Synthesis and Conductivity Studies of Poly(Methyl Methacrylate) (PMMA) by Co-Polymerization and Blending with Polyaniline (PANi)
Abstract
:1. Introduction
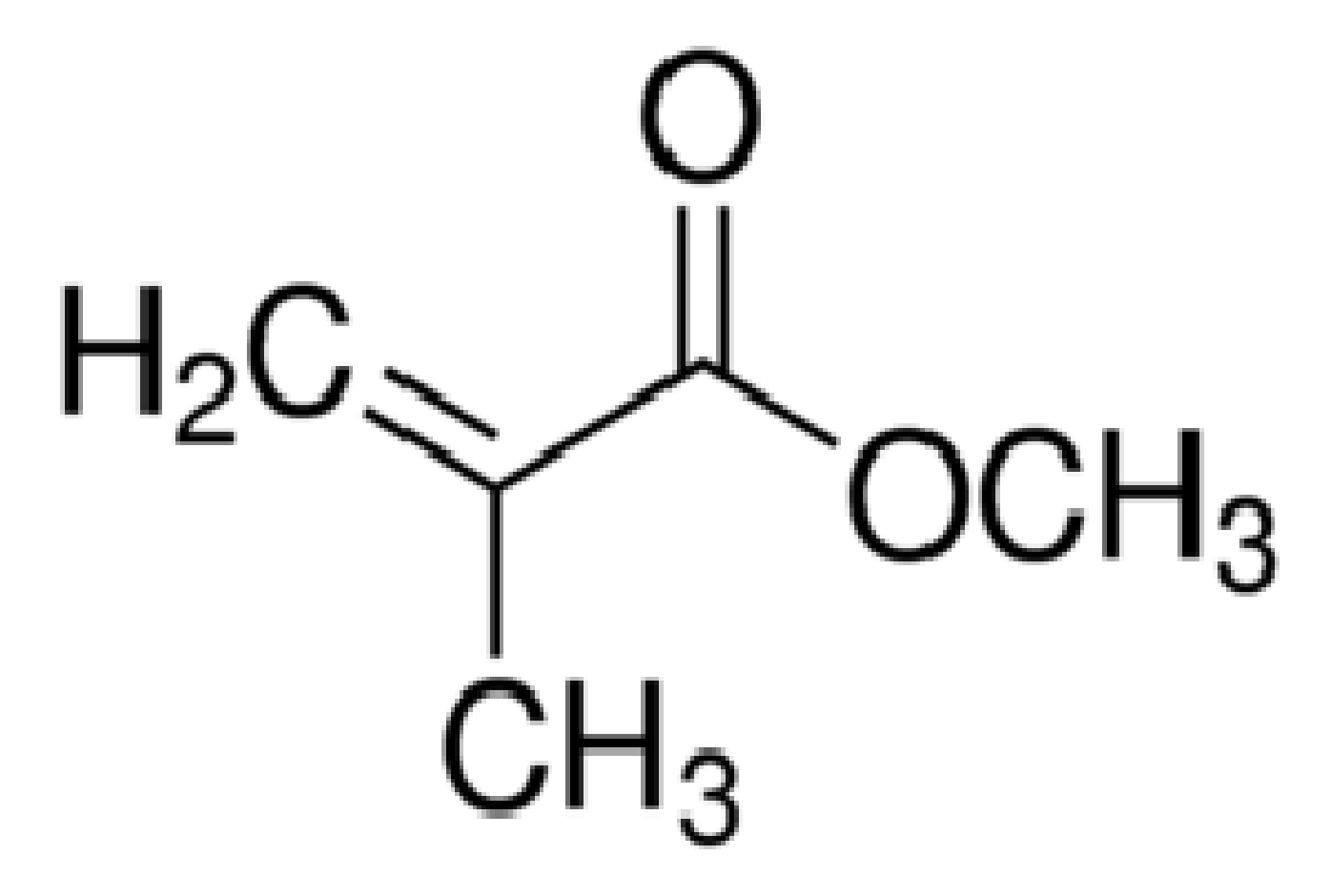
2. Poly(Methyl Methacrylate)
2.1. Conducting PMMA
2.2. Mechanical Properties
2.3. Dispersibility
3. Application of Conducting PMMA
3.1. Electrical and Electrochemistry
3.2. Coatings
3.3. Sensing
3.4. Medical
4. PANi-Based Blends and Composite
4.1. Solution Casting Method
4.2. Interfacial Polymerization
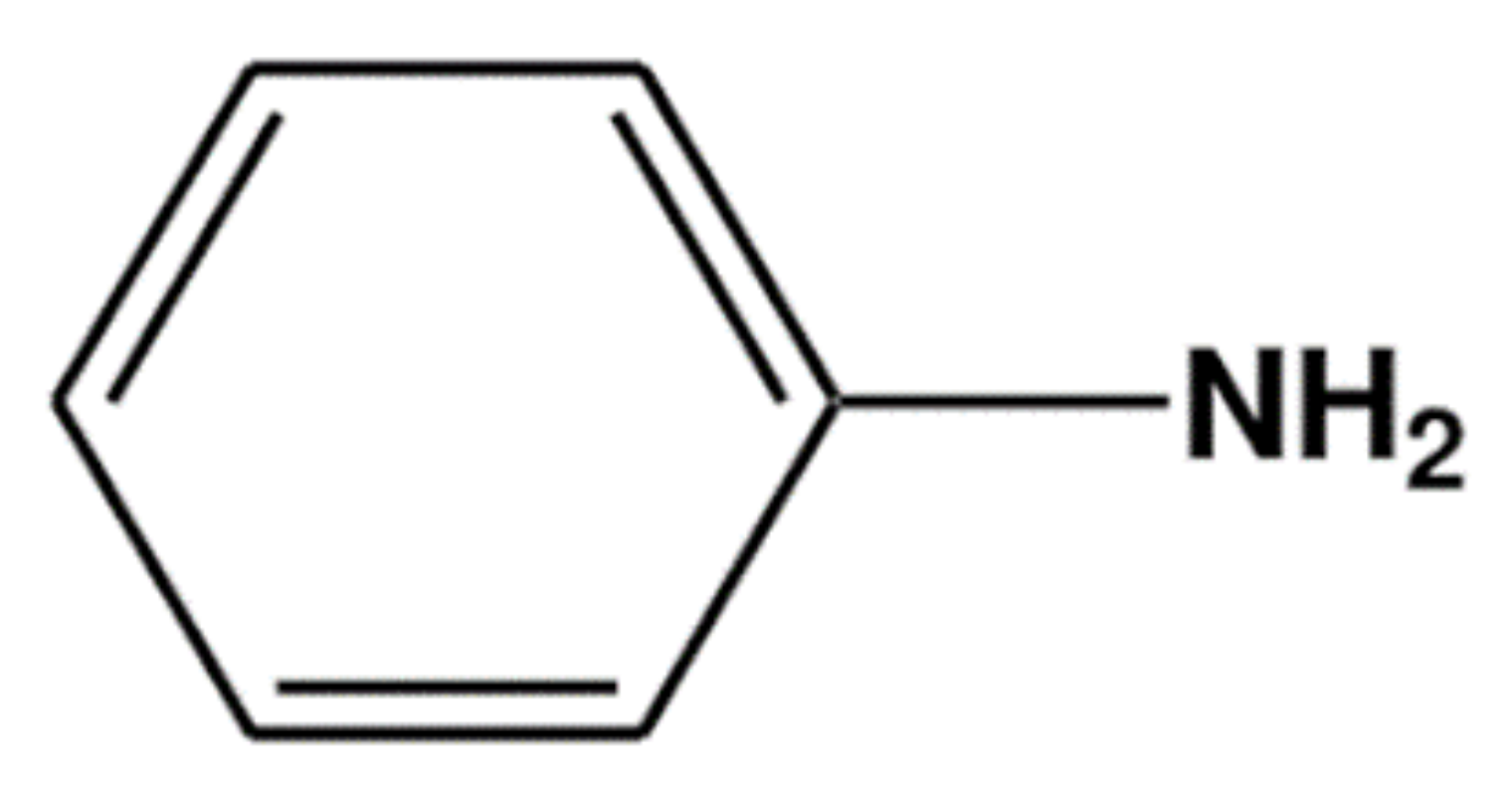
5. PANi Synthesis
5.1. Acid Dopants for PANi Synthesis
5.2. Emulsion Polymerization Method
5.3. Polymerization Temperature
5.4. Polymerization Yield
6. Co-Polymerization of PANi with Various Thermoplastics
6.1. Graft PANi Copolymer
6.2. Block PANi Copolymer
7. Conductivity
7.1. Doping
7.2. PANi/PMMA Ratio
8. Morphology
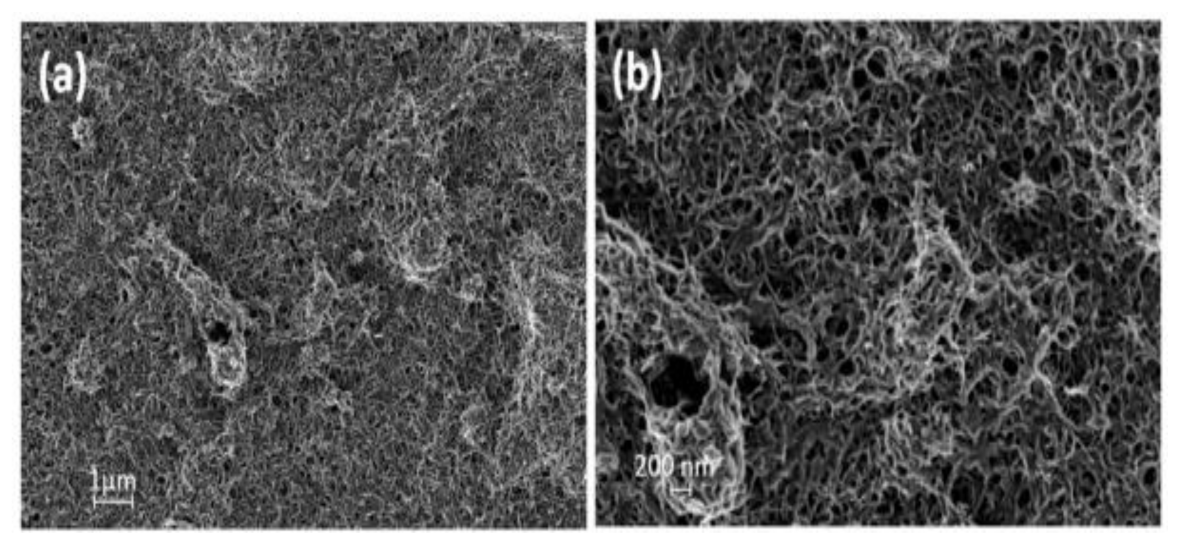
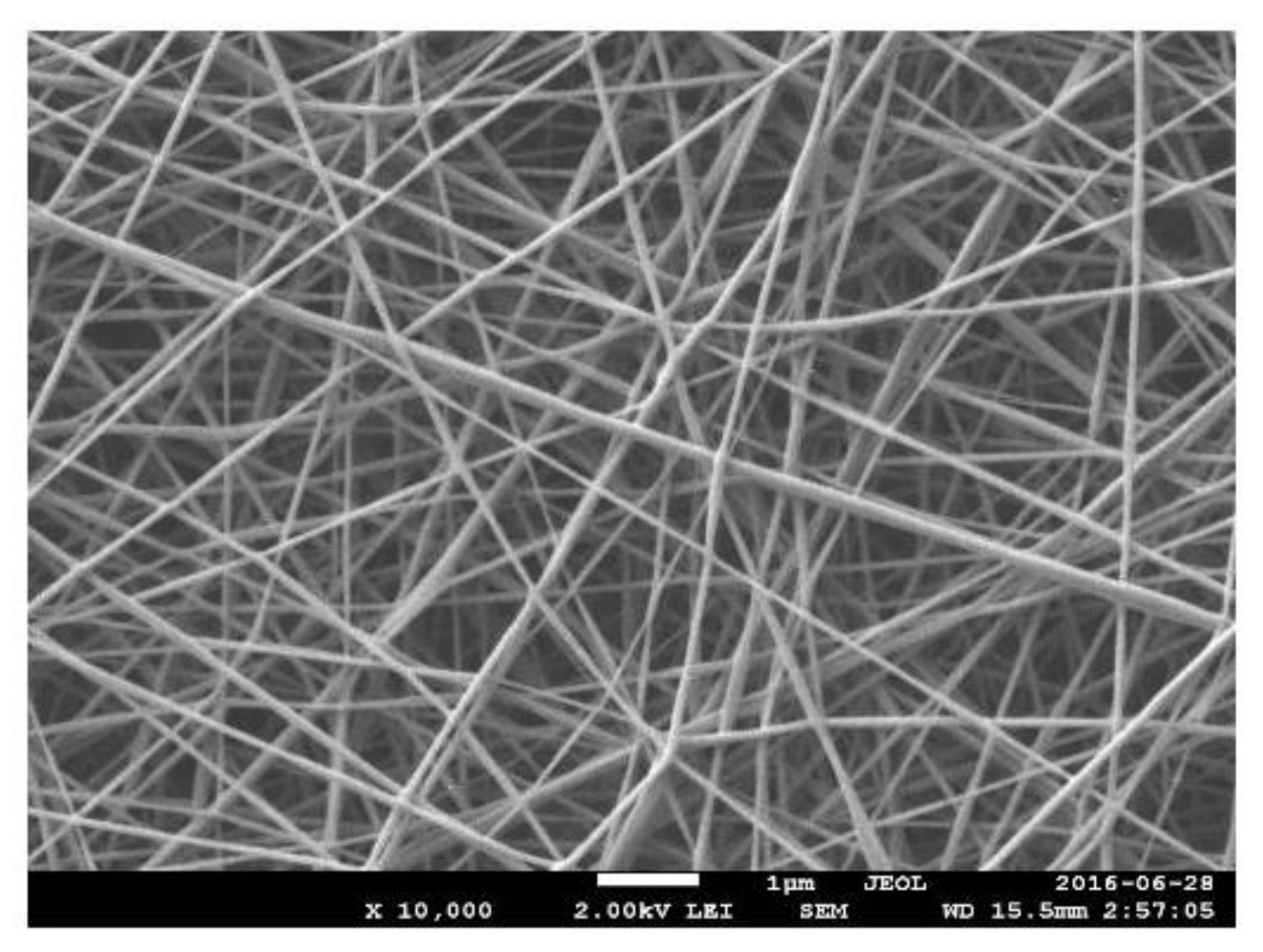
PMMA/PANi Nanofibers
9. Conclusions
- (1)
- For PMMA/PANi copolymer, the selection of the second monomer to copolymerize with PANi must consist in specific functional groups to incorporate into the backbone of PANi without reducing its crystallinity.
- (2)
- Solving the problem of incompatibility of the mixed polymer by optimizing certain parameters such as molecular weight and viscosity for both polymers and choosing the right solvent during mixing.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rohm, O.; Walter, B. Glass Substitute and Process of Preparing. U.S. Patent No. 2,193,742, 12 March 1940. [Google Scholar]
- Umar, A.; Karim, K.J.B.A.; Buang, N.A. A review of the properties and applications of poly (methyl methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar]
- Priyanka, D.; Choudhary, S.; Sengwa, R.J. Electrochemical performance of Li+-ion conducting solid polymer electrolytes based on PEO–PMMA blend matrix incorporated with various inorganic nanoparticles for the lithium ion batteries. Compos. Commun. 2018, 10, 11–17. [Google Scholar]
- Bergfelt, A.; Rubatat, L.; Mogensen, R.; Brandell, D.; Bowden, T. D8-poly(methyl methacrylate)-poly[(oligo ethylene glycol) methyl ether methacrylate] tri-block-copolymer electrolytes: Morphology, conductivity and battery performance. Polymer 2017, 131, 234–242. [Google Scholar] [CrossRef]
- Zhihua, L.; Gong, L. Research Progress on Applications of Polyaniline (PANI) for Electrochemical Energy Storage and Conversion. Materials 2020, 13, 548. [Google Scholar]
- Gholami Laelabadi, K.; Moradian, R.; Manouchehhri, I. One-step fabrication of flexible, cost/time effective and high energy storage reduced graphene oxide@ PANI supercapacitor. ACS Appl. Energy Mater. 2020, 3, 5301–5312. [Google Scholar] [CrossRef]
- Letheby, H. XXIX.—On the production of a blue substance by the electrolysis of sulphate of aniline. J. Chem. Soc. 1862, 15, 161–163. [Google Scholar] [CrossRef] [Green Version]
- Green, A.G.; Woodhead, A.E. CCXLIII.—Aniline-black and allied compounds. Part I. J. Chem. Soc. Trans. 1910, 97, 2388–2403. [Google Scholar] [CrossRef]
- Ankit, G.; Kumar, M. Synthesis of polyaniline without metal doping and its characterization. J. Mater. Sci. Surf. Eng. 2018, 6, 802–804. [Google Scholar]
- Alesary, H.F.; Ismail, H.K.; Khudhair, A.F.; Mohammed, M.Q. Effects of dopant ions on the properties of polyaniline conducting polymer. Orient. J. Chem. 2018, 34, 2525. [Google Scholar] [CrossRef] [Green Version]
- Roslan, N.C.; Aizamddin, M.F.; Omar, S.N.I.; Jani, N.A.; Halim, M.I.A.; Ariffin, Z.Z.; Mahat, M.M. Morphological and Conductivity Studies of Polyaniline Fabric Doped Phosphoric Acid. Malays. J. Anal. Sci. 2020, 24, 698–706. [Google Scholar]
- Usman, F.; Dennis, J.O.; Ahmed, A.Y.; Seong, K.C.; Fen, Y.W.; Sadrolhosseini, A.R.; Meriaudeau, F.; Kumar, P.; Ayodele, O.B. Structural characterization and optical constants of p-toluene sulfonic acid doped polyaniline and its composites of chitosan and reduced graphene-oxide. J. Mater. Res. Technol. 2020, 9, 1468–1476. [Google Scholar] [CrossRef]
- Nakova, A.; Ilieva, M.; Boiadjieva-Scherzer, T.; Tsakova, V. Electroless deposition of palladium nanoparticles on poly (3, 4-ethylene-dioxythiophene)—role of the electrode substrate. J. Solid State Electrochem. 2018, 22, 1901–1908. [Google Scholar] [CrossRef]
- Thanh-Hai, L.; Kim, Y.; Yoon, H. Electrical and electrochemical properties of conducting polymers. Polymers 2017, 9, 150. [Google Scholar]
- Tan, Y.; Liu, Y.; Kong, L.; Kang, L.; Xu, C.; Ran, F. In situ doping of PANI nanocomposites by gold nanoparticles for high-performance electrochemical energy storage. J. Appl. Polym. Sci. 2017, 134, 45309. [Google Scholar] [CrossRef]
- Ameen Sadia, M.; Akhtar, S.; Husain, M. A review on synthesis processing, chemical and conduction properties of polyaniline and its nanocomposites. Sci. Adv. Mater. 2010, 2, 441–462. [Google Scholar] [CrossRef]
- Jia, M.Y.; Zhang, Z.M.; Yu, L.M.; Wang, J. PANI-PMMA as cathodic electrode material and its application in cathodic polarization antifouling. Electrochem. Commun. 2017, 84, 57–60. [Google Scholar] [CrossRef]
- Banerjee, D.; Kar, A.K. Influence of polaron doping and concentration dependent FRET on luminescence of PAni–PMMA blends for application in PLEDs. Phys. Chem. Chem. Phys. 2018, 20, 23055–23071. [Google Scholar] [CrossRef]
- Smita, D.; Kar, A.K. Enhanced photoluminescence through Forster resonance energy transfer in Polypyrrole-PMMA blends for application in optoelectronic devices. Mater. Sci. Semicond. Process. 2019, 103, 104644. [Google Scholar]
- Vu, D.L.; Li, Y.Y.; Lin, T.H.; Wu, M.C. Fabrication and humidity sensing property of UV/ozone treated PANI/PMMA electrospun fibers. J. Taiwan Inst. Chem. Eng. 2019, 99, 250–257. [Google Scholar] [CrossRef]
- Zhang, H.D.; Tang, C.C.; Long, Y.Z.; Zhang, J.C.; Huang, R.; Li, J.J.; Gu, C.Z. High-sensitivity gas sensors based on arranged polyaniline/PMMA composite fibers. Sens. Actuators A Phys. 2014, 219, 123–127. [Google Scholar] [CrossRef]
