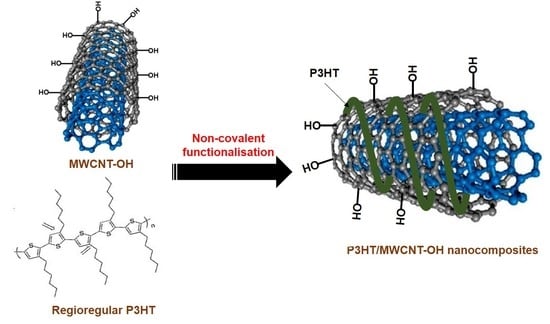
The Influence of Reaction Time on Non-Covalent Functionalisation of P3HT/MWCNT Nanocomposites
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
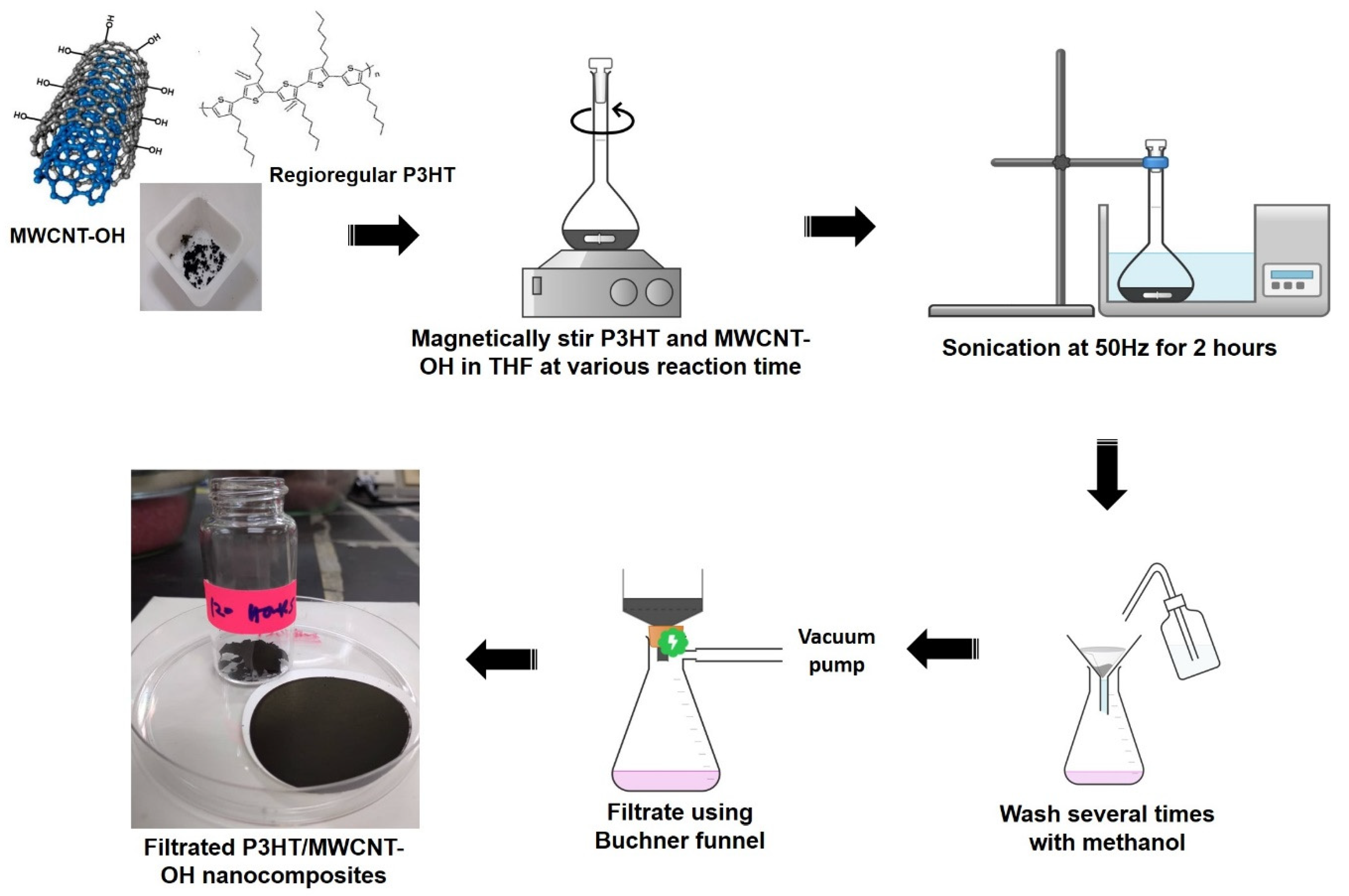
2.2. Preparation of P3HT/MWCNT-OH Nanocomposites
2.3. Characterisation Methods
3. Results and Discussion
3.1. Raman Spectroscopy Analysis
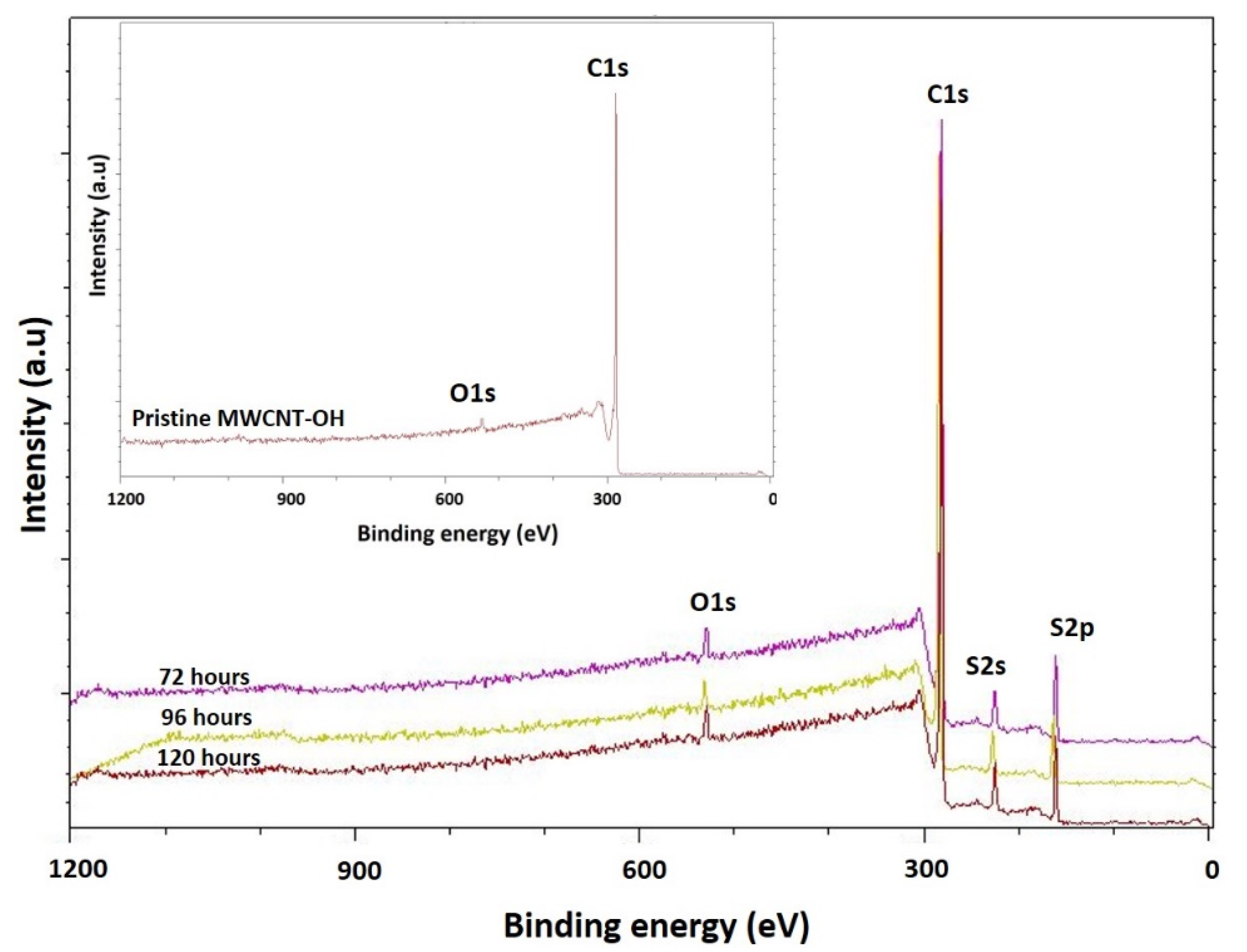
3.2. XPS Analysis
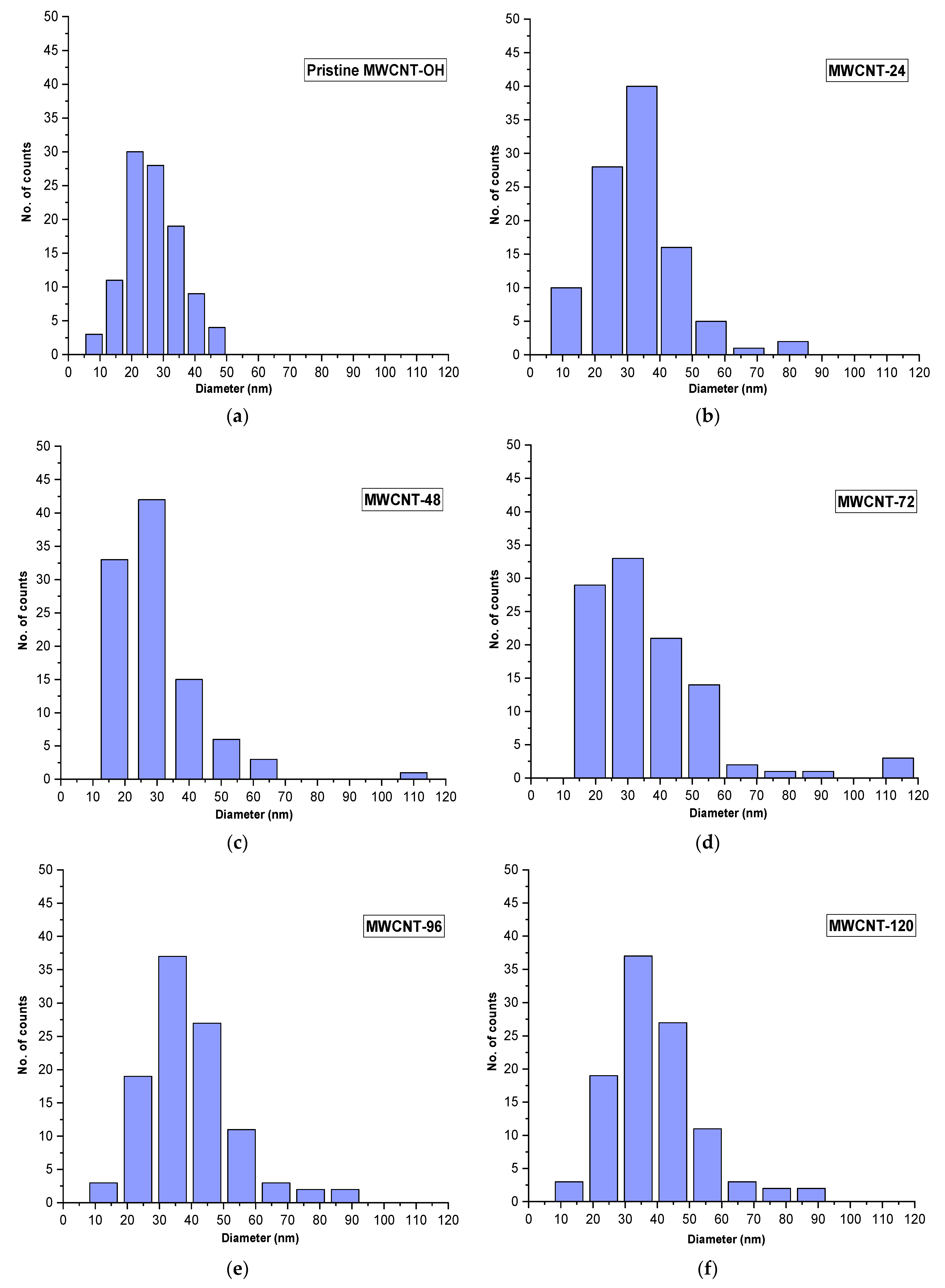
3.3. FESEM and Mean Diameter Size Distribution Analysis
3.4. High-Resolution Transmission Electron Microscopy Analysis
3.5. Thermogravimetric Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene,(CH) x. J. Chem. Soc. Chem. Commun. 1977, 578–580. [Google Scholar] [CrossRef]
- Kaloni, T.P.; Giesbrecht, P.K.; Schreckenbach, G.; Freund, M.S. Polythiophene: From fundamental perspectives to applications. Chem. Mater. 2017, 29, 10248–10283. [Google Scholar] [CrossRef]
- Mohd Nurazzi, N.; Muhammad Asyraf, M.R.; Khalina, A.; Abdullah, N.; Sabaruddin, F.A.; Kamarudin, S.H.; Ahmad, S.; Mahat, A.M.; Lee, C.L.; Aisyah, H.A. Fabrication, Functionalization, and Application of Carbon Nanotube-Reinforced Polymer Composite: An Overview. Polymers 2021, 13, 1047. [Google Scholar] [CrossRef]
- Le, T.H.; Kim, Y.; Yoon, H. Electrical and electrochemical properties of conducting polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef]
- Zhang, Y.; Bunes, B.R.; Wu, N.; Ansari, A.; Rajabali, S.; Zang, L. Sensing methamphetamine with chemiresistive sensors based on polythiophene-blended single-walled carbon nanotubes. Sens. Actuators B Chem. 2018, 255, 1814–1818. [Google Scholar] [CrossRef]
- Husain, A.; Ahmad, S.; Mohammad, F. Electrical conductivity and ammonia sensing studies on polythiophene/MWCNTs nanocomposites. Materialia 2020, 14, 100868. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Harussani, M.M.; Siti Zulaikha, N.D.; Norhana, A.H.; Syakir, M.I.; Norli, A. Composites based on conductive polymer with carbon nanotubes in DMMP gas sensors–an overview. Polimery 2021, 66, 85–97. [Google Scholar] [CrossRef]
- Norizan, M.N.; Zulaikha, N.D.S.; Norhana, A.B.; Syakir, M.I.; Norli, A. Carbon nanotubes-based sensor for ammonia gas detection–an overview. Polimery 2021, 66, 175–186. [Google Scholar] [CrossRef]
