Jojoba Oil: An Updated Comprehensive Review on Chemistry, Pharmaceutical Uses, and Toxicity
Abstract
:1. Introduction
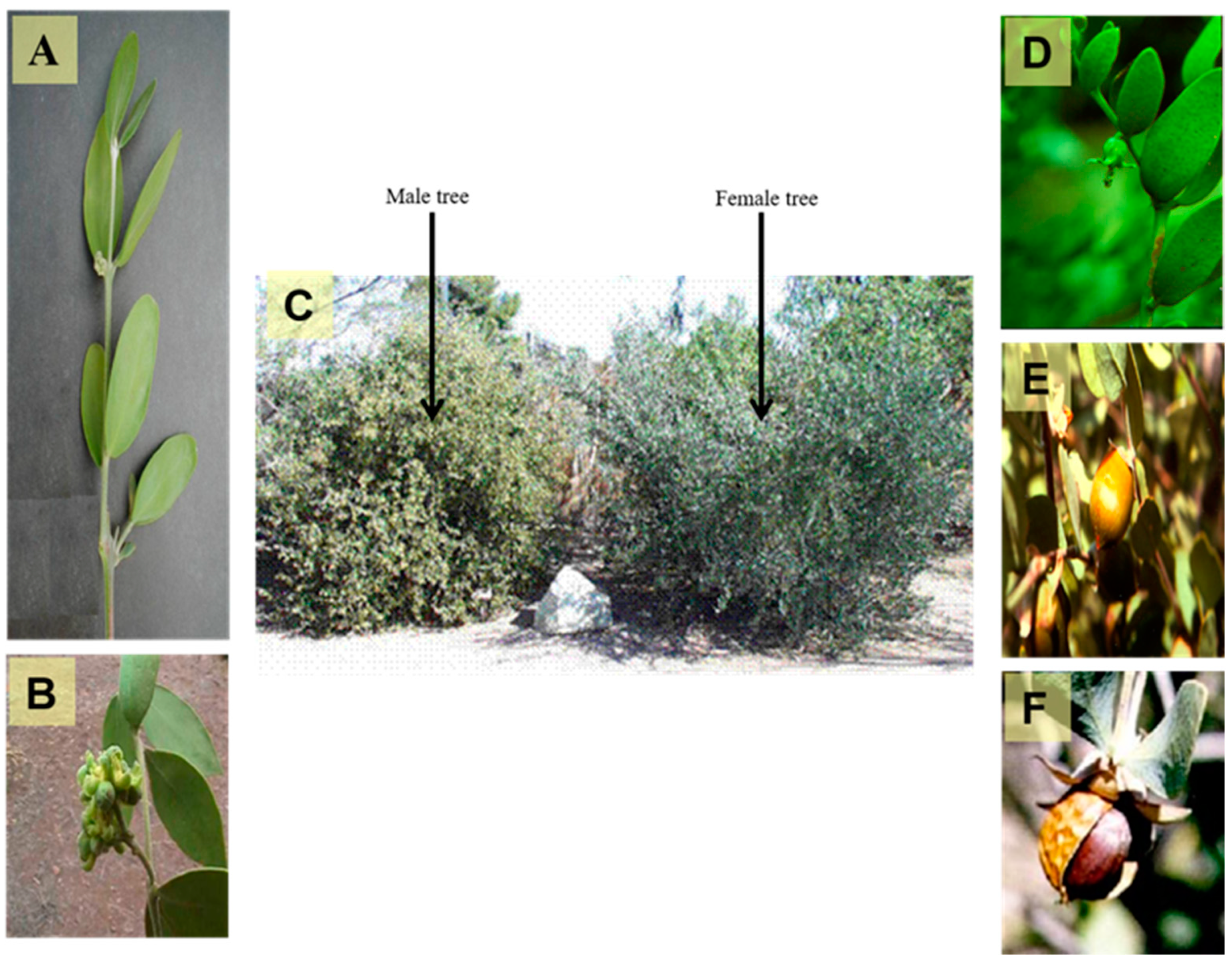
2. Common Names and Botanical Characteristics
3. Chemical Constituents
3.1. Jojoba Wax
3.1.1. Wax Esters
3.1.2. Free Fatty Acids and Alcohols
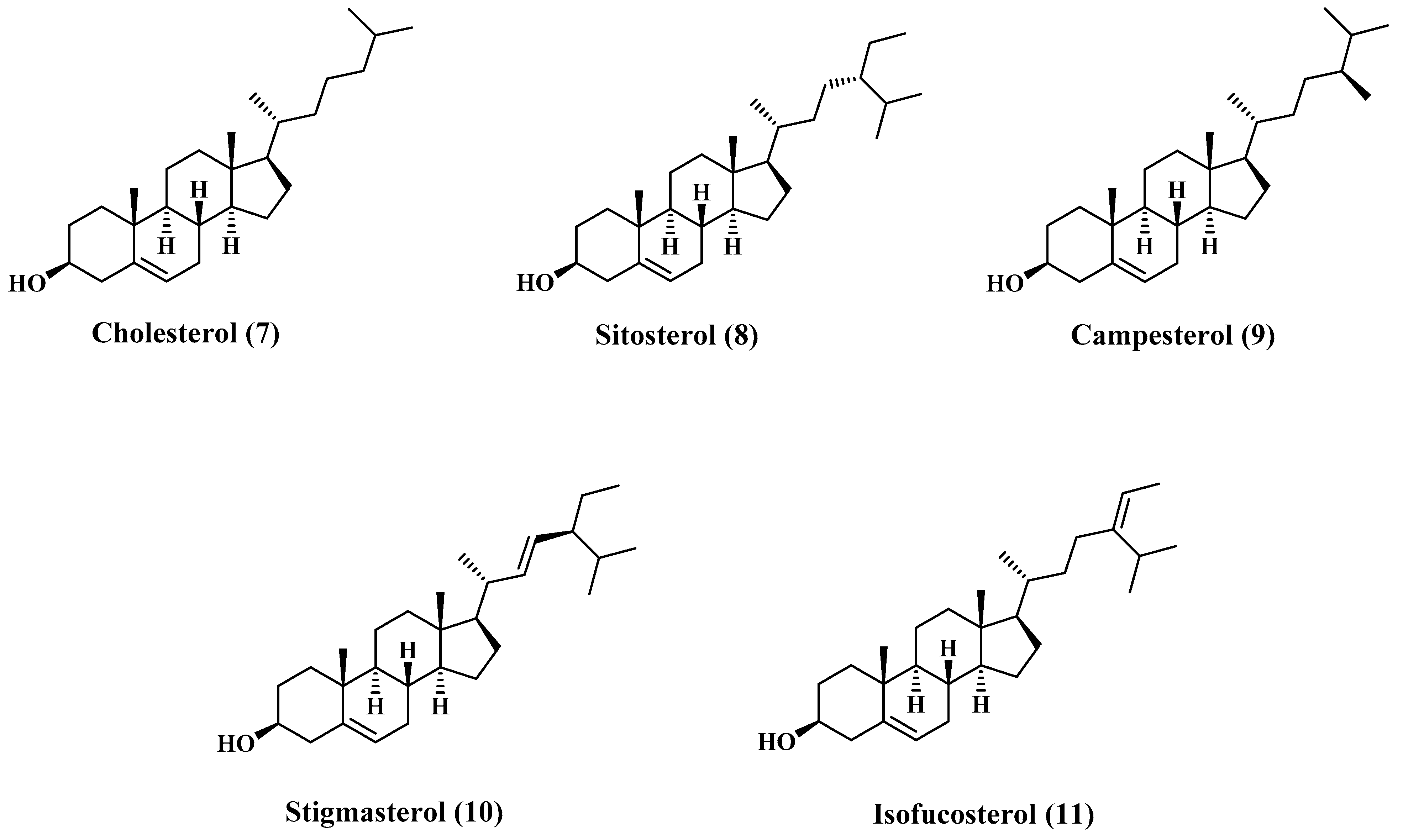
3.2. Sterols
3.3. Flavonoids, Phenolic, and Cyanogenic Compounds
3.4. Fat-Soluble Vitamins
4. Physical Characters of the Oil
5. Chemical Properties of the Oil
5.1. Cis/Trans Isomerization
5.2. Hydrogenation
5.3. Halogenation
5.4. Sulfurization and Sulfur Halogenation
5.5. Phosphonation
5.6. Oxidation, Epioxidation, and Ozonolysis
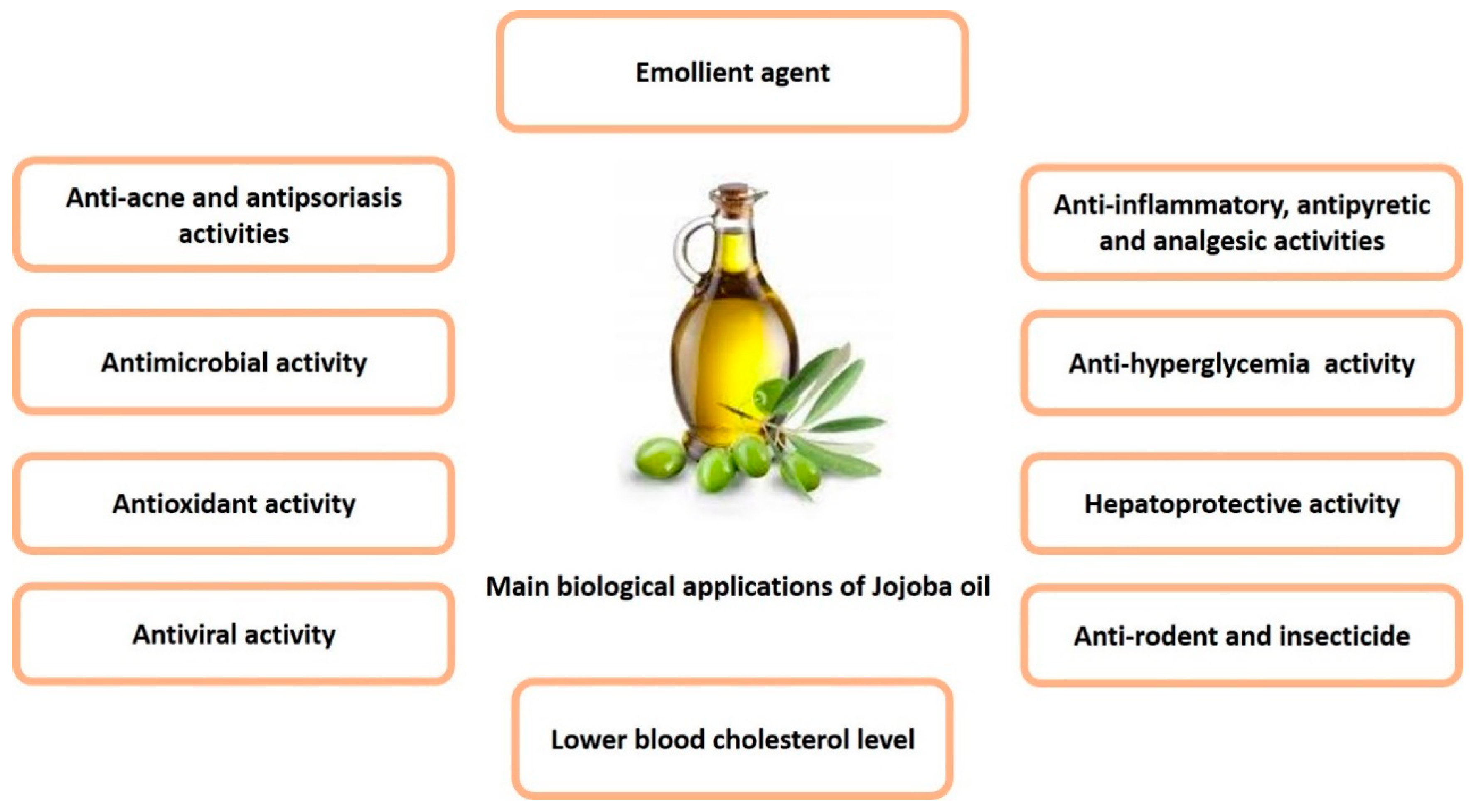
6. Biological Activity
6.1. Traditional and Folk Medicinal Uses
6.2. Pharmacological Uses
6.2.1. Emollient Agent
6.2.2. Anti-Acne and Antipsoriasis Activities
6.2.3. Anti-Inflammatory, Antipyretic, and Analgesic Activities
6.2.4. Antimicrobial Activity
6.2.5. Other Activities
7. Pharmaceutical Uses
7.1. Topical Preparations
7.2. Cosmetic Products
7.3. Transdermal and Intradermal Preparations
7.4. Parenteral Preparations
7.5. Inhalable Preparations
7.6. Anticancer Preparations
8. Industrial Applications
8.1. Synthesis Polyurethanes Polymers
8.2. Other Industrial Uses
9. Toxicity of Jojoba Oil
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spencer, G.F.; Plattner, R.D.; Miwa, T. Jojoba oil analysis by high pressure liquid chromatography and gas chromatography/mass spectrometry. J. Am. Oil Chem. Soc. 1977, 54, 187–189. [Google Scholar] [CrossRef]
- Khairi, M.M.A. Genetics and Breeding of Jojoba [Simmondsia chinensis (Link) Schneider]. In Advances in Plant Breeding Strategies: Industrial and Food Crops; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer: Cham, Switzerland, 2019; Volume 6, pp. 237–276. [Google Scholar] [CrossRef]
- Li, T.S.C. Medicinal Plants: Culture, Utilization, and Phytopharmacology; Technomic Pub. Co.: Lancaster, PA, USA, 2000; 517p. [Google Scholar]
- National Research Council (U.S.). Advisory Committee on Technology Innovation. Ad Hoc Panel. In Jojoba: New Crop for Arid Lands, New Raw Material for Industry; National Academy Press: Washington, DC, USA, 1985; 102p. [Google Scholar]
- Wisniak, J. The Chemistry and Technology of Jojoba Oil; American Oil Chemists’ Society: Champaign, IL, USA, 1987; 272p. [Google Scholar]
- Baldwin, A.R.; American Oil Chemists’ Society. Seventh International Conference on Jojoba and Its Uses: Proceedings; American Oil Chemists’ Society: Champaign, IL, USA, 1988; 453p. [Google Scholar]
- Habashy, R.R.; Abdel-Naim, A.B.; Khalifa, A.E.; Al-Azizi, M.M. Anti-inflammatory effects of jojoba liquid wax in experimental models. Pharmacol. Res. 2005, 51, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Ma, S.; Tominaga, T.; Yokoyama, K.; Kitatani, K.; Horikawa, K.; Suzuki, K. Acute Effects of Transdermal Administration of Jojoba Oil on Lipid Metabolism in Mice. Medicina 2019, 55, 594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Obaidi, J.R.; Halabi, M.F.; AlKhalifah, N.S.; Asanar, S.; Al-Soqeer, A.A.; Attia, M.F. A review on plant importance, biotechnological aspects, and cultivation challenges of jojoba plant. Biol. Res. 2017, 50, 25. [Google Scholar] [CrossRef] [Green Version]
- Pazyar, N.; Yaghoobi, R.; Ghassemi, M.R.; Kazerouni, A.; Rafeie, E.; Jamshydian, N. Jojoba in dermatology: A succinct review. G. Ital. Dermatol. Venereol. 2013, 148, 687–691. [Google Scholar] [PubMed]
