Smart Hydrogel Bilayers Prepared by Irradiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Smart Hydrogel Bilayers
2.3. Microstructure and Morphology Characterization
2.4. Swelling Test
2.5. Tensile Property Test
2.6. Antibacterial Test
2.7. Temperature Response Test
3. Results and Discussion
3.1. Construction and Characterization of Bilayered Hydrogel
3.2. The Properties of CS/Agar/P(NIPAM-co-AM) Hydrogel
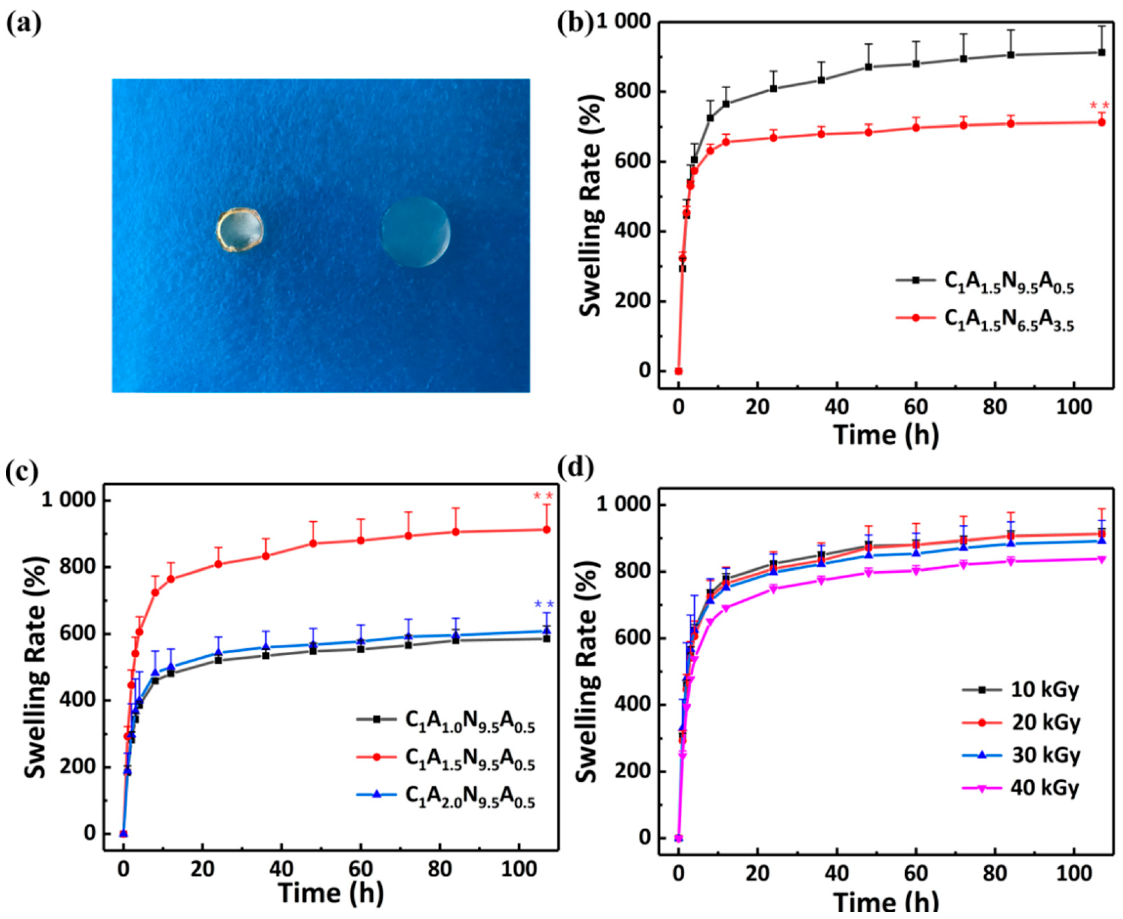
3.2.1. The Swelling Property of CS/Agar/P(NIPAM-co-AM) Hydrogel
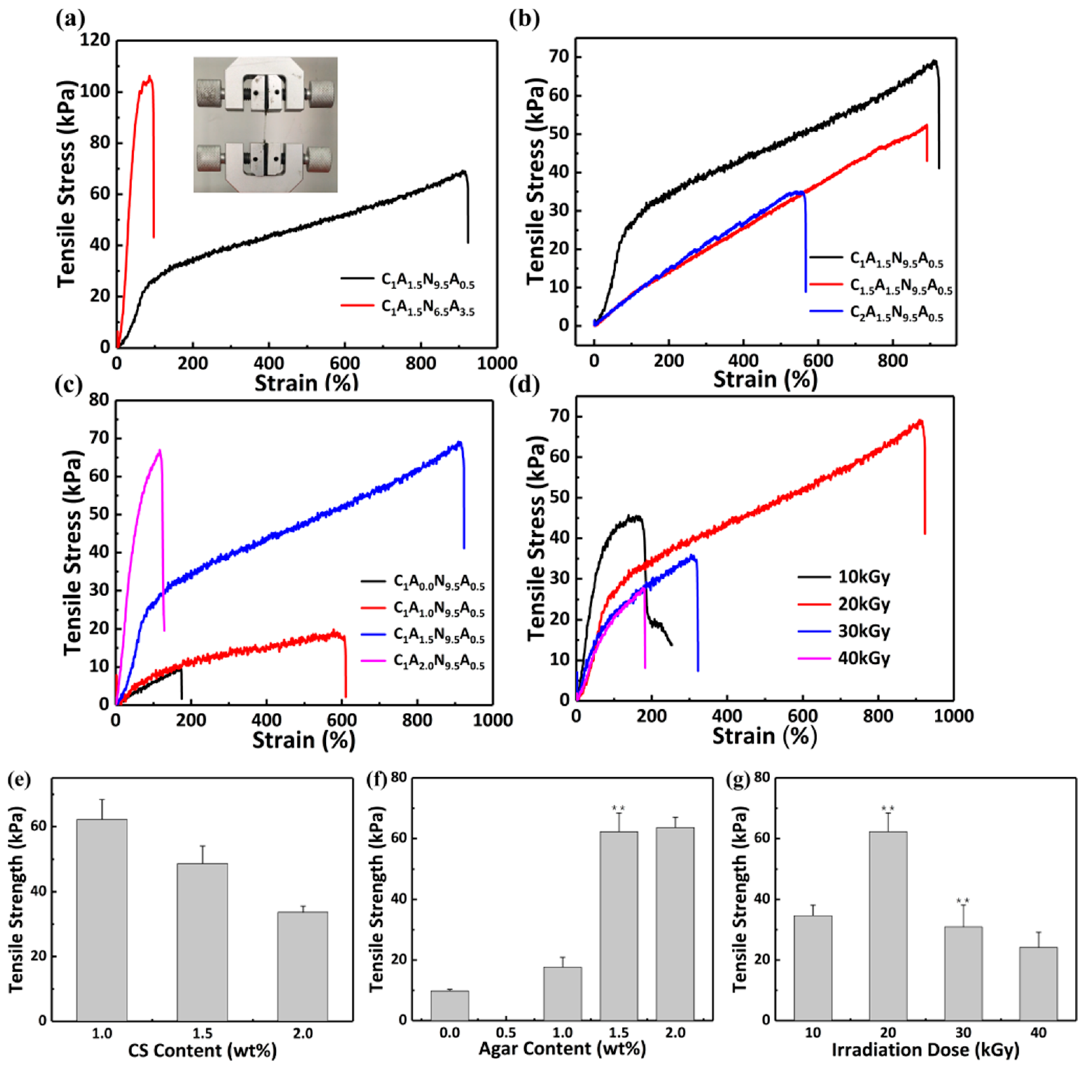
3.2.2. Mechanical Properties
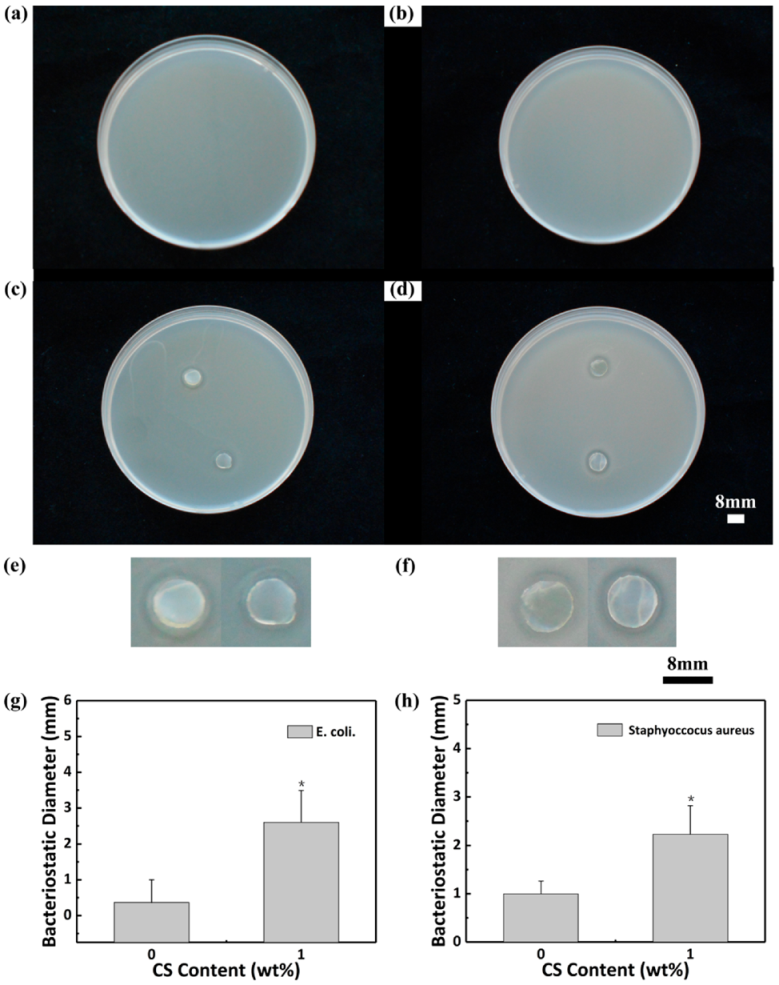
3.2.3. Antibacterial Test
3.3. Temperature Sensitivity and Temperature-Driven Deformation
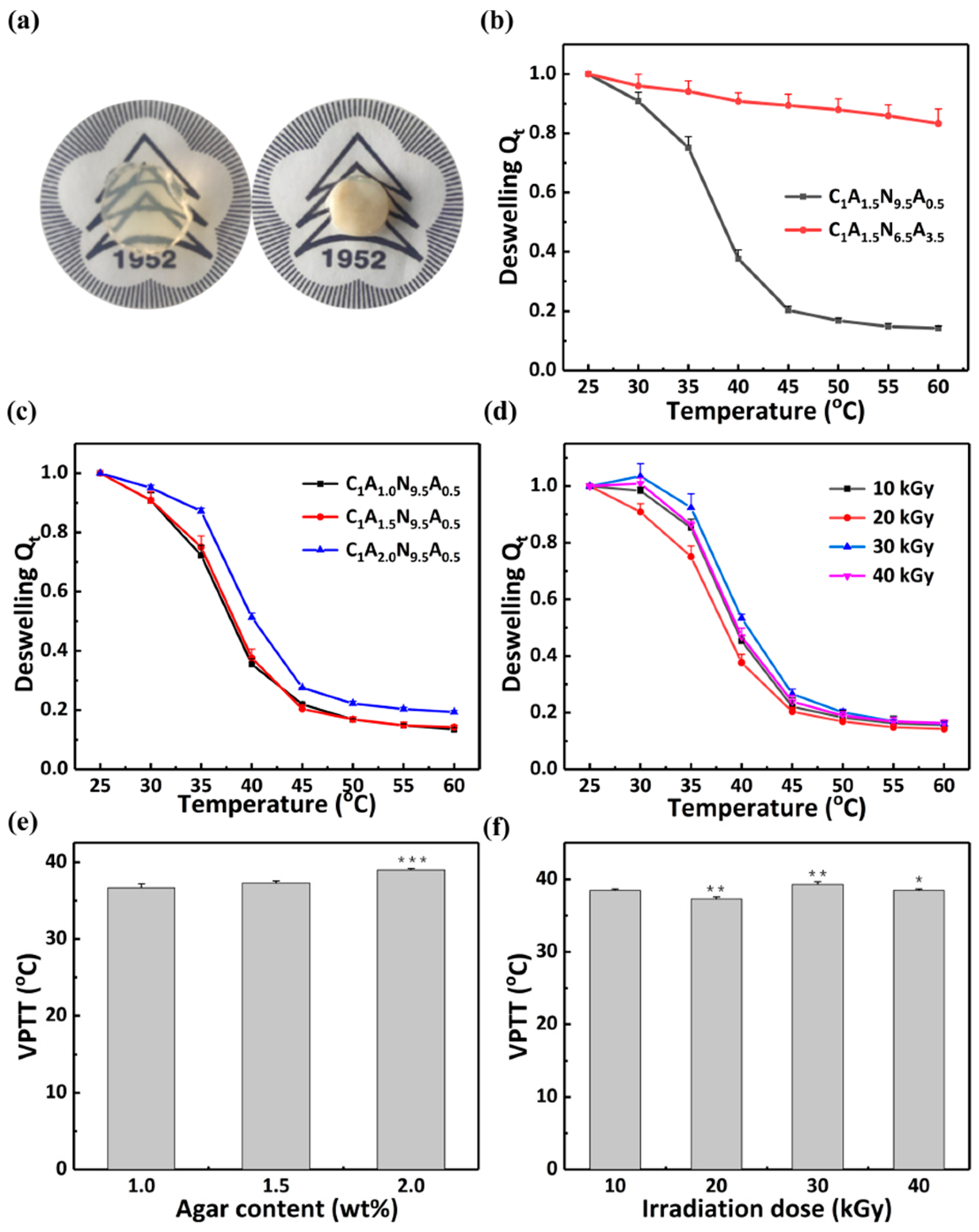
3.3.1. The VPTT of the Hydrogel
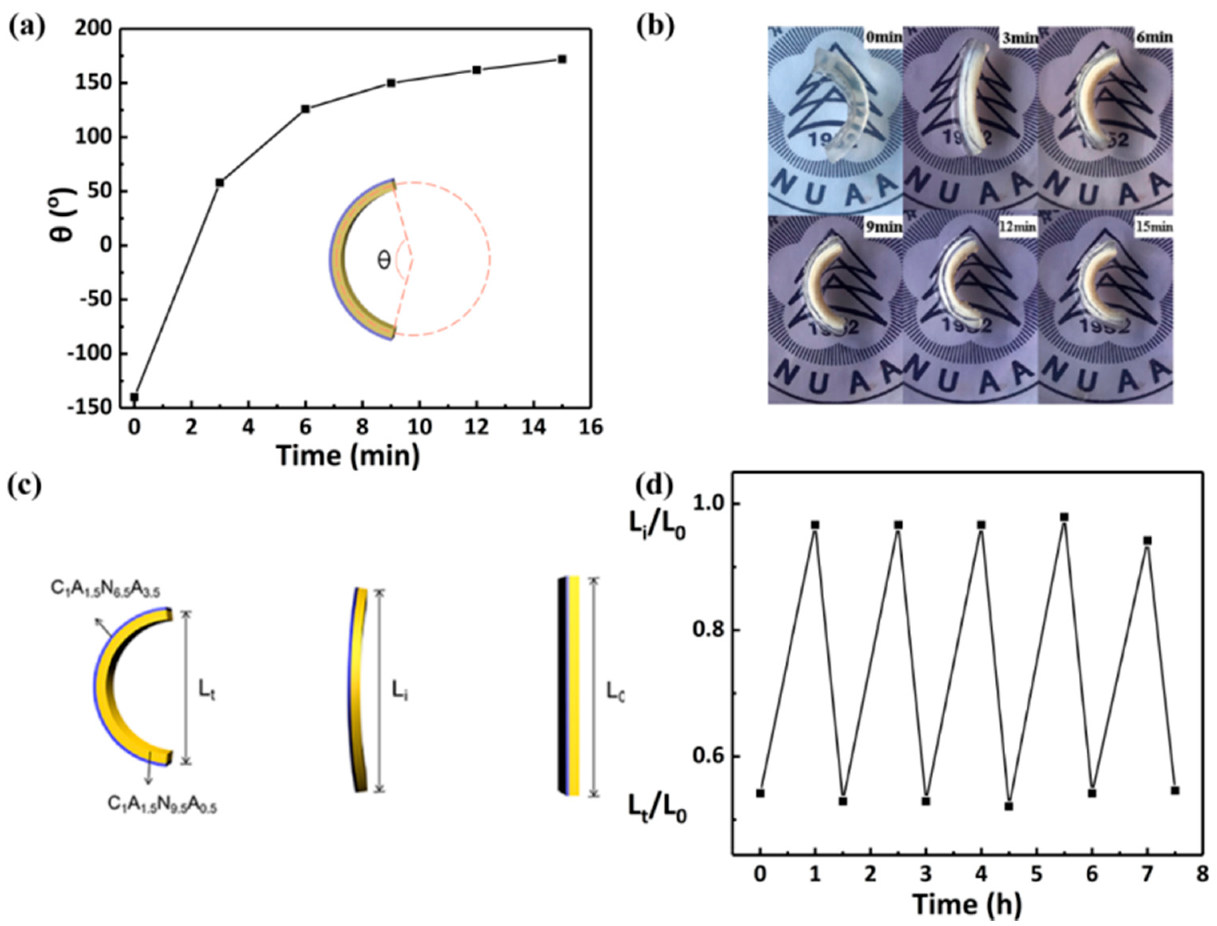
3.3.2. Temperature Responsive Behaviors of the Hydrogel Bilayers
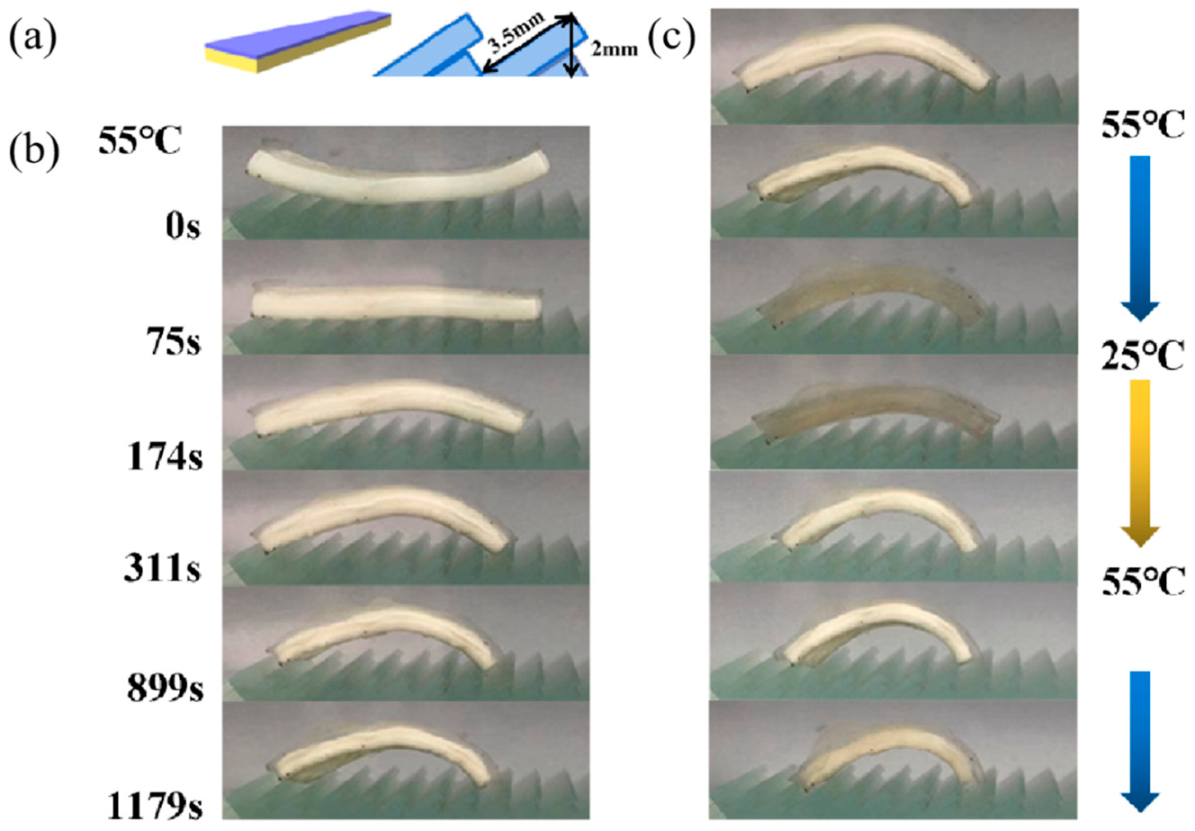
3.3.3. Thermo-Driven Move and Thermo-Deformation of Hydrogel Bilayers
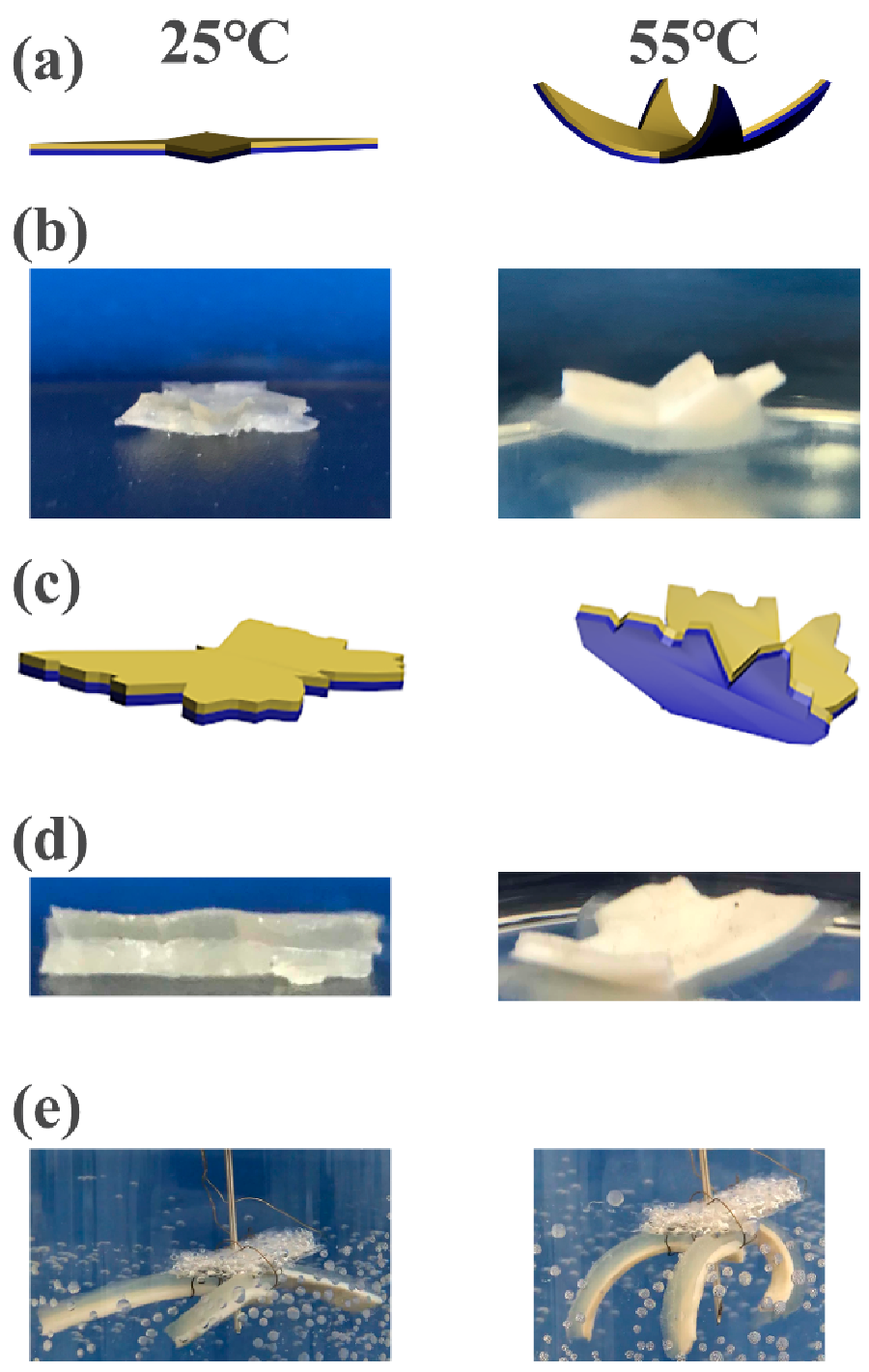
3.4. CS/Agar/MMT/PNIPAM Hydrogel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rotjanasuworapong, K.; Thummarungsan, N.; Lerdwijitjarud, W.; Sirivat, A. Facile formation of agarose hydrogel and electromechanical responses as electro-responsive hydrogel materials in actuator applications. Carbohydr. Polym. 2020, 247, 116709. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, Y.; Wang, T.; Sun, W.; Tong, Z. Ultrafast and programmable shape memory hydrogel of gelatin soaked in tannic acid solution. ACS Appl. Mater. Interfaces 2020, 12, 46701–46709. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, A.; Jain, V.; Kumar, P.; Unal, A.; Saxena, A. Soft hand exoskeleton for adaptive grasping using a compact differential mechanism. In Mechanism and Machine Science; Springer: Berlin/Heidelberg, Germany, 2020; pp. 733–746. [Google Scholar]