- Liang, Z.; Li, M.; Wang, Q.; Qin, Y.; Stuard, S.J.; Peng, Z.; Deng, Y.; Ade, H.; Ye, L.; Geng, Y. Optimization Requirements of Efficient Polythiophene: Nonfullerene Organic Solar Cells. Joule 2020, 4, 1278–1295. [Google Scholar] [CrossRef]
- Liang, Q.; Jiao, X.; Yan, Y.; Xie, Z.; Lu, G.; Liu, J.; Han, Y. Separating Crystallization Process of P3HT and O-IDTBR to Construct Highly Crystalline Interpenetrating Network with Optimized Vertical Phase Separation. Adv. Funct. Mater. 2019, 29, 1807591. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, Y.; Li, M.; Ye, L.; Geng, Y. Molecular Engineering and Morphology Control of Polythiophene: Nonfullerene Acceptor Blends for High-Performance Solar Cells. Adv. Energy Mater. 2020, 10, 2002572. [Google Scholar] [CrossRef]
- Iqbal, S.; Shah, J.; Kotnala, R.K.; Ahmad, S. Highly efficient low cost EMI shielding by barium ferrite encapsulated polythiophene nanocomposite. J. Alloy. Compd. 2019, 779, 487–496. [Google Scholar] [CrossRef]
- Dar, M.A.; Majid, K.; Najar, M.H.; Kotnala, R.K.; Shah, J.; Dhawan, S.K.; Farukh, M. Surfactant-assisted synthesis of polythiophene/Ni 0.5 Zn 0.5 Fe 2− x Ce x O 4 ferrite composites: Study of structural, dielectric and magnetic properties for EMI-shielding applications. Phys. Chem. Chem. Phys. 2017, 19, 10629–10643. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.H.; Park, J.J.; Housel, L.M.; Minnici, K.; Zhang, G.; Lee, S.R.; Lee, S.W.; Chen, Z.; Noda, S.; Takeuchi, E.S. Carbon Nanotube Web with Carboxylated Polythiophene “Assist” for High-Performance Battery Electrodes. ACS Nano 2018, 12, 3126–3139. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, P.; Yang, R. Enhanced electrochemical performance of core-shell Li4Ti5O12/PTh as advanced anode for rechargeable lithium-ion batteries. Ceram. Int. 2017, 43, 7600–7606. [Google Scholar] [CrossRef]
- Peymanfar, R.; Mohammadi, A.; Javanshir, S. Preparation of graphite-like carbon nitride/polythiophene nanocomposite and investigation of its optical and microwave absorbing characteristics. Compos. Commun. 2020, 21, 100421. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Lu, L. Synthesis and significantly enhanced microwave absorption properties of cobalt ferrite hollow microspheres with protrusions/polythiophene composites. J. Alloys Compd. 2017, 722, 158–165. [Google Scholar] [CrossRef]