- Rajashree, A.; Kondawar, S. Electrospun Poly (methyl Methacrylate)/Polyaniline Blend Nanofibres with Enhanced Toxic Gas Sensing at Room Temperature. J. Phys. Sci. 2018, 29, 101–119. [Google Scholar]
- Beregoi, M.; Busuioc, C.; Evanghelidis, A.; Matei, E.; Iordache, F.; Radu, M.; Dinischiotu, A.; Enculescu, I. Electrochromic properties of polyaniline-coated fiber webs for tissue engineering applications. Int. J. Pharm. 2016, 510, 465–473. [Google Scholar] [CrossRef]
- Beregoi, M.; Evanghelidis, A.; Ganea, P.; Iovu, H.; Matei, E.; Enculescu, I. One side polyaniline coated fibers based actuator. Univ. Politeh. Buchar. Sci. Bull. Ser. B Chem. Mater. Sci. 2017, 79, 119–130. [Google Scholar]
- Asima, N.; Sattar, R.; Siddiq, M. Preparation and properties of high performance multilayered PANi/PMMA/PPG-b-PEG-b-PPG/FGHMDA nanocomposites via in situ polymerization. Polym. Plast. Technol. Mater. 2019, 58, 282–294. [Google Scholar]
- Aksimentyeva, O.; Konopelnyk, O.; Opaynych, I.; Tsizh, B.; Ukrainets, A.; Ulansky, Y.; Martyniuk, G. Interaction of components and conductivity in polyaniline–polymethylmethacrylate nanocomposites. Rev. Adv. Mater. Sci. 2010, 23, 30–34. [Google Scholar]
- Moussa, M.A.; Ghoneim, A.M.; Abdel Rehim, M.H.; Khairy, S.A.; Soliman, M.A.; Turky, G.M. Relaxation dynamic and electrical mobility for poly (methyl methacrylate)-polyaniline composites. J. Appl. Polym. Sci. 2017, 134, 45415. [Google Scholar] [CrossRef]
- Jabur Akram, R. Effect of polyaniline on the electrical conductivity and activation energy of electrospun nylon films. Int. J. Hydrog. Energy 2018, 43, 530–536. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Jin, Y.; Qiu, Y. The optical and electrical characteristics of PMMA film prepared by spin coating method. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Beijing, China, 16–18 May 2015; Volume 87. [Google Scholar]
- Xavier, P.A.; Sreekumar, V.; Amrithesh, M.; Varghese, T. Structural, mechanical and electrical characterization of polyaniline/polymethylmethacrylate blends. In Proceedings of AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2020; Volume 2263. [Google Scholar]
- Feng, J.; Athanassiou, A.; Bonaccorso, F.; Fragouli, D. Enhanced electrical conductivity of poly (methyl methacrylate) filled with graphene and in situ synthesized gold nanoparticles. Nano Futures 2018, 2, 025003. [Google Scholar] [CrossRef]
- Amrithesh, M.; Aravind, S.; Jayalekshmi, S.; Jayasree, R.S. Enhanced luminescence observed in polyaniline–polymethylmethacrylate composites. J. Alloy. Compd. 2008, 449, 176–179. [Google Scholar] [CrossRef]
- Raja, L.; Daniel, S.C.G.K. Engineered Nanomaterials for Organic Light-Emitting Diodes (OLEDs). In Handbook of Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 312–323. [Google Scholar]
- Schenning, A.P.H.J.; Meijer, E.W. Functional Conjugated Polymers, Molecular Design of: Architecture. In The Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 3400–3407. [Google Scholar]
- Rozik Nehad, N.; Aman, I.; Azza, K.; Ward, A. Studies the behaviors of polyaniline on the properties of PS/PMMA blends. Proceedings of the Institution of Mechanical Engineers, Part L. J. Mater. Des. Appl. 2016, 230, 526–536. [Google Scholar]
- Saad Ali, S.; Pauly, A.; Brunet, J.; Varenne, C.; Ndiaye, A.L. MWCNTs/PMMA/PS composites functionalized PANI: Electrical characterization and sensing performance for ammonia detection in a humid environment. Sens. Actuators B Chem. 2020, 320, 128364. [Google Scholar] [CrossRef]
- Savest, N.; Plamus, T.; Kütt, K.; Kallavus, U.; Viirsalu, M.; Tarasova, E.; Vassiljeva, V.; Krasnou, I.; Krumme, A. Electrospun conductive mats from PANi-ionic liquid blends. J. Electrost. 2018, 96, 40–44. [Google Scholar] [CrossRef]
- Kenry; Liu, B. Recent advances in biodegradable conducting polymers and their biomedical applications. Biomacromolecules 2018, 19, 1783–1803. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Qiu, J.; Wu, X. Well-defined polyaniline nanotubes and nanofibers surface-modified with poly (methyl methacrylate) via in-situ radical polymerization. Mater. Lett. 2012, 77, 4–6. [Google Scholar]
- Ghorbani, M.; Fazli, S.; Soleimani Lashkenari, M. Fabrication of PMMA/PANI/Fe3O4 as a novel conducting hybrid coating. Polym. Plast. Technol. Eng. 2018, 57, 591–599. [Google Scholar] [CrossRef]
- Tomar, A.K.; Mahendia, S.; Chahal, R.; Kumar, S. Electrical studies of PMMA blended with iron loaded polyaniline. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2019; Volume 2093. [Google Scholar]
- Araújo, P.L.B.; Araújo, E.S.; Santos, R.F.S.; Pacheco, A.P.L. Synthesis and morphological characterization of PMMA/polyaniline nanofiber composites. Microelectron. J. 2005, 36, 1055–1057. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, E.; Kim, K.; Lee, B.H.; Choe, S. Polyaniline effect on the conductivity of the PMMA/Ag hybrid composite. Colloids Surf. A Physicochem. Eng. Asp. 2012, 396, 195–202. [Google Scholar] [CrossRef]
- Jum’h, I.; Mousa, M.S.; Mhawish, M.; Sbeih, S.; Telfah, A. Optical and structural properties of (PANI-CSA-PMMA)/NiNPs nanocomposites thin films for organic optical filters. J. Appl. Polym. Sci. 2020, 137, 48643. [Google Scholar] [CrossRef]
- Abutalib, M.M. Insights into the structural, optical, thermal, dielectric, and electrical properties of PMMA/PANI loaded with graphene oxide nanoparticles. Phys. B Condens. Matter. 2019, 552, 19–29. [Google Scholar] [CrossRef]
- Jankowska, K.; Zdarta, J.; Grzywaczyk, A.; Kijeńska-Gawrońska, E.; Biadasz, A.; Jesionowski, T. Electrospun poly (methyl methacrylate)/polyaniline fibres as a support for laccase immobilisation and use in dye decolourisation. Environ. Res. 2020, 184, 109332. [Google Scholar] [CrossRef]
