Native Spider Silk-Based Antimicrobial Hydrogels for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
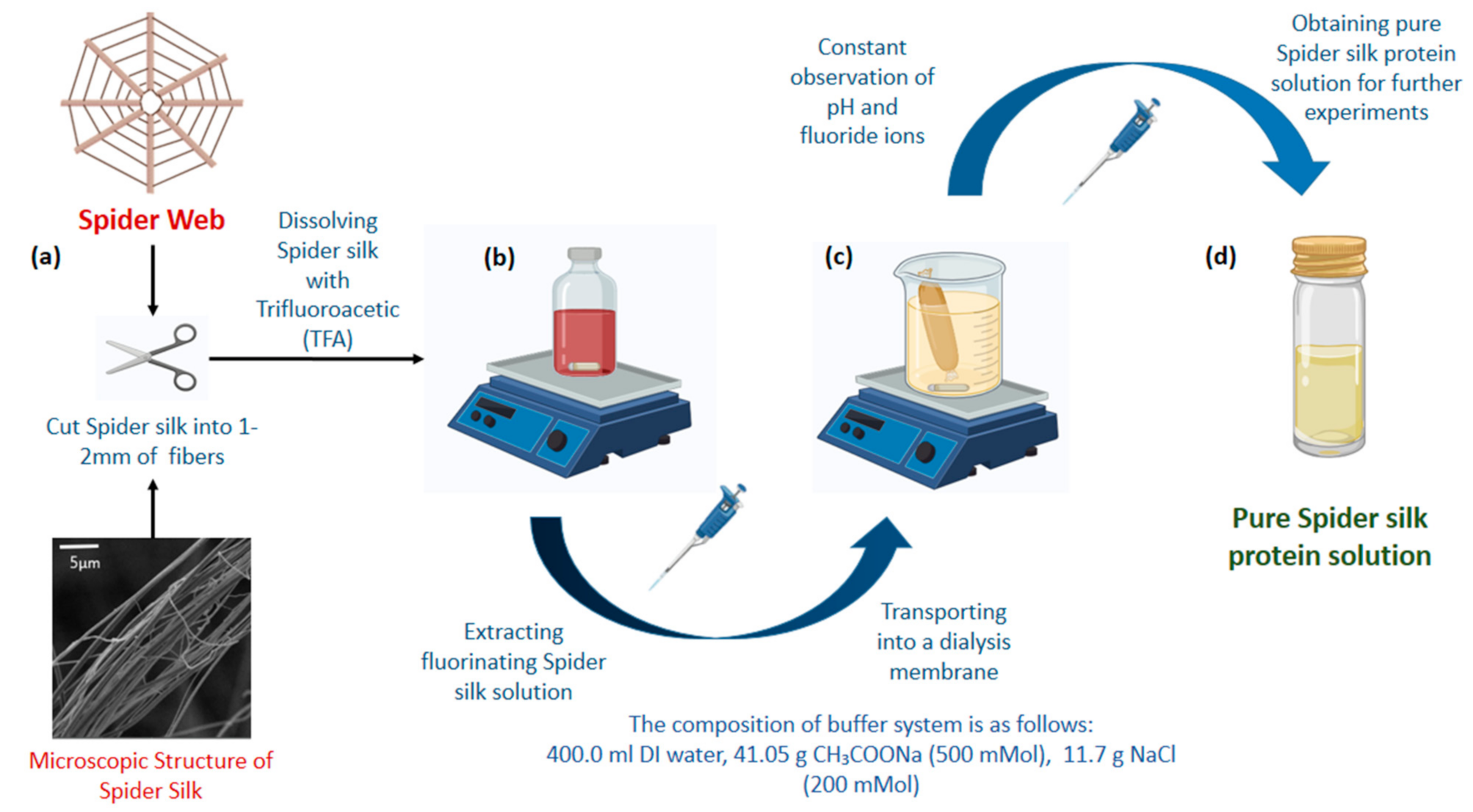
2.2. Preparation of Spider Silk Solution
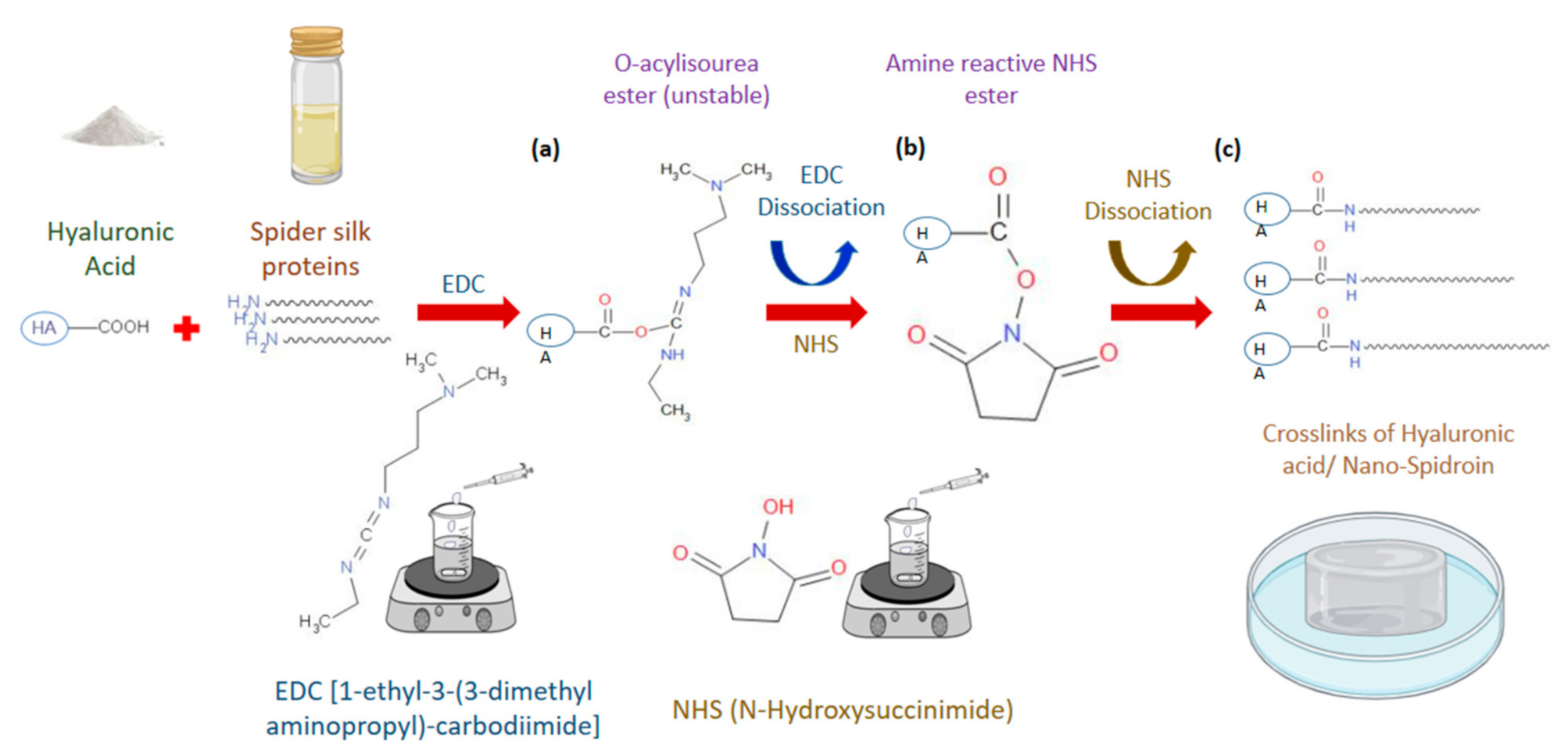
2.3. Preparation of Hyaluronic Acid/Spider Silk-Based Hydrogels
2.4. Freeze Drying of Hydrogels
2.5. Physical and Chemical Characterization of Hydrogels using Scanning Electron Microscopy
2.6. Fourier-Transform Infrared Spectroscopy
2.7. Contact Angle Measurements of Hyaluronic Acid /Spider Silk-Based Hydrogels
2.8. Rheological Properties of Hyaluronic acid /Spider Silk-Based Hydrogels
2.9. Swelling Ability of Hyaluronic Acid/Spider Silk-Based Hydrogels in Water
2.10. Gel Fraction and Crosslinking Density
2.11. Shrinking Ability of the Hyaluronic Acid/Spider Silk-Based Hydrogels
2.12. Drug Loading in Hyaluronic Acid/Spider Silk-Based Hydrogels
2.13. Drug Release Studies of Hyaluronic Acid/Spider Silk-Based Hydrogels
2.14. Conductivity of Hyaluronic Acid/Spider Silk-Based Hydrogels
2.15. Biodegradability Studies of Hyaluronic Acid/Spider Silk-Based Hydrogels
2.16. Antimicrobial Ability of Hyaluronic Acid/Spider Silk-Based Hydrogels
2.17. Cytotoxicity of Hyaluronic Acid/ Spider Silk-Based Hydrogels
3. Results and Discussion
3.1. Preparation of Hyaluronic Acid/Spider Silk-Based Hydrogels
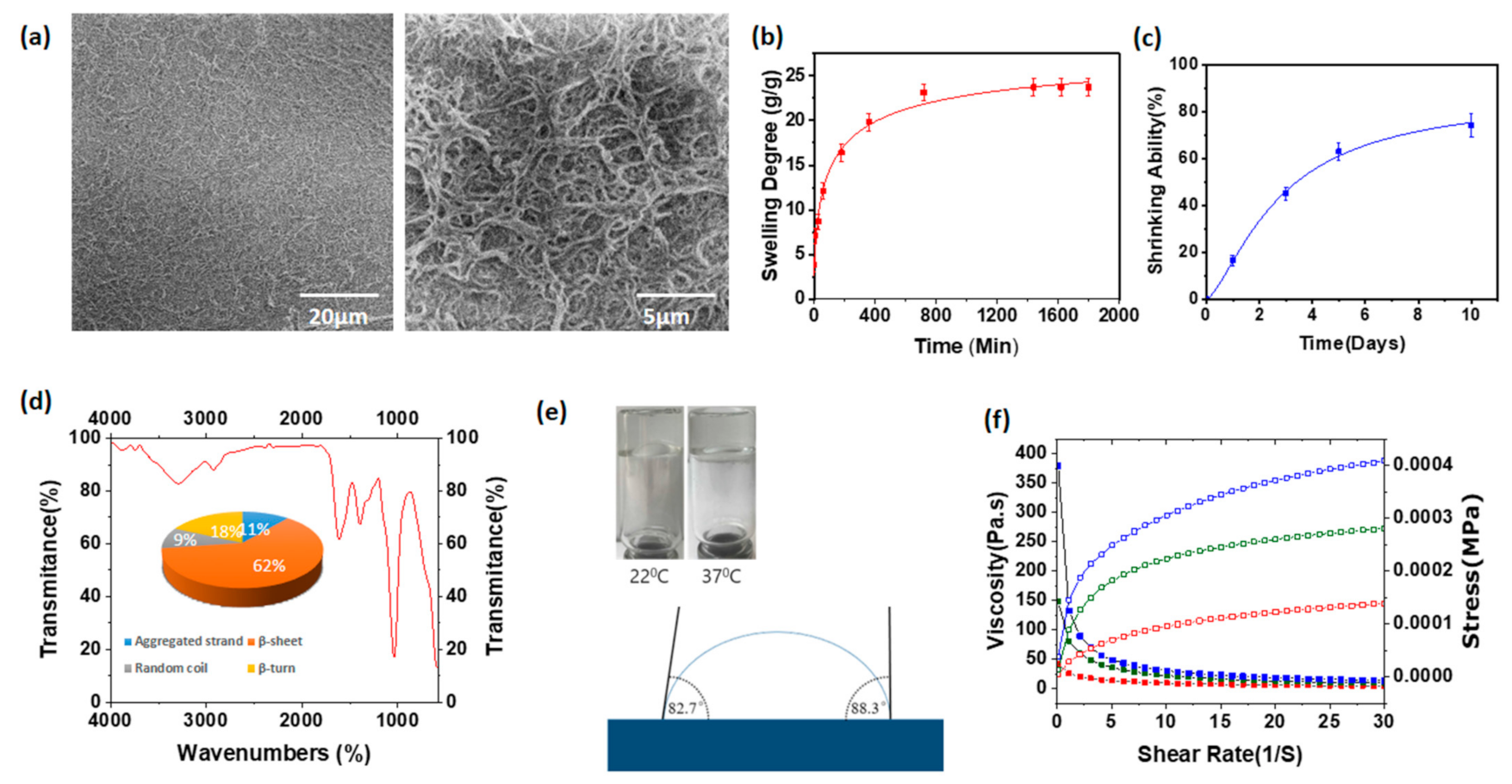
3.2. Scanning Electron Microscopic Images of Hyaluronic Acid/Spider Silk-Based Hydrogels
3.3. Swelling Degree of Hyaluronic Acid/Spider Silk-Based Hydrogels
3.4. Gel Fraction and Crosslinking Density of Hyaluronic Acid/Spider Silk-Based Hydrogels
3.5. Shrinking Ability of Hyaluronic Acid/Spider Silk-Based Hydrogels
3.6. Fourier-Transform Infrared Spectroscopy
3.7. Contact Angle Measurements and FLIP Test of Hyaluronic Acid/Spider Silk Hydrogels
3.8. Rheological Properties of Hyaluronic Acid/Spider Silk Hydrogels
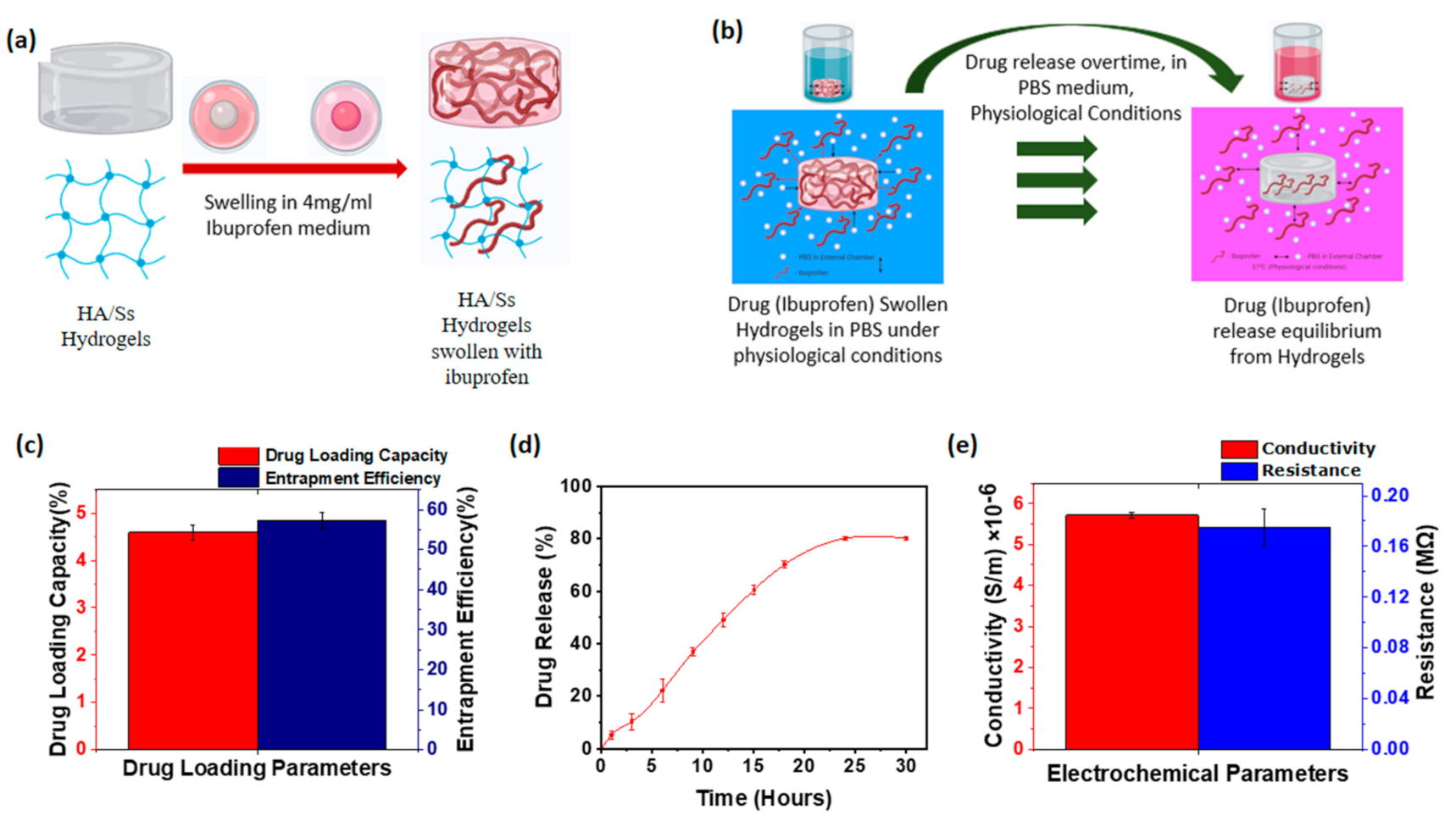
3.9. Drug Loading of Hyaluronic Acid/Spider Silk Hydrogels
3.10. Drug Release of Hyaluronic Acid/Spider Silk Hydrogels
3.11. Conductivity of Hyaluronic Acid/Spider Silk Hydrogels
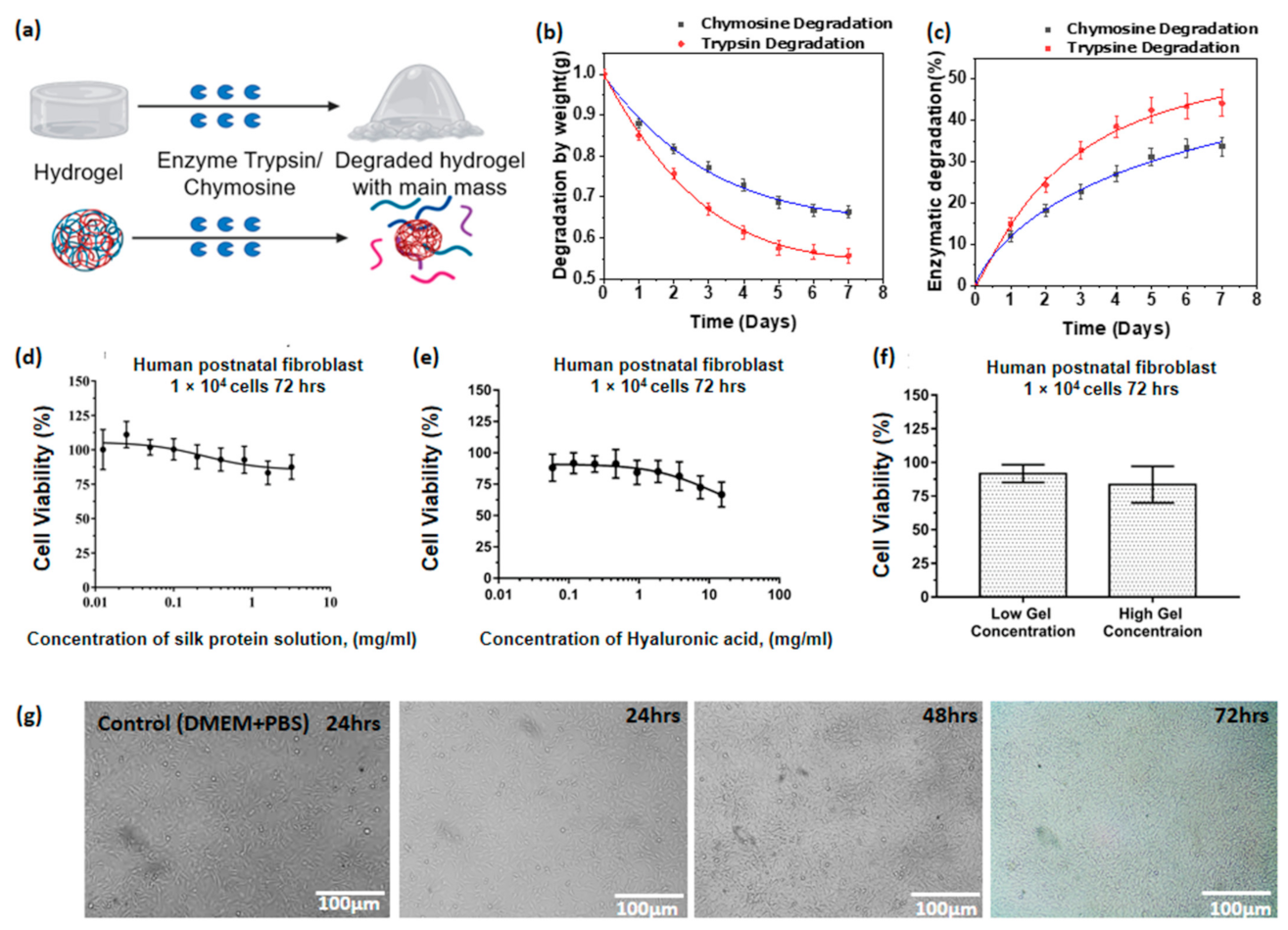
3.12. Enzymatic Degradability of Hyaluronic Acid/Spider Silk Hydrogels
3.13. Cell Viability Studies of Hyaluronic Acid/Spider Silk Hydrogels
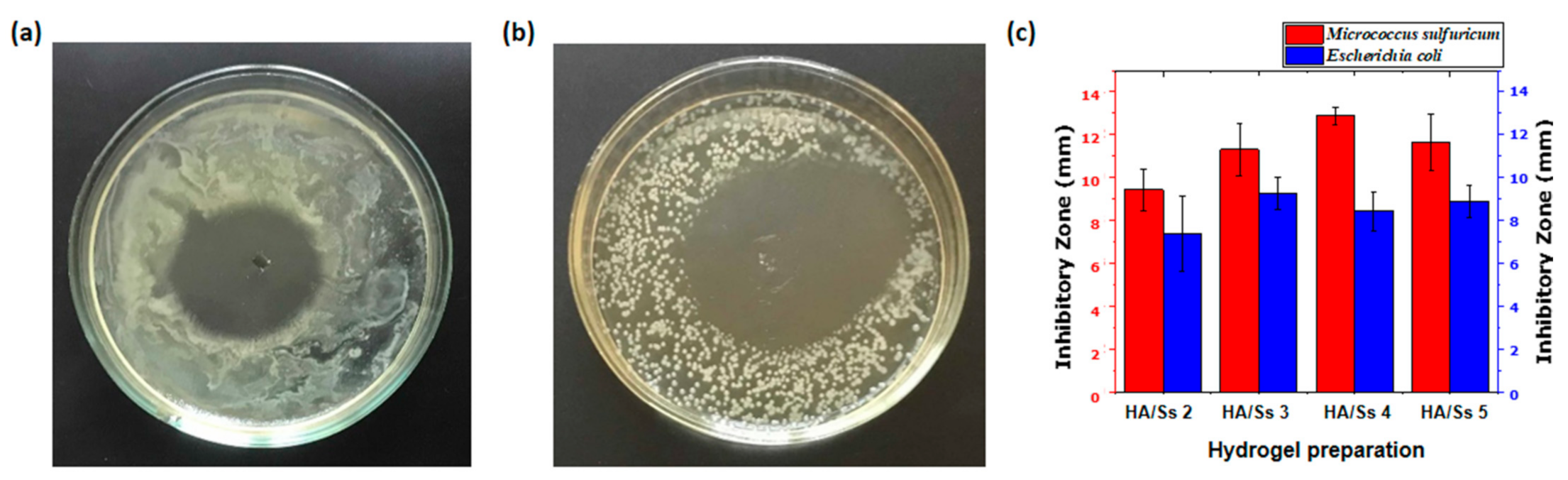
3.14. Antimicrobial Ability of Hyaluronic Acid/Spider Silk Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Fan, X.D. Synthesis, Properties and Controlled Release Behaviors of Hydrogel Networks Using Cyclodextrin as Pendant Groups. Biomaterials 2005, 26, 6367–6374. [Google Scholar] [CrossRef] [PubMed]
