Evaluation of the Physicochemical Properties of Chitosans in Inducing the Defense Response of Coffea arabica against the Fungus Hemileia vastatrix
Abstract
1. Introduction
2. Materials and Method
2.1. General Methods
2.1.1. Location of the Study Area
2.1.2. Plant Material
2.1.3. Biological Material
2.1.4. Chitosan Solutions
2.2. Chitosan Characterization
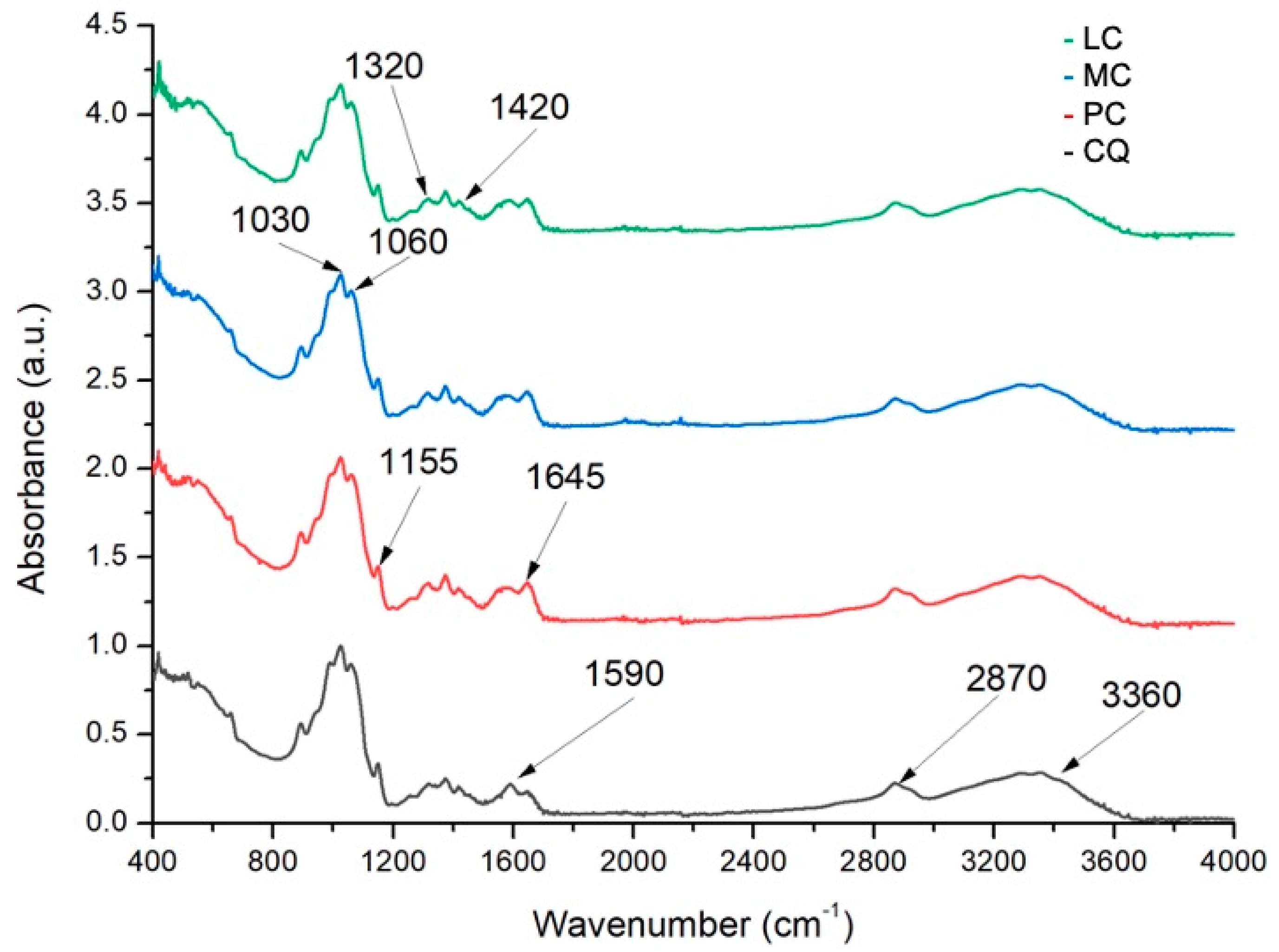
2.2.1. Degree of Deacetylation by Fourier Transform Infrared Spectroscopy (FTIR)
2.2.2. Viscosity Average Molecular Weight Determination by Chitosan Intrinsic Viscosity
2.3. Evaluation Chitosan Protection in Coffee Rust Control
2.3.1. Chitosan Solutions Preparations
2.3.2. Biological Effectiveness Test
2.3.3. Disease Incidence
2.3.4. Disease Severity
2.3.5. Defoliation
2.3.6. Disease Leaves
2.3.7. Area under the Curve Calculation for Disease Progression (AUDPC)
2.4. Evaluation of Chitosan in the Defense Response in the C. arabica–H. vastatrix Interaction
2.4.1. Enzymatic Extraction
2.4.2. β-1,3 Glucanase Activity Determination
2.4.3. Peroxidase Activity Determination
2.4.4. Total Phenolic Compounds Quantification
2.5. Statistical Analysis
3. Results
3.1. Chitosan Characterization
3.1.1. Degree of Deacetylation
3.1.2. Molecular Weight Viscosity Average
3.2. Chitosan Protection Evaluation in Coffee Rust Control
3.2.1. Biological Effectiveness Test
3.2.2. Defoliation
3.2.3. Disease Leaves
3.2.4. Area under the Disease Progress Curve (AUDPC)
3.3. Chitosan Evaluation in the Defense Response in the C. arabica–H. vastatrix Interaction
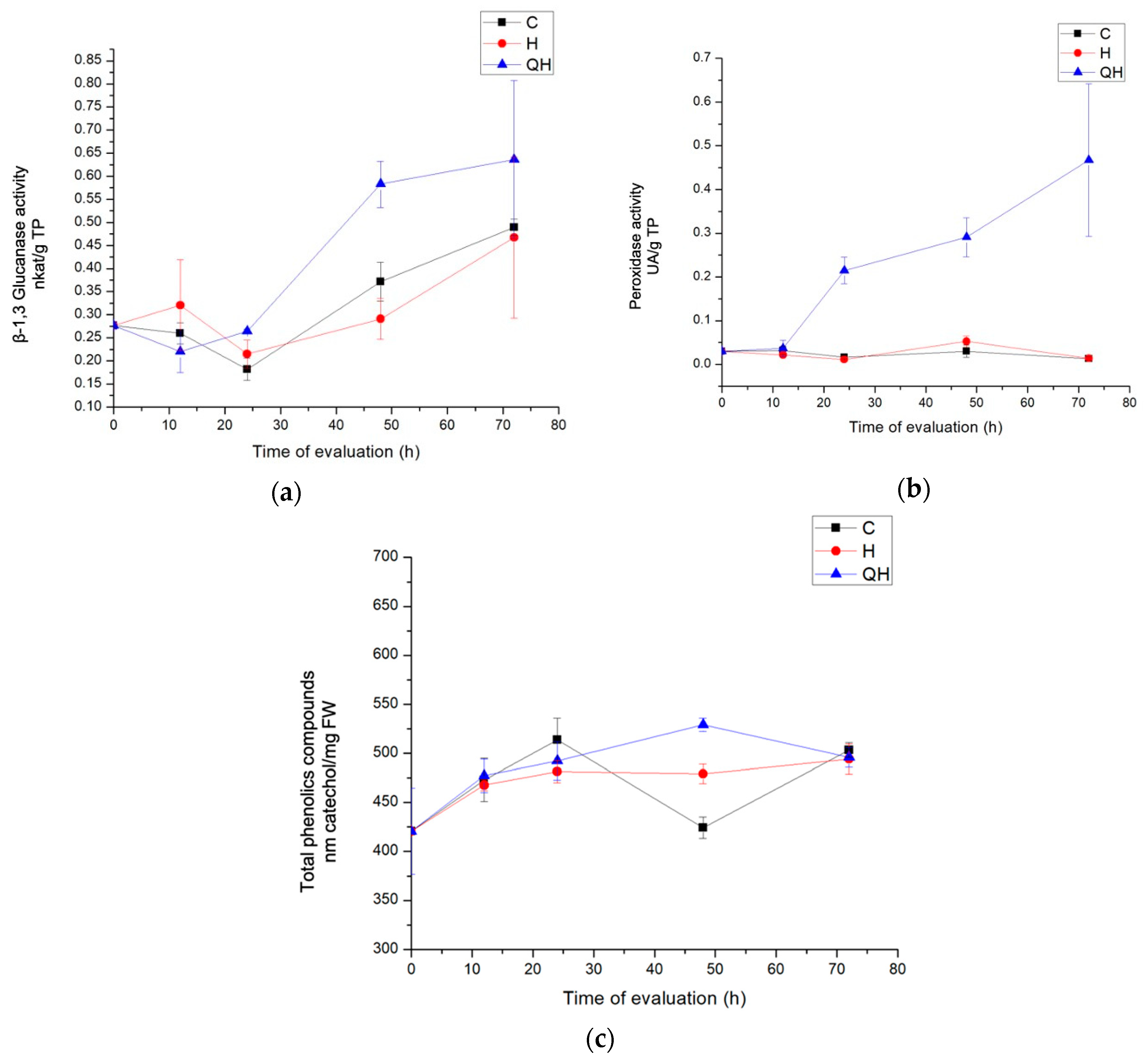
3.3.1. Enzyme Evaluation with β-1,3 Glucanase Activity
3.3.2. Peroxidase Activity Enzymes Evaluation