- Xiang, Y.; Li, B.; Zhang, Y.; Ma, S.; Li, B.; Gao, H.; Yu, B.; Li, J.; Zhou, F. Reversely Orthogonal Actuation of a Janus-Faced Film Based on Asymmetric Polymer Brush Modification. ACS Appl. Mater. Interfaces 2019, 11, 36073–36080. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Ko, H.; Ratri, M.C.; Kim, K.; Jung, Y.; Tae, G.; Shin, K. Formulation of Sugar/Hydrogel Inks for Rapid Thermal Response 4D Architectures with Sugar-derived Macropores. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Zhang, F.; Fan, J.; Zhang, P.; Liu, M.; Meng, J.; Jiang, L.; Wang, S. A monolithic hydro/organo macro copolymer actuator synthesized via interfacial copolymerization. NPG Asia Mater. 2017, 9, e380. [Google Scholar] [CrossRef]
- Gao, G.; Wang, Z.; Xu, D.; Wang, L.; Xu, T.; Zhang, H.; Chen, J.; Fu, J. Snap-buckling motivated controllable jumping of thermo-responsive hydrogel bilayers. ACS Appl. Mater. Interfaces 2018, 10, 41724–41731. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, W.; Yu, J.; Wang, Y.; Zhu, J.; Hu, Z. Mechanical strong stretchable conductive multi-stimuli-responsive nanocomposite double network hydrogel as biosensor and actuator. J. Biomater. Sci. Polym. Ed. 2020, 31, 1770–1792. [Google Scholar] [CrossRef]
- Huang, H.-W.; Tibbitt, M.W.; Huang, T.-Y.; Nelson, B.J. Matryoshka-inspired micro-origami capsules to enhance loading, encapsulation, and transport of drugs. Soft Robot. 2019, 6, 150–159. [Google Scholar] [CrossRef]
- Zheng, W.J.; An, N.; Yang, J.H.; Zhou, J.; Chen, Y.M. Tough Al-alginate/poly (N-isopropylacrylamide) hydrogel with tunable LCST for soft robotics. ACS Appl. Mater. Interfaces 2015, 7, 1758–1764. [Google Scholar] [CrossRef]