- Ranzato, E.; Martinotti, S.; Burlando, B. Wound healing properties of jojoba liquid wax: An in vitro study. J. Ethnopharmacol. 2011, 134, 443–449. [Google Scholar] [CrossRef]
- Bhatia, V.K.; Chaudhry, A.; Sivasankaran, G.A.; Bisht, R.P.S.; Kashyap, M. Modification of jojoba oil for lubricant formulations. J. Am. Oil Chem. Soc. 1990, 67, 1–7. [Google Scholar] [CrossRef]
- Miwa, T.K. Structural determination and uses of jojoba oil. J. Am. Oil Chem. Soc. 1984, 61, 407–410. [Google Scholar] [CrossRef]
- Quattrocchi, U. CRC World Dictionary of Medicinal and Poisonous Plants: Common Names, Scientific Names, Eponyms, Synonyms, and Etymology; CRC: Boca Raton, FL, USA, 2012. [Google Scholar]
- Stuessy, T.F. Plant Taxonomy: The Systematic Evaluation of Comparative Data; Columbia University Press: New York, NY, USA, 1990; 514p. [Google Scholar]
- Wunderlin, R.P. Guide to the Vascular Plants of Central Florida; University Presses of Florida: Gainesville, FL, USA, 1982; 472p. [Google Scholar]
- Ashour, M.L.; Ayoub, N.A.; Singab, A.N.B.; Al Azizi, M.M. Simmondsia chinensis (Jojoba): A comprehensive pharmacognostic study. J. Pharmacogn. Phytochem. 2013, 2, 97–120. [Google Scholar]
- Kramer, J.K.G.; Sauer, F.D.; Pigden, W.J. High and Low Erucic Acid Rapeseed Oils: Production, Usage, Chemistry, and Toxicological Evaluation; Academic Press: New York, NY, USA, 1983; 582p. [Google Scholar]
- Van Boven, M.; Daenens, P.; Maes, K.; Cokelaere, M. Content and composition of free sterols and free fatty alcohols in jojoba oil. J. Agric. Food Chem. 1997, 45, 1180–1184. [Google Scholar] [CrossRef]
- Van Boven, M.; Holser, R.; Cokelaere, M.; Flo, G.; Decuypere, E. Gas chromatographic analysis of simmondsins and simmondsin ferulates in jojoba meal. J. Agric. Food Chem. 2000, 48, 4083–4086. [Google Scholar] [CrossRef] [PubMed]
- Van Boven, M.; Leyssen, T.; Busson, R.; Holser, R.; Cokelaere, M.; Flo, G.; Decuypere, E. Identification of 4,5-didemethyl-4-O-alpha-D-glucopyranosylsimmondsin and pinitol alpha-D-galactosides in jojoba seed meal (Simmondsia chinensis). J. Agric. Food Chem. 2001, 49, 4278–4283. [Google Scholar] [CrossRef] [PubMed]
- Busson-Breysse, J.; Farines, M.; Soulier, J. Jojoba wax: Its esters and some of its minor components. J. Am. Oil Chem. Soc. 1994, 71, 999. [Google Scholar] [CrossRef]
- Tank Chintankumar, J.; Borkhataria Chetan, H.; Baria Ashok, H.; Patel Rakesh, P.; Tamizharasi, S.; Sureja, D.K.; Patel, I.D.; Parmar, G.R. Formulation and evaluation of aceclofenac loaded maltodextrin based proniosome. Int. J. ChemTech Res. 2009, 1, 567–573. [Google Scholar]
- Graille, J.; Pina, M.; Ploch, D. Routine analysis of jojoba wax fatty acids and alcohols by single column capillary GC. J. Am. Oil Chem. Soc. 1986, 63, 111–116. [Google Scholar] [CrossRef]
- Culling, C.F.A. Handbook of Histopathological and Histochemical Techniques: Including Museum Techniques, 3rd ed.; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Abdel-Mageed, W.M.; Bayoumi, S.A.; Salama, A.A.; Salem-Bekhit, M.M.; Abd-Alrahman, S.H.; Sayed, H.M. Antioxidant lipoxygenase inhibitors from the leaf extracts of Simmondsia chinensis. Asian Pac. J. Trop. Med. 2014, 7S1, S521–S526. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Mageed, W.M.; Bayoumi, S.A.; Al-Wahaibi, L.H.; Li, L.; Sayed, H.M.; Abdelkader, M.S.; El-Gamal, A.A.; Liu, M.; Zhang, J.; Zhang, L.; et al. Noncyanogenic Cyanoglucoside Cyclooxygenase Inhibitors from Simmondsia chinensis. Org. Lett. 2016, 18, 1728–1731. [Google Scholar] [CrossRef] [PubMed]