- Dehghani, Z.; Dadfarnia, S.; Haji Shabani, A.M.; Ehrampoush, M.H. Magnetic Multi-Walled Carbon Nanotubes Modified with Polythiophene as a Sorbent for Simultaneous Solid Phase Microextraction of Lead and Cadmium from Water and Food Samples. Anal. Bioanal. Chem. Res. 2020, 7, 509–523. [Google Scholar]
- Dutta, K.; Rana, D. Polythiophenes: An emerging class of promising water purifying materials. Eur. Polym. J. 2019, 116, 370–385. [Google Scholar] [CrossRef]
- Kao, E.; Liang, Q.; Bertholet, G.R.K.; Zang, X.; Park, H.S.; Bae, J.; Lu, J.; Lin, L. Electropolymerized polythiophene photoelectrodes for photocatalytic water splitting and hydrogen production. Sens. Actuators A Phys. 2018, 277, 18–25. [Google Scholar] [CrossRef]
- Ng, C.H.; Winther-Jensen, O.; Ohlin, C.A.; Winther-Jensen, B. Enhanced catalytic activity towards hydrogen evolution on polythiophene via microstructural changes. Int. J. Hydrogen Energy 2017, 42, 886–894. [Google Scholar] [CrossRef]
- Husain, A.; Ahmad, S.; Mohammad, F. Synthesis, characterisation and ethanol sensing application of polythiophene/graphene nanocomposite. Mater. Chem. Phys. 2020, 239, 122324. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Grace, A.N. Antistatic and microwave shielding performance of polythiophene-graphene grafted 3-dimensional carbon fibre composite. Diam. Relat. Mater. 2020, 106, 107871. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, L. Nanofibers for Gas Sensing. Adv. Fiber Sens. Technol. 2020, 133–153. [Google Scholar]
- Demon, S.Z.N.; Kamisan, A.I.; Abdullah, N.; Noor, S.A.M.; Khim, O.K.; Kasim, N.A.M.; Yahya, M.Z.A.; Manaf, N.A.A.; Azmi, A.F.M.; Halim, N.A. Graphene-based Materials in Gas Sensor Applications: A Review. Sens. Mater. 2020, 32, 759–777. [Google Scholar] [CrossRef]
- Shah, A.H. Applications of carbon nanotubes and their polymer nanocomposites for gas sensors. In Carbon Nanotubes-Current Progress of their Polymer Composites; IntechOpen (United Kingdom): London, UK, 2016; pp. 459–494. [Google Scholar]
- Hunter, G.W.; Akbar, S.; Bhansali, S.; Daniele, M.; Erb, P.D.; Johnson, K.; Liu, C.C.; Miller, D.; Oralkan, O.; Hesketh, P.J. Editors’ choice—Critical review—A critical review of solid state gas sensors. J. Electrochem. Soc. 2020, 167, 1–30. [Google Scholar] [CrossRef]
