Wear Assessment of Tibial Inserts Made of Highly Cross-Linked Polyethylene Supplemented with Dodecyl Gallate in the Total Knee Arthroplasty
Abstract
1. Introduction
2. Materials and Methods
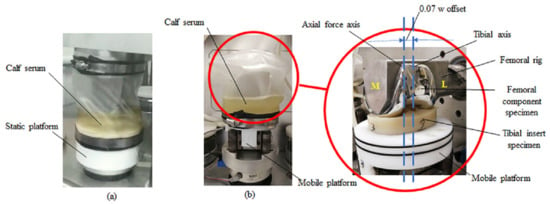
2.1. Wear Test
2.1.1. Prosthesis
2.1.2. Test Condition
2.2. Wear Assessment
2.2.1. Gravimetric Wear and Wear Rate
2.2.2. Wear Particle Analysis
3. Results
3.1. Gravimetric Wear and Wear Rate
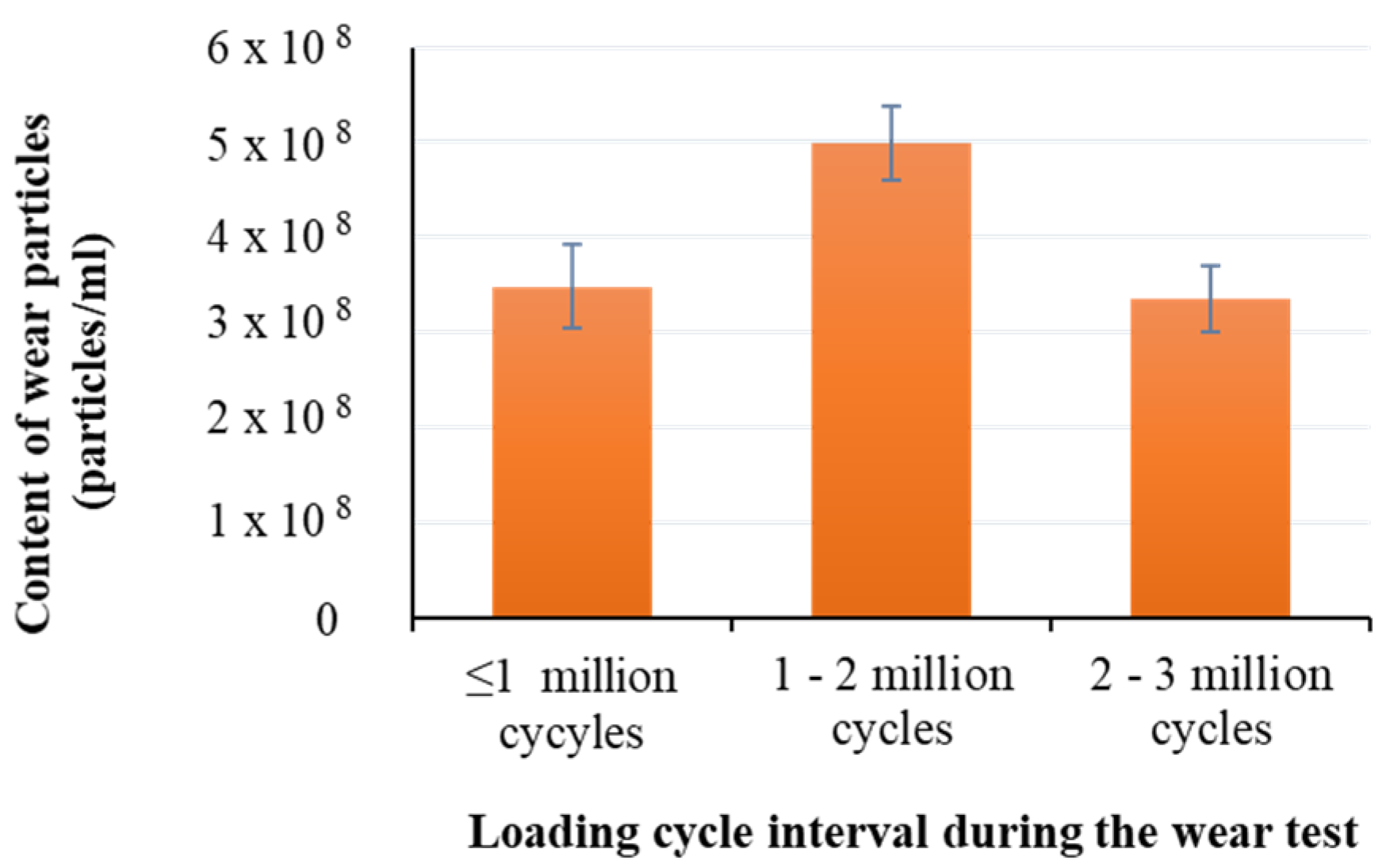
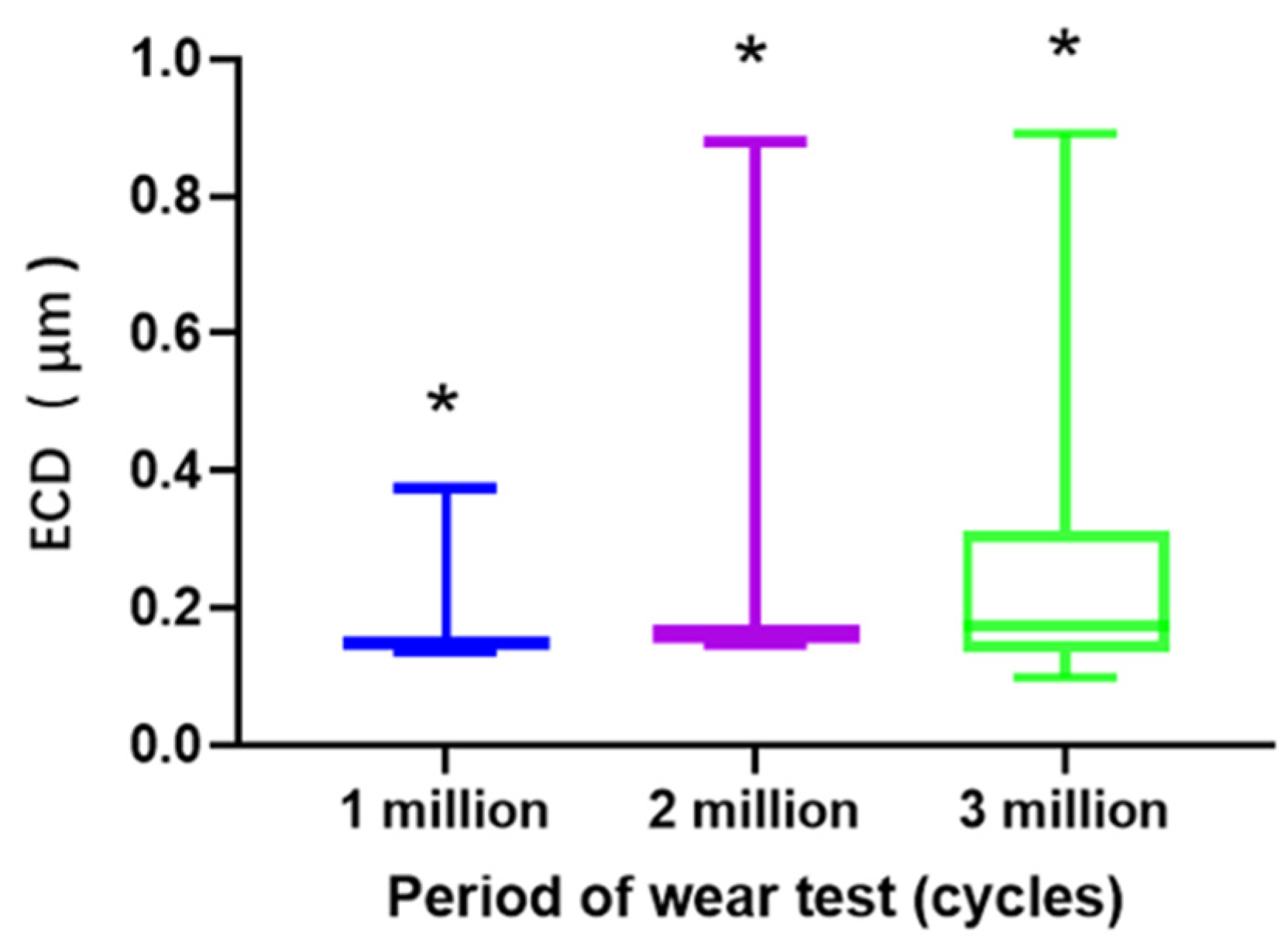
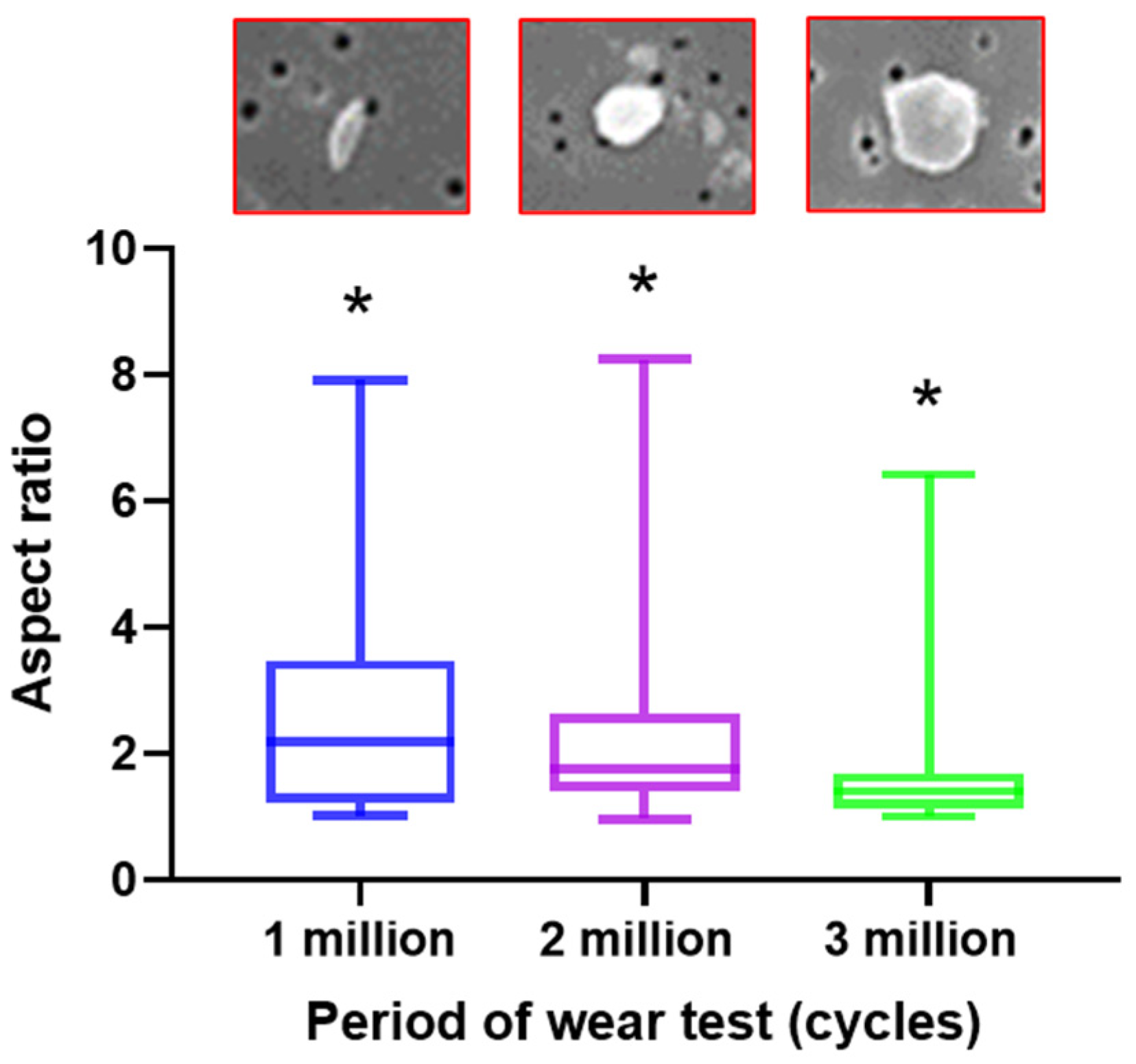
3.2. Measurement of Wear Particles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TKA | total knee arthroplasty |
| DG | dodecyl gallate |
| HXLPE | highly cross-linked polyethylene |
| HXLPE-DG | highly cross-linked polyethylene supplemented with dodecyl gallate |
| UHMWPE | Conventional ultra-high molecular weight polyethylene |
| HXLPE-VE | highly cross-linked polyethylene supplemented with vitamin E |
References
- Gandhi, R.; Dhotar, H.; Razak, F.; Tso, P.; Davey, J.R.; Mahomed, N.N. Predicting the longer term outcomes of total knee arthroplasty. Knee 2010, 17, 15–18. [Google Scholar] [CrossRef]
- Liang, H.; Bae, J.; Park, C.; Kim, K.; Bae, D.; Song, S. Comparison of mode of failure between primary and revision total knee arthroplasties. Orthop. Traumatol. Surg. Res. 2018, 104, 171–176. [Google Scholar] [CrossRef]