- Landis, P.S.; Craver, R.H.; Barton, D.E. Pyrolysis studies with jojoba oil. J. Agric. Food Chem. 1992, 40, 456–457. [Google Scholar] [CrossRef]
- Landis, P.S.; Craver, R.H. Solubility of jojoba oil in organic solvents. J. Am. Oil Chem. Soc. 1984, 61, 1879–1880. [Google Scholar] [CrossRef]
- Bower, D.I. An Introduction to Polymer Physics; Cambridge University Press: Cambridge, UK, 2002; 444p. [Google Scholar]
- Wisniak, J.; Liberman, D. Some physical properties of Simmondsia oil. J. Am. Oil Chem. Soc. 1975, 52, 259–261. [Google Scholar] [CrossRef]
- Knoepfler, N.B.; Vix, H.L.E. Vegetable Oils, Review of Chemistry and Research Potential of Simmondsia Chinensis (Jojoba) Oil. J. Agric. Food Chem. 1958, 6, 118–121. [Google Scholar] [CrossRef]
- Wisniak, J. Potential uses of jojoba oil and meal—A review. Ind. Crop. Prod. 1994, 3, 43–68. [Google Scholar] [CrossRef]
- Shani, A. Functionalization at the double bond region of jojoba oil: I. bromine derivatives. J. Am. Oil Chem. Soc. 1981, 58, 845–850. [Google Scholar] [CrossRef]
- Shani, A. Functionalization at the double bond region of jojoba oil: II. Diels-alder adducts of jojobatetraene. J. Am. Oil Chem. Soc. 1982, 59, 228–230. [Google Scholar] [CrossRef]
- Wisniak, J.; Alfandary, P. Geometrical Isomerization of Jojoba Oil. Ind. Eng. Chem. Prod. Res. Dev. 1975, 14, 177–180. [Google Scholar] [CrossRef]
- Galun, A.B.; Grinberg, S.; Kampf, A.; Shaubi, E. Oxidation and halogenation of jojoba wax. J. Am. Oil Chem. Soc. 1984, 61, 1088–1089. [Google Scholar] [CrossRef]
- Galun, A.B.; Shaubi, E. Thermal isomerization of jojoba wax. J. Am. Oil Chem. Soc. 1984, 61, 564–569. [Google Scholar] [CrossRef]
- Wisniak, J.; Holin, M. Hydrogenation of Jojoba Oil. Ind. Eng. Chem. Prod. Res. Dev. 1975, 14, 226–231. [Google Scholar] [CrossRef]
- Simpson, T.D.; Miwa, T.K. X-ray study of hydrogenated jojoba wax. J. Am. Oil Chem. Soc. 1977, 54, 54. [Google Scholar] [CrossRef]
- Soontag, N.O.V. Halogenation. J. Am. Oil Chem. Soc. 1963, 40, 199–203. [Google Scholar] [CrossRef]
- Wisniak, J.; Alfandary, P. Sperm Whale Oil Replacements from Halogenation of Jojoba Oil. Ind. Eng. Chem. Prod. Res. Dev. 1979, 18, 358–364. [Google Scholar] [CrossRef]
- Hagemann, J.W.; Rothfus, J.A. Oxidative stability of wax esters by thermogravimetric analysis. J. Am. Oil Chem. Soc. 1979, 56, 629–631. [Google Scholar] [CrossRef]
- Meier, L.; Stange, R.; Michalsen, A.; Uehleke, B. Clay jojoba oil facial mask for lesioned skin and mild acne--results of a prospective, observational pilot study. Forsch. Komplementmedizin 2012, 19, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Lovell, C.R. Plants and the Skin; Blackwell Scientific Publications: Oxford, UK, 1993; 272p. [Google Scholar]
- Baccouch, N.; Ben Salah, H.; Belhadj, S.; Hentati, O.; Abdennabi, R.; Gharsallah, N.; Elfeki, A.; Ayedi, M.; Allouche, N. Chemical characterization and biological activities of Simmondsia chinensis (Link) C. K. Schneid seeds oil. Cell. Mol. Biol. 2018, 64, 11–16. [Google Scholar] [CrossRef]
- Belhadj, S.; Hentati, O.; Hamdaoui, G.; Fakhreddine, K.; Maillard, E.; Dal, S.; Sigrist, S. Beneficial Effect of Jojoba Seed Extracts on Hyperglycemia-Induced Oxidative Stress in RINm5f Beta Cells. Nutrients 2018, 10, 384. [Google Scholar] [CrossRef] [Green Version]
- Belhadj, S.; Dal, S.; Khaskhoussi, F.; Maillard-Pedracini, E.; Hentati, O.; Sigrist, S. Anorexic and metabolic effect of jojoba: Potential treatment against metabolic syndrome and hepatic complications. Nutr. Metab. 2020, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.A.; Yermanos, D.M. Effects of ingestion of jojoba oil on blood cholesterol levels and lipoprotein patterns in New Zealand white rabbits. Biochem. Biophys. Res. Commun. 1981, 102, 1409–1415. [Google Scholar] [CrossRef]