- Gulrez, S.K.H.; Al-Assaf, S.O.G. Hydrogels: Methods of Preparation, Characterisation and Applications. In Progress in Molecular and Environmental Bioengineering—From Analysis and Modeling to Technology Applications; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Ko, D.Y.; Shinde, U.P.; Yeon, B.; Jeong, B. Recent progress of in situ formed gels for biomedical applications. Prog. Polym. Sci. 2013, 38, 672–701. [Google Scholar] [CrossRef]
- Ramazani, A.; Aghahosseini, H. The Biological Properties of Hydrogels Based on Natural Polymers. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Nadolna, K.; Owczarek, A. The Physical and Chemical Properties of Hydrogels Based on Natural Polymers. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Capanema, N.S.V.; Mansur, A.A.P.; de Jesus, A.C.; Carvalho, S.M.; de Oliveira, L.C.; Mansur, H.S. Superabsorbent Crosslinked Carboxymethyl Cellulose-PEG Hydrogels for Potential Wound Dressing Applications. Int. J. Biol. Macromol. 2018, 106, 1218–1234. [Google Scholar] [CrossRef]
- Ikada, Y. Absorbable Hydrogels for Medical Use. In Gels Handbook; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar] [CrossRef]
- Ou, X.; Han, Q.; Dai, H.H.; Wang, J. Molecular Dynamic Simulations of the Water Absorbency of Hydrogels. J. Mol. Model. 2015, 21, 1–10. [Google Scholar] [CrossRef]
- Khansari, M.M.; Sorokina, L.V.; Mukherjee, P.; Mukhtar, F.; Shirdar, M.R.; Shahidi, M.; Shokuhfar, T. Classification of Hydrogels Based on Their Source: A Review and Application in Stem Cell Regulation. JOM 2017, 69, 1340–1347. [Google Scholar] [CrossRef]
- Singh, M.R.; Patel, S.; Singh, D. Natural Polymer-Based Hydrogels as Scaffolds for Tissue Engineering. In Nanobiomaterials in Soft Tissue Engineering: Applications of Nanobiomaterials; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-Based Hydrogels As Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef]
- Zhao, W.; Jin, X.; Cong, Y.; Liu, Y.; Fu, J. Degradable natural polymer hydrogels for articular cartilage tissue engineering. J. Chem. Technol. Biotechnol. 2012, 88, 327–339. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking Method of Hyaluronic-Based Hydrogel for Biomedical Applications. J. Tissue Eng. 2017, 8. [Google Scholar] [CrossRef]
- Hennink, W.; van Nostrum, C. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 13–36. [Google Scholar] [CrossRef]
- Samorezov, J.E.; Morlock, C.M.; Alsberg, E. Dual Ionic and Photo-Crosslinked Alginate Hydrogels for Micropatterned Spatial Control of Material Properties and Cell Behavior. Bioconjug. Chem. 2015, 26, 1339–1347. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, Y.; Wang, L.; Xu, L.; Zhai, M.; Wei, S. Radiation synthesis and characterization of nanosilver/gelatin/carboxymethyl chitosan hydrogel. Radiat. Phys. Chem. 2012, 81, 553–560. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.; Felt, O.; Peppas, N.