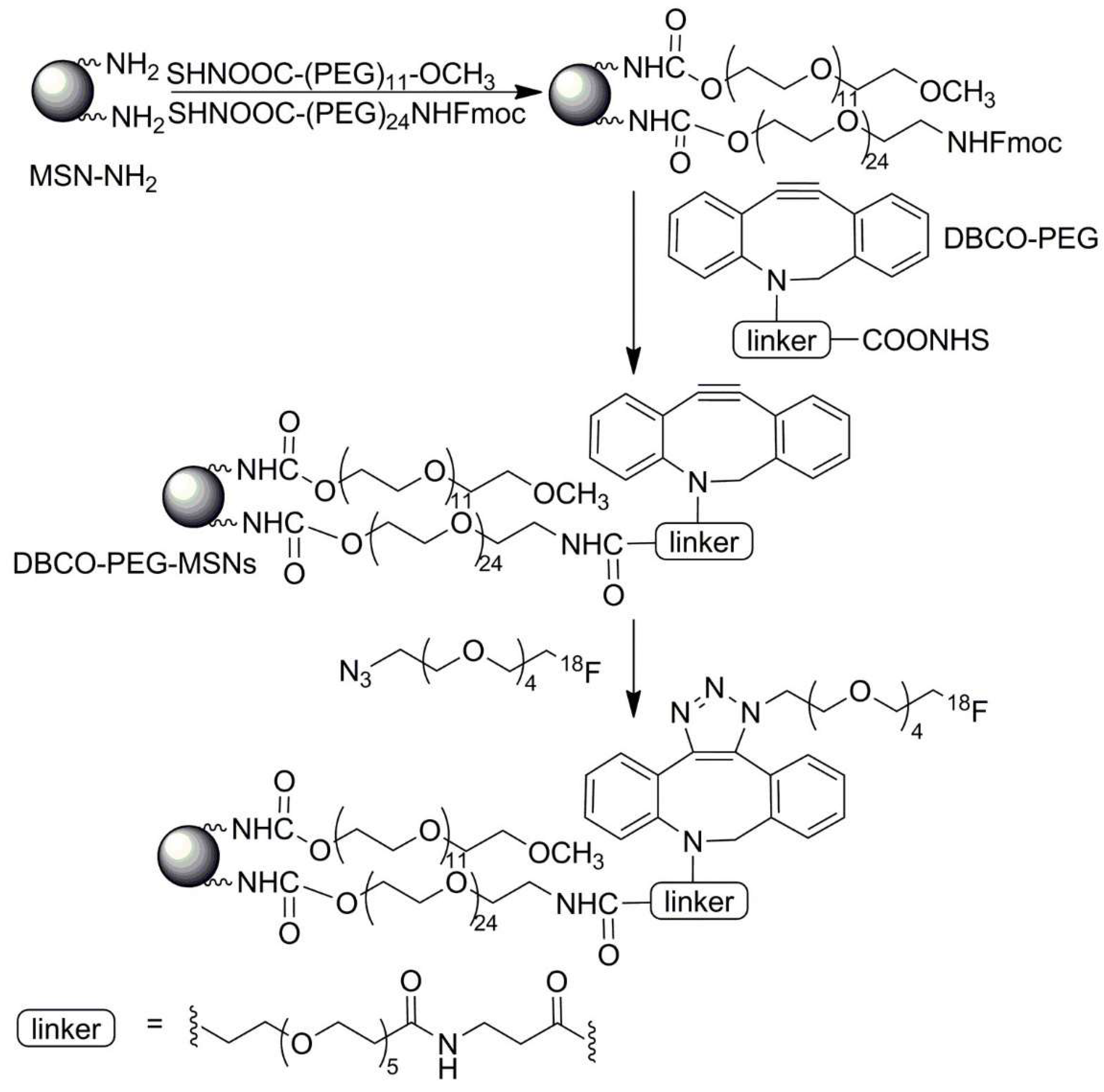
| No. | Chemical Composition | Functional Chemical Group(s) | Reference |
|---|---|---|---|
| Copolymers obtained exclusively via synthetic routes | |||
| 1 | Poly(oligoethylene glycol) methyl ether methacrylate-co-poly(propyl methacrylate) | –OH, –COOH | [167] |
| 2 | Poly(diisopropylaminoethyl methacrylate)- poly(ethylene glycol)-poly(methacrylphosphoryl choline) | –OH, –PO42−, –N(CH3)3 | [168] |
| 3 | Poly(d,l-lactides) and copolymers with PEO or poly(2-methyl-2-oxazoline) | –OH, –COOH (after hydrolysis) | [169,170,171] |
| 4 | Poly(ethylene glycol) methyl ether-Dlabile-poly(β-amino ester)-Dlabile-poly(ethylene glycol) methyl ether | without reactive functions | [172] |
| 5 | Poly(anhydride-co-imides): poly(trimellitic anhydride-glycine/sebacic acid); poly(sebacic anhydride); poly(sebacic anhydride) and poly(1,6-bis-p-carboxyphenoxy)hexane | without reactive functions | [39,40,173] |
| 6 | Polyglycerol-co-polycaprolactone | –OH | [174] |
| 7 | Poly(tetraethylene glycolyl poly(trimethylene carbonate) grafted poly(2-nitrobenzyl methacrylate) linked by disulfide bond)-co-(5-methyl-5-propargyloxycarbonyl-1,3-dioxan-2-one); poly(ethylene glycol)-b-poly(5-methyl-5-propargyl-1,3-dioxan-2-one) | 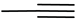 | [73,175] |
| 8 | Poly(methyl-benzyloxycarbonyl) carbonate; Poly(ethylene glycol)-b-polycarbonate with benzyloxycarbonyl group; poly(ethylene glycol)-poly(2-methyl-2-benzyloxycarbonyl-propylene carbonate) | 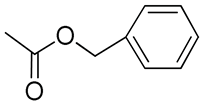 | [52,70,80,81] |
| 9 | Poly(ethylene glycol)-poly(2-methyl-2-benzyloxy-methylene carbonate); | 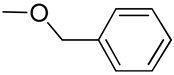 | [79] |
| 10 | Poly(ethylene glycol)-poly(2-methyl-2-carbonyl-oxy-methylene alkyne carbonate); | 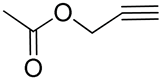 | [73,78] |
| 11 | Poly(trimetylene carbonate) with 4,5-dimethoxy-2-nitrobenzyl group | 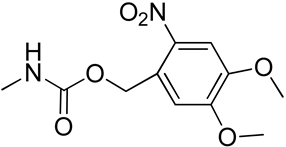 | [76] |