- Abou-Zeid, S.M.; Tahoun, E.A.; AbuBakr, H.O. Ameliorative effects of jojoba oil on fipronil-induced hepatorenal- and neuro-toxicity: The antioxidant status and apoptotic markers expression in rats. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef]
- Garti, N.; Shevachman, M.; Shani, A. Solubilization of lycopene in jojoba oil microemulsion. J. Am. Oil Chem. Soc. 2004, 81, 873–877. [Google Scholar] [CrossRef]
- Shevachman, M.; Shani, A.; Garti, N. Formation and investigation of microemulsions based on jojoba oil and nonionic surfactants. J. Am. Oil Chem. Soc. 2004, 81, 1143–1152. [Google Scholar] [CrossRef]
- Schwarz, J.S.; Weisspapir, M.R.; Shani, A.; Amselem, S. Enhanced antiinflammatory activity of diclofenac in jojoba oil submicron emulsion cream. J. Appl. Cosmetol. 1996, 14, 19–24. [Google Scholar]
- Thakur, N.K.; Bharti, P.; Mahant, S.; Rao, R. Formulation and characterization of benzoyl peroxide gellified emulsions. Sci. Pharm. 2012, 80, 1045–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramez, S.A.; Soliman, M.M.; Fadel, M.; Nour El-Deen, F.; Nasr, M.; Youness, E.R.; Aboel-Fadl, D.M. Novel methotrexate soft nanocarrier/fractional erbium YAG laser combination for clinical treatment of plaque psoriasis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Abdel-Hamid, S.; Mofath, N.H.; Fadel, M.; Alyoussef, A.A. Jojoba Oil Soft Colloidal Nanocarrier of a Synthetic Retinoid: Preparation, Characterization and Clinical Efficacy in Psoriatic Patients. Curr. Drug Deliv. 2017, 14, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Archana, C.; Amaldoss, M.J.N. Synthesis and Characterization of Valacyclovir HCl Hybrid Solid Lipid Nanoparticles by Using Natural Oils. Recent Pat. Drug Deliv. Formul. 2019, 13, 46–61. [Google Scholar] [CrossRef]
- Estanqueiro, M.; Conceição, J.; Amaral, M.H.; Sousa Lobo, J.M. Characterization, sensorial evaluation and moisturizing efficacy of nanolipidgel formulations. Int. J. Cosmet. Sci. 2014, 36, 159–166. [Google Scholar] [CrossRef]
- Shahin, M.; Abdel Hady, S.; Hammad, M.; Mortada, N. Novel Jojoba Oil-Based Emulsion Gel Formulations for Clotrimazole Delivery. AAPS PharmSciTech 2011, 12, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Shahin, M.; Hady, S.A.; Hammad, M.; Mortada, N. Optimized formulation for topical administration of clotrimazole using Pemulen polymeric emulsifier. Drug Dev. Ind. Pharm. 2011, 37, 559–568. [Google Scholar] [CrossRef] [PubMed]
- El Laithy, H.M.; El-Shaboury, K.M.F. The development of Cutina lipogels and gel microemulsion for topical administration of fluconazole. AAPS PharmSciTech 2002, 3, E35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Dias, T.C.S.; Baby, A.R.; Kaneko, T.M.; Velasco, M.V.R. Protective effect of conditioning agents on Afro-ethnic hair chemically treated with thioglycolate-based straightening emulsion. J. Cosmet. Dermatol. 2008, 7, 120–126. [Google Scholar] [CrossRef]
- Touitou, E.; Godin, B. Skin nonpenetrating sunscreens for cosmetic and pharmaceutical formulations. Clin. Dermatol. 2008, 26, 375–379. [Google Scholar] [CrossRef]
- Touitou, E.; Godin, B. New approaches for UV-induced photodamage protection. J. Appl. Cosmetol. 2006, 24, 139. [Google Scholar]
- Geeta, A.; Sanju, D.; HariKumar, S.L. Natural Oils as Skin Permeation Enhancers for Transdermal Delivery of Olanzapine: In Vitro and In Vivo Evaluation. Curr. Drug Deliv. 2012, 9, 172–181. [Google Scholar] [CrossRef]