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Shu, X.Z.; Liu, Y.; Palumbo, F.; Prestwich, G.D. Disulfide-crosslinked hyaluronan-gelatin hydrogel films: A covalent mimic of the extracellular matrix for in vitro cell growth. Biomaterials 2003, 24, 3825–3834. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Klein, M.; Poverenov, E. Natural biopolymer-based hydrogels for use in food and agriculture. J. Sci. Food Agric. 2020, 100, 2337–2347. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Sundaramurthi, D.; Krishnan, U.M.; Sethuraman, S. Electrospun Nanofibers as Scaffolds for Skin Tissue Engineering. Polym. Rev. 2014, 54, 348–376. [Google Scholar] [CrossRef]
- Duncan, R.; Kopeček, J. Soluble synthetic polymers as potential drug carriers. Adv. Polym. Sci. 1984, 57, 51–101. [Google Scholar]
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Prog. Polym. Sci. 2012, 37, 237–280. [Google Scholar] [CrossRef]
- Maitz, M. Applications of synthetic polymers in clinical medicine. Biosurface Biotribology 2015, 1, 161–176. [Google Scholar] [CrossRef]
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Madhavi, K.; Reddy, A. Potential Danger of Plastics to Marine Environment. Cont. J. Environ. Sci. 2013, 7, 8–12. [Google Scholar]
- Bhatia, S.; Bhatia, S. Natural Polymers vs. Synthetic Polymer. In Natural Polymer Drug Delivery Systems; J.B. Metzler: Stuttgart, Germany, 2016. [Google Scholar] [CrossRef]
- Olatunji, O. Natural Polymers: Industry Techniques and Applications; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Reddy, B.; Eoff, L.; Dalrymple, E.D.; Black, K.; Brown, D.; Rietjens, M. A Natural Polymer-Based Cross-Linker System for Conformance Gel Systems. SPE J. 2003, 8, 99–106. [Google Scholar] [CrossRef]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of Collagen I Hydrogels for Bioengineered Tissue Microenvironments: Characterization of Mechanics, Structure, and Transport. Tissue Eng. Part B Rev. 2014, 20, 683–696. [Google Scholar] [CrossRef]
- Kapoor, S.; Kundu, S.C. Silk protein-based hydrogels: Promising advanced materials for biomedical applications. Acta Biomater. 2016, 31, 17–32. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L. Cellulose-based hydrogels: Present status and application prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Klaassen, C.D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; Panel, C.I.R.E.; Andersen, F.A. Final Report of the Safety Assessment of Hyaluronic Acid, Potassium Hyaluronate, and Sodium Hyaluronate. Int. J. Toxicol. 2009, 28, 5–67. [Google Scholar] [CrossRef]
- Roman, M. Toxicity of Cellulose Nanocrystals: A Review. Ind. Biotechnol. 2015, 11, 25–33. [Google Scholar] [CrossRef]
- Blamires, S.J.; Blackledge, T.A.; Tso, I.-M. Physicochemical Property Variation in Spider Silk: Ecology, Evolution, and Synthetic Production. Annu. Rev. Entomol. 2017, 62, 443–460. [Google Scholar] [CrossRef]
