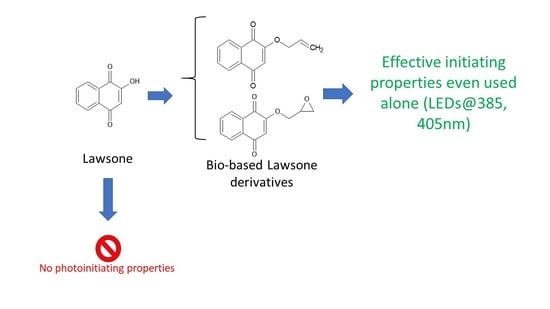
Lawsone Derivatives as Efficient Photopolymerizable Initiators for Free-Radical, Cationic Photopolymerizations, and Thiol—Ene Reactions
Abstract
:1. Introduction
2. Experimental Section
Materials
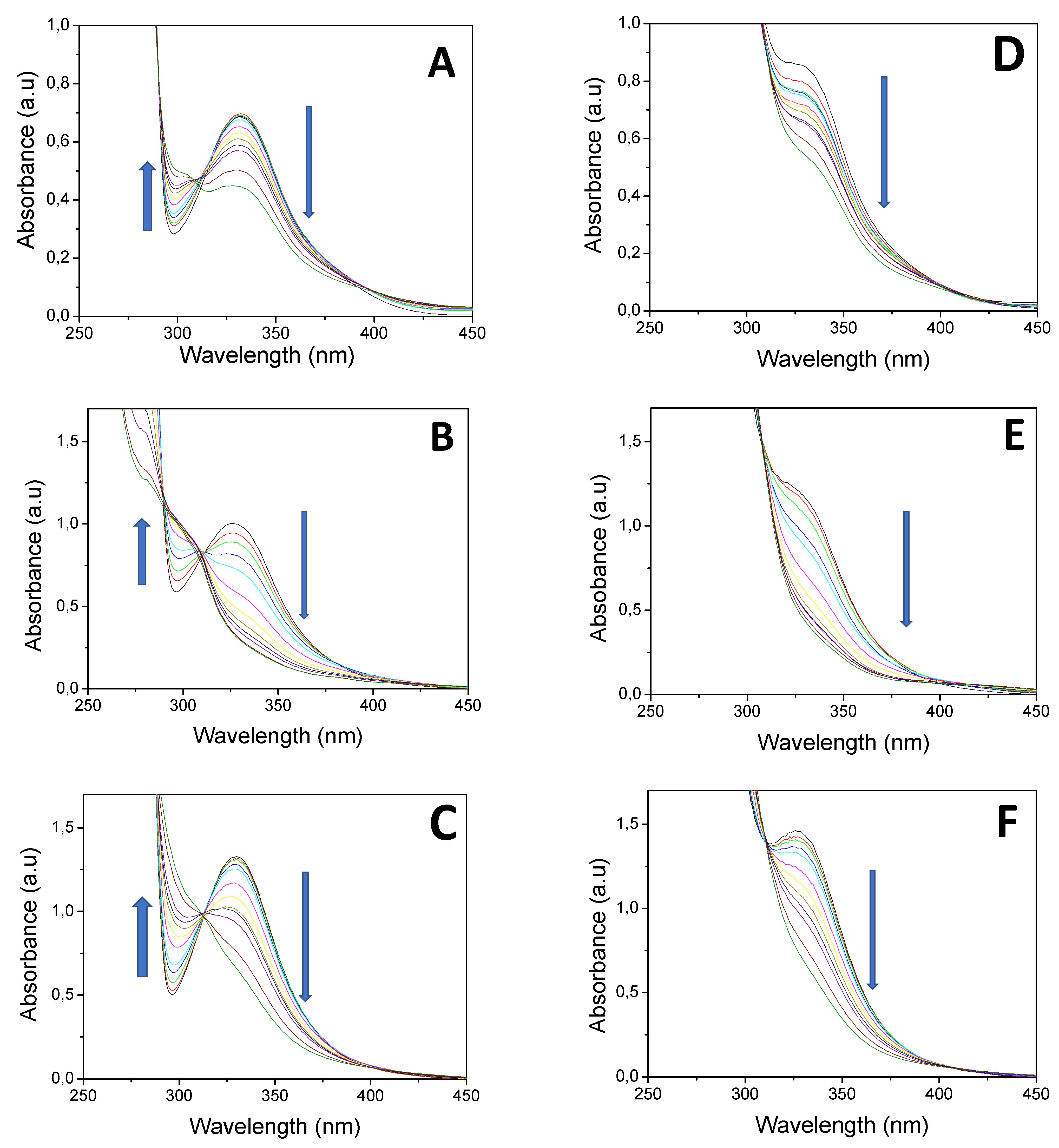
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated Polymerization: Advances, Challenges, and Opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Allonas, X.; Burget, D. Photopolymerization reactions under visible lights: Principle, mechanisms and examples of applications. Progr. Org. Coat. 2003, 47, 16–36. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, J.; Dumur, F.; Tehfe, M.A.; Morlet-Savary, F.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Visible light sensitive photoinitiating systems: Recent progress in cationic and radical photopolymerization reactions under soft conditions Progr. Polym. Sci. 2015, 41, 32–66. [Google Scholar] [CrossRef]
- Steyrer, B.; Neubauer, P.; Liska, R.; Stampfl, J. Visible Light Photoinitiator for 3D-Printing of Tough Methacrylate Resins. Materials 2017, 10, 1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fouassier, J.P.; Lalevée, J. Photopolymerisation Initiating Systems; Royal Society of Chemical: London, UK, 2018. [Google Scholar]
- Shao, J.; Huang, Y.; Fan, Q. Visible light initiating systems for photopolymerization: Status, development and challenges. Polym. Chem. 2014, 5, 4195–4210. [Google Scholar] [CrossRef]
- Noirbent, G.; Dumur, F. Recent Advances on Copper Complexes as Visible Light Photoinitiators and (Photo) Redox Initiators of Polymerization. Catalysts 2020, 10, 953. [Google Scholar] [CrossRef]
- Pigot, C.; Noirbent, G.; Brunel, D.; Dumur, F. Recent advances on push–pull organic dyes as visible light photoinitiators of polymerization. Eur. Polym. J. 2020, 133, 109797. [Google Scholar] [CrossRef]