- Ray, S.; Easteal, A.J.; Cooney, R.P.; Edmonds, N.R. Structure and properties of melt-processed PVDF/PMMA/polyaniline blends. Mater. Chem. Phys. 2009, 113, 829–838. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Yu, L.; Jiang, T. Hydrophobic polystyrene/electro-spun polyaniline coatings for corrosion protection. Synth. Met. 2017, 234, 166–174. [Google Scholar] [CrossRef]
- Jia, M.-Y.; Waterhouse, G.I.N.; Zhang, J.Y.; Zhang, Z.M.; Wang, J.; Yu, L.-M. Comparison of the corrosion protection of electro-spun and drop-cast polyaniline microfiber coatings on carbon steel. Synth. Met. 2018, 246, 204–212. [Google Scholar] [CrossRef]
- Salvatierra, R.V.; Zitzer, G.; Savu, S.A.; Alves, A.P.; Zarbin, A.J.G.; Chassé, T.; Casu, M.B.; Rocco, M.L.M. Carbon nanotube/polyaniline nanocomposites: Electronic structure, doping level and morphology investigations. Synth. Met. 2015, 203, 16–21. [Google Scholar] [CrossRef]
- Zainal, A.N.F.; Chan, C.H. Crystallization and melting behavior of compatibilized polymer blends. In Compatibilization of Polymer Blends; Elsevier: Amsterdam, The Netherlands, 2020; pp. 391–433. [Google Scholar]
- Farrell, T.; Wang, K.; Lin, C.W.; Kaner, R.B. Organic dispersion of polyaniline and single-walled carbon nanotubes and polyblends with poly (methyl methacrylate). Polymer 2017, 129, 1–4. [Google Scholar] [CrossRef]
- Kumar, A.; Kumari, Y.; Kumar Jangir, L.; Kumar, V. Structural and morphological study of poly (methyl methacrylate)/polyaniline composite membranes. Mater. Today Proc. 2020, 31, 674–678. [Google Scholar] [CrossRef]
- Fattoum, A.; Othman, Z.B.; Arous, M. Dc and Ac conductivity of polyaniline/poly (methyl methacrylathe) blends below the percolation threshold. Mater. Chem. Phys. 2012, 135, 117–122. [Google Scholar] [CrossRef]
- Dimitriev, O.P.; Kopylov, O.N.; Tracz, A. Mechanisms of polyaniline film formation via solution casting: Intra-chain contraction versus inter-chain association. Eur. Polym. J. 2015, 66, 119–128. [Google Scholar] [CrossRef]
- Zeng, F.; Qin, Z.; Liang, B.; Liu, N.; Zhou, Z.; Zhu, M. Polyaniline nanostructures tuning with oxidants in interfacial polymerization system. Prog. Nat. Sci. Mater. Int. 2015, 25, 512–519. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Fan, J.; Wang, S. Recent progress in interfacial polymerization. Mater. Chem. Front. 2017, 1, 1028–1040. [Google Scholar] [CrossRef]
- Li, T.; Qin, Z.; Liang, B.; Tian, F.; Zhao, J.; Liu, N.; Zhu, M. Morphology-dependent capacitive properties of three nanostructured polyanilines through interfacial polymerization in various acidic media. Electrochim. Acta 2015, 177, 343–351. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, Y.; Dong, Y.; He, F.; Zhao, X.; Yin, J. Low-Temperature Interfacial Polymerization and Enhanced Electro-Responsive Characteristic of Poly (ionic liquid) s@ polyaniline Core-shell Microspheres. Macromol. Rapid Commun. 2019, 40, 1800351. [Google Scholar] [CrossRef]
- Zhai, D.; Zhu, M.; Chen, S.; Yin, Y.; Shang, X.; Li, L.; Zhou, G.; Peng, J. Effect of Block Sequence in All-Conjugated Triblock Copoly (3-alkylthiophene) s on Control of the Crystallization and Field-Effect Mobility. Macromolecules 2020, 53, 5775–5786. [Google Scholar] [CrossRef]
- Bortamuly, R.; Konwar, G.; Boruah, P.K.; Das, M.R.; Mahanta, D.; Saikia, P. CeO2-PANI-HCl and CeO2-PANI-PTSA composites: Synthesis, characterization, and utilization as supercapacitor electrode materials. Ionics 2020, 26, 5747–5756. [Google Scholar] [CrossRef]
- Banerjee, J.; Dutta, K.; Kader, M.A.; Nayak, S.K. An overview on the recent developments in polyaniline-based supercapacitors. Polym. Adv. Technol. 2019, 30, 1902–1921. [Google Scholar] [CrossRef]
- Wang, H.; Wen, H.; Hu, B.; Fei, G.; Shen, Y.; Sun, L.; Yang, D. Facile approach to fabricate waterborne polyaniline nanocomposites with environmental benignity and high physical properties. Sci. Rep. 2017, 7, 43694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Zhang, J.; Ye, F.; Wang, W.; Wang, G.; Zhang, Z.; Li, S.; Zhou, Y.; Cai, J. Vulcanization treatment: An effective way to improve the electrochemical cycle stability of polyaniline in supercapacitors. J. Power Sources 2019, 443, 227246. [Google Scholar] [CrossRef]
- Waghuley, S.A. Applications of Polyaniline-Based Blends, Composites, and Nanocomposites. In Polyaniline Blends, Composites, and Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2018; pp. 257–277. [Google Scholar]
- Lai, X.; Hu, G.; Peng, Z.; Tong, H.; Lu, Y.; Wang, Y.; Qi, X.; Xue, Z.; Huang, Y.; Du, K.; et al. Surface structure decoration of high capacity Li1.2Mn0.54Ni0.13Co0.13O2 cathode by mixed conductive coating of Li1.4Al0.4Ti1.6 (PO4)3 and polyaniline for lithium-ion batteries. J. Power Sources 2019, 431, 144–152. [Google Scholar] [CrossRef]
- Zohra, H.F.; Naar, N. Improvement of the structural and electrical properties of PMMA/PANI-MA blends synthesized by interfacial in situ polymerization in a continuous organic phase. Polym. Bull. 2020, 1–27. [Google Scholar]
- Yin, C.; Gao, L.; Zhou, F.; Duan, G. Facile synthesis of polyaniline nanotubes using self-assembly method based on the hydrogen bonding: Mechanism and application in gas sensing. Polymers 2017, 9, 544. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Chen, S.; Wang, Y.; Peng, X.; Zhang, W.; Chen, J. Excellent Electrochemical Performances of Cabbage-like Polyaniline Fabricated by Template Synthesis. J. Power Sources 2016, 321, 94–101. [Google Scholar] [CrossRef]
- Zhao, Y.; Quan, X.; Li, C. Facile preparation of etched halloysite@ polyaniline nanorods and their enhanced electrochemical capacitance performance. Electrochim. Acta 2019, 321, 134715. [Google Scholar] [CrossRef]
- Singh, R.; Choudhary, R.B.; Kandulna, R. Optical Band Gap Tuning and Thermal Properties of PMMA-ZnO Sensitized Polymers for Efficient Exciton Generation in Solar Cell Application. Mater. Sci. Semicond. Process. 2019, 103, 104623. [Google Scholar] [CrossRef]
- Della Pina, C.; Busacca, C.; Frontera, P.; Antonucci, P.L.; Scarpino, L.A.; Sironi, A.; Falletta, E. Advances in Poly(4-Aminodiphenylaniline) Nanofibers Preparation by Electrospinning Technique. J. Nanosci. Nanotechnol. 2016, 16, 5369–5377. [Google Scholar] [CrossRef]
