The Effect of Stereocomplex Polylactide Particles on the Stereocomplexation of High Molecular Weight Polylactide Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PLLA and PDLA Synthesis
2.3. Stereocomplex Formation of PLA
2.4. Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garlotta, D. A literature review of poly(lactic acid). Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Ikada, Y.; Tsuji, H. Biodegradable polyesters for medical and ecological applications. Macromol. Rapid Commun. 2000, 21, 117–132. [Google Scholar] [CrossRef]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.H. Stereocomplex formation between enantiomeric poly(lactides). Macromolecules 1987, 20, 904–906. [Google Scholar] [CrossRef]
- De Jong, S.J.; van Dijk-Wolthuis, W.N.E.; Kettenes-van den Bosch, J.J.; Schuyl, P.J.W.; Hennink, W.E. Monodisperese enantiomeric lactic acid oligomers: Preparation, characterization, and stereocomplex formation. Macromolecules 1998, 31, 6397–6402. [Google Scholar] [CrossRef]
- Yamane, H.; Sasai, K. Effect of the addition of poly(D-lactic acid) on thermal property of poly(L-lactic acid). Polymer 2003, 44, 2569–2575. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acid)s. 6. Binary blends from copolymers. Macromolecules 1992, 25, 5719–5723. [Google Scholar] [CrossRef]
- Fukushima, K.; Kimura, Y. An efficient solid-state polycondensation method for synthesizing stereocomplexed poly(lactic acid)s with high molecular weight. J. Polym. Sci. A Polym. Chem. 2008, 46, 3714–3722. [Google Scholar] [CrossRef]
- Purnama, P.; Kim, S.H. Stereocomplex formation of high-molecular-weight polylactide using supercritical fluid. Macromolecules 2010, 43, 1137–1142. [Google Scholar] [CrossRef]
- Purnama, P.; Kim, S.H. Rapid stereocomplex formation of polylactide using supercritical fluid technology. Polym. Int. 2021, 61, 939–942. [Google Scholar] [CrossRef]
- Purnama, P.; Kim, S.H. Stereocomplex formation of polylactide using microwave irradiation. Polym. Int. 2013, 63, 741–745. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acids). 9. Stereocomplexation from the melt. Macromolecules 1993, 26, 6918–6926. [Google Scholar] [CrossRef]