- Kristufek, S.L.; Wacker, K.T.; Tsao, Y.-Y.T.; Su, L.; Wooley, K.L. Monomer design strategies to create natural product-based polymer materials. Nat. Prod. Rep. 2017, 34, 433–459. [Google Scholar] [CrossRef]
- Needles, H.L. Riboflavin-sensitized photopolymerizations of acrylic monomers in the presence of proteins or amino acids. J. Polym. Sci. B Polym. Lett. 1967, 5, 595–600. [Google Scholar] [CrossRef]
- Crivello, J.V.; Bulut, U. Curcumin: A naturally occurring long-wavelength photosensitizer for diaryliodonium salts. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 5217–5231. [Google Scholar] [CrossRef]
- Zhao, J.; Lalevée, J.; Lu, H.; MacQueen, R.; Kable, S.H.; Schmidt, T.W.; Stenzel, M.H.; Xiao, P. A new role of curcumin: As a multicolor photoinitiator for polymer fabrication under household UV to red LED bulbs. Polym. Chem. 2015, 6, 5053–5061. [Google Scholar] [CrossRef]
- Condat, M.; Mazeran, P.E.; Malval, J.P.; Lalevée, J.; Morlet-Savary, F.; Renard, E.; Langlois, V.; Andalloussi, S.A.; Versace, D.L. Photoinduced curcumin derivative-coatings with antibacterial properties. RSC Adv. 2015, 5, 85214–85224. [Google Scholar] [CrossRef]
- Versace, D.-L.; Moran, G.; Belqat, M.; Spangenberg, A.; Méallet-Renault, R.; Abbad-Andaloussi, S.; Brezová, V.; Malval, J.-P. Highly Virulent Bactericidal Effects of Curcumin-Based μ-Cages Fabricated by Two-Photon Polymerization. ACS Appl. Mater. Interfaces 2020, 12, 5050–5057. [Google Scholar] [CrossRef]
- Breloy, L.; Ouarabi, C.A.; Brosseau, A.; Dubot, P.; Brezova, V.; Andaloussi, S.A.; Malval, J.-P.; Versace, D.-L. β-Carotene/Limonene Derivatives/Eugenol: Green Synthesis of Antibacterial Coatings under Visible-Light Exposure. ACS Sustain. Chem. Eng. 2019, 7, 19591–19604. [Google Scholar] [CrossRef]