- Sidwaba, U.; Feleni, U.; Makelane, H.; Nxusani, E.; Wilson, L.; Qakala, S.; Iwuoha, E. A novel polyaniline nanocomposite with doping effects of poly(methyl methacrylate) and TiO2 nanoparticles. In Journal of Nano Research; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2016; Volume 44, pp. 281–292. [Google Scholar]
- Tan, W.; Fu, R.; Ji, H.; Wu, D.; Xu, Y.; Kong, Y. Comparison of supercapacitive behaviors of polyaniline doped with two low-molecular-weight organic acids: D-tartaric acid and citric acid. Adv. Polym. Technol. 2018, 37, 3038–3044. [Google Scholar] [CrossRef]
- Biswas, S.; Jeong, H.S.; Jeong, J.B.; Cho, B.; Lee, S.; Lee, D.W.; Kim, H. Synthesis and charge transport of polymer nanocomposite of polyaniline: Polystyrene sulfonate. J. Nanosci. Nanotechnol. 2019, 19, 4638–4642. [Google Scholar] [CrossRef]
- Mukherjee, P.S.; Gupta, K.; Chakraborty, G.; Meikap, A.K. Response of magnetic field and temperature on electrical transport of polyaniline–malic acid nanocomposite. Polym. Compos. 2016, 37, 1042–1048. [Google Scholar] [CrossRef]
- Ingle, R.V.; Shaikh, S.F.; Bhujbal, P.K.; Pathan, H.M.; Tabhane, V.A. Polyaniline doped with protonic acids: Optical and morphological studies. ES Mater. Manuf. 2020, 8, 54–59. [Google Scholar] [CrossRef]
- Golba, S.; Popczyk, M.; Miga, S.; Jurek-Suliga, J.; Zubko, M.; Kubisztal, J.; Balin, K. Impact of Acidity Profile on Nascent Polyaniline in the Modified Rapid Mixing Process—Material Electrical Conductivity and Morphological Study. Materials 2020, 13, 5108. [Google Scholar] [CrossRef]
- Noby, H.; El-Shazly, A.H.; Elkady, M.F.; Ohshima, M. Strong acid doping for the preparation of conductive polyaniline nanoflowers, nanotubes, and nanofibers. Polymer 2019, 182, 121848. [Google Scholar] [CrossRef]
- Cabuk, M.; Gündüz, B. Controlling the optical properties of polyaniline doped by boric acid particles by changing their doping agent and initiator concentration. Appl. Surf. Sci. 2017, 424, 345–351. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Katunin, A. The effect of reaction medium on the conductivity and morphology of polyaniline doped with camphorsulfonic acid. Synth. Met. 2016, 214, 45–49. [Google Scholar] [CrossRef]
- Chen, X.P.; Liang, Q.H.; Jiang, J.K.; Wong, C.K.Y.; Leung, S.Y.Y.; Ye, H.Y.; Yang, D.G.; Ren, T.L. Functionalization-induced changes in the structural and physical properties of amorphous polyaniline: A first-principles and molecular dynamics study. Sci. Rep. 2016, 6, 20621. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.; Varghese, J.; Sebastian, M.T. Self assembled polyaniline nanofibers with enhanced electromagnetic shielding properties. RSC Adv. 2015, 5, 20459–20466. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, Y.; Liu, J.; Ma, G.; Huang, M. Wormlike acid-doped polyaniline: Controllable electrical properties and theoretical investigation. J. Phys. Chem. C 2018, 122, 2032–2040. [Google Scholar] [CrossRef]
- Mazzeu, M.A.C.; Faria, L.K.; Cardoso, A.D.M.; Gama, A.M.; Baldan, M.R.; Gonçalves, E.S. Structural and morphological characteristics of polyaniline synthesized in pilot scale. J. Aerosp. Technol. Manag. 2017, 9, 39–47. [Google Scholar] [CrossRef]
- Lee, H.J.; Hur, S.O.; Ahn, M.K.; Changez, M.; Lee, J.S. In situ formation of molecular-scale ordered polyaniline films by zinc coordination. Nanoscale 2017, 9, 6545–6550. [Google Scholar] [CrossRef]
- Bhadra, J.; Al-Thani, N.J.; Madi, N.K.; Al-Maadeed, M.A. Effects of aniline concentrations on the electrical and mechanical properties of polyaniline polyvinyl alcohol blends. Arab. J. Chem. 2017, 10, 664–672. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Li, Y.; Feng, Y.; Hu, W.; Feng, W. Hierarchical graphene oxide/polyaniline nanocomposites prepared by interfacial electrochemical polymerization for flexible solid-state supercapacitors. J. Mater. Chem. A 2015, 3, 2135–2143. [Google Scholar] [CrossRef]
- Qiu, S.; Chen, C.; Cui, M.; Li, W.; Zhao, H.; Wang, L. Corrosion protection performance of waterborne epoxy coatings containing self-doped polyaniline nanofiber. Appl. Surf. Sci. 2017, 407, 213–222. [Google Scholar] [CrossRef]
- Yan, T.; Zhang, M.; Jiang, J.; Chen, N. Synthesis of DBSA-doped polyaniline by emulsion polymerization and PANI/PLA electrospun fiber membrane conductivity. J. Text. Inst. 2019, 110, 274–281. [Google Scholar] [CrossRef]
- Jun, C.S.; Kwon, S.H.; Choi, H.J.; Seo, Y. Polymeric nanoparticle-coated Pickering emulsion-synthesized conducting polyaniline hybrid particles and their electrorheological study. ACS Appl. Mater. Interfaces 2017, 9, 44811–44819. [Google Scholar] [CrossRef]
- Bilal, S.; Gul, S.; Holze, R. An impressive emulsion polymerization route for the synthesis of highly soluble and conducting polyaniline salts. Synth. Met. 2015, 206, 131–144. [Google Scholar] [CrossRef]
- Palaniappan, S.; John, A. Polyaniline materials by emulsion polymerization pathway. Prog. Polym. Sci. 2008, 33, 732–758. [Google Scholar] [CrossRef]
- Nazari, H.; Arefinia, R. An investigation into the relationship between the electrical conductivity and particle size of polyaniline in nano scale. Int. J. Polym. Anal. Charact. 2019, 24, 178–190. [Google Scholar] [CrossRef]
- Chajanovsky, I.; Suckeveriene, R.Y. Preparation of Hybrid Polyaniline/Nanoparticle Membranes for Water Treatment Using an Inverse Emulsion Polymerization Technique under Sonication. Processes 2020, 8, 1503. [Google Scholar] [CrossRef]
- Mohammad, M.; Kamarudin, S.; Mohamed, N.H.; Asim, N.; Sopian, K. Homogenization Effect on Nanostructure and Conductivity of Polyaniline Nanofibre Synthesis by Mini-Emulsion Polymerization Technique. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Melbourne, Australia, 15–17 November 2017; IOP Publishing: Bristol, UK, 2017; Volume 293. [Google Scholar]