- Tsuji, H.; Hyon, S.H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acid)s. 3. Calorimetric studies on blend films cast from dilute solution. Macromolecules 1991, 24, 5651–5656. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acid)s. XI. Mechanical properties and morphology of solution-cast films. Polymer 1999, 40, 6699–6708. [Google Scholar] [CrossRef]
- Fukushima, K.; Kimura, Y. A novel synthetic approach to stereo-block poly(lactic acid). Macromol. Symp. 2005, 224, 133–143. [Google Scholar] [CrossRef]
- Anderson, K.S.; Hillmyer, M.A. Melt preparation and nucleation efficiency of polylactide stereocomplex crystallites. Polymer 2006, 47, 2030–2035. [Google Scholar] [CrossRef]
- Tsuji, H. Poly(lactide) stereocomplexes: Formation, structure, properties, degradation, and applications. Macromol. Biosci. 2005, 5, 569–597. [Google Scholar] [CrossRef]
- Tsuji, H.; Horii, F.; Nakagawa, M.; Ikada, Y.; Odani, H.; Kitamaru, R. Stereocomplex formation between enantiomeric poly(lactic acid)s. 7. Phase structure of the stereocomplex crystallized from a dilute acetonitrile solution as studied by high-resolution solid-state carbon-13 NMR spectroscopy. Macromolecules 1992, 25, 4114–4118. [Google Scholar] [CrossRef]
- Tsuji, H.; Mizuno, A.; Ikada, Y. Enhanced crystallization of poly(L-lactide-co-ε-caprolactone) during storage at room temperature. J. Appl. Polym. Sci. 2000, 76, 947–953. [Google Scholar] [CrossRef]
- Ji, N.; Hu, G.; Li, J.; Ren, J. Influence of poly(lactide) stereocomplexes as nucleating agents on the crystallization behavior of poly(lactide)s. RSC Adv. 2019, 9, 6221–6227. [Google Scholar] [CrossRef] [Green Version]
- Purnama, P.; Samsusi, M.; Iswaldi, I. Properties Enhancement of High Molecular Weight Polylactide Using Stereocomplex Polylactide as a Nucleating Agent. Polymers 2021, 13, 1725. [Google Scholar] [CrossRef]
- Kassos, N.; Kelly, K.L.; Gough, T.; Gill, A.A. Acceleration of crystallization rate in injection moulded PLLA by stereocomplex formation. Mater. Res. Express 2020, 7, 105308. [Google Scholar] [CrossRef]
- Park, H.-S.; Hong, C.-K. Relationship between the stereocomplex crystallization behavior and mechanical properties of PLLA/PDLA blends. Polymers 2021, 13, 1851. [Google Scholar] [CrossRef]