- Duan, X.; Yu, J.; Zhu, Y.; Zheng, Z.; Liao, Q.; Xiao, Y.; Li, Y.; He, Z.; Zhao, Y.; Wang, H. Large-Scale Spinning Approach to Engineering Knittable Hydrogel Fiber for Soft Robots. ACS Nano 2020, 14, 14929–14938. [Google Scholar] [CrossRef]
- Chun, K.-Y.; Kim, S.H.; Shin, M.K.; Kwon, C.H.; Park, J.; Kim, Y.T.; Spinks, G.M.; Lima, M.D.; Haines, C.S.; Baughman, R.H. Hybrid carbon nanotube yarn artificial muscle inspired by spider dragline silk. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Haines, C.S.; Lima, M.D.; Li, N.; Spinks, G.M.; Foroughi, J.; Madden, J.D.; Kim, S.H.; Fang, S.; De Andrade, M.J.; Göktepe, F. Artificial muscles from fishing line and sewing thread. Science 2014, 343, 868–872. [Google Scholar] [CrossRef]
- Iamsaard, S.; Aßhoff, S.J.; Matt, B.; Kudernac, T.; Cornelissen, J.J.; Fletcher, S.P.; Katsonis, N. Conversion of light into macroscopic helical motion. Nat. Chem. 2014, 6, 229–235. [Google Scholar] [CrossRef]
- Ninawe, P.R.; Parulekar, S.J. Drug loading into and drug release from pH- and temperature-responsive cylindrical hydrogels. Biotechnol. Prog. 2011, 27, 1442–1454. [Google Scholar] [CrossRef]
- El-Sawy, N.M.; Raafat, A.I.; Badawy, N.A.; Mohamed, A.M. Radiation development of pH-responsive (xanthan-acrylic acid)/MgO nanocomposite hydrogels for controlled delivery of methotrexate anticancer drug. Int. J. Biol. Macromol. 2020, 142, 254–264. [Google Scholar] [CrossRef]
- Khozemy, E.E.; Nasef, S.M.; Mahmoud, G.A. Synthesis and characterization of antimicrobial nanocomposite hydrogel based on wheat flour and poly (vinyl alcohol) using γ-irradiation. Adv. Polym. Technol. 2018, 37, 3252–3261. [Google Scholar] [CrossRef]
- Martínez-López, A.L.; Carvajal-Millan, E.; Micard, V.; Rascón-Chu, A.; Brown-Bojorquez, F.; Sotelo-Cruz, N.; López-Franco, Y.L.; Lizardi-Mendoza, J. In vitro degradation of covalently cross-linked arabinoxylan hydrogels by bifidobacteria. Carbohydr. Polym. 2016, 144, 76–82. [Google Scholar] [CrossRef]