- Singu, B.S.; Srinivasan, P.; Yoon, K.R. Emulsion polymerization method for polyaniline-multiwalled carbon nanotube nanocomposites as supercapacitor materials. J. Solid State Electrochem. 2016, 20, 3447–3457. [Google Scholar] [CrossRef]
- Saeb, M.R.; Zarrintaj, P.; Khandelwal, P.; Chauhan, N.P.S. Synthetic route of polyaniline (I): Conventional oxidative polymerization. In Fundamentals and Emerging Applications of Polyaniline; Elsevier: Amsterdam, The Netherlands, 2019; pp. 17–41. [Google Scholar]
- Amorim, D.R.B.; da Silva Guimarães, I.; Fugikawa-Santos, L.; Vega, M.L.; da Cunha, H.N. Effect of temperature on the electrical conductivity of polyaniline/cashew gum blends. Mater. Chem. Phys. 2020, 253, 123383. [Google Scholar] [CrossRef]
- Wang, H.L.; Romero, R.J.; Mattes, B.R.; Zhu, Y.; Winokur, M.J. Effect of processing conditions on the properties of high molecular weight conductive polyaniline fiber. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 194–204. [Google Scholar] [CrossRef]
- Farrokhzad, H.; Darvishmanesh, S.; Genduso, G.; Van Gerven, T.; Van Der Bruggen, B. Development of bivalent cation selective ion exchange membranes by varying molecular weight of polyaniline. Electrochim. Acta 2015, 158, 64–72. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, A.; Wang, Q.; Fan, X.; Cavaco-Paulo, A.; Zhang, Y. Conductive cotton prepared by polyaniline in situ polymerization using laccase. Appl. Biochem. Biotechnol. 2014, 174, 820–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yılmaz, F.; Küçükyavuz, Z. The influence of polymerization temperature on structure and properties of polyaniline. e-Polymers 2009, 9. [Google Scholar] [CrossRef] [Green Version]
- Maity, P.C.; Khandelwal, M. Synthesis time and temperature effect on polyaniline morphology and conductivity. Am. J. Mater. Synth. Process. 2016, 1, 37–42. [Google Scholar]
- Bláha, M.; Varga, M.; Prokeš, J.; Zhigunov, A.; Vohlídal, J. Effects of the polymerization temperature on the structure, morphology and conductivity of polyaniline prepared with ammonium peroxodisulfate. Eur. Polym. J. 2013, 49, 3904–3911. [Google Scholar] [CrossRef]
- Park, C.H.; Jang, S.K.; Kim, F.S. Conductivity enhancement of surface-polymerized polyaniline films via control of processing conditions. Appl. Surf. Sci. 2018, 429, 121–127. [Google Scholar] [CrossRef]
- Raza, A.; Nasir, A.; Tahir, M.; Taimur, S.; Yasin, T.; Nadeem, M. Synthesis and EMI shielding studies of polyaniline grafted conducting nanohybrid. J. Appl. Polym. Sci. 2021, 138, 49680. [Google Scholar] [CrossRef]
- Ibrahim, K.A. Synthesis and characterization of polyaniline and poly (aniline-co-o-nitroaniline) using vibrational spectroscopy. Arab. J. Chem. 2017, 10, S2668–S2674. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Wang, X.; Guo, J.; Liu, P. Effect of the oxidant/monomer ratio and the washing post-treatment on electrochemical properties of conductive polymers. Ind. Eng. Chem. Res. 2014, 53, 13680–13689. [Google Scholar] [CrossRef]
- Navarchian, A.H.; Hasanzadeh, Z.; Joulazadeh, M. Effect of polymerization conditions on reaction yield, conductivity, and ammonia sensing of polyaniline. Adv. Polym. Technol. 2013, 32. [Google Scholar] [CrossRef]
- Stejskal, J.; Bober, P.; Trchová, M.; Horský, J.; Pilař, J.; Walterová, Z. The oxidation of aniline with p-benzoquinone and its impact on the preparation of the conducting polymer, polyaniline. Synth. Met. 2014, 192, 66–73. [Google Scholar] [CrossRef]
- Lauter, V.; Lauter, H.; Glavic, A.; Toperverg, B. Reflectivity, Off-Specular Scattering, and GISANS Neutrons. In Reference Module in Materials Science and Materials Engineering; Hashmi, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–27. [Google Scholar]
- Shen, J.; Shahid, S.; Sarihan, A.; Patterson, D.A.; Emanuelsson, E.A. Effect of polyacid dopants on the performance of polyaniline membranes in organic solvent nanofiltration. Sep. Purif. Technol. 2018, 204, 336–344. [Google Scholar] [CrossRef]
- Abd El-Mageed, H.R.; Abd El-Salam, H.M.; Abdel-Latif, M.K.; Mustafa, F.M. Preparation and spectroscopic properties, density functional theory calculations and nonlinear optical properties of poly (acrylic acid-co-acrylamide)-graft-polyaniline. J. Mol. Struct. 2018, 1173, 268–279. [Google Scholar] [CrossRef]
- Abbaspoor, S.; Agbolaghi, S.; Nazari, M.; Abbasi, F. Disperse-within-disperse patterning on ternary/binary mixed-brush single crystals using polyaniline, polystyrene and poly (methyl methacrylate) grafts. J. Polym. Res. 2017, 24, 160. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, J.U.; Jo, J.W.; Bae, S.; Kim, K.T.; Jo, W.H. In-situ preparation of graphene/poly (styrenesulfonic acid-graft-polyaniline) nanocomposite via direct exfoliation of graphite for supercapacitor application. Carbon 2016, 105, 191–198. [Google Scholar] [CrossRef]
- Massoumi, B.; Mohammad-Rezaei, R.; Jaymand, M. Chemical and electrochemical grafting of polyaniline onto poly (vinyl chloride): Synthesis, characterization, and materials properties. Polym. Adv. Technol. 2016, 27, 1056–1063. [Google Scholar] [CrossRef]
- Fazullin, D.D.; Mavrin, G.V.; Sokolov, M.P.; Shaikhiev, I.G. Infrared spectroscopic studies of the PTFE and nylon membranes modified polyaniline. Mod. Appl. Sci. 2015, 9, 242. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, R.; Karimi, M.; Mofrad, R.T.; Asadpour-Zeynali, K.; Entezami, A.A. Electroactive nanofibers of poly (2-hydroxyethyl methacrylate-graft-aniline) copolymers and their blends with polycaprolactone. Polym. Plast. Technol. Eng. 2015, 54, 21–32. [Google Scholar] [CrossRef]
- Nazari, M.; Agbolaghi, S.; Gheybi, H.; Abbaspoor, S.; Abbasi, F. A focus on the features of polyaniline nanofibres prepared via developing the single crystals of their block copolymers with poly (ethylene glycol). Bull. Mater. Sci. 2018, 41, 29. [Google Scholar] [CrossRef] [Green Version]