- Esquisabelr, A.; Hernáandez, M.; Igartuaa, M.; Gascóan, R.; Calvo, B.; Pedraz, J.L. Production of BCG alginate-PLL microcapsules by emulsification/internal gelation. J. Microencapsul. 1997, 14, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Esquisabel, A.; Hernández, R.M.; Igartua, M.; Gascón, A.R.; Calvo, B.; Pedraz, J.L. Preparation and stability of agarose microcapsules containing BCG. J. Microencapsul. 2002, 19, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Venturini, C.G.; Bruinsmann, F.A.; Oliveira, C.P.; Contri, R.V.; Pohlmann, A.R.; Guterres, S.S. Vegetable Oil-Loaded Nanocapsules: Innovative Alternative for Incorporating Drugs for Parenteral Administration. J. Nanosci. Nanotechnol. 2016, 16, 1310–1320. [Google Scholar] [CrossRef] [PubMed]
- Gogoll, K.; Stein, P.; Lee, K.D.; Arnold, P.; Peters, T.; Schild, H.; Radsak, M.; Langguth, P. Solid nanoemulsion as antigen and immunopotentiator carrier for transcutaneous immunization. Cell. Immunol. 2016, 308, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Flores-Villaseñor, S.E.; Peralta-Rodríguez, R.D.; Padilla-Vaca, F.; Meléndez-Ortiz, H.I.; Ramirez-Contreras, J.C.; Franco, B. Preparation of Peppermint Oil-Based Nanodevices Loaded with Paclitaxel: Cytotoxic and Apoptosis Studies in HeLa Cells. AAPS PharmSciTech 2019, 20, 198. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Afonin, K.A.; Viard, M.; Herrero, V.; Kasprzak, W.; Kagiampakis, I.; Kim, T.; Koyfman, A.Y.; Puri, A.; Stepler, M.; et al. Bolaamphiphiles as carriers for siRNA delivery: From chemical syntheses to practical applications. J. Control. Release 2015, 213, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Xie, F.; Sun, Y.; Yu, X.; Xiao, Z.; Fang, R.; Li, J.; Li, Q.; Du, L.; Jin, Y. Inhalable Jojoba Oil Dry Nanoemulsion Powders for the Treatment of Lipopolysaccharide- or H2O2-Induced Acute Lung Injury. Pharmaceutics 2021, 13, 486. [Google Scholar] [CrossRef]
- Raquez, J.M.; Deléglise, M.; Lacrampe, M.F.; Krawczak, P. Thermosetting (bio)materials derived from renewable resources: A critical review. Prog. Polym. Sci. 2010, 35, 487–509. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Adhikari, B. Polyurethanes from seed oil-based polyols: A review of synthesis, mechanical and thermal properties. Ind. Crop. Prod. 2019, 142, 111841. [Google Scholar] [CrossRef]
- Mokhtari, C.; Malek, F.; Caillol, S.; Negrell, C. Synthesis of Bio-Based Polyurethanes from Jojoba Oil. Eur. J. Lipid Sci. Technol. 2018, 120, 1700414. [Google Scholar] [CrossRef]
- Mokhtari, C.; Malek, F.; Manseri, A.; Caillol, S.; Negrell, C. Reactive jojoba and castor oils-based cyclic carbonates for biobased polyhydroxyurethanes. Eur. Polym. J. 2019, 113, 18–28. [Google Scholar] [CrossRef]
- Mokhtari, C.; Malek, F.; Halila, S.; Belgacem, M.-N.; Khiari, R. New Biobased Polyurethane Materials from Modified Vegetable Oil. J. Renew. Mater. 2021, 9, 1213–1223. [Google Scholar] [CrossRef]
- Daugherty, P.M.; Sineath, H.H.; Wastler, T.A. Industrial raw materials of plant origin. IV. A survey of Simmondsia chinensis (Jojoba). Econ. Bot. 1958, 12, 296–304. [Google Scholar] [CrossRef]
- Wisniak, J. Chemistry and Technology of Jojoba Oil: State of the Art. In Sixth International Conference on Jojoba and Its Uses: Proceedings Beer-Shiva, Israel, 21–26 October 1984; American Oil Chemists’ Society: Beer-Shiva, Israel, 1984. [Google Scholar]