- Hu, J.; Quan, Y.; Lai, Y.; Zheng, Z.; Hu, Z.; Wang, X.; Dai, T.; Zhang, Q.; Cheng, Y. A smart aminoglycoside hydrogel with tunable gel degradation, on-demand drug release, and high antibacterial activity. J. Control. Release 2017, 247, 145–152. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Kim, H.-J.; Rhim, J.-W. Effect of sulfur nanoparticles on properties of alginate-based films for active food packaging applications. Food Hydrocoll. 2021, 110, 106155. [Google Scholar] [CrossRef]
- Jiang, Q.; Lin, Z.; Gu, B.; Pang, C.; Wang, X. Green synthesis and immobilization of AgNPs by lumpy corn stalk as interlayer filling material for durable antibacterial. Ind. Crop Prod. 2020, 158, 112987. [Google Scholar] [CrossRef]
- Incoronato, A.; Conte, A.; Buonocore, G.; Del Nobile, M.A. agar hydrogel with silver nanoparticles to prolong the shelf life of Fior di Latte cheese. J. Dairy Sci. 2011, 94, 1697–1704. [Google Scholar] [CrossRef]
- Estrada-Villegas, G.; Morselli, G.; Oliveira, M.; Gonzalez-Perez, G.; Lugão, A. PVGA/Alginate-AgNPs hydrogel as absorbent biomaterial and its soil biodegradation behavior. Polym. Bull. 2020, 77, 4147–4166. [Google Scholar] [CrossRef]
- Fierascu, I.; Ditu, L.-M.; Sutan, A.N.; Drăghiceanu, O.A.; Fierascu, R.C.; Avramescu, S.M.; Lungulescu, E.-M.; Nicula, N.; Soare, L.C. Influence of gamma irradiation on the biological properties of Asplenium scolopendrium L. hydroalcoholic extracts. Radiat. Phys. Chem. 2021, 181, 109175. [Google Scholar] [CrossRef]