- Norizan, M.N.; Moklis, M.H.; Demon, S.Z.N.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon nanotubes: Functionalisation and their application in chemical sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef]
- Ahmadi, M.; Zabihi, O.; Masoomi, M.; Naebe, M. Synergistic effect of MWCNTs functionalization on interfacial and mechanical properties of multi-scale UHMWPE fibre reinforced epoxy composites. Compos. Sci. Technol. 2016, 134, 1–11. [Google Scholar] [CrossRef]
- Maruyama, B.; Alam, K. Carbon nanotubes and nanofibers in composite materials. Sampe J. 2002, 38, 59–70. [Google Scholar]
- Collins, P.G.; Avouris, P. Nanotubes for Electronics–Scientific American. San Fr. Nat. Publ. Group 2000, 283, 62–69. [Google Scholar]
- Abdullah, A.; Mohamad, I.S.; Bani Hashim, A.Y.; Abdullah, N.; Poh, B.W.; Md Isa, M.H.; Zainal Abidin, S. Thermal conductivity and viscosity of deionised water and ethylene glycol-based nanofluids. J. Mech. Eng. Sci. 2016, 10, 2249–2261. [Google Scholar] [CrossRef]
- Kou, J.; Qian, H.; Lu, H.; Liu, Y.; Xu, Y.; Wu, F.; Fan, J. Optimizing the design of nanostructures for improved thermal conduction within confined spaces. Nanoscale Res. Lett. 2011, 6, 1–8. [Google Scholar] [CrossRef]
- Elashmawi, I.S.; Gaabour, L.H. Raman, morphology and electrical behavior of nanocomposites based on PEO/PVDF with multi-walled carbon nanotubes. Results Phys. 2015, 5, 105–110. [Google Scholar] [CrossRef]
- Mescoloto, A.D.F.; Pulcinelli, S.H.; Santilli, C.V.; Gonçalves, V.C. Structural and thermal properties of carboxylic acid functionalized polythiophenes. Polímeros 2014, 24, 25–30. [Google Scholar] [CrossRef]
- Bachhav, S.; Patil, D. Preparation and Characterization of Multiwalled Carbon Nanotubes-Polythiophene Nanocomposites and its Gas Sensitivity Study at Room Temperature. J. Nanostructures 2017, 7, 247–257. [Google Scholar]
- Ribeiro, B.; Botelho, E.C.; Costa, M.L.; Bandeira, C.F. Carbon nanotube buckypaper reinforced polymer composites: A review. Polímeros 2017, 27, 247–255. [Google Scholar] [CrossRef]
- Mallakpour, S.; Soltanian, S. Surface functionalization of carbon nanotubes: Fabrication and applications. Rsc Adv. 2016, 6, 109916–109935. [Google Scholar] [CrossRef]
- Karim, M.R. Synthesis and characterizations of poly (3-hexylthiophene) and modified carbon nanotube composites. J. Nanomater. 2012, 2012, 174353. [Google Scholar] [CrossRef]
