Study on the Corrosion Resistance of Graphene Oxide-Based Epoxy Zinc-Rich Coatings
Abstract
1. Introduction
2. Experimental
2.1. Materials
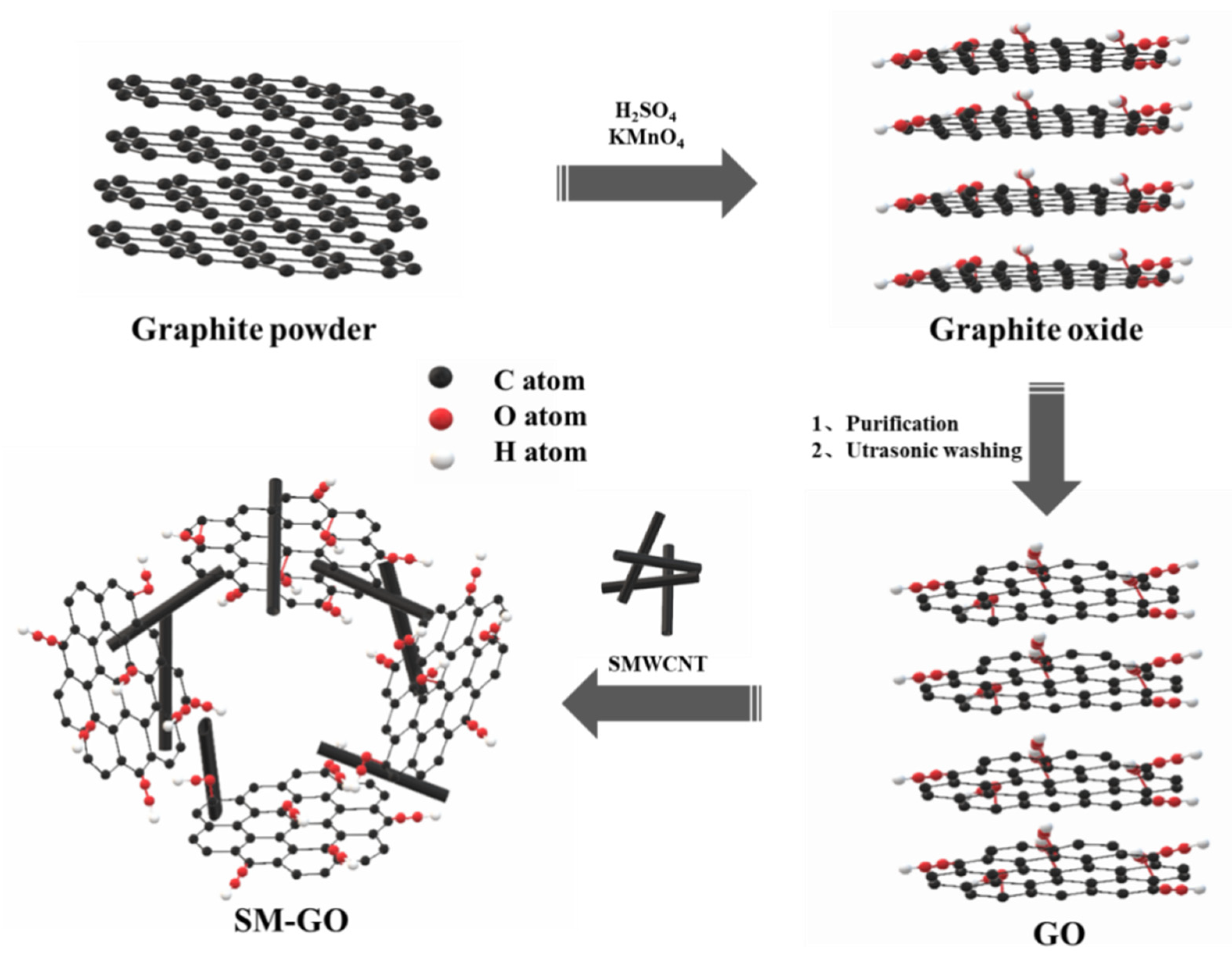
2.2. Preparation of SMWCNTs, GO and SM-GO
2.2.1. Preparation of SMWCNTs
2.2.2. Preparation of GO and SM-GO
2.3. Preparation of the Coating Samples