- Eljarrat-Binstock, E.; Bentolila, A.; Kumar, N.; Harel, H.; Domb, A.J. Preparation, characterization, and sterilization of hydrogel sponges for iontophoretic drug-delivery use. Polym. Adv. Technol. 2007, 18, 720–730. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.; Zhu, C.; Liu, L. Synthesis and characterization of temperature-sensitive and biodegradable hydrogel based on N-isopropylacrylamide. Open Chem. 2010, 8, 426–433. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, T.; Sun, W. Construction of biocompatible bilayered light-driven actuator composed of rGO/PNIPAM and PEGDA hydrogel. J. Appl. Polym. Sci. 2020, 137, 49375. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.; Dutta, P. Chitosan based ZnO nanoparticles loaded gallic-acid films for active food packaging. Food Chem. 2020, 334, 127605. [Google Scholar] [CrossRef]
- Ahmad, U.; Sohail, M.; Ahmad, M.; Minhas, M.U.; Khan, S.; Hussain, Z.; Kousar, M.; Mohsin, S.; Abbasi, M.; Shah, S.A. Chitosan based thermosensitive injectable hydrogels for controlled delivery of loxoprofen: Development, characterization and in-vivo evaluation. Int. J. Biol. Macromol. 2019, 129, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Luo, C.; Luo, F. Preparation and properties of self-healable and conductive PVA-agar hydrogel with ultra-high mechanical strength. Eur. Polym. J. 2020, 124, 109465. [Google Scholar] [CrossRef]
- Shin, B.; Kim, J.; Vales, T.P.; Yang, S.K.; Kim, J.K.; Sohn, H.; Kim, H.J. Thermoresponsive drug controlled release from chitosan-based hydrogel embedded with poly (N-isopropylacrylamide) nanogels. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1907–1914. [Google Scholar] [CrossRef]
- Xu, Z.; Tang, E.; Zhao, H. An environmentally sensitive silk fibroin/chitosan hydrogel and its drug release behaviors. Polymers 2019, 11, 1980. [Google Scholar] [CrossRef]