- Römer, L.; Scheibel, T. The elaborate structure of spider silk. Prion 2008, 2, 154–161. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, B.Y.; Kim, D.H.; Jin, B.R. Recombinant spider silk fibroin protein produces a non-cytotoxic and non-inflammatory response. J. Asia Pac. Entomol. 2016, 19, 1015–1018. [Google Scholar] [CrossRef]
- Li, G.; Li, F.; Zheng, Z.; Luo, T.; Liu, J.; Wu, J.; Wang, X.; Kaplan, D.L. Silk microfiber-reinforced silk composite scaffolds: Fabrication, mechanical properties, and cytocompatibility. J. Mater. Sci. 2015, 51, 3025–3035. [Google Scholar] [CrossRef]
- Salehi, S.; Koeck, K.; Scheibel, T. Spider Silk for Tissue Engineering Applications. Molecules 2020, 25, 737. [Google Scholar] [CrossRef]
- Kiseleva, A.P.; Krivoshapkin, P.V.; Krivoshapkina, E.F. Recent Advances in Development of Functional Spider Silk-Based Hybrid Materials. Front. Chem. 2020, 8, 554. [Google Scholar] [CrossRef]
- Kluge, J.A.; Rabotyagova, O.; Leisk, G.G.; Kaplan, D.L. Spider silks and their applications. Trends Biotechnol. 2008, 26, 244–251. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Stoppel, W.L.; Raia, N.; Kimmerling, E.; Wang, S.; Ghezzi, C.E.; Kaplan, D.L. 2.12 Silk Biomaterials. In Comprehensive Biomaterials II; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Borkner, C.B.; Lentz, S.; Müller, M.; Fery, A.; Scheibel, T. Ultrathin Spider Silk Films: Insights into Spider Silk Assembly on Surfaces. ACS Appl. Polym. Mater. 2019, 1, 3366–3374. [Google Scholar] [CrossRef]
- Johansson, U.; Ria, M.; Åvall, K.; Shalaly, N.D.; Zaitsev, S.V.; Berggren, P.-O.; Hedhammar, M. Pancreatic Islet Survival and Engraftment Is Promoted by Culture on Functionalized Spider Silk Matrices. PLoS ONE 2015, 10, e0130169. [Google Scholar] [CrossRef]
- Xia, X.-X.; Qian, Z.-G.; Ki, C.S.; Park, Y.H.; Kaplan, D.L.; Lee, S.Y. Native-sized recombinant spider silk protein produced in metabolically engineered Escherichia coli results in a strong fiber. Proc. Natl. Acad. Sci. USA 2010, 107, 14059–14063. [Google Scholar] [CrossRef]
- Lazaris, A.; Arcidiacono, S.; Huang, Y.; Zhou, J.-F.; Duguay, F.; Chretien, N.; Welsh, E.A.; Soares, J.W.; Karatzas, C.N. Spider Silk Fibers Spun from Soluble Recombinant Silk Produced in Mammalian Cells. Science 2002, 295, 472–476. [Google Scholar] [CrossRef]
- Tokareva, O.; Michalczechen-Lacerda, V.A.; Rech, E.L.; Kaplan, D.L. Recombinant DNA production of spider silk proteins. Microb. Biotechnol. 2013, 6, 651–663. [Google Scholar] [CrossRef]
- Whittall, D.R.; Baker, K.V.; Breitling, R.; Takano, E. Host Systems for the Production of Recombinant Spider Silk. Trends Biotechnol. 2021, 39, 560–573. [Google Scholar] [CrossRef]