- Su, X.; Feng, L.; Yu, D. Formation of stereocomplex crystal and its effect on the morphology and property of PDLA/PLLA blends. Polymers 2020, 12, 2515. [Google Scholar] [CrossRef] [PubMed]
- Nijenhuis, A.J.; Grijpma, D.W.; Pennings, A.J. Lewis acid catalyzed polymerization of L-lactide. Kinetics and mechanism of the bulk polymerization. Macromolecules 1992, 25, 6419–6424. [Google Scholar] [CrossRef]
- Purnama, P.; Jung, Y.; Hong, C.H.; Han, D.S.; Kim, S.H. Synthesis of poly(D-lactide) with different molecular weight via melt-polymerization. Macromol. Res. 2012, 20, 515–519. [Google Scholar] [CrossRef]
- Pack, J.W.; Kim, S.H.; Park, S.Y.; Lee, Y.W.; Kim, Y.H. Ring-opening polymerization of L-lactide and preparation of its microsphere in supercritical fluids. Macromol. Biosci. 2004, 4, 340–345. [Google Scholar] [CrossRef]
- Chamberlain, B.M.; Jazdzewski, B.A.; Pink, M.; Hillmyer, M.A.; Tolman, W.B. Controlled polymerization of DL-lactide and ε-Caprolactone by structurally well-defined alkoxo-bridged di- and triyttrium(III) complexes. Macromolecules 2000, 33, 3970–3977. [Google Scholar] [CrossRef]
- Gregg, C.J.; Stein, F.P.; Radosz, M. Phase Behavior of telechelic polyisobutylene in subcritical and supercritical fluids. 4. SAFT association parameters from FTIR for blank, monohydroxy, and dihydroxy PIB 200 in ethane, carbon dioxide, and chlorodifluoromethane. J. Phys. Chem. B 1999, 103, 1167–1175. [Google Scholar] [CrossRef]
- Sarasua, J.R.; Arraiza, A.L.; Balerdi, P.; Maiza, I. Crystallization and thermal behavior of optically pure polylactides and their blends. J. Mater. Sci. 2005, 40, 1855–1862. [Google Scholar] [CrossRef]
- Loomis, G.L.; Murdoch, J.R.; Gardner, K.H. Polylactide stereocomplexes. Polymer. Prepr. 1990, 31, 55. [Google Scholar]
- Fukushima, K.; Kimura, Y. Stereocomplexed polylactides (Neo-PLA) as high-performance bio-based polymers: Their formation, properties, and application. Polym. Int. 2006, 55, 626–642. [Google Scholar] [CrossRef]
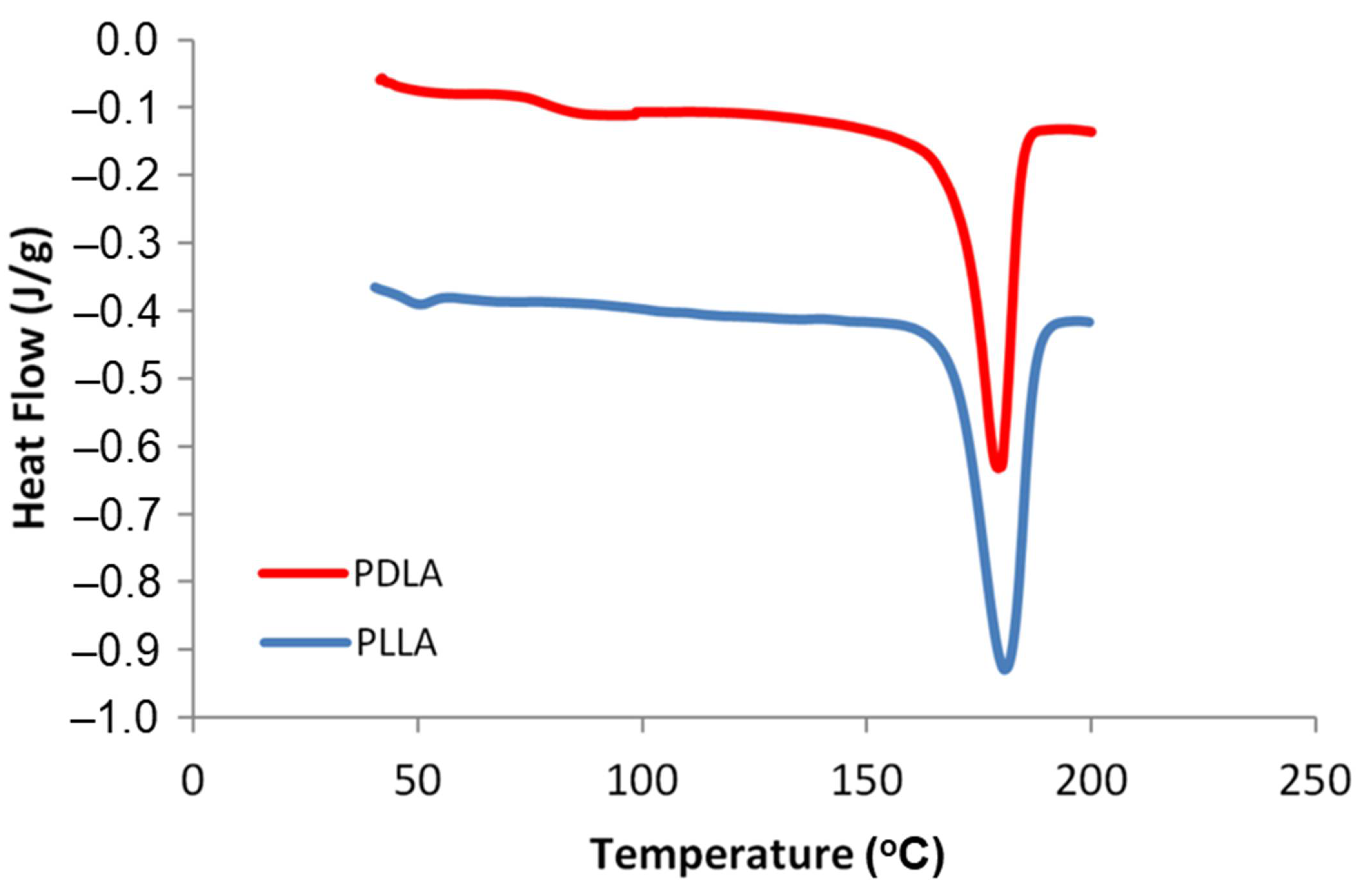


| Materials | Yield (%) | Mn | Mw | MWD |
|---|---|---|---|---|
| PLLA | 95.16 ± 3.84 | 195,322 | 395,141 | 2.02 |
| PDLA | 94.40 ± 4.82 | 170,035 | 459,042 | 2.70 |