| 12 | Poly(ethylene glycol)-b-polycarbonate with catechol bearing moiety | 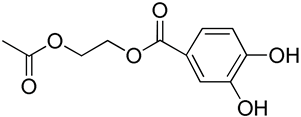 | [75] |
| 13 | Poly(ethylene glycol)-b-polycarbonate with benzyloxy-p-chloromethyl group in each repeating unit | 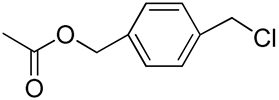 | [74] |
| 14 | Poly(3,4-dihydroxybutyric acid carbonate) | –COOH | [176] |
| 15 | Poly(ethylene glycol)-b-poly(5-allyloxycarbonyl-trimethylene carbonate) | 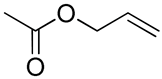 | [72,177] |
| 16 | Poly(ethylene glycol)-b-poly(4-(hydroxymethyl) phenylboronic acid pinacol ester carbonate) | 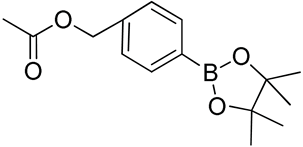 | [178] |
| 17 | Poly(trimethylene carbonate) triol functionalized vinyl sulfone | 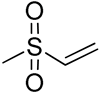 | [71] |
| 18 | Poly(ethylene glycol)-b-poly(trimetylene-3-hydroxypropoxybenzaldehyde) | 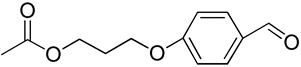 | [179] |
| 19 | Poly(ethylene glycol)-b-polycarbonate functionalized urea | 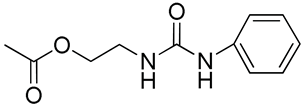 | [66] |
| 20 | Poly(ethylene glycol)-b-2-(2,4- dinitrophenylthio)ethyl-2-oxo-1,3-dioxane-5-carbonate | 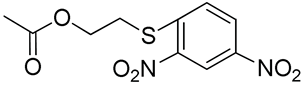 | [64] |
| 21 | Poly(ethylene glycol)-b-cholesteryl 2-(2-oxo-5-carboxyloyloxy)ethyl polycarbonate | 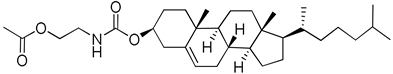 | [63] |
| 22 | Polycarbonate ester-co-poly(ε-caprolactone-co-9-phenyl-2,4,8,10- tetraoxaspiro-[5,5]undecane-3-one) containing hydroxyl groups | 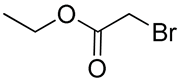 –OH | [61] |
| 23 | Polycarbonate bearing carbohydrate function | 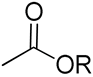 R-diacetonide sugar R-diacetonide sugar | [60] |
| 24 | Poly-α,β-(N-(2-hydroxyethyl)-l-aspartamide)-g-poly(1,3-trimethylene carbonate) | –(CH2)2OH | [180] |
| Natural polymers and copolymers (and/or natural polymers conjugated with synthetic polymers) | |||
| 25 | Proteins and proteins linked with oligosaccharides | –COOH, –NH2, –OH | [181,182,183,184,185,186,187,188,189,190,191,192] |