- Yong, T.M.; Young, E.C.; Molloy, I.B.; Fisher, B.M.; Keeney, B.J.; Moschetti, W.E. Long-Term Implant Survivorship and Modes of Failure in Simultaneous Concurrent Bilateral Total Knee Arthroplasty. J. Arthroplast. 2020, 35, 139–144. [Google Scholar] [CrossRef]
- Visco, A.; Campo, N.; Brancato, V.; Trimarchi, M. Influence of α-tocopherol load and annealing treatment on the wear resistance of biomedical UHMWPE irradiated with electron beam. Int. J. Polym. Anal. Charact. 2013, 18, 545–556. [Google Scholar] [CrossRef]
- Yousef, S.; Visco, A.; Galtieri, G.; Nocita, D.; Espro, C. Wear behaviour of UHMWPE reinforced by carbon nanofiller and paraffin oil for joint replacement. Mater. Sci. Eng. C 2017, 73, 234–244. [Google Scholar] [CrossRef]
- Fu, J.; Shen, J.; Gao, G.; Xu, Y.; Hou, R.; Cong, Y.; Cheng, Y. Natural polyphenol-stabilised highly crosslinked UHMWPE with high mechanical properties and low wear for joint implants. J. Mater. Chem. B 2013, 1, 4727–4735. [Google Scholar] [CrossRef]
- Ansari, F.; Ries, M.D.; Pruitt, L. Effect of processing, sterilization and crosslinking on UHMWPE fatigue fracture and fatigue wear mechanisms in joint arthroplasty. J. Mech. Behav. Biomed. Mater. 