2.4. Characterization Methods
3. Results and Discussion
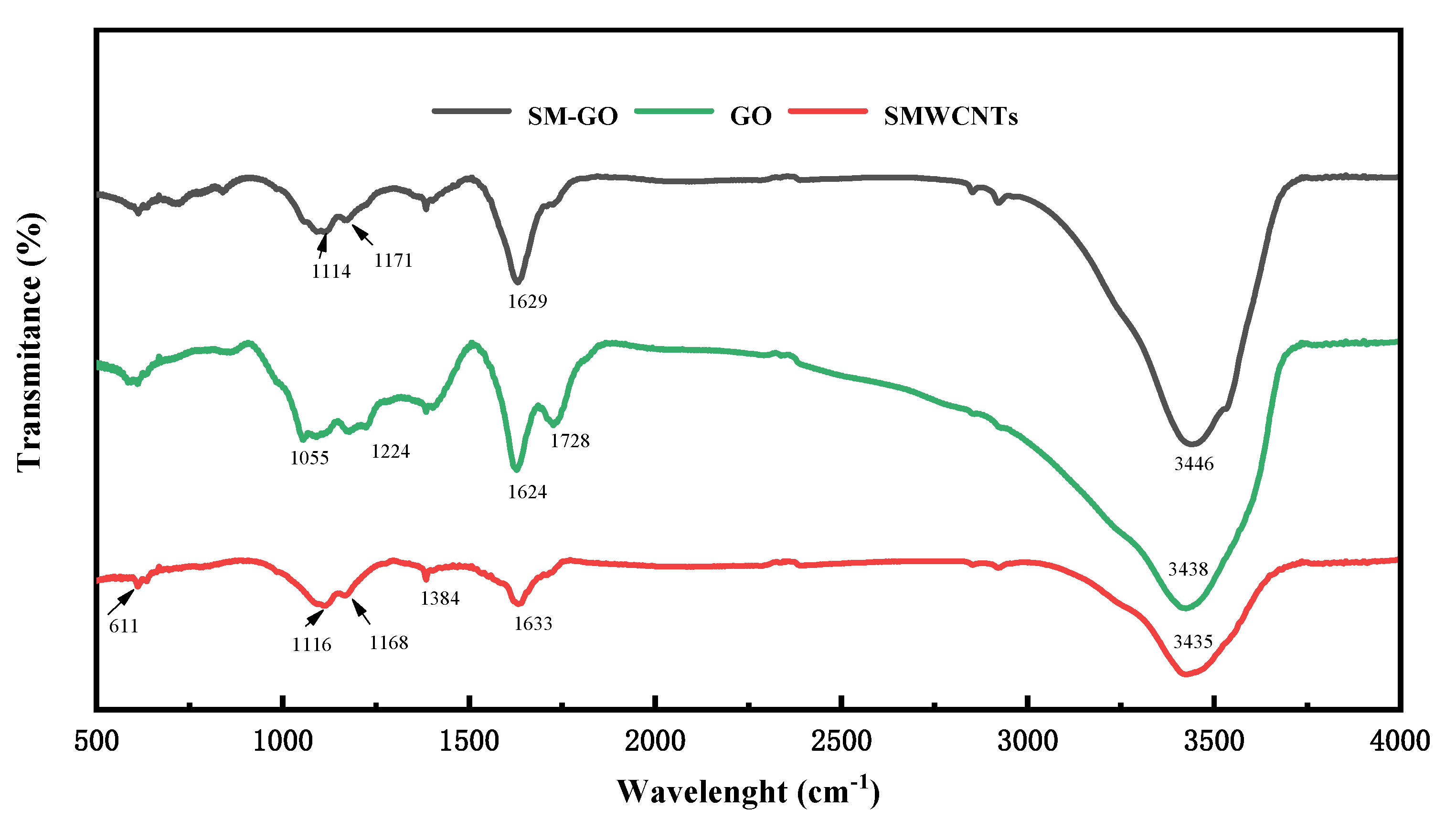
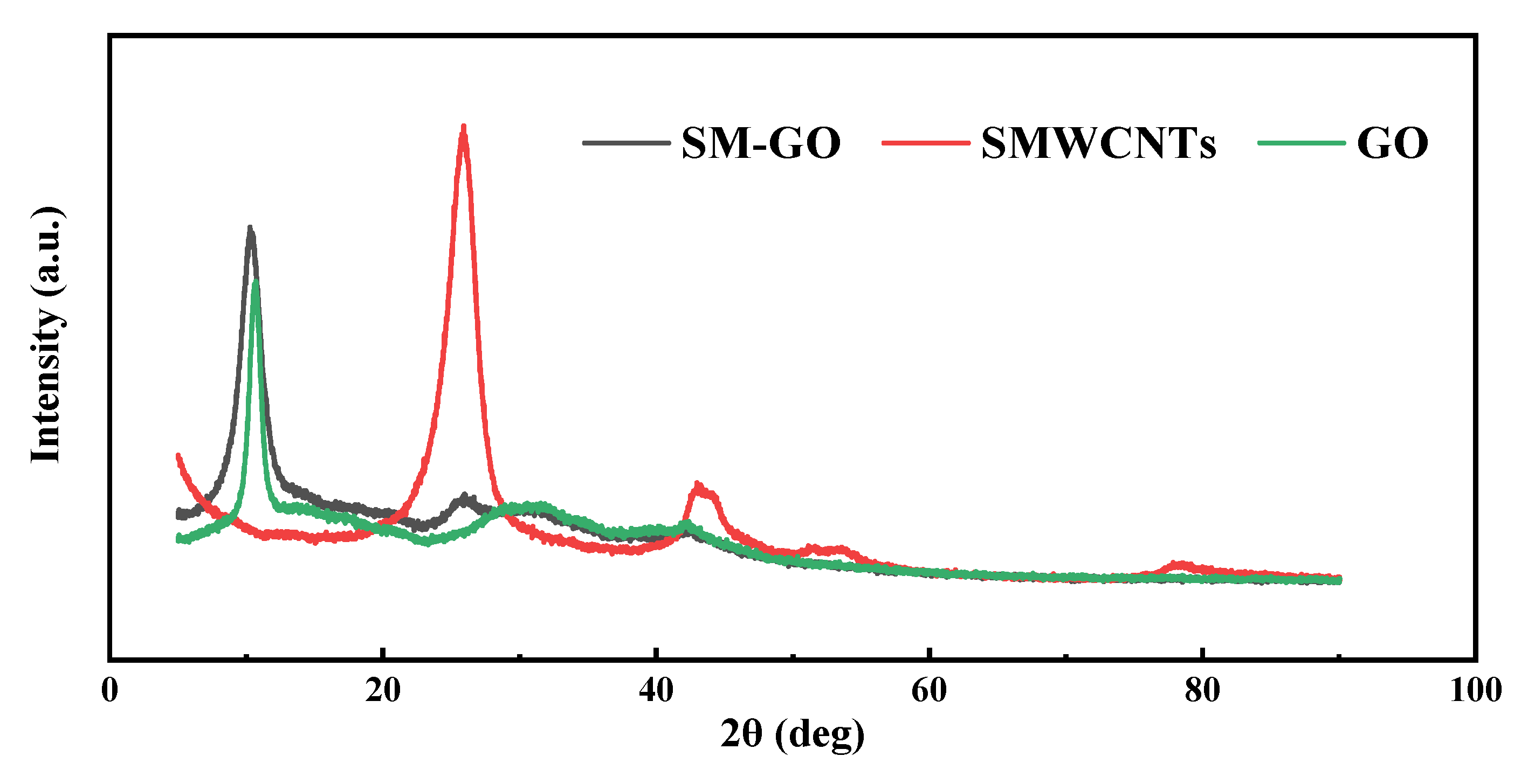