- Wang, B.; Wu, X.; Li, J.; Hao, X.; Lin, J.; Cheng, D.; Lu, Y. Thermosensitive behavior and antibacterial activity of cotton fabric modified with a chitosan-poly (N-isopropylacrylamide) interpenetrating polymer network hydrogel. Polymers 2016, 8, 110. [Google Scholar] [CrossRef]
- Nasef, S.M.; Khozemy, E.E.; Kamoun, E.A.; El-Gendi, H. Gamma radiation-induced crosslinked composite membranes based on polyvinyl alcohol/chitosan/AgNO3/vitamin E for biomedical applications. Int. J. Biol. Macromol. 2019, 137, 878–885. [Google Scholar] [CrossRef]
- Zhuang, Z.; Wu, L.; Ma, X.; Diao, W.; Fang, Y. High-strength, tough, rapidly self-recoverable, and fatigue-resistant hydrogels based on multi-network and multi-bond toughening mechanism. J. Appl. Polym. Sci. 2018, 135, 46847. [Google Scholar] [CrossRef]
- Ammar, N.E.B.; Saied, T.; Barbouche, M.; Hosni, F.; Hamzaoui, A.H.; Şen, M. A comparative study between three different methods of hydrogel network characterization: Effect of composition on the crosslinking properties using sol–gel, rheological and mechanical analyses. Polym. Bull. 2018, 75, 3825–3841. [Google Scholar] [CrossRef]
- Singh, B.; Varshney, L.; Sharma, V. Design of sterile mucoadhesive hydrogels for use in drug delivery: Effect of radiation on network structure. Colloids Surf. B Biointerfaces 2014, 121, 230–237. [Google Scholar] [CrossRef]
- Jeon, O.; Song, S.J.; Lee, K.-J.; Park, M.H.; Lee, S.-H.; Hahn, S.K.; Kim, S.; Kim, B.-S. Mechanical properties and degradation behaviors of hyaluronic acid hydrogels cross-linked at various cross-linking densities. Carbohydr. Polym. 2007, 70, 251–257. [Google Scholar] [CrossRef]
- Zou, W.; Chen, Y.; Zhang, X.; Li, J.; Sun, L.; Gui, Z.; Du, B.; Chen, S. Cytocompatible chitosan based multi-network hydrogels with antimicrobial, cell anti-adhesive and mechanical properties. Carbohydr. Polym. 2018, 202, 246–257. [Google Scholar] [CrossRef]