2016, 53, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Markhoff, J.; Zietz, C.; Fabry, C.; Fulda, G.; Bader, R. Influence of analyzed lubricant volumes on the amount and characteristics of generated wear particles from three different types of polyethylene liner materials. J. Biomed. Mater. Res. B 2017, 106, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Visco, A.; Torrisi, L.; Campo, N.; Emanuele, U.; Trifirò, A.; Trimarchi, M. Mechanical performance of electron-beam-irradiated UHMWPE in vacuum and in air. J. Biomed. Mater. Res. B 2009, 89, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Dumbleton, J.H.; D’Antonio, J.A.; Manley, M.T.; Capello, W.N.; Wang, A. The basis for a second-generation highly cross-linked UHMWPE. Clin. Orthop. Relat. Res. 2006, 453, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Bracco, P.; Oral, E. Vitamin E-stabilized UHMWPE for total joint implants: A review. Clin. Orthop. Relat. Res. 2011, 469, 2286–2293. [Google Scholar] [CrossRef]
- Kurtz, S.; Dumbleton, J.; Siskey, R.; Wang, A.; Manley, M. Trace concentrations of vitamin E protect radiation crosslinked UHMWPE from oxidative degradation. J. Biomed. Mater. Res. A 2009, 90, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Lerf, R.; Zurbrügg, D.; Delfosse, D. Use of vitamin E to protect cross-linked UHMWPE from oxidation. Biomaterials 2010, 31, 3643–3648. [Google Scholar] [CrossRef] [PubMed]