- Bisht, R.P.S.; Sivasankaran, G.A.; Bhatia, V.K. Additive properties of jojoba oil for lubricating oil formulations. Wear 1993, 161, 193–197. [Google Scholar] [CrossRef]
- Anand, O.; Chhibber, V. Vegetable oil derivatives: Environment-friendly lubricants and fuels. J. Synth. Lubr. 2006, 23, 91–107. [Google Scholar] [CrossRef]
- Nasser, R.; Nassar, A.; Ahmed, N. Jojoba Polymers As Lubricating Oil Additives. Pet. Coal 2015, 57, 120–129. [Google Scholar]
- Abdelmoez, W.; Tayeb, A.M.; Mustafa, A.; Abdelhamid, M. Green Approach for Biodiesel Production from Jojoba Oil Supported by Process Modeling and Simulation. Int. J. Chem. React. Eng. 2016, 14, 185–193. [Google Scholar] [CrossRef]
- Sarojini, G.; Kannan, P.; Pravin, G. Production of biodiesel from jojoba oil using ultra sonicator. J. Environ. Biol. 2019, 40, 802–806. [Google Scholar] [CrossRef]
- Kozliak, E.; Mota, R.; Rodriguez, D.; Overby, P.; Kubátová, A.; Stahl, D.; Niri, V.; Ogden, G.; Seames, W. Non-catalytic cracking of jojoba oil to produce fuel and chemical by-products. Ind. Crop. Prod. 2013, 43, 386–392. [Google Scholar] [CrossRef]
- Al Omari, S.A.B.; Hamdan, M.O.; Selim, M.Y.E.; Elnajjar, E. Combustion of jojoba-oil/diesel blends in a small scale furnace. Renew. Energy 2019, 131, 678–688. [Google Scholar] [CrossRef]
- Nashy, E.-S.H.A.; Megahed, M.G.; Abd El-Ghaffar, M.A. Preparation of Fat-Liquor Based on Jojoba Oil Under Phase Transfer Catalysis. J. Am. Oil Chem. Soc. 2011, 88, 1239–1246. [Google Scholar] [CrossRef]
- Santos, E.P.; Dutra, A.J.B.; Oliveira, J.F. The effect of jojoba oil on the surface properties of calcite and apatite aiming at their selective flotation. Int. J. Miner. Process. 2015, 143, 34–38. [Google Scholar] [CrossRef]
- Di Berardino, L.; Di Berardino, F.; Castelli, A.; Della Torre, F. A case of contact dermatitis from jojoba. Contact Dermat. 2006, 55, 57–58. [Google Scholar] [CrossRef] [PubMed]
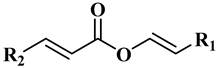 | |
|---|---|
| R1 = C20H41, R2 = C17 H35 | Docosenyl eicosenoate (1) |
| R1 = C18H37, R2 = C17 H35 | Eicosenyl eicosenoate (2) |
| R1 = C18H37, R2 = C19 H39 | Eicosenyl docosenoate (3) |
| R1 = C16H33, R2 = C19 H39 | Docosenyl docosenoate (4) |
| R1 = C18H37, R2 = C17 H33 (C9) | Eicosenyl oleate (5) |
| R1 = C20H41, R2 = C17 H33 (C9) | Docosenyl oleate (6) |
| Alcohols | (%) | Acids | (%) |
|---|---|---|---|
| Tetradecanol | trace | Dodecanoic | trace |
| Hexadecanol | 0.1 | Tetradecanoic | trace |
| Heptadec-8-enol | trace | Pentadecanoic | trace |
| Octadecanol | 0.2 | Hexadecanoic | 1.2 |
| Octadec-9-enol | 0.7 | Hexadec-7-onoic | 0.1 |
| Octadec-11-enol | 0.4 | Hexadec-9-enoic | 0.2 |
| Eicosanol | trace | Heptadecenoic | trace |
| Eicos-11-enol | 43.8 | Octadecanoic | 0.1 |
| Hecos-12-enol | trace | Octadec-9-enoic | 10.1 |
| Docosanol | 1.0 | Octadec-11-enoic | 1.1 |
| Docos-12-enol | 44.9 | Octadecadienoic | 0.1 |
| Tetracos-15-enol | 8.9 | Octadecatrienoic | trace |
| Hexacosenol | trace | Nonadecenoic | trace |
| Eicosanoic | 0.1 | ||
| Eicos-l1-enoic | 71.3 | ||
| Eicosadienoic | trace | ||
| Docosanoic | 0.2 | ||
| Docos-13-enoic | 13.6 | ||
| Tricosenoic | trace | ||
| Tetracosenoic | trace | ||
| Tetracos-15-enoic | 1.3 |
| Sterol | Sterol Fraction (%) | Total Wax (mg/kg Seed) |
|---|---|---|
| Unidentified | 0.4 | 16 |
| Stigmasta-5,25-dien-3β-ol | 0.6 | 24 |
| Fucosterol | 0.6 | 24 |
| Isofucosterol | 4.1 | 163 |
| Cholesterol | 0.8 | 32 |
| Stigmasterol | 6.7 | 266 |
| Campesterol | 16.9 | 672 |
| Sitosterol | 69.9 | 2780 |
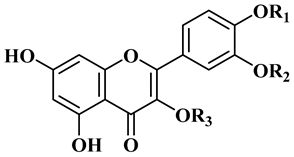 | |||
|---|---|---|---|
| R1 | R2 | R3 | |