| 26 | Functionalized chitosan-substitution of amine group of chitosan’ monomer unit in oligosaccharide chain in position R1: leucine conjugated chitosan; (5β-cholanic acid) glycol chitosan; octanoyl functionalized chitosan; thioglycolic acid conjugated chitosan; urocanic acid functionalized amine group of chitosan; position R1—salbutamol group; position R2—guanidine group | 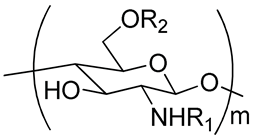 (a) 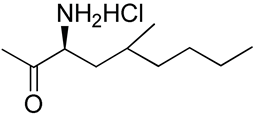 R2 = H (b) 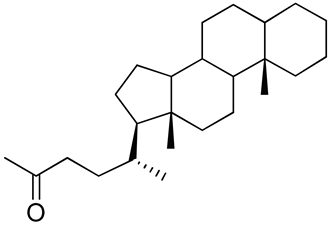 R2 = CH2CH2OH (c) 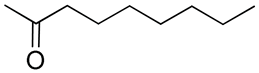 R2 = H or CO(CH2)6CH3 (d) 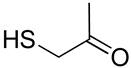 R2 = CH2CH2OH (e) 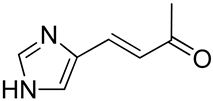 R2 = H (f) 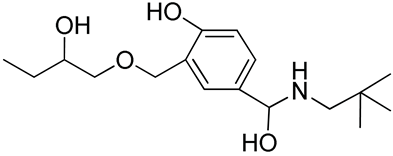 R2 = R2 = 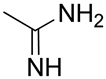 | [193,194,195,196,197,198,199] |
| 27 | Apolipoproteins e.g., 1,2-dimyristoyl-sn-glycero-3-phosphocholine | –NH2, –OH, –PO42−, –N(CH3)3 | [200] |
| 28 | Nucleic acids with synthetic polymers | –OH, –NH2, –PO42− | [201] |
| 29 | Oligosaccharides: dextran, cyclodextrins | –OH | [202,203,204,205] |
| 30 | Synthetic polymers copolymerized with oligosaccharides: Simple sugars conjugated with PEO-PPO; Pullulan-b-poly(N-vinylpyrrolidone); Alginate-g-poly(oligoethylene glycol methacrylate); PDMAEMA-βCDs; Poly(ethylene glycol)-bpoly(glycidyl methacrylate) with βCD tags; Folic acid-poly(6-O-methacryloyl-d-galactopyranose)-b-poly(2-diisopropylamino)ethyl methacrylate-co-pyridyl disulfide methylacrylate; | (a) –OH (b) –OH (c) –OH, –COO- (d) –OH, –N(CH3)3+Cl− (e) –ethylene oxide, –OH (f) –OH, –NH2, –COOH | [206,207,208,209,210,211] |
| 31 | Dihydrolipoic acid-poly(ethylene glycol) shell QDs ended 4-formyl benzoyl group | –CHO | [212] |
| Type (Material) of Particle | Attached Ligand | Target Cells, Tissue, Tumor, Factor in the Body, Disease, etc. | Reference |
|---|---|---|---|
| PEGylated silica mesoporous nanoparticles with Dibenzocyclooctyne (DBCO) | [(18F)]fluoro pentaethylene glycolic azide | Solid tumor | [238] |