- Nazari, M.; Agbolaghi, S.; Abbaspoor, S.; Gheybi, H.; Abbasi, F. Arrangement of conductive rod nanobrushes via conductive–dielectric–conductive sandwiched single crystals of poly (ethylene glycol) and polyaniline block copolymers. Macromolecules 2015, 48, 8947–8957. [Google Scholar] [CrossRef]
- Huang, K.; Canterbury, D.P.; Rzayev, J. Organosoluble polypyrrole nanotubes from core–shell bottlebrush copolymers. Chem. Commun. 2010, 46, 6326–6328. [Google Scholar] [CrossRef]
- Hu, J.; Liu, S. Responsive polymers for detection and sensing applications: Current status and future developments. Macromolecules 2010, 43, 8315–8330. [Google Scholar] [CrossRef]
- Wang, L.; Muslim, A.; Turdi, D.; Salam, M.; Tursun, Z. Controllable length adjustment of polyaniline particle with temperature sensitive block copolymer as template. Polym. Adv. Technol. 2021, 32, 411–419. [Google Scholar] [CrossRef]
- Shimano, J.; Alan, Y.; MacDiarmid, G. Polyaniline, a dynamic block copolymer: Key to attaining its intrinsic conductivity? Synth. Met. 2001, 123, 251–262. [Google Scholar] [CrossRef]
- Kuila, B.K.; Stamm, M. Fabrication of oriented polyaniline nanostructures using block copolymer nanotemplates and their optical, electrochemical and electric properties. J. Mater. Chem. 2010, 20, 6086–6094. [Google Scholar] [CrossRef]
- Chotsuwan, C.; Asawapirom, U.; Shimoi, Y.; Akiyama, H.; Ngamaroonchote, A.; Jiemsakul, T.; Jiramitmongkon, K. Investigation of the electrochromic properties of tri-block polyaniline-polythiophene-polyaniline under visible light. Synth. Met. 2017, 226, 80–88. [Google Scholar] [CrossRef]
- Marcasuzaa, P.; Reynaud, S.; Ehrenfeld, F.; Khoukh, A.; Desbrieres, J. Chitosan-graft-polyaniline-based hydrogels: Elaboration and properties. Biomacromolecules 2010, 11, 1684–1691. [Google Scholar] [CrossRef]
- Jung, J.W.; Lee, J.U.; Jo, W.H. High-efficiency polymer solar cells with water-soluble and self-doped conducting polyaniline graft copolymer as hole transport layer. J. Phys. Chem. C 2010, 114, 633–637. [Google Scholar] [CrossRef]
- Bae, W.J.; Kim, K.H.; Jo, W.H.; Park, Y.H. Exfoliated nanocomposite from polyaniline graft copolymer/clay. Macromolecules 2004, 37, 9850–9854. [Google Scholar] [CrossRef]
- Tan, J.; Xie, Z.; Zhang, Z.; Sun, Y.; Shi, W.; Ge, D. Dopamine modified polyaniline with improved adhesion, dispersibility, and biocompatibility. J. Mater. Sci. 2018, 53, 447–455. [Google Scholar] [CrossRef]
- Bae, W.J.; Jo, W.H.; Park, Y.H. Preparation of polystyrene/polyaniline blends by in situ polymerization technique and their morphology and electrical property. Synth. Met. 2003, 132, 239–244. [Google Scholar] [CrossRef]
- Albertsson, A.-C.; Liu, Y. Comparison between physical blending and copolymerization of poly (trimethylene carbonate) and poly (adipic anhydride) with special regard to compatibility, morphology and degradation. J. Macromol. Sci. Part A Pure Appl. Chem. 1997, 34, 1457–1482. [Google Scholar] [CrossRef]
- Stockton, W.B.; Rubner, M.F. Electrically conducting compatible blends of polyaniline/poly (Vinyl Pyrrolidone). MRS Online Proc. Libr. 1993, 328, 257–262. [Google Scholar] [CrossRef]
- Gheybi, H.; Abbasian, M.; Moghaddam, P.N.; Entezami, A.A. Chemical modification of polyaniline by N-grafting of polystyrene synthesized via ATRP. J. Appl. Polym. Sci. 2007, 106, 3495–3501. [Google Scholar] [CrossRef]
- Edmondson, S.; Gilbert, M. The chemical nature of plastics polymerization. Brydson’s Plast. Mater. 2017, 19–37. [Google Scholar]
- Chen, M.; Zhong, M.; Johnson, J.A. Light-controlled radical polymerization: Mechanisms, methods, and applications. Chem. Rev. 2016, 116, 10167–10211. [Google Scholar] [CrossRef] [Green Version]
- Su, W.-F. Ionic Chain Polymerization. In Principles of Polymer Design and Synthesis; Springer: Heidelberg/Berlin, Germany, 2013; pp. 185–218. [Google Scholar]
- Sun, L.; Campbell, M.G.; Dincă, M. Electrically conductive porous metal–organic frameworks. Angew. Chem. Int. Ed. 2016, 55, 3566–3579. [Google Scholar] [CrossRef]
- Zhuiykov, S. Nanostructured Semiconductors; Woodhead Publishing: Cambridge, UK, 2018. [Google Scholar]
- Bandeira, R.M.; Bezerra Amorim, D.R.; Vega, M.L.; Elias de Matos, J.M.; Ribeiro dos Santos Júnior, J.; Nunes da Cunha, H. Charge transport in benzoic acid-doped polyaniline films. Mater. Chem. Phys. 2020, 260, 124083. [Google Scholar] [CrossRef]
- Wang, Y. Preparation and application of polyaniline nanofibers: An overview. Polym. Int. 2018, 67, 650–669. [Google Scholar] [CrossRef]
- Wu, R.; Yuan, H.; Liu, C.; Lan, J.L.; Yang, X.; Lin, Y.H. Flexible PANI/SWCNT thermoelectric films with ultrahigh electrical conductivity. RSC Adv. 2018, 8, 26011–26019. [Google Scholar] [CrossRef] [Green Version]
- Rahayu, I.; Eddy, D.R.; Novianty, A.R.; Rukiah; Anggreni, A.; Bahti, H.; Hidayat, S. The effect of hydrochloric acid-doped polyaniline to enhance the conductivity. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Semarang, Indonesia, 7–8 September 2018; IOP Publishing: Bristol, UK, 2019; Volume 509. [Google Scholar]
- Mondal, S.; Rana, U.; Malik, S. Graphene quantum dot-doped polyaniline nanofiber as high performance supercapacitor electrode materials. Chem. Commun. 2015, 51, 12365–12368. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, B.; Ni, T.; Dai, T.; Lu, Y. One-step synthesis of iodine doped polyaniline-reduced graphene oxide composite hydrogel with high capacitive properties. Compos. Sci. Technol. 2015, 109, 12–17. [Google Scholar] [CrossRef]
- Ai, Y.; Wu, M.; Li, L.; Zhao, F.; Zeng, B. Highly selective and effective solid phase microextraction of benzoic acid esters using ionic liquid functionalized multiwalled carbon nanotubes-doped polyaniline coating. J. Chromatogr. A 2016, 1437, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Hu, Y.; Wang, L.; Guo, Q.; Chen, S.; Chen, S.; Hou, H.; Song, Y. Macroporous carbon/nitrogen-doped carbon nanotubes/polyaniline nanocomposites and their application in supercapacitors. Electrochim. Acta 2016, 189, 158–165. [Google Scholar] [CrossRef]