3.3.3. Total Phenolic Compounds Quantification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khosravi, F.; Dehestani, A.; Gharanjik, S. Molecular responses of Phytophthora capsici-challenged cucumber (Cucumis sativus L.) plants as influenced by resistance inducer application. J. Plant Mol. Breed. 2017, 5, 1–10. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef] [PubMed]
- Orzali, L.; Corsi, B.; Forni, C.; Riccioni, L. Chitosan in Agriculture: A New Challenge for Managing Plant Disease. Biol. Act. Appl. Mar. Polysacch. 2017, 17–36. [Google Scholar] [CrossRef]
- Beatrice, C.; Linthorst, J.M.H.; Cinzia, F.; Luca, R. Enhancement of PR1 and PR5 gene expressions by chitosan treatment in kiwifruit plants inoculated with Pseudomonas syringae pv. actinidiae. Eur. J. Plant Pathol. 2017, 148, 163–179. [Google Scholar] [CrossRef]
- Kuyyogsuy, A.; Deenamo, N.; Khompatara, K.; Ekchaweng, K.; Churngchow, N. Chitosan enhances resistance in rubber tree (Hevea brasiliensis), through the induction of abscisic acid (ABA). Physiol. Mol. Plant Pathol. 2018, 102, 67–78. [Google Scholar] [CrossRef]
- Prodhan, Y.; Issak, M.; Munemasa, S.; Nakamura, Y.; Murata, Y. Salicylic acid receptor NPR1 is involved in guard cell chitosan signaling. Biosci. Biotechnol. Biochem. 2020, 84, 963–969. [Google Scholar] [CrossRef]
- Xoca-Orozco, L.Á.; Aguilera-Aguirre, S.; Vega-Arreguín, J.; Acevedo-Hernández, G.; Tovar-Pérez, E.; Stoll, A.; Herrera-Estrella, L.; Chacón-López, A. Activation of the phenylpropanoid biosynthesis pathway reveals a novel action mechanism of the elicitor effect of chitosan on avocado fruit epicarp. Food Res. Int. 2019, 121, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Kiprushkina, E.I.; Shestopalova, I.A.; Pekhotina, A.M.; Kuprina, E.E.; Nikitina, O.V. Protective-stimulating properties of chitosan in the vegetation and storing tomatoes. Prog. Chem. Appl. Chitin Deriv. 