- Janudin, N.; Abdullah, L.C.; Abdullah, N.; Md Yasin, F.; Saidi, N.M.; Kasim, N.A.M. Comparison and characterization of acid functionalization of multi walled carbon nanotubes using various methods. Solid State Phenom. 2017, 264, 83–86. [Google Scholar] [CrossRef]
- Mohamad Saidi, N.; Kasim, N.A.M.; Osman, M.J.; Janudin, N.; Mohamad, I.S.; Abdullah, N. The Influences of Chemical and Mechanical Treatment on the Morphology of Carbon Nanofibers. Solid State Phenom. 2017, 264, 107–111. [Google Scholar] [CrossRef]
- Janudin, N.; Abdullah, L.C.; Abdullah, N.; Yasin, F.M.; Saidi, N.M.; Kasim, N.A.M. Characterization of Amide and Ester Functionalized Multiwalled Carbon Nanotubes. Asian J. Chem. 2018, 30, 1613–1616. [Google Scholar] [CrossRef]
- Janudin, N.; Abdullah, N.; Wan Yunus, W.M.Z.; Yasin, F.M.; Yaacob, M.H.; Mohamad Saidi, N.; Mohd Kasim, N.A. Effect of functionalized carbon nanotubes in the detection of benzene at room temperature. J. Nanotechnol. 2018, 2018, 2107898. [Google Scholar] [CrossRef]
- Ma, P.C.; Siddiqui, N.A.; Marom, G.; Kim, J.K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Ahir, S.V.; Terentjev, E.M. Dispersion rheology of carbon nanotubes in a polymer matrix. Phys. Rev. B 2006, 73, 125422. [Google Scholar] [CrossRef]
- Bose, S.; Khare, R.A.; Moldenaers, P. Assessing the strengths and weaknesses of various types of pre-treatments of carbon nanotubes on the properties of polymer/carbon nanotubes composites: A critical review. Polymer 2010, 51, 975–993. [Google Scholar] [CrossRef]
- Sa’aya, N.S.N.; Demon, S.Z.N.; Abdullah, N.; Abd Shatar, V.F.K.E.; Halim, N.A. Optical and Morphological Studies of Multiwalled Carbon Nanotube-incorporated Poly (3-hexylthiophene-2, 5-diyl) Nanocomposites. Sens. Mater. 2019, 31, 2997–3006. [Google Scholar]
- Rathore, P.; Negi, C.M.S.; Yadav, A.; Verma, A.S.; Gupta, S.K. Influence of MWCNT doping on performance of polymer bulk heterojunction based devices. Optik 2018, 160, 131–137. [Google Scholar] [CrossRef]
- Abdulla, S.; Mathew, T.L.; Pullithadathil, B. Highly sensitive, room temperature gas sensor based on polyaniline-multiwalled carbon nanotubes (PANI/MWCNTs) nanocomposite for trace-level ammonia detection. Sens. Actuators B Chem. 2015, 221, 1523–1534. [Google Scholar] [CrossRef]