| Supramolecular nanoparticles composed of poly(ethylene imine) | Trans-cyclooctene (TCO) | Solid tumor | [237] |
| Liposomes | Muromonab-CD3 (monoclonal antibody) | Autoimmune disorder | [239] |
| Fab fragment of antibody | [240,241] | ||
| Poly(glycidol methacrylate) particles loaded with Docetaxel | Transferrin | Membrane bound transferrin receptors on prostate cancer | [242] |
| Liposomes | Internalizing RGD (arginine-glycine-aspartate) motif | α√β3 integrin receptor on angiogenic endothelial cells | [241] |
| Gold nanoparticles with carboxyl ended linker | Anti-17β-estradiol IgG antibodies | 17β-estradiol | [243] |
| Gold nanoparticles with dual functionalities | Glucose and folic acid | Folate receptor/epidermal growth factor receptor on cancer cells | [236] |
| Functionalized microbubbles | Tetrazine | Endothelial growth factor intravascular VEGFR2 receptors and introduced bound antibodies (TCO-anti-VEGFR2) | [220] |
| Magnetic supraparticles core and poly-(methylacrylic acid-co-N,N-bis(acryloyl) cystamine) shell nanoparticles with streptavidin | Biotin labeled multiple targeting ligands | Folate and integrin receptors of HeLa cells | [244] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basinska, T.; Gadzinowski, M.; Mickiewicz, D.; Slomkowski, S. Functionalized Particles Designed for Targeted Delivery. Polymers 2021, 13, 2022. https://doi.org/10.3390/polym13122022
Basinska T, Gadzinowski M, Mickiewicz D, Slomkowski S. Functionalized Particles Designed for Targeted Delivery. Polymers. 2021; 13(12):2022. https://doi.org/10.3390/polym13122022
Chicago/Turabian StyleBasinska, Teresa, Mariusz Gadzinowski, Damian Mickiewicz, and Stanislaw Slomkowski. 2021. "Functionalized Particles Designed for Targeted Delivery" Polymers 13, no. 12: 2022. https://doi.org/10.3390/polym13122022
APA StyleBasinska, T., Gadzinowski, M., Mickiewicz, D., & Slomkowski, S. (2021). Functionalized Particles Designed for Targeted Delivery. Polymers, 13(12), 2022. https://doi.org/10.3390/polym13122022