- Oral, E.; Christensen, S.D.; Malhi, A.S.; Wannomae, K.K.; Muratoglu, O.K. Wear resistance and mechanical properties of highly cross-linked, ultrahigh–molecular weight polyethylene doped with vitamin E. J. Arthroplast. 2006, 21, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Mallegol, J.; Carlsson, D.; Deschenes, L. Post-γ-irradiation reactions in vitamin E stabilised and unstabilised HDPE. Nucl. Instrum. Meth. B 2001, 185, 283–293. [Google Scholar] [CrossRef]
- Bracco, P.; Brunella, V.; Zanetti, M.; Luda, M.; Costa, L. Stabilisation of ultra-high molecular weight polyethylene with vitamin E. Polym. Degrad. Stab. 2007, 92, 2155–2162. [Google Scholar] [CrossRef]
- Gallo, J.; Goodman, S.B.; Konttinen, Y.T.; Wimmer, M.A.; Holinka, M. Osteolysis around total knee arthroplasty: A review of pathogenetic mechanisms. Acta Biomater. 2013, 9, 8046–8058. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Okubo, Y.; Teramura, S.; Niwa, Y.; Ibaraki, K.; Kawasaki, T.; Hamada, D.; Uetsuki, K.; Tomita, N. The antioxidant and non-antioxidant contributions of vitamin E in vitamin E blended ultra-high molecular weight polyethylene for total knee replacement. J. Mech. Behav. Biomed. Mater. 2014, 31, 21–30. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, Z.-Y.; Lan, R.-T.; Xu, L.; Gao, Y.; Zhao, B.; Xu, J.-Z.; Gul, R.M.; Li, Z.-M. Enhanced oxidation stability of highly cross-linked ultrahigh molecular weight polyethylene by tea polyphenols for total joint implants. Mater. Sci. Eng. C 2019, 94, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Sieving, A.; Wu, B.; Mayton, L.; Nasser, S.; Wooley, P.H. Morphological characteristics of total joint arthroplasty-derived ultra-high molecular weight polyethylene (UHMWPE) wear debris that provoke inflammation in a murine model of inflammation. J. Biomed. Mater. Res. A 2003, 64, 457–464. [Google Scholar] [CrossRef]
- ISO 14243-1 Implants for Surgery—Wear of Total Knee-Joint Prostheses. In Part 1: Loading and Displacement Parameters for Wear-Testing Machines with Load Control and Corresponding Environmental Conditions for Test; ISO Copyright Office: Geneva, Switzerland, 2009.
- Affatato, S.; Bersaglia, G.; Rocchi, M.; Taddei, P.; Fagnano, C.; Toni, A. Wear behaviour of cross-linked polyethylene assessed in vitro under severe conditions. Biomaterials 2005, 26, 3259–3267. [Google Scholar] [CrossRef]
- ISO 14243-2 Implants for Surgery—Wear of Total Knee-Joint Prostheses. In Part 2: Methods of Measurement; ISO Copyright Office: Geneva, Switzerland, 2016.