- Karim, M.R.; Lee, C.J.; Lee, M.S. Synthesis and characterization of conducting polythiophene/carbon nanotubes composites. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 5283–5290. [Google Scholar] [CrossRef]
- Gómez, S.; Rendtorff, N.M.; Aglietti, E.F.; Sakka, Y.; Suárez, G. Surface modification of multiwall carbon nanotubes by sulfonitric treatment. Appl. Surf. Sci. 2016, 379, 264–269. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, G.; Roche, P.; Leclerc, M. A calorimetric study of the phase transitions in poly (3-hexylthiophene). Polymer 1995, 36, 2211–2214. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.; Wang, Y.; Iqbal, Z.; Chhowalla, M.; Mitra, S. A fullerene–single wall carbon nanotube complex for polymer bulk heterojunction photovoltaic cells. J. Mater. Chem. 2007, 17, 2406–2411. [Google Scholar] [CrossRef]
- Giulianini, M.; Waclawik, E.R.; Bell, J.M.; Scarselli, M.; Castrucci, P.; De Crescenzi, M.; Motta, N. Microscopic and spectroscopic investigation of poly (3-hexylthiophene) interaction with carbon nanotubes. Polymers 2011, 3, 1433–1446. [Google Scholar] [CrossRef]
- Saini, V.; Li, Z.; Bourdo, S.; Dervishi, E.; Xu, Y.; Ma, X.; Kunets, V.P.; Salamo, G.J.; Viswanathan, T.; Biris, A.R. Electrical, optical, and morphological properties of P3HT-MWNT nanocomposites prepared by in situ polymerization. J. Phys. Chem. C 2009, 113, 8023–8029. [Google Scholar] [CrossRef]
- Fujigaya, T.; Nakashima, N. Non-covalent polymer wrapping of carbon nanotubes and the role of wrapped polymers as functional dispersants. Sci. Technol. Adv. Mater. 2015, 16, 024802. [Google Scholar] [CrossRef] [PubMed]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Kang, E.T.; Neoh, K.G.; Tan, K.L. X-ray photoelectron spectroscopic studies of poly (2, 2’-bithiophene) and its complexes. Phys. Rev. B 1991, 44, 10461. [Google Scholar] [CrossRef]
- Karim, M.R.; Yeum, J.H.; Lee, M.S.; Lim, K.T. Synthesis of conducting polythiophene composites with multi-walled carbon nanotube by the γ-radiolysis polymerization method. Mater. Chem. Phys. 2008, 112, 779–782. [Google Scholar] [CrossRef]
- Swathy, T.S.; Antony, M.J. Tangled silver nanoparticles embedded polythiophene-functionalized multiwalled carbon nanotube nanocomposites with remarkable electrical and thermal properties. Polymer 2020, 189, 122171. [Google Scholar] [CrossRef]