| Quercetin (12) | H | H | H |
| Isorhamnetin (13) | H | Me | H |
| Quercetin 3-methyl ether (14) | H | H | Me |
| Quercetin 3,3′-methyl ether (15) | H | Me | Me |
| isorhamnetin 3-O-glucoside (16) | H | Me | Glc |
| Quercetin-3-O-glucoside (17) | H | H | Glc |
| Typhaneoside (18) | Me | H | 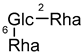 |
| Isorhamnetin 3-O-rutinoside (19) | Me | H | 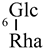 |
| Quercetin 3-O-rutinoside (20) | H | H | 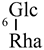 |
| Solvent | mL of Solvent | Observation b |
|---|---|---|
| Water | 5.0 | I |
| 10.0 | I | |
| Acetic acid | 10.0 | I |
| 40.0 | I | |
| 50.0 | S | |
| Methanol | 1.0 | I |
| 10.0 | I | |
| 40.0 | S | |
| Ethanol | 1.0 | I |
| 5.0 | I | |
| 20.0 | S | |
| t-Amyl Alcohol | 1.0 | S |
| 1-Butanol | 1.0 | S |
| Acetone | 1.0 | I |
| 3.0 | I | |
| 8.0 | I | |
| Benzene | 1.0 | I |
| Toulene | 1.0 | I |
| Carbon Tetrachloride | 1.0 | I |
| s-Tetrachlocthane | 1.0 | I |
| Diethylether | 1.0 | I |
| Tetrahydrofuran | 1.0 | I |
| Hexane | 1.0 | I |
| Cyclohexane | 1.0 | I |
| Dimethylformamide | 1.0 | I |
| 10.0 | I | |
| 30.0 | S | |
| Dimethylsulfoxide | 1.0 | I |
| 5.0 | I | |
| 20.0 | S | |
| Acetonitrile | 1.0 | I |
| 10.0 | I | |
| 30.0 | S | |
| Aniline | 2.0 | S |
| m-Cresol | 2.0 | S |
| Freezing point, °C | 10.6–7.0 |
| Melting point, °C | 6.8–7.0 |
| Boiling point at 757 mm under N2, °C | 389 |
| Heat of fusion by DSC, Cal/g | 21 |
| Refractive index at 25 °C | 1.465 |
| Dielectric constant, 27 °C | 2.680 |
| Specific conductivity, 27 °C, mho/cm | 8.86.10–13 |
| Specific gravity, 25/25 °C | 0.863 |
| MV-1 rotor in MY cup, cp | 35 |
| Plate and cone with PK-1, cp | 33 |
| Brookfield, spindle #1, 25 °C, cp | 37 |
| Cannon–Fenske, 25 °C, cp | 50 |
| Cannon–Fenske, 100 °C centistokes | 27 |
| Smoke point, °C | 195 |
| Flash point, °C | 295 |
| Fire point, °C | 338 |
| Iodine value | 82 |
| Saponification value | 92 |
| Acid value | <2 |
| Acetyl value | 2 |
| Unsaponifiable matter, % | 51 |
| Total acids, % | 52 |
| Iodine value of alcohols | 77 |
| Iodine value of acids | <76 |
| Average molecular weight of wax esters | 606 |
| Dosage Form | Drug | Ingredients | Use/effect of Jojoba Oil | Ref. |
|---|---|---|---|---|
| Microemulsion | Antioxidant lycopene | Jojoba oil, alcohols, nonionic surfactant (Brij 96V) | To solubilize lycopene | [51] |
| Microemulsion | - | Jojoba oil, alcohols, different nonionic surfactants, namely Brij 96V and Tweens, and water | To study the effect of Jojoba oil content on the type of the microemulsion | [52] |
| Sub-micron emulsion | Diclofenac (Diethyl ammonium) | Jojoba oil, purified egg lecithin, Cremophor EL surfactant, and water | To increase the anti-inflammatory effect of topical preparations of diclofenac | [53] |
| Gellified emulsion | Anti-acne agent, Benzoyl peroxide | Lipophilic surfactant (Span 60), jojoba oil, hydrophilic surfactant (Tween 20), propylene glycol, methyl paraben, propyl paraben, disodium EDTA, butylated hydroxy toluene, Carbopol 940, and water | To decrease the skin irritation and dryness caused by benzoyl peroxide | [54] |
| Microemulsion | Methotrexate | Jojoba oil, Tween 80, Span-85 and water | Treatment of psoriasis vulgaris. | [55] |
| Microemulsion | Synthetic retinoid tazarotene | Jojoba wax, labrasol/plurol isostearique and water | Treatment of psoriasis and increase in skin deposition of tazarotene | [56] |