- Heidebrecht, A.; Scheibel, T. Recombinant Production of Spider Silk Proteins. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Xu, J.; Dong, Q.; Yu, Y.; Niu, B.; Ji, D.; Li, M.; Huang, Y.; Chen, X.; Tan, A. Mass spider silk production through targeted gene replacement in Bombyx mori. Proc. Natl. Acad. Sci. USA 2018, 115, 8757–8762. [Google Scholar] [CrossRef]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The Biomedical Use of Silk: Past, Present, Future. Adv. Health Mater. 2019, 8, e1800465. [Google Scholar] [CrossRef]
- King, G.F. The wonderful world of spiders: Preface to the special Toxicon issue on spider venoms. Toxicon 2004, 43, 471–475. [Google Scholar] [CrossRef]
- Aukerman, M. In Praise of Wiggle Room: Locating Comprehension in Unlikely Places. Lang. Arts 2008, 86, 52–60. [Google Scholar]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.; Jia, X. Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomater. 2014, 10, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. A clinical evaluation of the comparable efficacy of hyaluronic acid-based foam and ceramide-containing emulsion cream in the treatment of mild-to-moderate atopic dermatitis. J. Cosmet. Dermatol. 2011, 10, 185–188. [Google Scholar] [CrossRef]
- Jegasothy, S.M.; Zabolotniaia, V.; Bielfeldt, S. Efficacy of a New Topical Nano-hyaluronic Acid in Humans. J. Clin. Aesthetic Dermatol. 2014, 7, 27–29. [Google Scholar]
- Pirnazar, P.; Wolinsky, L.; Nachnani, S.; Haake, S.; Pilloni, A.; Bernard, G.W. Bacteriostatic Effects of Hyaluronic Acid. J. Periodontol. 1999, 70, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Dereure, O.; Czubek, M.; Combemale, P. Efficacy and safety of hyaluronic acid in treatment of leg ulcers: A double-blind RCT. J. Wound Care 2012, 21, 131–139. [Google Scholar] [CrossRef]
- Aya, K.L.; Stern, R. Hyaluronan in wound healing: Rediscovering a major player. Wound Repair Regen. 2014, 22, 579–593. [Google Scholar] [CrossRef]
- Anisha, B.; Biswas, R.; Chennazhi, K.; Jayakumar, R. Chitosan–hyaluronic acid/nano silver composite sponges for drug resistant bacteria infected diabetic wounds. Int. J. Biol. Macromol. 2013, 62, 310–320. [Google Scholar] [CrossRef]
- Valverde, A.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Olivenza, M.A.P.; Blanco, M.B.G.; Díaz-Fuentes, M.; Vilas-Vilela, J.L. Antibacterial hyaluronic acid/chitosan multilayers onto smooth and micropatterned titanium surfaces. Carbohydr. Polym. 2019, 207, 824–833. [Google Scholar] [CrossRef]
- Bonafè, F.; Govoni, M.; Giordano, E.; Caldarera, C.M.; Guarnieri, C.; Muscari, C. Hyaluronan and cardiac regeneration. J. Biomed. Sci. 2014, 21, 1–13. [Google Scholar] [CrossRef]
- Kallestrup, E.B.; Jørgensen, S.S.; Nordling, J.; Hald, T. Treatment of interstitial cystitis with Cystistat®, A hyaluronic acid product. Scand. J. Urol. Nephrol. 2005, 39, 143–147. [Google Scholar] [CrossRef]