- Sautrot-Ba, P.; Malval, J.-P.; Weiss-Maurin, M.; Paul, J.; Blacha-Grzechnik, A.; Tomane, S.; Mazeran, P.-E.; Lalevée, J.; Langlois, V.; Versace, D.-L. Paprika, Gallic Acid, and Visible Light: The Green Combination for the Synthesis of Biocide Coatings. ACS Sustain. Chem. Eng. 2018, 6, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Sautrot-Ba, P.; Jockusch, S.; Malval, J.-P.; Brezová, V.; Rivard, M.; Abbad-Andaloussi, S.; Blacha-Grzechnik, A.; Versace, D.-L. Quinizarin Derivatives as Photoinitiators for Free-Radical and Cationic Photopolymerizations in the Visible Spectral Range. Macromolecules 2020, 53, 1129–1141. [Google Scholar] [CrossRef]
- Sautrot-Ba, P.; Brezová, V.; Malval, J.-P.; Chiappone, A.; Breloy, L.; Abbad-Andaloussi, S.; Versace, D.-L. Purpurin derivatives as visible-light photosensitizers for 3D printing and valuable biological applications. Polym. Chem. 2021, 12, 2627–2642. [Google Scholar] [CrossRef]
- Zhang, J.; Lalevée, J.; Zhao, J.; Graff, B.; Stenzel, M.H.; Xiao, P. Dihydroxyanthraquinone derivatives: Natural dyes as blue-light-sensitive versatile photoinitiators of photopolymerization. Polym. Chem. 2016, 7, 7316–7324. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Garra, P.; Dumur, F.; Graff, B.; Fouassier, J.P.; Lalevée, J. Flavones as natural photoinitiators for light mediated free-radical polymerization via light emitting diodes. J. Polym. Sci. 2020, 58, 254–262. [Google Scholar] [CrossRef]
- Condat, M.; Babinot, J.; Tomane, S.; Malval, J.-P.; Kang, I.-K.; Spillebout, F.; Mazeran, P.-E.; Lalevée, J.; Andalloussi, S.A.; Versace, D.-L. Development of photoactivable glycerol-based coatings containing quercetin for antibacterial applications. RSC Adv. 2016, 6, 18235–18245. [Google Scholar] [CrossRef]