2017, 22, 77–81. [Google Scholar] [CrossRef]
- Liu, Y.; Wisniewski, M.; Kennedy, J.F.; Jiang, Y.; Tang, J.; Liu, J. Chitosan and oligochitosan enhance ginger (Zingiber officinale Roscoe) resistance to rhizome rot caused by Fusarium oxysporum in storage. Carbohydr. Polym. 2016, 151, 474–479. [Google Scholar] [CrossRef]
- Jannat, R.; Shaha, M.; Rubayet, M.T.; Sultana, S. Role of Chitosan in Induction of Defense Response against Phomopsis vexans and Augmentation of Growth and Yield of Eggplant. Glob. J. Sci. Front. Res. C Biol. Sci. 2018, 18, 6–13. [Google Scholar]
- Chandra, S.; Chakraborty, N.; Panda, K.; Acharya, K. Chitosan-induced immunity in Camellia sinensis (L.) O. Kuntze against blister blight disease is mediated by nitric-oxide. Plant Physiol. Biochem. 2017, 115, 298–307. [Google Scholar] [CrossRef]
- Lucini, L.; Baccolo, G.; Rouphael, Y.; Colla, G.; Bavaresco, L.; Trevisan, M. Chitosan treatment elicited defence mechanisms, pentacyclic triterpenoids and stilbene accumulation in grape (Vitis vinifera L.) bunches. Phytochemistry 2018, 156, 1–8. [Google Scholar] [CrossRef]
- Jia, X.; Meng, Q.; Zeng, H.; Wang, W.; Yin, H. Chitosan oligosaccharide induces resistance to Tobacco mosaic virus in Arabidopsis via the salicylic acid-mediated signalling pathway. Sci. Rep. 2016, 6, 26144. [Google Scholar] [CrossRef]