- Kogan, G.; Šoltés, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2006, 29, 17–25. [Google Scholar] [CrossRef]
- Di Simone, M.; Baldi, F.; Vasina, V.; Bacci, M.L.; Scorrano, F.; Poggioli, G.; Ferrieri, A. Barrier effect of Esoxx® on esophageal mucosal damage: Experimental study on ex-vivo swine model. Clin. Exp. Gastroenterol. 2012, 5, 103. [Google Scholar] [CrossRef]
- Pyo, J.-S.; Cho, W.J. Systematic Review and Meta-Analysis of Intravesical Hyaluronic Acid and Hyaluronic Acid/Chondroitin Sulfate Instillation for Interstitial Cystitis/Painful Bladder Syndrome. Cell. Physiol. Biochem. 2016, 39, 1618–1625. [Google Scholar] [CrossRef]
- Xu, X.; Jha, A.; Harrington, D.A.; Farach-Carson, M.; Jia, X. Hyaluronic acid-based hydrogels: From a natural polysaccharide to complex networks. Soft Matter 2012, 8, 3280–3294. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, L.; Bao, Y.; Yan, X.; Yin, Y.; Li, Y.; Wang, X.; Huang, Z.; Xu, P. Preparation and characterization of injectable chitosan–hyaluronic acid hydrogels for nerve growth factor sustained release. J. Bioact. Compat. Polym. 2017, 32, 146–162. [Google Scholar] [CrossRef]
- Lou, J.; Stowers, R.; Nam, S.; Xia, Y.; Chaudhuri, O. Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3D cell culture. Biomaterials 2018, 154, 213–222. [Google Scholar] [CrossRef]
- Segura, T.; Anderson, B.C.; Chung, P.H.; Webber, R.E.; Shull, K.R.; Shea, L.D. Crosslinked hyaluronic acid hydrogels: A strategy to functionalize and pattern. Biomaterials 2005, 26, 359–371. [Google Scholar] [CrossRef]
- Hua, J.; Li, Z.; Xia, W.; Yang, N.; Gong, J.; Zhang, J.; Qiao, C. Preparation and properties of EDC/NHS mediated crosslinking poly (gamma-glutamic acid)/epsilon-polylysine hydrogels. Mater. Sci. Eng. C 2016, 61, 879–892. [Google Scholar] [CrossRef]
- Madurga, R.; Ganan-Calvo, A.; Plaza, G.R.; Guinea, G.V.; Elices, M.; Pérez-Rigueiro, J. Straining flow spinning: Production of regenerated silk fibers under a wide range of mild coagulating chemistries. Green Chem. 2017, 19, 3380–3389. [Google Scholar] [CrossRef]
- Mezger, T.G. The Rheology Handbook; Vincentz Network: Hanover, Germany, 2019. [Google Scholar] [CrossRef]
- Yang, T. Mechanical and Swelling Properties of Hydrogels. Ph.D. Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2012. [Google Scholar]
- Nawaz, S.; Khan, S.; Farooq, U.; Haider, M.S.; Ranjha, N.M.; Rasul, A.; Nawaz, A.; Arshad, N.; Hameed, R. Biocompatible hydrogels for the controlled delivery of anti-hypertensive agent: Development, characterization and in vitro evaluation. Des. Monomers Polym. 2018, 21, 18–32. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, T.; Liu, Y.; Leng, J. Microwave synthesis and actuation of shape memory polycaprolactone foams with high speed. Sci. Rep. 2015, 5, 11152. [Google Scholar] [CrossRef]