- Breloy, L.; Negrell, C.; Mora, A.S.; Li, W.S.J.; Brezová, V.; Caillol, S.; Versace, D.L. Vanillin derivative as performing type I photoinitiator. Eur. Polym. J. 2020, 132, 109727. [Google Scholar] [CrossRef]
- Peng, X.; Zhu, D.; Xiao, P. Naphthoquinone derivatives: Naturally derived molecules as blue-light-sensitive photoinitiators of photopolymerization. Eur. Polym. J. 2020, 127, 109569. [Google Scholar] [CrossRef]
- Sritrairat, N.; Nukul, N.; Inthasame, P.; Sansuk, A.; Prasirt, J.; Leewatthanakorn, T.; Piamsawad, U.; Dejrudee, A.; Panichayupakaranant, P.; Pangsomboon, K.; et al. Antifungal activity of lawsone methyl ether in comparison with chlorhexidine. Oral Path. Med. 2011, 40, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Rahmoun, N.M.; Boucherit-Otmani, Z.; Boucherit, K.; Benabdallah, M.; Villemin, D.; Choukchou-Braham, N. Antibacterial and antifungal activity of lawsone and novel naphthoquinone derivatives. Med. Mal. Infect. 2012, 42, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Ostovari, A.; Hoseinieh, S.M.; Peikari, M.; Shadizadeh, S.R.; Hashemi, S.J. Corrosion inhibition of mild steel in 1M HCl solution by henna extract: A comparative study of the inhibition by henna and its constituents (Lawsone, Gallic acid, α-d-Glucose and Tannic acid). Corros. Sci. 2009, 51, 1935–1949. [Google Scholar] [CrossRef]
- Zaware, S.B.; Gonnade, R.G.; Srinivas, D.; Khan, A.; Rane, S.Y. Antioxidant and anticancer activities of supramolecularly controlled magnetostructural halo-oximes of lawsone. New J. Chem. 2011, 35, 1615–1623. [Google Scholar] [CrossRef]
- Jelly, R.; Lewis, S.W.; Lennard, C.; Lim, K.F.; Almog, J. Lawsone: A novel reagent for the detection of latent fingermarks on paper surfaces. Chem. Comm. 2008, 3513–3515. [Google Scholar] [CrossRef] [Green Version]