- Falcón-Rodríguez, A.B.; Costales, D.; Cabrera, J.C.; Martínez-Téllez, M.Á. Chitosan physico–chemical properties modulate defense responses and resistance in tobacco plants against the oomycete Phytophthora nicotianae. Pestic. Biochem. Physiol. 2011, 100, 221–228. [Google Scholar] [CrossRef]
- ASERCA. Panorama Agropecuario Semanal de Café. Available online: https://info.aserca.gob.mx/panorama/semanal.asp?de=cafe (accessed on 15 March 2021).
- Porto, B.N.; Caixeta, E.T.; Mathioni, S.M.; Vidigal, P.M.P.; Zambolim, L.; Zambolim, E.M.; Donofrio, N.; Polson, S.W.; Maia, T.A.; Chen, C.; et al. Genome sequencing and transcript analysis of Hemileia vastatrix reveal expression dynamics of candidate effectors dependent on host compatibility. PLoS ONE 2019, 14, e0215598. [Google Scholar] [CrossRef]
- Monteiro, A.C.A.; de Resende, M.L.V.; Valente, T.C.T.; Ribeiro-Junior, P.M.; Pereira, V.F.; da Costa, J.R.; da Silva, J.A.G. Manganese Phosphite in Coffee Defence against Hemileia vastatrix, the Coffee Rust Fungus: Biochemical and Molecular Analyses. J. Phytopathol. 2016, 164, 1043–1053. [Google Scholar] [CrossRef]
- Quiroz-Figueroa, F.; Méndez-Zeel, M.; Larqué-Saavedra, A.; Loyola-Vargas, V. Picomolar concentrations of salicylates induce cellular growth and enhance somatic embryogenesis in Coffea arabica tissue culture. Plant Cell Rep. 2001, 20, 679–684. [Google Scholar] [CrossRef]
- Polo-Ibáñez, A.R.; Vargas-Vélez, I.C. Eliminación de Fosfatos y nitratos de Agua Residual Municipal Mediante un Inóculo Optimizado de Chlorella sp. en un Sistema de Fotobiorreactores Verticales con Columna de Burbujeo a Escala Piloto. Bachelor’s thesis, Universidad de la Costa, Barranquilla, Colombia, 2019. Available online: https://repositorio.cuc.edu.co (accessed on 23 June 2020).