- Dumitru, A.; Vulpe, S.; Radu, A.; Antohe, S. Influence of nitrogen environment on the performance of conducting polymers/CNTs nanocomposites modified anodes for microbial fuel cells (MFCs). Rom. J. Phys 2018, 63, 605–625. [Google Scholar]
- Erten-Ela, S.; Cogal, S.; Cogal, G.C.; Oksuz, A.U. Highly conductive polymer materials based multi-walled carbon nanotubes as counter electrodes for dye-sensitized solar cells. Fuller. Nanotub. Carbon Nanostruct. 2016, 24, 380–384. [Google Scholar] [CrossRef]
- Husain, A.; Ahmad, S.; Mohammad, F. Polythiophene/graphene/zinc tungstate nanocomposite: Synthesis, characterization, DC electrical conductivity and cigarette smoke sensing application. Polym. Polym. Compos. 2020. [Google Scholar] [CrossRef]
- Husain, A.; Shariq, M.U.; Mohammad, F. DC electrical conductivity and liquefied petroleum gas sensing application of polythiophene/zinc oxide nanocomposite. Materialia 2020, 9, 100599. [Google Scholar] [CrossRef]
- Hacaloglu, J.; Yigit, S.; Akbulut, U.; Toppare, L. Thermal degradation of polythiophene natural rubber and polythiophene-synthetic rubber conducting polymer composites. Polymer 1997, 38, 5119–5124. [Google Scholar] [CrossRef]
- Peng, Z.; Holm, A.H.; Nielsen, L.T.; Pedersen, S.U.; Daasbjerg, K. Covalent sidewall functionalization of carbon nanotubes by a “formation− degradation” approach. Chem. Mater. 2008, 20, 6068–6075. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Jia, J.; Hou, C.; Hao, X.; Zhang, H. Preparation of hydroxyl and (3-aminopropyl) triethoxysilane functionalized multiwall carbon nanotubes for use as conductive fillers in the polyurethane composite. Polym. Compos. 2018, 39, 1212–1222. [Google Scholar] [CrossRef]
- Liang, S.; Li, G.; Tian, R. Multi-walled carbon nanotubes functionalized with a ultrahigh fraction of carboxyl and hydroxyl groups by ultrasound-assisted oxidation. J. Mater. Sci. 2016, 51, 3513–3524. [Google Scholar] [CrossRef]
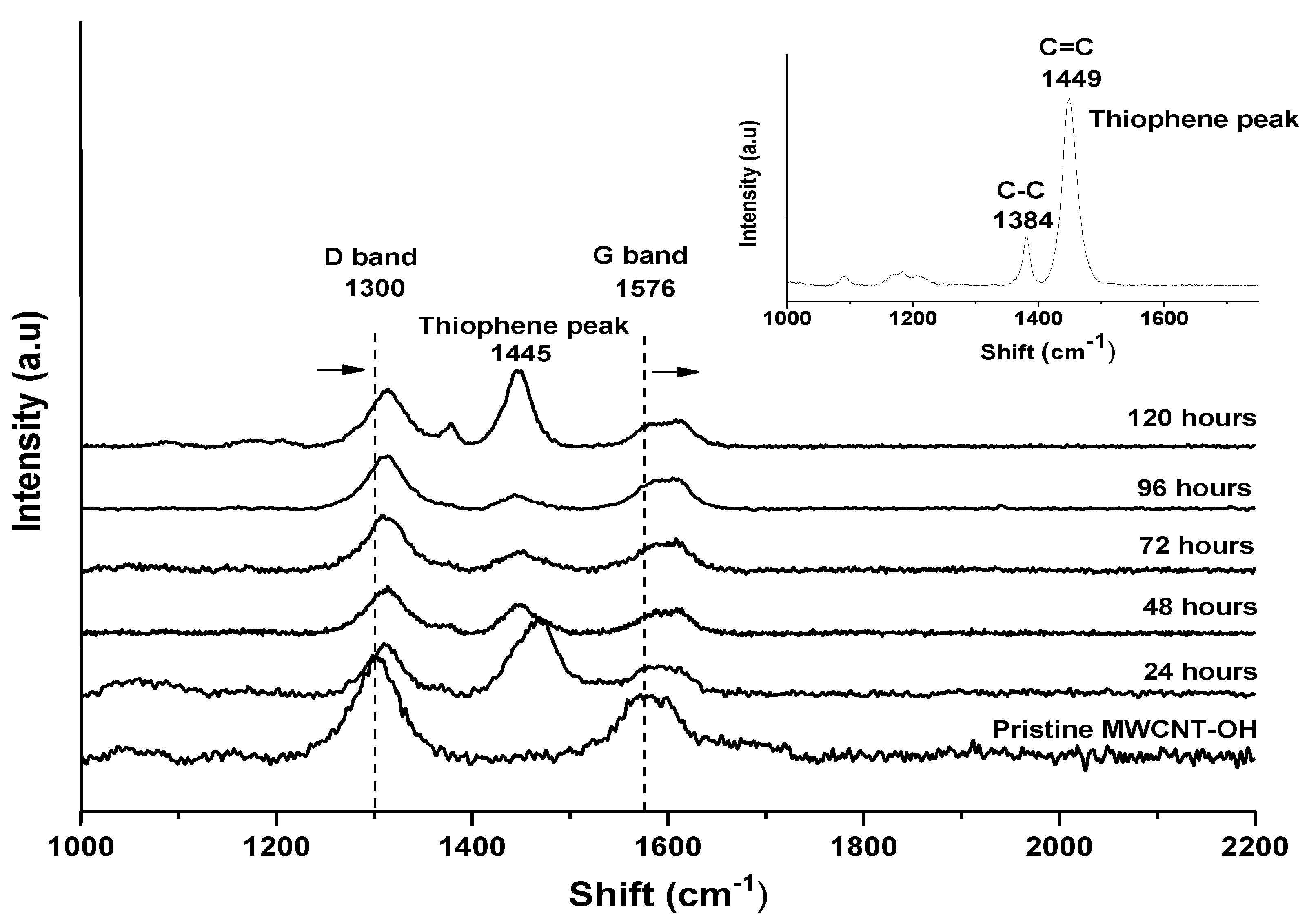





| Sample | ID | IG | ID/IG |
|---|---|---|---|
| Pristine MWCNT-OH | 1297 | 1571 | 0.83 |
| MWCNT-24 | 1312 | 1458 | 0.90 |
| MWCNT-48 | 1313 | 1414 | 0.93 |
| MWCNT-72 | 1372 | 1456 | 0.94 |
| MWCNT-96 | 1390 | 1431 | 0.97 |
| MWCNT-120 | 1398 | 1427 | 0.98 |
| Sample | Peak | Binding Energy (eV) | Atomic (%) | Ref. |