- Rehm, D.; Weller, A. Kinetics of Fluorescence Quenching by Electron and H-Atom Transfer. Isr. J. Chem. 1970, 8, 259–271. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]
- Head-Gordon, M.; Pople, J.A.; Frisch, M.J. MP2 energy evaluation by direct methods. Chem. Phys. Lett. 1988, 153, 503–506. [Google Scholar] [CrossRef]
- Head-Gordon, M.; Head-Gordon, T. Analytic MP2 frequencies without fifth-order storage. Theory and application to bifurcated hydrogen bonds in the water hexamer. Chem. Phys. Lett. 1994, 220, 122–128. [Google Scholar] [CrossRef]
- Kendall, R.A.; Jr, T.H.D.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef] [Green Version]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Barone, V. Recent Advances in Density Functional Methods, Part I; Chong, D.P., Ed.; World Scientific Publ. Co.: Singapore, 1996. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Neda, M.; Okinaga, K.; Shibata, M. High-performance bio-based thermosetting resins based on bismaleimide and allyl-etherified eugenol derivatives. Mater. Chem. Phys. 2014, 148, 319–327. [Google Scholar] [CrossRef]
- Leemhuis, M.; Akeroyd, N.; Kruijtzer, J.A.W.; van Nostrum, C.F.; Hennink, W.E. Synthesis and characterization of allyl functionalized poly(α-hydroxy)acids and their further dihydroxylation and epoxidation. Eur. Polym. J. 2008, 44, 308–317. [Google Scholar] [CrossRef]
- Allushi, A.; Kutahya, C.; Aydogan, C.; Kreutzer, J.; Yilmaz, G.; Yagci, Y. Conventional Type II photoinitiators as activators for photoinduced metal-free atom transfer radical polymerization. Polym. Chem. 2017, 8, 1972–1977. [Google Scholar] [CrossRef]
- Breloy, L.; Losantos, R.; Sampedro, D.; Marazzi, M.; Malval, J.-P.; Heo, Y.; Akimoto, J.; Ito, Y.; Brezová, V.; Versace, D.-L. Allyl amino-thioxanthone derivatives as highly efficient visible light H-donors and co-polymerizable photoinitiators. Polym. Chem. 2020, 11, 4297–4312. [Google Scholar] [CrossRef]