| Solid lipid nanoparticles | Valacyclovir hydrochloride | Glyceryl monostearate. jojoba oil, polyethylene Glycol 400, Tween 80, and water | To benefit from jojoba oil moisturizing and stabilizing activity in the treatment of viral infections in humans | [57] |
| Nanostructured lipid carriers | - | Glyceryl behenate, jojoba oil, Tween 80, cetrimide, glycerine, Carbopol 934 or Carbopol 980, triethanolamine, and water | To improve symptoms of some skin disorders like eczema | [58] |
| Emulgels | Clotrimazole | Jojoba oil, hydroxypropyl methylcellulose (HPMC) Carbopol 934, Span 60, Brij 35, triethanolamine, propylene glycol, and water | An excipient for different topical antifungal preparations | [59] |
| Hydrophobically modified co-polymers of acrylic acid, namely Pemulen TR1 and TR2, jojoba oil, and water | [60] | |||
| Cutina lipogels | Fluconazole | Cutina, Jojoba oil | An excipient for fluconazole topical drug delivery | [61] |
| Microemulsion gel | Jojoba oil, Brij 96, Capmul and, water | |||
| Straightening emulsions | - | Jojoba oil, ammonium thioglycolate, self-emulsifying wax, oleth-3, mineral oil, propylene glycol, aqua, and preservative blend | As a conditioning agent added to the emulsion | [62] |
| Skin non-penetrating sunscreens | - | Jojoba oil, methoxycinnamate | To link UV sunscreen molecules as methoxycinnamate to jojoba oil to form new filters | [63,64] |
| Transdermal patch | Olanzapine | Jojoba oil, Eudragit polymer | As a penetration enhancer in transdermal delivery | [65] |
| Small-sized agarose microcapsules | Bacillus Calmette–Guérin (BCG) vaccine | Agarose, jojoba oil | An excipient | [66,67] |
| Small-diameter alginate beads | Calcium alginate matrix, jojoba oil | |||
| Nanocapsules | Jojoba oil, Poly(€-caprolactone) Tween 80, and Span 60 | To study physical stability and the hemocompatibility of jojoba oil-based nanocapsules for parenteral administration | [68] | |
| Solid nanoemulsion | Imiquimod, a Toll-like receptor 7 (TLR7) agonist + SIINFEKL antigen | Jojoba oil, sucrose fatty ester S-1670 and water | An excipient | [69] |
| O/W microemulsions | Paclitaxel | Jojoba oil, d-α-tocopherol polyethylene glycol 1000 succinate (TPGS-1000), isobutanol, and water | As an excipient to load paclitaxel for cancer treatment | [70] |
| Charged micelles | Small interfering RNAs (siRNAs) | Cationic lipids Bolaamphiphiles (GLH-58 and GLH-60) synthesized from jojoba oil | Starting material for the synthesis of lipids | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gad, H.A.; Roberts, A.; Hamzi, S.H.; Gad, H.A.; Touiss, I.; Altyar, A.E.; Kensara, O.A.; Ashour, M.L. Jojoba Oil: An Updated Comprehensive Review on Chemistry, Pharmaceutical Uses, and Toxicity. Polymers 2021, 13, 1711. https://doi.org/10.3390/polym13111711
Gad HA, Roberts A, Hamzi SH, Gad HA, Touiss I, Altyar AE, Kensara OA, Ashour ML. Jojoba Oil: An Updated Comprehensive Review on Chemistry, Pharmaceutical Uses, and Toxicity. Polymers. 2021; 13(11):1711. https://doi.org/10.3390/polym13111711
Chicago/Turabian StyleGad, Heba A., Autumn Roberts, Samirah H. Hamzi, Haidy A. Gad, Ilham Touiss, Ahmed E. Altyar, Osama A. Kensara, and Mohamed L. Ashour. 2021. "Jojoba Oil: An Updated Comprehensive Review on Chemistry, Pharmaceutical Uses, and Toxicity" Polymers 13, no. 11: 1711. https://doi.org/10.3390/polym13111711
APA StyleGad, H. A., Roberts, A., Hamzi, S. H., Gad, H. A., Touiss, I., Altyar, A. E., Kensara, O. A., & Ashour, M. L. (2021). Jojoba Oil: An Updated Comprehensive Review on Chemistry, Pharmaceutical Uses, and Toxicity. Polymers, 13(11), 1711. https://doi.org/10.3390/polym13111711