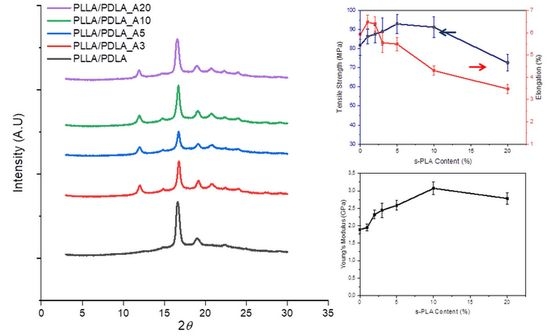
| Materials | Tm1 (°C) | ∆Hm1 (J/g) | Tm2 (°C) | ∆Hm2 (J/g) | ∆Hm (J/g) | ∆Hm0 (J/g) | X (%) | s-PLA Degree (%) |
|---|---|---|---|---|---|---|---|---|
| PLLA/PDLA | 177.85 | 39.85 | - | - | 39.89 | 106.00 | 37.59 | 0 |
| PLLA/PDLA_A1 | 177.48 | 32.87 | 226.73 | 15.05 | 47.92 | 117.31 | 40.85 | 31.41 |
| PLLA/PDLA_A3 | 179.87 | 27.86 | 226.39 | 22.63 | 50.49 | 122.14 | 41.34 | 44.83 |
| PLLA/PDLA_A5 | 180,26 | 27.80 | 219.28 | 26.85 | 54.66 | 123.69 | 44.34 | 49.13 |
| PLLA/PDLA_A10 | 179.47 | 31.32 | 221.38 | 23.39 | 54.71 | 121.39 | 45.07 | 42.75 |
| PLLA/PDLA_A20 | 176.98 | 26.92 | 224.47 | 13.12 | 40.04 | 117.80 | 34.00 | 32.77 |
| PLLA/PDLA_B10 | 178.50 | 28.08 | 221.99 | 23.06 | 51.14 | 122.23 | 41.84 | 45.09 |
| PLLA/PDLA_C10 | 179.67 | 26.88 | 223.43 | 25.30 | 52.19 | 123.46 | 42.27 | 48.49 |
| Materials | Elongation at Break (%) | Tensile Strength (MPa) | Young’s Modulus (GPa) |
|---|---|---|---|
| PLLA/PDLA | 5.90 ± 0.23 | 81.85 ± 5.32 | 1.89 ± 0.11 |
| PLLA/PDLA_A10 | 4.30 ± 0.20 | 91.27 ± 5.48 | 3.07 ± 0.18 |
| PLLA/PDLA_B10 | 4.22 ± 0.22 | 83.84 ± 3.02 | 2.76 ± 0.13 |
| PLLA/PDLA_C10 | 4.12 ± 0.23 | 64.95 ± 4.29 | 2.50 ± 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samsuri, M.; Iswaldi, I.; Purnama, P. The Effect of Stereocomplex Polylactide Particles on the Stereocomplexation of High Molecular Weight Polylactide Blends. Polymers 2021, 13, 2018. https://doi.org/10.3390/polym13122018
Samsuri M, Iswaldi I, Purnama P. The Effect of Stereocomplex Polylactide Particles on the Stereocomplexation of High Molecular Weight Polylactide Blends. Polymers. 2021; 13(12):2018. https://doi.org/10.3390/polym13122018
Chicago/Turabian StyleSamsuri, Muhammad, Ihsan Iswaldi, and Purba Purnama. 2021. "The Effect of Stereocomplex Polylactide Particles on the Stereocomplexation of High Molecular Weight Polylactide Blends" Polymers 13, no. 12: 2018. https://doi.org/10.3390/polym13122018
APA StyleSamsuri, M., Iswaldi, I., & Purnama, P. (2021). The Effect of Stereocomplex Polylactide Particles on the Stereocomplexation of High Molecular Weight Polylactide Blends. Polymers, 13(12), 2018. https://doi.org/10.3390/polym13122018