- Görner, H. Photoreactions of 1,4-Naphthoquinones: Effects of Substituents and Water on the Intermediates and Reactivity. Photochem. Photobiol. 2005, 81, 376–383. [Google Scholar] [CrossRef] [PubMed]
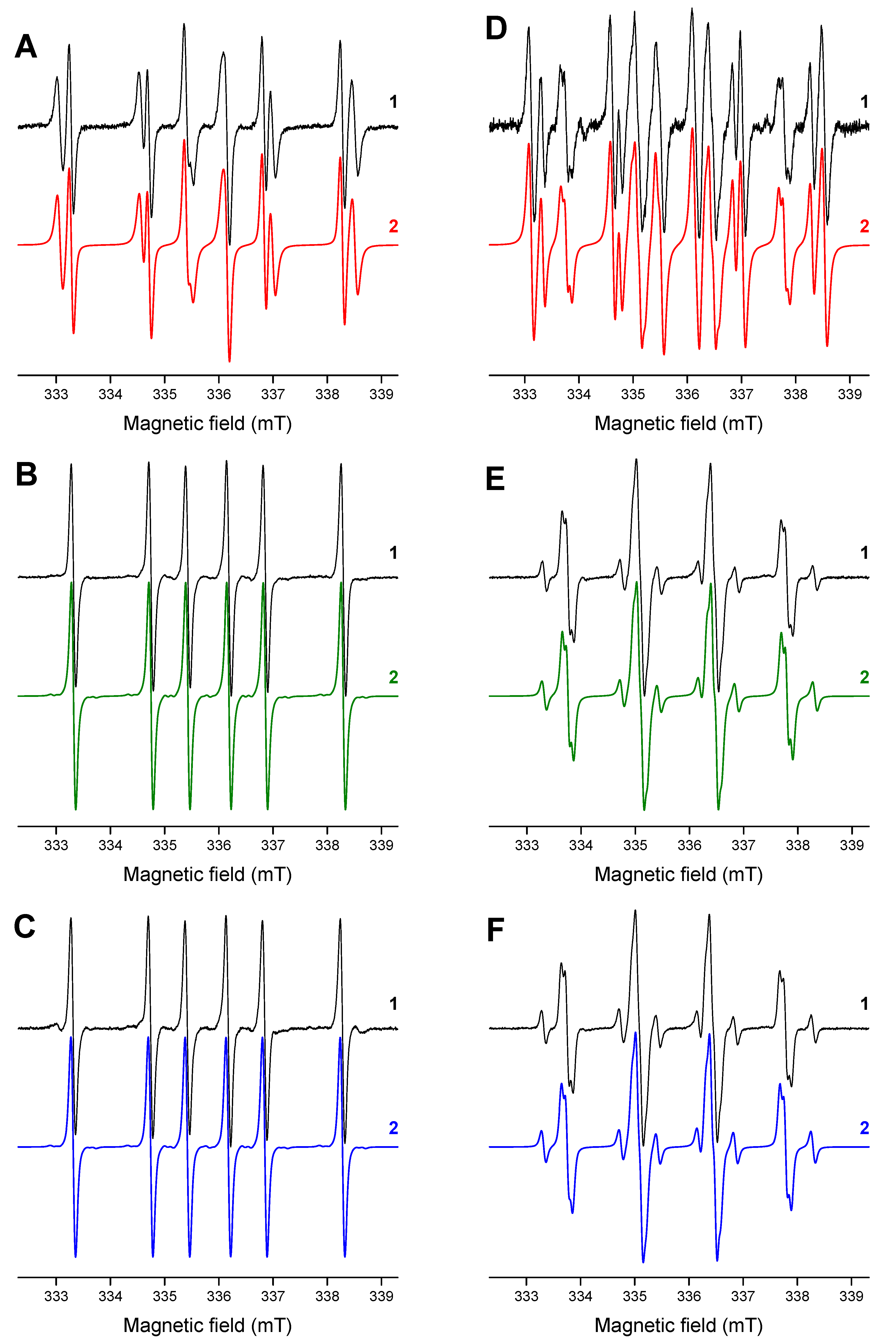
- Frontana, C.; González, I. Effects of the molecular structure on the electrochemical properties of naturally occurring α-hydroxyquinones. An electrochemical and ESR study. J. Electroanal. Chem. 2007, 603, 155–165. [Google Scholar] [CrossRef]
- Criqui, A.; Lalevée, J.; Allonas, X.; Fouassier, J.-P. Electron Spin Resonance Spin Trapping Technique: Application to the Cleavage Process of Photoinitiators. Macromol. Chem. Phys. 2008, 209, 2223–2231. [Google Scholar] [CrossRef]
- Breloy, L.; Brezová, V.; Malval, J.-P.; de Anda, A.R.; Bourgon, J.; Kurogi, T.; Mindiola, D.J.; Versace, D.-L. Well-Defined Titanium Complex for Free-Radical and Cationic Photopolymerizations under Visible Light and Photoinduction of Ti-Based Nanoparticles. Macromolecules 2019, 52, 3716–3729. [Google Scholar] [CrossRef]
- Christmann, J.; Allonas, X.; Ley, C.; Croutxé-Barghorn, C. The role of ketyl radicals in free radical photopolymerization: New experimental and theoretical insights. Polym. Chem. 2019, 10, 1099–1109. [Google Scholar] [CrossRef]
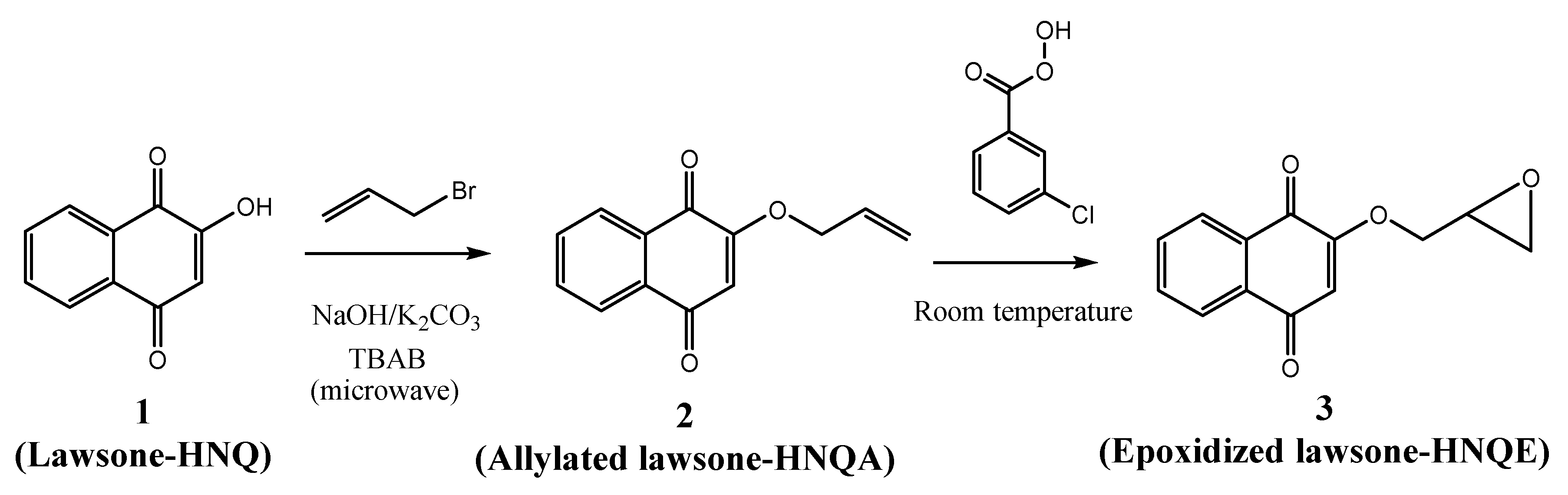

| Name | Chemical Structure |
|---|---|
| 2-hydroxy-1,4-naphthoquinone (HNQ, Lawsone) |  |
| 2-(allyloxy) 1,4-naphthoquinone (HNQA) | 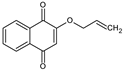 |
| 2-(oxiran-2yl methoxy) 1,4-naphthoquinone (HNQE) | 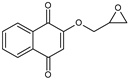 |
| 3,4- epoxycyclohexane)methyl-3,4-epoxycyclohexylcarboxylate (EPOX) |  |