- de Queiroz-Antonino, R.; Lia-Fook, B.R.; de Oliveira-Lima, V.A.; de Farias-Rached, R.I.; Nascimento-Lima, E.P.; da Silva-Lima, R.J.; Peniche-Covas, C.A.; Lia-Fook, M.V. Preparation and Characterization of Chitosan Obtained from Shells of Shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef]
- Zając, A.; Hanuza, J.; Wandas, M.; Dymińska, L. Determination of N-acetylation degree in chitosan using Raman spectroscopy. Spectrochim Acta A Mol. Biomol. Spectrosc. 2015, 134, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Z.; Chen, X.G.; Liu, N.; Wang, S.X.; Liu, C.S.; Meng, X.H.; Liu, C.G. Protonation constants of chitosan with different molecular weight and degree of deacetylation. Carbohydr. Polym. 2006, 65, 194–201. [Google Scholar] [CrossRef]
- Qun, G.; Ajun, W. Effects of molecular weight, degree of acetylation and ionic strength on surface tension of chitosan in dilute solution. Carbohydr. Polym. 2006, 64, 29–36. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhang, X.; Merewitz, E.; Peng, Y.; Ma, X.; Huang, L.; Yan, Y. Metabolic Pathways Regulated by Chitosan Contributing to Drought Resistance in White Clover. J. Proteome Res. 2017, 16, 3039–3052. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Santiago, A. Efectividad Biológica del Producto Bactrone en el Control de Roya (Hemileia vastatrix) en el Cultivo de Café. Available online: https://lidag.com/wp-content/uploads/2019/06/Bactrone-en-cafe-LIDAG.pdf (accessed on 20 March 2021).
- Nahar, M.S.; Naher, N.; Alam, M.J.; Hussain, M.J.; Yasmin, L.; Mian, M.Y.; Rosa, C. Survey, morphology and white mold disease of country bean (Lablab purpureus L.) caused by Sclerotinia sclerotiorum (Lib.) de Bary in-relation to soil physico-chemical properties and weather conditions in Bangladesh. Crop Prot. 2020, 135, 104825. [Google Scholar] [CrossRef]
- SENASICA. ROYA DEL CAFETO Hemileia vastatrix Berkeley & Broome Ficha Técnica No. 40. 2016. Available online: https://prod.senasica.gob.mx/SIRVEF/ContenidoPublico/Roya%20cafeto/Fichas%20tecnicas/Ficha%20Técnica%20de%20Roya%20del%20cafeto.pdf (accessed on 20 March 2021).