- ISO 17853 Wear of Implant Materials. In Polymer and metal Wear Particles—Isolation and Characterization; ISO Copyright Office: Geneva, Switzerland, 2011.
- Shanbhag, A.S.; Jacobs, J.J.; Glant, T.T.; Gilbert, J.L.; Black, J.; Galante, J.O. Composition and morphology of wear debris in failed uncemented total hip replacement. Bone Joint. J. 1994, 76, 60–67. [Google Scholar] [CrossRef]
- Paulus, A.C.; Franke, M.; Kraxenberger, M.; Schröder, C.; Jansson, V.; Utzschneider, S. PMMA third-body wear after unicondylar knee arthroplasty decuples the UHMWPE wear particle generation in vitro. Biomed. Res. Int. 2015, 1–7. [Google Scholar] [CrossRef]
- ASTM F1877-05 Standard Practice for Characterization of Particles; ASTM International: Conshohocken, PA, USA, 2010.
- Haider, H.; Weisenburger, J.N.; Kurtz, S.M.; Rimnac, C.M.; Freedman, J.; Schroeder, D.W.; Garvin, K.L. Does vitamin E–Stabilized ultrahigh-molecular-weight polyethylene address concerns of cross-linked polyethylene in total knee arthroplasty? J. Arthroplast. 2012, 27, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Ries, M.D.; Scott, M.L.; Jani, S. Relationship between gravimetric wear and particle generation in hip simulators: Conventional compared with cross-linked polyethylene. J. Bone Joint. Surg. Am. 2001, 83-A, S116–S122. [Google Scholar] [CrossRef] [PubMed]
- Deen, J.T.; Clay, T.B.; Iams, D.A.; Horodyski, M.; Parvataneni, H.K. Proximal tibial resorption in a modern total knee prosthesis. Arthroplast. Today 2018, 4, 244–248. [Google Scholar] [CrossRef] [PubMed]


| Number of Cycles | Gravimetric Wear (mg) | Wear Rate (mg/Million Cycles) | Correlation Coefficient (R2) |
|---|---|---|---|
| 0.5 million | 3.30 ± 0.51 | - | - |
| 1 million | 6.24 ± 2.51 | - | - |
| 2 million | 10.40 ± 1.50 | - | - |
| 3 million | 16.00 ± 0.94 | 3.92 ± 0.19 | 0.96 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Wang, J.-Y.; Su, J.; Wang, J.-J.; Yan, S.-T.; Luan, Y.-C.; Cheng, C.-K. Wear Assessment of Tibial Inserts Made of Highly Cross-Linked Polyethylene Supplemented with Dodecyl Gallate in the Total Knee Arthroplasty. Polymers 2021, 13, 1847. https://doi.org/10.3390/polym13111847
Zhang M, Wang J-Y, Su J, Wang J-J, Yan S-T, Luan Y-C, Cheng C-K. Wear Assessment of Tibial Inserts Made of Highly Cross-Linked Polyethylene Supplemented with Dodecyl Gallate in the Total Knee Arthroplasty. Polymers. 2021; 13(11):1847. https://doi.org/10.3390/polym13111847
Chicago/Turabian StyleZhang, Min, Jia-Yu Wang, Jian Su, Jian-Jun Wang, Shi-Tong Yan, Yi-Chao Luan, and Cheng-Kung Cheng. 2021. "Wear Assessment of Tibial Inserts Made of Highly Cross-Linked Polyethylene Supplemented with Dodecyl Gallate in the Total Knee Arthroplasty" Polymers 13, no. 11: 1847. https://doi.org/10.3390/polym13111847
APA StyleZhang, M., Wang, J.-Y., Su, J., Wang, J.-J., Yan, S.-T., Luan, Y.-C., & Cheng, C.-K. (2021). Wear Assessment of Tibial Inserts Made of Highly Cross-Linked Polyethylene Supplemented with Dodecyl Gallate in the Total Knee Arthroplasty. Polymers, 13(11), 1847. https://doi.org/10.3390/polym13111847