| Bis(4-methylphenyl) iodonium hexafluorophosphate (Iod) | 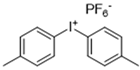 |
| Trimethylolpropane tris(3-mercaptopropionate) (trithiol |  |
| Soybean oil acrylate (SOA) | 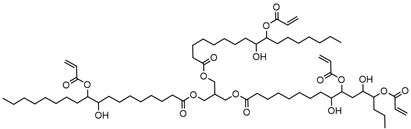 |
| λmax (nm) | λmaxEm (nm) | ES (eV) | Eox (V/SCE) | ERed (V/SCE) | ΔGeT (S) (eV) | |
|---|---|---|---|---|---|---|
| HNQ | 335 | - | - | +1.82 | - | |
| HNQE | 330 | 411 | 3.024 | +1.44 | −1.372 | |
| HNQA | 329 | 410 | 3.016 | +1.33 | −1.499 | |
| Iod | −0.2 |
 | a (mT) | |||||
|---|---|---|---|---|---|---|
| H(2) | H(3) | H(5) | H(6) | H(7) | H(8) | |
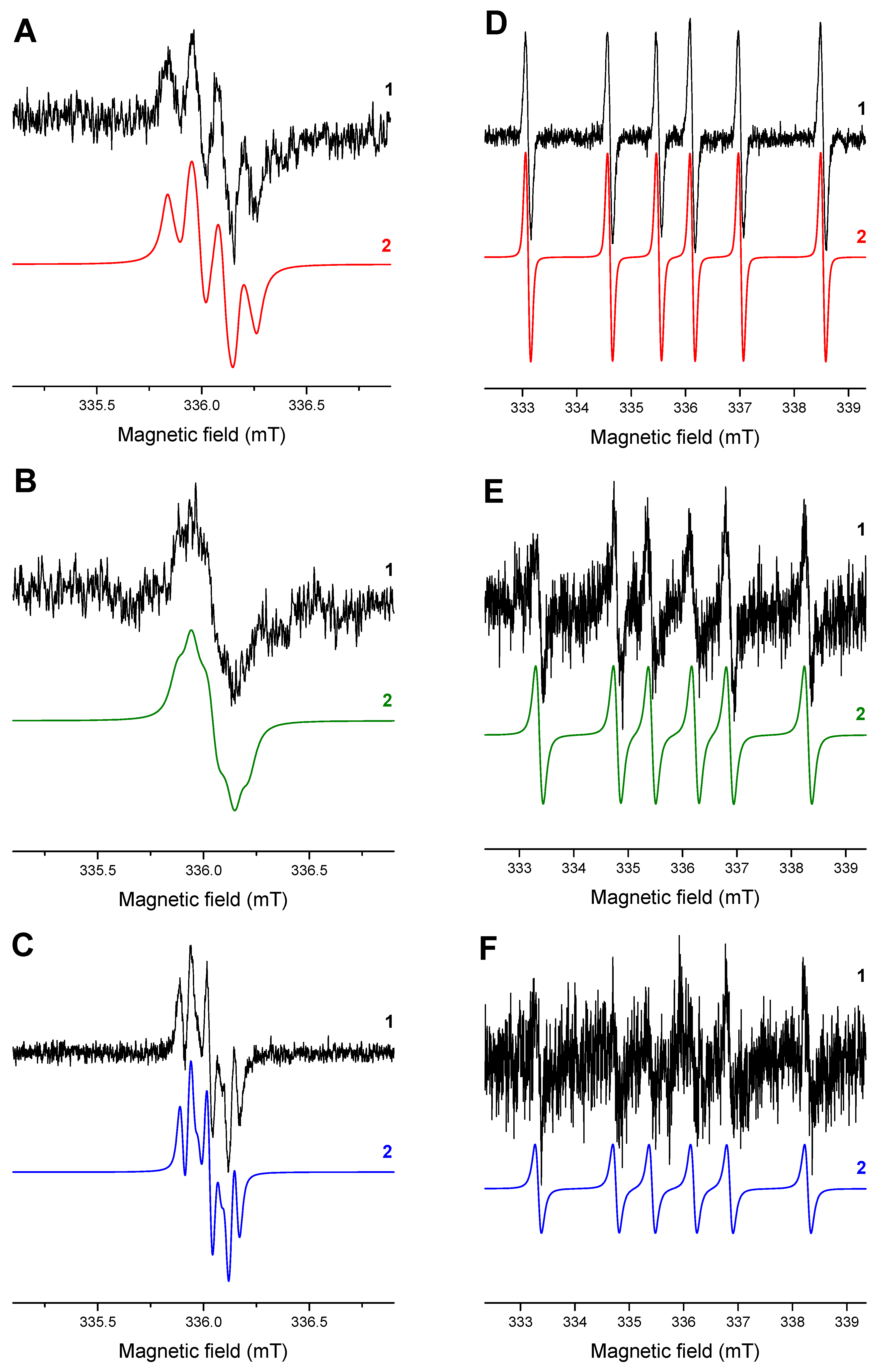
| HNQ•− (R=hydroxy) | −0.064 (OH) 0.039 | −0.154 0.142 | 0.002 <0.02 | −0.123 0.114 | −0.040 <0.02 | −0.076 0.091 |
| HNQA•− (R=allyloxy) | −0.014, 0.023 (CH2) <0.02 | −0.235 0.098 | −0.015 <0.02 | −0.086 0.083 | −0.055 0.057 | −0.045 0.053 |
| HNQE•− (R=oxiran-2-yl-methoxy) | −0.012, 0.030 (CH2) <0.02 | −0.238 0.083 | −0.015 <0.02 | −0.086 0.073 | −0.054 0.054 | −0.045 0.044 |
| Radical Added to DMPO Spin Trap | aN, mT | a, mT | g-Factor |
|---|---|---|---|
| •C(OH)R1R2 | 1.515 ± 0.005 | 2.411 ± 0.005 (Hβ) | 2.0059 ± 0.0001 |
| •CR | 1.436 ± 0.003 | 2.090 ± 0.010 (Hβ) | 2.0060 ± 0.0001 |
| •Phenyl(4-methyl) | 1.438 ± 0.005 | 2.116 ± 0.005 (Hβ) 0.773 ± 0.03 (2 × 13C), 0.604 ± 0.01 (13C), 0.532 ± 0.01 (13C) | 2.0060 ± 0.0001 |
| •SR’ | 1.369 ± 0.003 | 1.308 ± 0.003 (Hβ) 0.080 ± 0.003 (Hγ) 0.072 ± 0.003 (Hγ) | 2.0062 ± 0.0001 |
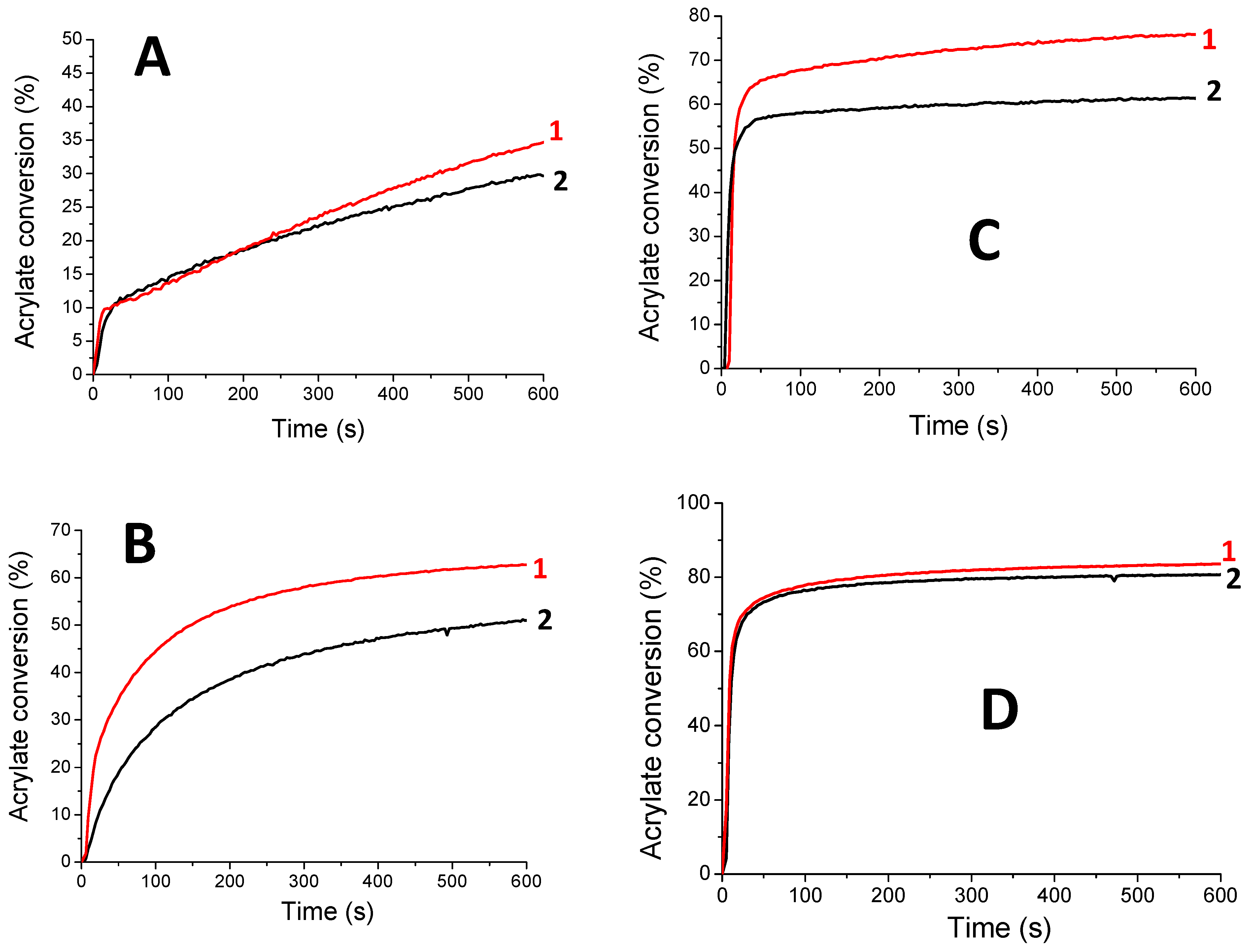
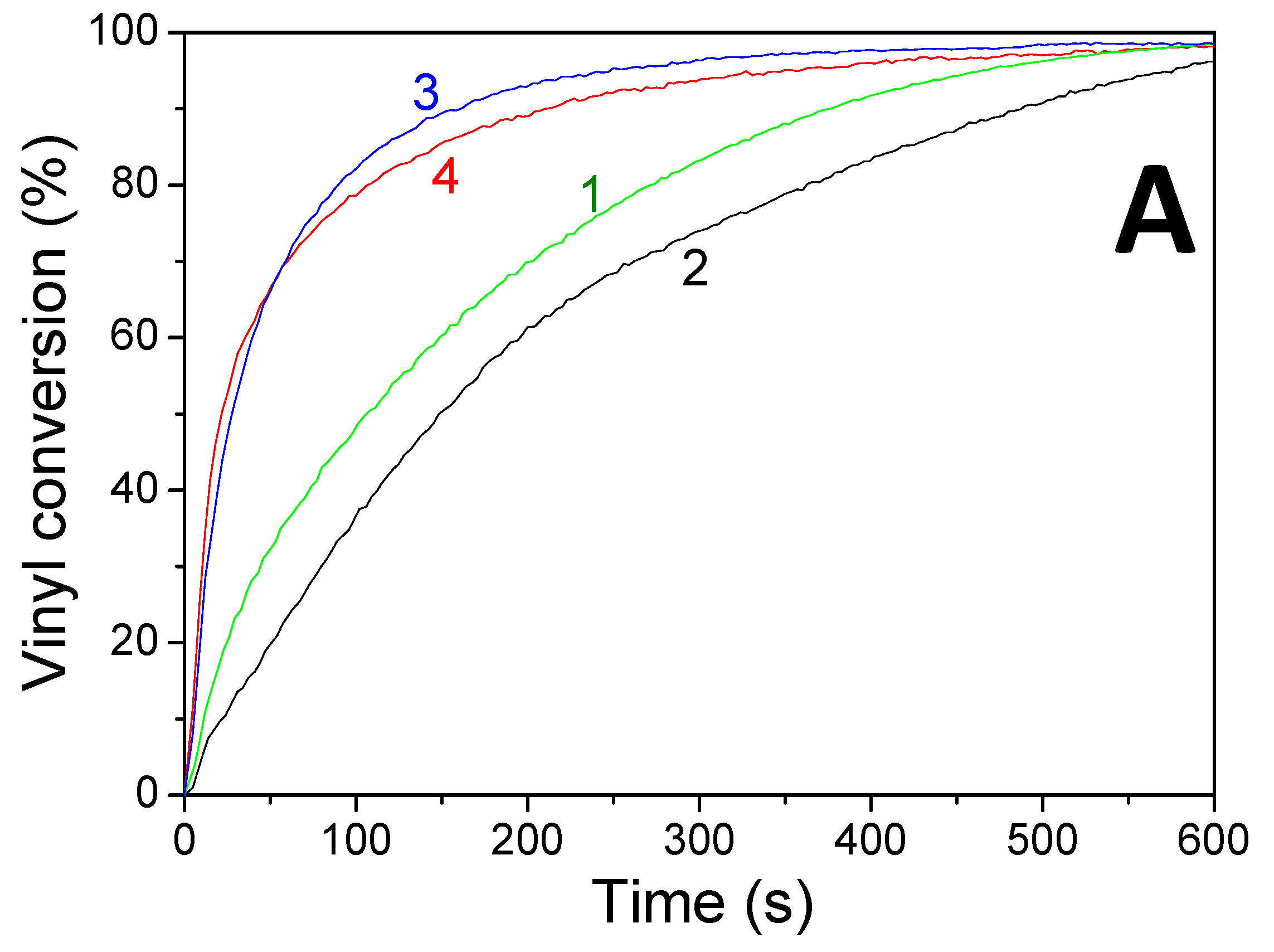
| Acrylate Conversions (%) | 100xRp/[M0] (s−1) | |||
|---|---|---|---|---|
| Photoinitiating Systems | LED@385 nm | LED@405 nm | 385 nm | 405 nm |
| HNQE/SOA | 51 | 63 | 0.5 | 1.3 |
| HNQE/Iod/SOA | 81 | 83 | 5 | 5.7 |
| HNQ/SOA | - | - | - | - |
| HNQ/Iod/SOA | - | - | - | - |