- Shaner, G.; Finney, R. The effect of nitrogen fertilization on the expression of slow-mildewing resistance in Knox wheat. Phytopathology 1977, 67, 1021–1056. [Google Scholar] [CrossRef]
- Oliveira, J.T.A.; Barreto, A.L.H.; Vasconcelos, I.M.; Eloy, Y.R.G.; Gondim, D.M.F. Role of Antioxidant Enzymes, Hydrogen Peroxide and PRProteins in the Compatible and Incompatible Interactions of Cowpea (Vigna unguiculata) Genotypes with the Fungus Colletotrichum gloeosporioides. J. Plant Physiol. Pathol. 2014, 2. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Basa, S.; Nampally, M.; Honorato, T.; Das, S.N.; Podile, A.R.; El Gueddari, N.E.; Moerschbacher, B.M. The Pattern of Acetylation Defines the Priming Activity of Chitosan Tetramers. J. Am. Chem. Soc. 2020, 142, 1975–1986. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar-Ahmed, K.B.; Khan, M.M.A.; Siddiqui, H.; Jahan, A. Chitosan and its oligosaccharides, a promising option for sustainable crop production—A review. Carbohydr. Polym. 2020, 227, 115331. [Google Scholar] [CrossRef]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in Plant Protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef] [PubMed]
- Malerba, M.; Cerana, R. Recent Applications of Chitin- and Chitosan-Based Polymers in Plants. Polymers 2019, 11, 839. [Google Scholar] [CrossRef]
- Dima, J.B.; Sequeiros, C.; Zaritky, N.E. Optimización de la obtención de quitosano de crustáceos patagónicos (Puerto Madryn, Chubut): Desarrollo de micropartículas y evaluación de su acción bactericida en patógenos de usual frecuencia en maricultura. Rev. Asociación Argent. Ing. Quím. 2013, 57, 1–19. [Google Scholar]
- Khalil-Bagy, H.M.M.; Ibtesam, B.F.M.; Abou-Zaid, E.A.A.; Sabah, B.M.; Nashwa, S.M.A. Control of green mold disease using chitosan and its effect on orange properties during cold storage. Arch. Phytopathol. Plant Prot. 2020, 1–16. [Google Scholar] [CrossRef]
- No, H.K.; Kim, S.H.; Lee, S.H.; Park, N.Y.; Prinyawiwatkul, W. Stability and antibacterial activity of chitosan solutions affected by storage temperature and time. Carbohydr. Polym. 2006, 65, 174–178. [Google Scholar] [CrossRef]
- Luján-Hidalgo, M.C.; Jiménez-Aguilar, L.A.; Ruiz-Lau, N.; Reyes-Zambrano, S.J.; Gutiérrez-Miceli, F.A. Cambios bioquímicos en respuesta al ataque de roya en plantaciones de café. Polibotanica 2020, 49, 149–160. [Google Scholar] [CrossRef]
- Honorato-Júnior, J.; Zambolim, L.; Aucique-Pérez, C.E.; Resende, R.S.; Rodrigues, F.A. Photosynthetic and antioxidative alterations in coffee leaves caused by epoxiconazole and pyraclostrobin sprays and Hemileia vastatrix infection. Pestic. Biochem. Physiol. 2015, 123, 31–39. [Google Scholar] [CrossRef]
- Salcedo-Sarmiento, S.; Aucique-Pérez, C.E.; Silveira, P.R.; Colmán, A.A.; Silva, A.L.; Corrêa-Mansur, P.S.; Rodrigues, F.Á.; Evans, H.C.; Barreto, R.W. Elucidating the interactions between the rust Hemileia vastatrix and a Calonectria mycoparasite and the coffee plant. iScience 2021, 24, 102352. [Google Scholar] [CrossRef] [PubMed]
- Sathiyabama, M.; Bernstein, N.; Anusuya, S. Chitosan elicitation for increased curcumin production and stimulation of defence response in turmeric (Curcuma longa L.). Ind. Crops. Prod. 2016, 89, 94. [Google Scholar] [CrossRef]