- El-Khodary, S.A.; El-Enany, G.M.; El-Okr, M.; Ibrahim, M. Modified iron doped polyaniline/sulfonated carbon nanotubes for all symmetric solid-state supercapacitor. Synth. Met. 2017, 233, 41–51. [Google Scholar] [CrossRef]
- Alghunaim, N.S.; El-Khodary, S.A.; Ibrahim, M.; El-Enany, G.M. Spectroscopic analyses of iron doped protonated polyaniline/graphene oxide system. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 216, 349–358. [Google Scholar] [CrossRef]
- Arulmani, S.; Wu, J.J.; Anandan, S. Ultrasound promoted transition metal doped polyaniline nanofibers: Enhanced electrode material for electrochemical energy storage applications. Ultrason. Sonochem. 2019, 51, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Asen, P.; Shahrokhian, S.; Zad, A.I. Transition metal ions-doped polyaniline/graphene oxide nanostructure as high performance electrode for supercapacitor applications. J. Solid State Electrochem. 2018, 22, 983–996. [Google Scholar] [CrossRef]
- Elnaggar, E.M.; Kabel, K.I.; Farag, A.A.; Al-Gamal, A.G. Comparative study on doping of polyaniline with graphene and multi-walled carbon nanotubes. J. Nanostruct. Chem. 2017, 7, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Mo, Y.; Meng, W.; Xia, Y.; Du, X. Redox-Active Gel Electrolyte Combined with Branched Polyaniline Nanofibers Doped with Ferrous Ions for Ultra-High-Performance Flexible Supercapacitors. Polymers 2019, 11, 1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taheri, N.N.; Ramezanzadeh, B.; Mahdavian, M.; Bahlakeh, G. In-situ synthesis of Zn doped polyaniline on graphene oxide for inhibition of mild steel corrosion in 3 wt.% chloride solution. J. Ind. Eng. Chem. 2018, 63, 322–339. [Google Scholar] [CrossRef]
- Pirhady Tavandashti, N.; Ghorbani, M.; Shojaei, A.; Gonzalez-Garcia, Y.; Terryn, H.; Mol, J.M.C. pH responsive Ce (III) loaded polyaniline nanofibers for self-healing corrosion protection of AA2024-T3. Prog. Org. Coat. 2016, 99, 197–209. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Zhang, J.; Song, P.; Xiao, Z.; Liang, C.; Qiu, H.; Kong, J.; Gu, J. Fabrication and investigation on the ultra-thin and flexible Ti3C2Tx/co-doped polyaniline electromagnetic interference shielding composite films. Compos. Sci. Technol. 2019, 183, 107833. [Google Scholar] [CrossRef]
- Khan, M.D.A.; Akhtar, A.; Nabi, S.A. Investigation of the electrical conductivity and optical property of polyaniline-based nanocomposite and its application as an ethanol vapor sensor. New J. Chem. 2015, 39, 3728–3735. [Google Scholar] [CrossRef]
- Ebrahimi, G.; Neshati, J.; Rezaei, F. An investigation on the effect of H3PO4 and HCl-doped polyaniline nanoparticles on corrosion protection of carbon steel by means of scanning kelvin probe. Prog. Org. Coat. 2017, 105, 1–8. [Google Scholar] [CrossRef]
- Taş, R.; Gülen, M.; Can, M.; Sönmezoʇlu, S. Effects of solvent and copper-doping on polyaniline conducting polymer and its application as a counter electrode for efficient and cost-effective dye-sensitized solar cells. Synth. Met. 2016, 212, 75–83. [Google Scholar] [CrossRef]
- Dominic, J.; David, T.; Vanaja, A.; Muralidharan, G.; Maheswari, N.; Kumar, K.K.S. Supercapacitor Perform. Study Lithium Chloride Doped Polyaniline. Appl. Surf. Sci. 2018, 460, 40–47. [Google Scholar] [CrossRef]
- Martynyuk, G.V.; Aksimentyeva, O.I. Features of charge transport in polymer composites polymethylmethacrylate-polyaniline. Phys. Chem. Solid State 2020, 21, 319–324. [Google Scholar] [CrossRef]
- Sahu, S.; Sahoo, A.P.; Shubhadarshinee, L.; Ramakrishna, D.S.; Barick, A.K. Effect of polyaniline-coated carbon nanotube and nanosilver hybrid nanoparticles on the dielectric properties of poly (methyl methacrylate) nanocomposites. Polym. Compos. 2018, 39, E1294–E1305. [Google Scholar] [CrossRef]
- Tarawneh, M.A.; Saraireh, S.A.; Chen, R.S.; Ahmad, S.H.; Al-Tarawni, M.A.M.; Al-Tweissi, M.; Yu, L.J. Mechanical, thermal, and conductivity performances of novel thermoplastic natural rubber/graphene nanoplates/polyaniline composites. J. Appl. Polym. Sci. 2020, 137, 48873. [Google Scholar] [CrossRef]
- Andriianova, A.N.; Yuliya, N.B.; Akhat, G.M. Effect of structural factors on the physicochemical properties of functionalized polyanilines. RSC Adv. 2020, 10, 7468–7491. [Google Scholar] [CrossRef]
- Rashid, I.A.; Irfan, M.S.; Gill, Y.Q.; Nazar, R.; Saeed, F.; Afzal, A.; Ehsan, H.; Qaiser, A.A.; Shakoor, A. Stretchable strain sensors based on polyaniline/thermoplastic polyurethane blends. Polym. Bull. 2020, 77, 1081–1093. [Google Scholar] [CrossRef]
- Tan, H.-X.; Xu, X. Conductive properties and mechanism of various polymers doped with carbon nanotube/polyaniline hybrid nanoparticles. Compos. Sci. Technol. 2016, 128, 155–160. [Google Scholar] [CrossRef]
- Gill, Y.Q.; Ehsan, H.; Irfan, M.S.; Saeed, F.; Shakoor, A. Synergistic augmentation of polypropylene composites by hybrid morphology polyaniline particles for antistatic packaging applications. Mater. Res. Express 2020, 7, 015331. [Google Scholar] [CrossRef]
- Mazlan, N.A.; Jamil, M.S.; Sambasevam, K.P. Synthesis and fabrication of polyaniline/eggshell composite in ammonia detection. J. Met. Mater. Miner. 2020, 30. [Google Scholar] [CrossRef]
- Beesabathuni, S.N.; Stockham, J.G.; Kim, J.H.; Lee, H.B.; Chung, J.H.; Shen, A.Q. Fabrication of conducting polyaniline microspheres using droplet microfluidics. RSC Adv. 2013, 3, 24423–24429. [Google Scholar] [CrossRef]
- Ibrahim, N.I.; Ahmed, S.W. A comparative study of polyaniline/MWCNT with polyaniline/SWCNT nanocomposite films synthesized by microwave plasma polymerization. Synth. Met. 2019, 250, 49–54. [Google Scholar] [CrossRef]
- Ahmad, A.; Yunos, M.Z.; Harun, Z.; Hassan, M.F.; Adzila, S.; Arifin, A.M.T.; Rahman, M.N.A.; Haq, R.H.A. Effect of polyaniline on surface properties of polysulfone membrane. Chem. Eng. Trans. 2017, 56, 691–696. [Google Scholar]