|---|---|---|---|---|
| Pristine P3HT | C 1s | 284.6 | - | [59] |
| O 1s | ~530.0 | - | ||
| S 2s | 229.1 | - | ||
| S 2p | 165.1, 165.8 | - | ||
| Pristine MWCNT-OH | C 1s | 284.0 | 97.25 | Current work |
| O 1s | 531.0 | 2.75 | ||
| S 2s | - | - | ||
| S 2p | - | - | ||
| MWCNT-72 | C 1s | 284.0 | 80.5 | |
| O 1s | 531.0 | 2.7 | ||
| S 2s | 227.0 | 8.5 | ||
| S 2p | 163.0 | 8.3 | ||
| MWCNT-96 | C 1s | 281.0 | 78.2 | |
| O 1s | 529.0 | 2.78 | ||
| S 2s | 225.0 | 6.6 | ||
| S 2p | 161.0 | 12.5 | ||
| MWCNT-120 | C 1s | 281.0 | 77.07 | |
| O 1s | 529.0 | 2.63 | ||
| S 2s | 225.0 | 6.5 | ||
| S 2p | 161.0 | 13.8 |
| Sample | Diameter (nm) | Standard Deviation |
|---|---|---|
| Pristine MWCNT-OH | 27 | 8.10 |
| MWCNT-24 | 33 | 12.48 |
| MWCNT-48 | 35 | 14.80 |
| MWCNT-72 | 42 | 18.30 |
| MWCNT-96 | 45 | 12.58 |
| MWCNT-120 | 50 | 19.24 |
| Sample | Tonset (°C) | Tmax (°C) | T10% (°C) | T25% (°C) | Weight Loss (%) at 500 °C |
|---|---|---|---|---|---|
| Pristine P3HT | - | 487.2 | 462.9 | 479.6 | 55.0 |
| Pristine MWCNT-OH | 310.5 | - | - | - | 1.3 |
| MWCNT-24 | 342.7 | 481.0 | 364.8 | 478.4 | 33.8 |
| MWCNT-48 | 351.3 | 480.7 | 446.1 | 483.5 | 31.7 |
| MWCNT-72 | 351.5 | 481.0 | 450.2 | 488.9 | 31.0 |
| MWCNT-96 | 354.5 | 480.2 | 455.4 | 490.0 | 30.5 |
| MWCNT-120 | 355.5 | 479.5 | 461.5 | 493.8 | 26.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurazzi, N.M.; Abdullah, N.; Demon, S.Z.N.; Halim, N.A.; Mohamad, I.S. The Influence of Reaction Time on Non-Covalent Functionalisation of P3HT/MWCNT Nanocomposites. Polymers 2021, 13, 1916. https://doi.org/10.3390/polym13121916
Nurazzi NM, Abdullah N, Demon SZN, Halim NA, Mohamad IS. The Influence of Reaction Time on Non-Covalent Functionalisation of P3HT/MWCNT Nanocomposites. Polymers. 2021; 13(12):1916. https://doi.org/10.3390/polym13121916
Chicago/Turabian StyleNurazzi, N.M., N. Abdullah, S.Z.N. Demon, N.A. Halim, and I.S. Mohamad. 2021. "The Influence of Reaction Time on Non-Covalent Functionalisation of P3HT/MWCNT Nanocomposites" Polymers 13, no. 12: 1916. https://doi.org/10.3390/polym13121916
APA StyleNurazzi, N. M., Abdullah, N., Demon, S. Z. N., Halim, N. A., & Mohamad, I. S. (2021). The Influence of Reaction Time on Non-Covalent Functionalisation of P3HT/MWCNT Nanocomposites. Polymers, 13(12), 1916. https://doi.org/10.3390/polym13121916