| HNQA/SOA | 30 | 34 | 0.5 | 0.9 |
| HNQA/Iod/SOA | 62 | 76 | 3.8 | 3.3 |
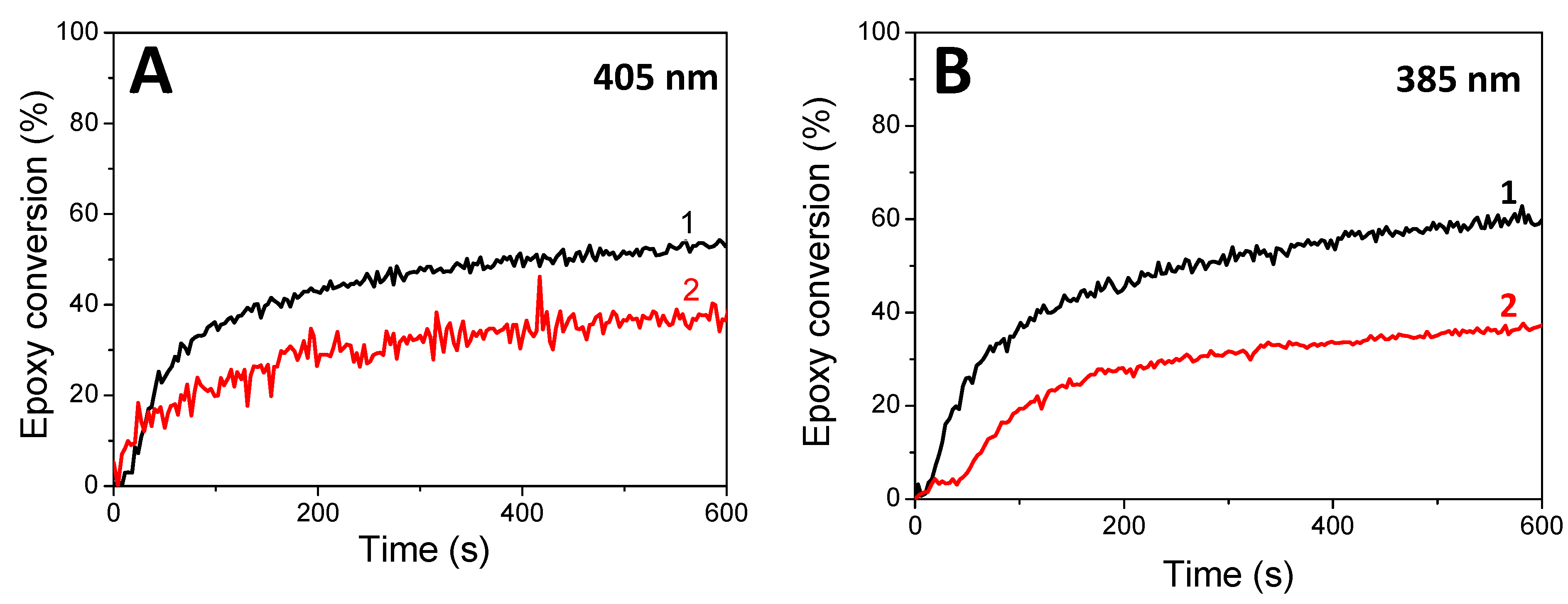
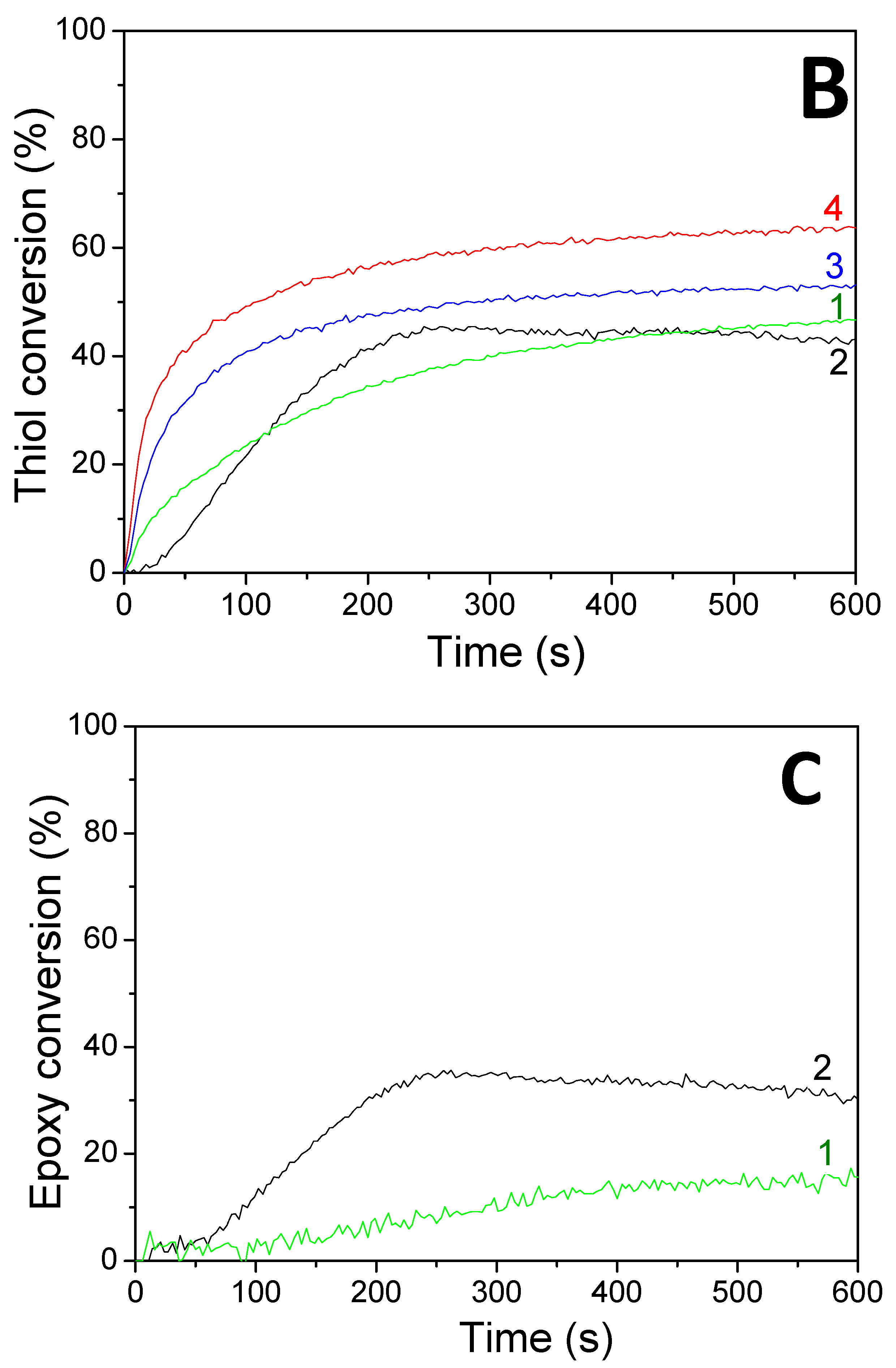
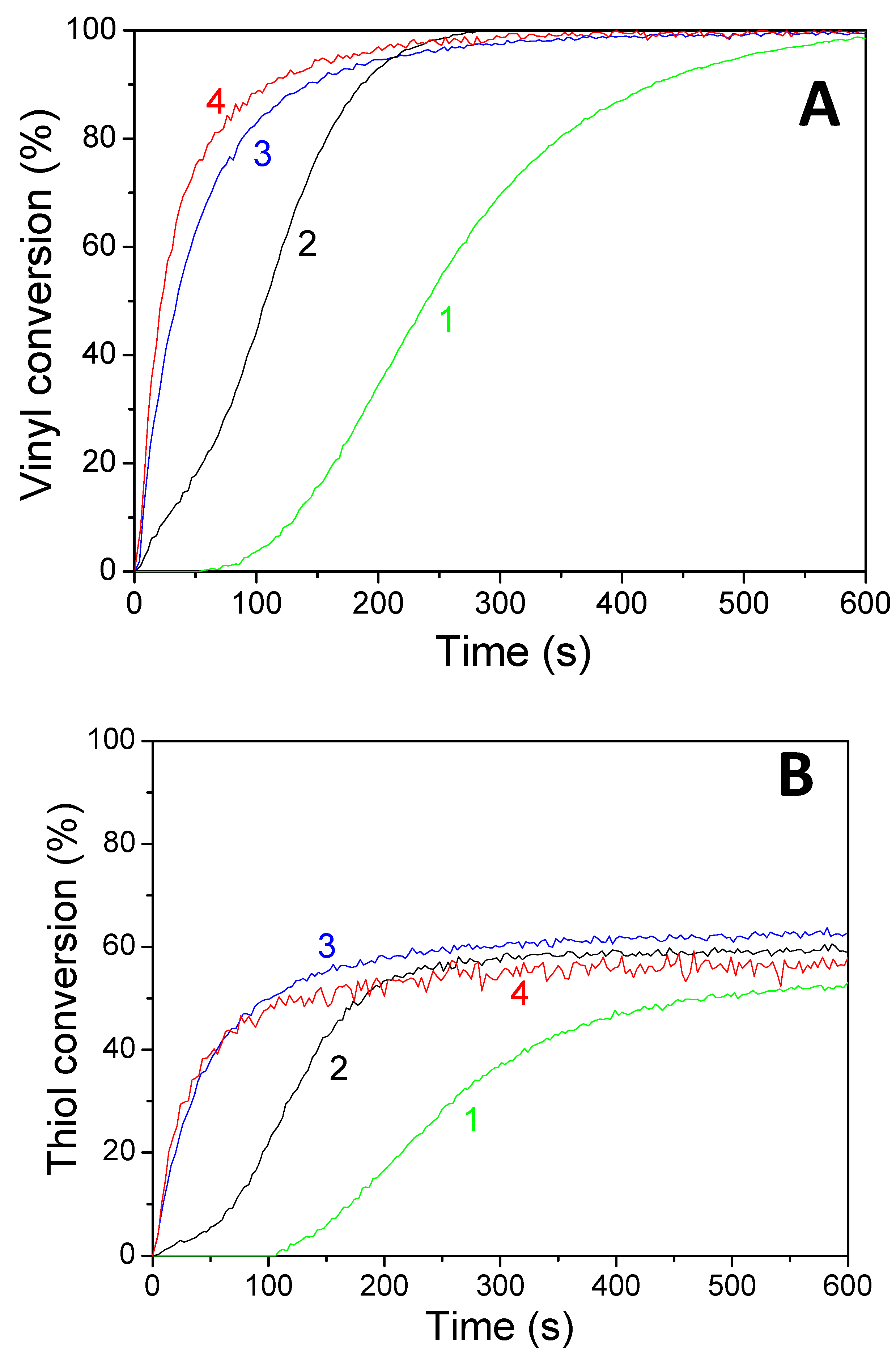
| Vinyl Conversions (%) | Thiol Conversions (%) | Epoxy Conversions (%) | ||||
|---|---|---|---|---|---|---|
| Photosensitive Formulations | LED@ 385 nm | LED@ 405 nm | LED@ 385 nm | LED@ 405 nm | LED@ 385 nm | LED@405 nm |
| HNQA/Iod/(limonene 1,2 epoxide/trithiol) | 96 a 98 b | 98 a 98 b | 43 a 64 b | 47 a 53 b | 30 a | 16 a |
| HNQE/Iod/(limonene1,2 epoxide/trithiol) | 98 a 99 b | 100 a 100 b | 53 a 62 b | 59 a 58 b | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elian, C.; Brezová, V.; Sautrot-Ba, P.; Breza, M.; Versace, D.-L. Lawsone Derivatives as Efficient Photopolymerizable Initiators for Free-Radical, Cationic Photopolymerizations, and Thiol—Ene Reactions. Polymers 2021, 13, 2015. https://doi.org/10.3390/polym13122015
Elian C, Brezová V, Sautrot-Ba P, Breza M, Versace D-L. Lawsone Derivatives as Efficient Photopolymerizable Initiators for Free-Radical, Cationic Photopolymerizations, and Thiol—Ene Reactions. Polymers. 2021; 13(12):2015. https://doi.org/10.3390/polym13122015
Chicago/Turabian StyleElian, Christine, Vlasta Brezová, Pauline Sautrot-Ba, Martin Breza, and Davy-Louis Versace. 2021. "Lawsone Derivatives as Efficient Photopolymerizable Initiators for Free-Radical, Cationic Photopolymerizations, and Thiol—Ene Reactions" Polymers 13, no. 12: 2015. https://doi.org/10.3390/polym13122015
APA StyleElian, C., Brezová, V., Sautrot-Ba, P., Breza, M., & Versace, D.-L. (2021). Lawsone Derivatives as Efficient Photopolymerizable Initiators for Free-Radical, Cationic Photopolymerizations, and Thiol—Ene Reactions. Polymers, 13(12), 2015. https://doi.org/10.3390/polym13122015