- He, M.; Chen, H.; Zhang, X.; Wang, C.; Xu, C.; Xue, Y.; Wang, J.; Zhou, P.; Zhao, Q. Construction of novel cellulose/chitosan composite hydrogels and films and their applications. Cellulose 2018, 25, 1987–1996. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Y.; Jia, F.; Li, S.; Kong, Y.; Zhang, E. Synthesis and characterization of temperature-sensitive poly (N-isopropylacryamide-co-acrylamide)/montmorillonite nanocomposite hydrogels. Int. J. Polym. Mater. 2013, 62, 34–38. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Wang, J.; Ge, L.; Zhu, J. Development of a thermally responsive nanogel based on chitosan–poly (N-isopropylacrylamide-co-acrylamide) for paclitaxel delivery. J. Pharm. Sci. 2014, 103, 2012–2021. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.; Bucatariu, S.-M.; Doroftei, F.; Fundueanu, G. Smart composite materials based on chitosan microspheres embedded in thermosensitive hydrogel for controlled delivery of drugs. Carbohydr. Polym. 2017, 157, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Ha, S.; Lee, J.; Premkumar, T.; Song, C. UV-mediated synthesis of pNIPAM-crosslinked double-network alginate hydrogels: Enhanced mechanical and shape-memory properties by metal ions and temperature. Polymer 2018, 149, 206–212. [Google Scholar] [CrossRef]
- Li, Y.L.; Ouyang, N.; Ke, A.R.; Lin, S.B. Synthesis and properties of P(NIPAAm-co-AM)/SiO2 hybrid temperature-sensitive hydrogels. J. Appl. Polym. Sci. 2013, 128, 761–766. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Hsieh, A.-C. A gelatin-g-poly (N-isopropylacrylamide) biodegradable in situ gelling delivery system for the intracameral administration of pilocarpine. Biomaterials 2012, 33, 2372–2387. [Google Scholar] [CrossRef]
- Vernon, B.; Kim, S.W.; Bae, Y.H. Thermoreversible copolymer gels for extracellular matrix4. J. Biomed. Mater. Res. 2000, 51, 69–79. [Google Scholar] [CrossRef]