- Omidian, H.; Hashemi, S.A.; Askari, F.; Nafisi, S. Swelling and Crosslink Density Measurements for Hydrogels. Iran. J. Polym. Sci. Technol. 1994, 5, 1–10. [Google Scholar]
- Gallagher, S.; Florea, L.; Fraser, K.J.; Diamond, D. Swelling and Shrinking Properties of Thermo-Responsive Polymeric Ionic Liquid Hydrogels with Embedded Linear pNIPAAM. Int. J. Mol. Sci. 2014, 15, 5337–5349. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, V.L.; Choudhari, P.B.; Bhatia, N.M.; Bhatia, M.S. Characterization of Pharmaceutical Nanocarriers: In Vitro and in Vivo Studies. In Nanomaterials for Drug Delivery and Therapy; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Xiao, X.; Wu, G.; Zhou, H.; Qian, K.; Hu, J. Preparation and Property Evaluation of Conductive Hydrogel Using Poly (Vinyl Alcohol)/Polyethylene Glycol/Graphene Oxide for Human Electrocardiogram Acquisition. Polymers 2017, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Numata, K.; Yamazaki, S.; Katashima, T.; Chuah, J.; Naga, N.; Sakai, T. Silk-Pectin Hydrogel with Superior Mechanical Properties, Biodegradability, and Biocompatibility. Macromol. Biosci. 2014, 14, 799–806. [Google Scholar] [CrossRef]
- Garrard, A. Ibuprofen. In Encyclopedia of Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Rainsford, K.D. Ibuprofen: Pharmacology, efficacy and safety. Inflammopharmacology 2009, 17, 275–342. [Google Scholar] [CrossRef]
- Banga, A.K.; Chien, Y.W. Hydrogel-Based Lontotherapeutic Delivery Devices for Transdermal Delivery of Peptide/Protein Drugs. Pharm. Res. Off. J. Am. Assoc. Pharm. Sci. 1993, 10, 697–702. [Google Scholar] [CrossRef]
- Palza, H.; Zapata, P.A.; Angulo-Pineda, C. Electroactive Smart Polymers for Biomedical Applications. Materials 2019, 12, 277. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J. Families of serine peptidases. Methods Enzymol. 1994, 244, 19–61. [Google Scholar] [CrossRef]
- Koshikawa, N.; Hasegawa, S.; Nagashima, Y.; Mitsuhashi, K.; Tsubota, Y.; Miyata, S.; Miyagi, Y.; Yasumitsu, H.; Miyazaki, K. Expression of Trypsin by Epithelial Cells of Various Tissues, Leukocytes, and Neurons in Human and Mouse. Am. J. Pathol. 1998, 153, 937–944. [Google Scholar] [CrossRef]
- Foltmann, B. Chymosin: A short review on foetal and neonatal gastric proteases. Scand. J. Clin. Lab. Investig. 1992, 52, 65–79. [Google Scholar] [CrossRef]
- Kitamura, N.; Tanimoto, A.; Hondo, E.; Andren, A.; Cottrell, D.F.; Sasaki, M.; Yamada, J. Immunohistochemical Study of the Ontogeny of Prochymosin- and Pepsinogen-producing Cells in the Abomasum of Sheep. Anat. Histol. Embryol. 2001, 30, 231–235. [Google Scholar] [CrossRef]