- Mohan, K.; Bora, A.; Nath, B.C.; Gogoi, P.; Saikia, B.J.; Dolui, S.K. A highly stable and efficient quasi solid state dye sensitized solar cell based on Polymethyl methacrylate (PMMA)/Polyaniline Nanotube (PANI-NT) gel electrolyte. Electrochim. Acta 2016, 222, 1072–1078. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Yu, L.; Tang, Q. Electrospinning of polyaniline microfibers for anticorrosion coatings: An avenue of enhancing anticorrosion behaviors. Synth. Met. 2016, 212, 84–90. [Google Scholar] [CrossRef]
- Bhadra, J.; Al-Thani, N.J.; Madi, N.K.; Al-Maadeed, M.A. High performance sulfonic acid doped polyaniline–polystyrene blend ammonia gas sensors. J. Mater. Sci. Mater. Electron. 2016, 27, 8206–8216. [Google Scholar] [CrossRef]
- Simsek, M.; Von Kruechten, L.; Buchner, M.; Duerkop, A.; Baeumner, A.J.; Wongkaew, N. An efficient post-doping strategy creating electrospun conductive nanofibers with multi-functionalities for biomedical applications. J. Mater. Chem. C 2019, 7, 9316–9325. [Google Scholar] [CrossRef]
- Abdali, H.; Ajji, A. Preparation of electrospun nanocomposite nanofibers of polyaniline/poly (methyl methacrylate) with amino-functionalized graphene. Polymers 2017, 9, 453. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Mirzaei, A.; Bonyani, M.; Kim, K.H.; Kim, H.W.; Kim, S.S. Advances in electrospun nanofiber fabrication for polyaniline (PANI)-based chemoresistive sensors for gaseous ammonia. TrAC Trends Anal. Chem. 2020, 129, 115938. [Google Scholar] [CrossRef]
| Applications | Material | References |
|---|---|---|
| Electrode Material | PMMA/PANi Coating | [17] |
| Polymer Light Emitting Dioeds (PLED) | PMMA/PANi Blends | [18] |
| Electrochemical Device | PMMA/PANi nanocomposites | [45] |
| Dye Decoloration | PMMA/PANi fibers | [46] |
| Packaging | PMMA/PANi/PVDF Blends | [47] |
| Humidity Sensors | PMMA/PANi fibers | [20] |
| Gas Sensor | PMMA/PANi Fibers | [21,22] |
| Smart Actuators | PANi-coated PMMA fiber webs | [23,24] |
| Corrosion Protector | PMMA/PANi Coating | [48,49] |
| Type of Acid | Molarity (M) | References |
|---|---|---|
| Hydrochloric acid (HCl) | 1.5 0.2 | [71] [72] |
| Sulphuric acid (H2SO4) | 1 | [73] |
| Tartaric acid (TA) | 1 | [74] |
| Citric acid (CA) | 0.001 | [68] |
| Camphorsulfonic acid (CSA) | 0.001 | [75] |
| Malic acid (MA) | 0.001 | [76] |
| Acid | PANi Structure | Thickness (nm) |
|---|---|---|
| 0.1 M HCl | Nanotubes and Nanorods | ≈40 |
| 2 M HCl | Nanoflowers and Nanofibers | ≈40 |
| 5 M HCl | Nanosheets | ≈40 |
| 1.1 M H2SO4 | Nanofibers | - |
| Sample | Structure | Diameter (nm) |
|---|---|---|
| Pure PANi | Nanofibers | 20–30 |
| MWNT | Nanotubes | 100–120 |
| PANI-MWNT-4 | PANi corals | 150–300 |
| Parameter | Polymerization Yield (%) |
|---|---|
| Oxidant (mmol) | |
| 0.25 | ≈38.0 |
| 0.5 | ≈39.0 |
| 0.75 | ≈41.0 |
| 1 | ≈44.0 |
| 1.25 | ≈50.0 |
| 1.5 | ≈45.0 |
| 1.75 | ≈42.0 |
| Aniline (mmol) | |
| 1.09 | ≈50.0 |
| 5.47 | ≈57.5 |
| 10.95 | ≈70.0 |
| 16.42 | ≈62.5 |
| 21.9 | ≈50.0 |
| 27.38 | ≈37.5 |
| H2SO4 (mmol) | |
| 3.75 | ≈31.0 |
| 5 | ≈32.0 |
| 7.5 | ≈36.0 |
| 12.5 | ≈50.0 |
| 17.5 | ≈40.0 |
| 22.5 | ≈36.0 |
| 25 | ≈35.0 |
| Type of Copolymer | Advantages | Disadvantages |
|---|---|---|
| Block |
|
|
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Graft |
|
|
|
| |
|
| |
|
|
| Copolymerization | Blends | |
|---|---|---|
| Advantages |
|
|
|
| |
|
| |
|
| |
| Disadvantages |
|
|
|
| |
|
| |
|
| |
|
|
| HCl Molarity (M) | Conductivity (S·cm−1) |
|---|---|
| 0.25 | 4.34 × 10−2 |
| 0.50 | 7.33 × 10−2 |
| 0.75 | 3.31 × 10−2 |
| 1.00 | 7.08 × 10−2 |
| 1.25 | 6.27 × 10−2 |
| 1.50 | 1.66 × 10−2 |
| Dopants | Conductivity (S·cm−1) | References |
|---|---|---|
| HCl | 9.3 × 10−1 | [159] |
| H3PO4 | 5.6 × 10−1 | [159] |
| Graphene | 1.2 × 10−1 | [153] |
| CNTs | 1.2 × 10−2 | [153] |
| Iodine | 4.8 × 10−1 | [146] |
| Copper | 2.9 × 10−1 | [160] |
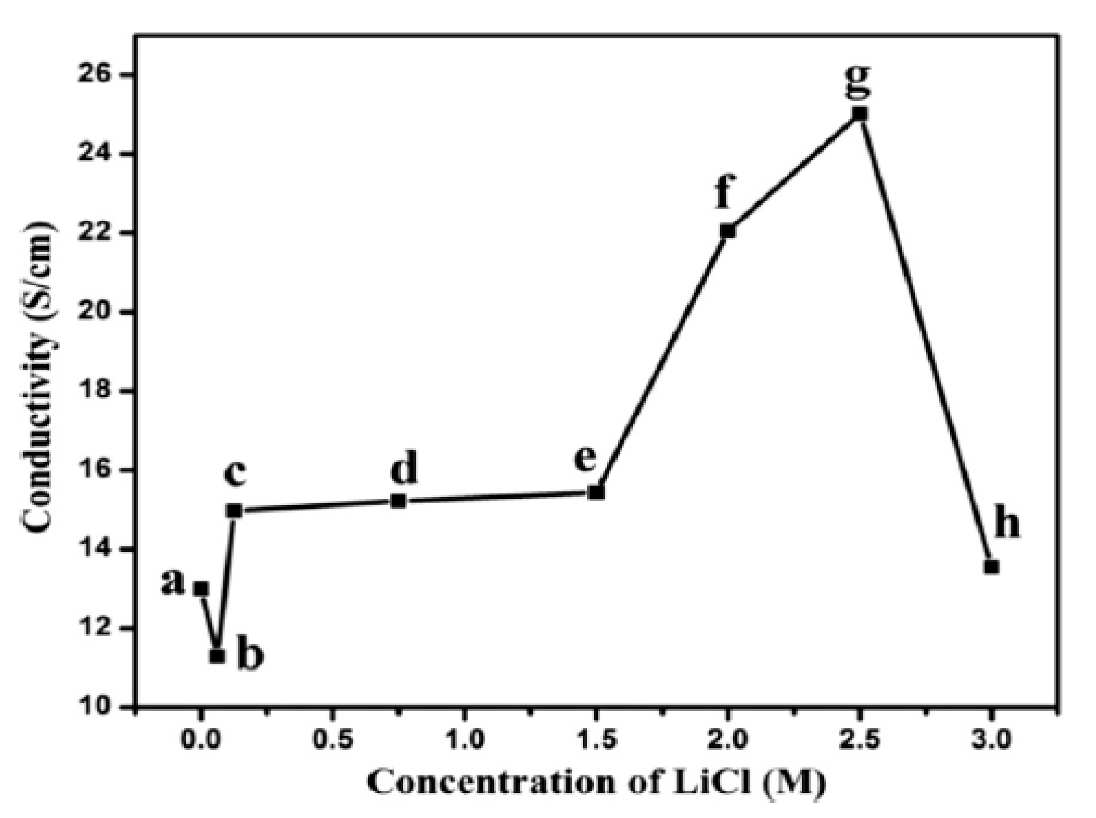
| LiCl | 25 | [161] |
| PMMA/PANi Ratio | AC Conductivity,σac (S/cm) | Dielectric Permittivity, E0 (έ) | Dielectric Loss (tan d) |
|---|---|---|---|
| Pure PANi PMMA/0.101 wt% PANi | ≈10−2 ≈10−6 | 103–104 101–102 | 100–101 ≈10−2 |
| PMMA/0.101 wt% PANi@AgNP | ≈10−5 | ≈102 | 10−2–10−1 |
| PMMA/0.101 wt% PANi@f-CNT | ≈10−1 | ≈104 | ≈101 |
| Sample | Structure and Morphology Properties | |
|---|---|---|
| References | ||
| [45] | [163] | |
| Pure PMMA | Transparent, soft, uniform (smooth surface) | - |
| Pure PANi | Nanofiber morphology (rough surface) | Globular microstructure, strong aggregation |
| PMMA/PANi Blend | No cracks (Improved surface roughness) | Low aggregation |
| Summary | Remarks |
|---|---|
| Factors affecting Conductivity |
|
| Challenges |
|
| Future Directions |
|
| Applications |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu Hassan Shaari, H.; Ramli, M.M.; Mohtar, M.N.; Abdul Rahman, N.; Ahmad, A. Synthesis and Conductivity Studies of Poly(Methyl Methacrylate) (PMMA) by Co-Polymerization and Blending with Polyaniline (PANi). Polymers 2021, 13, 1939. https://doi.org/10.3390/polym13121939
Abu Hassan Shaari H, Ramli MM, Mohtar MN, Abdul Rahman N, Ahmad A. Synthesis and Conductivity Studies of Poly(Methyl Methacrylate) (PMMA) by Co-Polymerization and Blending with Polyaniline (PANi). Polymers. 2021; 13(12):1939. https://doi.org/10.3390/polym13121939
Chicago/Turabian StyleAbu Hassan Shaari, Helyati, Muhammad Mahyiddin Ramli, Mohd Nazim Mohtar, Norizah Abdul Rahman, and Azizan Ahmad. 2021. "Synthesis and Conductivity Studies of Poly(Methyl Methacrylate) (PMMA) by Co-Polymerization and Blending with Polyaniline (PANi)" Polymers 13, no. 12: 1939. https://doi.org/10.3390/polym13121939
APA StyleAbu Hassan Shaari, H., Ramli, M. M., Mohtar, M. N., Abdul Rahman, N., & Ahmad, A. (2021). Synthesis and Conductivity Studies of Poly(Methyl Methacrylate) (PMMA) by Co-Polymerization and Blending with Polyaniline (PANi). Polymers, 13(12), 1939. https://doi.org/10.3390/polym13121939