- Schmaljohann, D.; Oswald, J.; Jørgensen, B.; Nitschke, M.; Beyerlein, D.; Werner, C. Thermo-responsive PNiPAAm-g-PEG films for controlled cell detachment. Biomacromolecules 2003, 4, 1733–1739. [Google Scholar] [CrossRef]
- Luo, L.-J.; Nguyen, D.D.; Lai, J.-Y. Benzoic acid derivative-modified chitosan-g-poly (N-isopropylacrylamide): Methoxylation effects and pharmacological treatments of Glaucoma-related neurodegeneration. J. Control. Release 2020, 317, 246–258. [Google Scholar] [CrossRef]
- Luo, L.-J.; Nguyen, D.D.; Lai, J.-Y. Long-acting mucoadhesive thermogels for improving topical treatments of dry eye disease. Mater. Sci. Eng. C 2020, 115, 111095. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Luo, L.-J.; Nguyen, D.D. Multifunctional glutathione-dependent hydrogel eye drops with enhanced drug bioavailability for glaucoma therapy. Chem. Eng. J. 2020, 402, 126190. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, W.; An, H.; Chang, S.; Yang, S.; Huang, Y.; Zhang, X.; Hu, X.; Zhang, H. Smart Hydrogel Bilayers Prepared by Irradiation. Polymers 2021, 13, 1753. https://doi.org/10.3390/polym13111753
Huo W, An H, Chang S, Yang S, Huang Y, Zhang X, Hu X, Zhang H. Smart Hydrogel Bilayers Prepared by Irradiation. Polymers. 2021; 13(11):1753. https://doi.org/10.3390/polym13111753
Chicago/Turabian StyleHuo, Weixian, Heng An, Shuquan Chang, Shengsheng Yang, Yin Huang, Xiaohong Zhang, Xiaodan Hu, and Haiqian Zhang. 2021. "Smart Hydrogel Bilayers Prepared by Irradiation" Polymers 13, no. 11: 1753. https://doi.org/10.3390/polym13111753
APA StyleHuo, W., An, H., Chang, S., Yang, S., Huang, Y., Zhang, X., Hu, X., & Zhang, H. (2021). Smart Hydrogel Bilayers Prepared by Irradiation. Polymers, 13(11), 1753. https://doi.org/10.3390/polym13111753