- Yegorov, Y.E.; Moldaver, M.V.; Vishnyakova, K.S.; Terekhov, S.M.; Dashinimaev, E.B.; Cheglakov, I.B.; Toropygin, I.Y.; Yarygin, K.N.; Chumakov, P.M.; Korochkin, L.I.; et al. Enhanced control of proliferation in telomerized cells. Russ. J. Dev. Biol. 2007, 38, 76–89. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A.; Caterson, E. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Gedikli, S.; Güngör, G.; Toptaş, Y.; Sezgin, D.E.; Demirbilek, M.; Yazıhan, N.; Çelik, P.A.; Denkbaş, E.B.; Bütün, V.; Çabuk, A. Optimization of hyaluronic acid production and its cytotoxicity and degradability characteristics. Prep. Biochem. Biotechnol. 2018, 48, 610–618. [Google Scholar] [CrossRef]
- Boeckel, D.G.; Shinkai, R.S.A.; Grossi, M.L.; Teixeira, E. In vitro evaluation of cytotoxicity of hyaluronic acid as an extracellular matrix on OFCOL II cells by the MTT assay. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, e423–e428. [Google Scholar] [CrossRef]
- Zamboni, F.; Okoroafor, C.; Ryan, M.P.; Pembroke, J.T.; Strozyk, M.; Culebras, M.; Collins, M.N. On the bacteriostatic activity of hyaluronic acid composite films. Carbohydr. Polym. 2021, 260, 117803. [Google Scholar] [CrossRef]
| Sample | Spider Silk | Hyaluruonic ACID | EDC/NHS | MES Buffer (0.1 mol/L) |
|---|---|---|---|---|
| HA/Ss 1 | 18 mg | 100 mg | 1 ml | 8 mL |
| HA/Ss 2 | 18 mg | 150 mg | 1 ml | 8 mL |
| HA/Ss 3 | 18 mg | 200 mg | 1 ml | 8 mL |
| HA/Ss 4 | 22.5 mg | 150 mg | 1 ml | 7.5 mL |
| HA/Ss 5 | 27 mg | 150 mg | 1 ml | 7.5 mL |
| No. | Hydrogel Preparation | Gel Fraction (%) | Crosslinking Density ×10−4 mol/cm−3 |
|---|---|---|---|
| 01 | HA/Ss 1 | 79.83 | 7.27 |
| 02 | HA/Ss 2 | 79.86 | 8.11 |
| 03 | HA/Ss 3 | 80.09 | 7.81 |
| 04 | HA/Ss 4 | 81.91 | 8.20 |
| 05 | HA/Ss 5 | 83.86 | 8.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Withanage, S.; Savin, A.; Nikolaeva, V.; Kiseleva, A.; Dukhinova, M.; Krivoshapkin, P.; Krivoshapkina, E. Native Spider Silk-Based Antimicrobial Hydrogels for Biomedical Applications. Polymers 2021, 13, 1796. https://doi.org/10.3390/polym13111796
Withanage S, Savin A, Nikolaeva V, Kiseleva A, Dukhinova M, Krivoshapkin P, Krivoshapkina E. Native Spider Silk-Based Antimicrobial Hydrogels for Biomedical Applications. Polymers. 2021; 13(11):1796. https://doi.org/10.3390/polym13111796
Chicago/Turabian StyleWithanage, Sinith, Artemii Savin, Valeria Nikolaeva, Aleksandra Kiseleva, Marina Dukhinova, Pavel Krivoshapkin, and Elena Krivoshapkina. 2021. "Native Spider Silk-Based Antimicrobial Hydrogels for Biomedical Applications" Polymers 13, no. 11: 1796. https://doi.org/10.3390/polym13111796
APA StyleWithanage, S., Savin, A., Nikolaeva, V., Kiseleva, A., Dukhinova, M., Krivoshapkin, P., & Krivoshapkina, E. (2021). Native Spider Silk-Based Antimicrobial Hydrogels for Biomedical Applications. Polymers, 13(11), 1796. https://doi.org/10.3390/polym13111796