- Wulanjari, D.; Wijaya, K.A.; Rosyady, M.G.; Wafa, A. Polyphenol Content and Enhancing Plant resistance of Lowland Arabica Coffee. E3S Web Conf. 2020, 142, 01006. [Google Scholar] [CrossRef]
- Melo, G.A.; Shimizu, M.M.; Mazzafera, P. Polyphenoloxidase activity in coffee leaves and its role in resistance against the coffee leaf miner and coffee leaf rust. Phytochemistry 2006, 67, 277–285. [Google Scholar] [CrossRef] [PubMed]

| Value | Symptom |
|---|---|
| 0 | 0% of leaf area damaged |
| 1 | Chlorotic spots on the leaf surface |
| 2 | 2% of leaf area damaged |
| 3 | 7% of leaf area damaged |
| 4 | 20% of leaf area damaged |
| 5 | 45% of leaf area damaged |
| 6 | More than 70% of the leaf area damaged |
| Sample | [η] | Mv (kD) | DD (%) |
|---|---|---|---|
| PC | 593.5 ± 2.87 | 260.6 ± 23.96 | 77.22 ± 3.05 |
| MC | 829.9 ± 3.49 | 452.8 ± 36.99 | 76.81 ± 2.87 |
| LC | 704.9 ± 3.06 | 295.4 ± 54.86 | 79.69 ± 0.54 |
| CQ | 631.1 ± 10.90 | 314.4 ± 12.31 | 90.35 ± 2.57 |
| Treatment * | Defoliation (%) * | Diseased Leaves (%) * | AUDPC * | Disease Incidence (%) |
|---|---|---|---|---|
| C | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 |
| H | 18.83 ± 5.81 b | 34.83 ± 8.23 bcd | 184 ± 2.58 e | 100 |
| LH1 | 17.93 ± 8.24 b | 36.56 ± 7.01 cd | 166 ± 5.86 de | 100 |
| LH5 | 24.67 ± 7.81 b | 39.05 ± 10.60 d | 180 ± 6.66 e | 100 |
| MH1 | 20.51 ± 6.05 b | 33.12 ± 6.55 bcd | 179 ± 7.97 e | 100 |
| MH5 | 16.72 ± 6.07 b | 38.69 ± 10.47 d | 178 ± 6.99 de | 100 |
| PH1 | 15.58 ± 5.19 b | 37.50 ± 8.32 cd | 153 ± 2.41 cd | 100 |
| PH5 | 19.55 ± 6.75 b | 22.23 ± 8.43 b | 76 ± 27.70 b | 80 |
| CH1 | 15.88 ± 5.79 b | 42.64 ± 8.07 d | 79 ± 24.77 b | 90 |
| CH5 | 14.47 ± 5.81 b | 24.47 ± 4.99 bc | 136 ± 20.97 c | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Velázquez, J.C.; Haro-González, J.N.; García-Morales, S.; Espinosa-Andrews, H.; Navarro-López, D.E.; Montero-Cortés, M.I.; Qui-Zapata, J.A. Evaluation of the Physicochemical Properties of Chitosans in Inducing the Defense Response of Coffea arabica against the Fungus Hemileia vastatrix. Polymers 2021, 13, 1940. https://doi.org/10.3390/polym13121940
López-Velázquez JC, Haro-González JN, García-Morales S, Espinosa-Andrews H, Navarro-López DE, Montero-Cortés MI, Qui-Zapata JA. Evaluation of the Physicochemical Properties of Chitosans in Inducing the Defense Response of Coffea arabica against the Fungus Hemileia vastatrix. Polymers. 2021; 13(12):1940. https://doi.org/10.3390/polym13121940
Chicago/Turabian StyleLópez-Velázquez, Julio César, José Nabor Haro-González, Soledad García-Morales, Hugo Espinosa-Andrews, Diego Eloyr Navarro-López, Mayra Itzcalotzin Montero-Cortés, and Joaquín Alejandro Qui-Zapata. 2021. "Evaluation of the Physicochemical Properties of Chitosans in Inducing the Defense Response of Coffea arabica against the Fungus Hemileia vastatrix" Polymers 13, no. 12: 1940. https://doi.org/10.3390/polym13121940
APA StyleLópez-Velázquez, J. C., Haro-González, J. N., García-Morales, S., Espinosa-Andrews, H., Navarro-López, D. E., Montero-Cortés, M. I., & Qui-Zapata, J. A. (2021). Evaluation of the Physicochemical Properties of Chitosans in Inducing the Defense Response of Coffea arabica against the Fungus Hemileia vastatrix. Polymers, 13(12), 1940. https://doi.org/10.3390/polym13121940