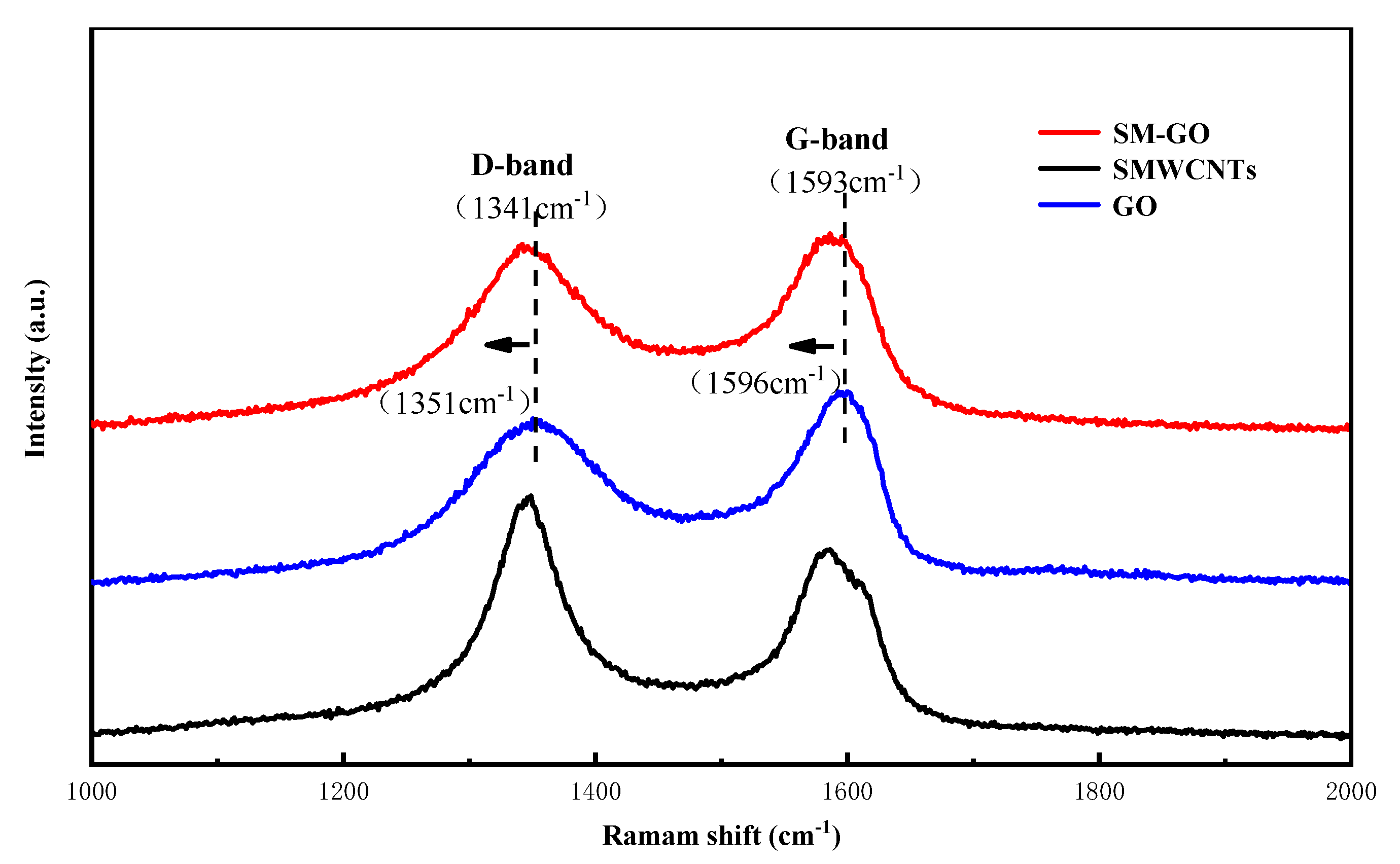
3.1. Characterization Results of GO, SMWCNTs and SM-GO
3.2. Characterization of Corrosion Resistance of Coatings
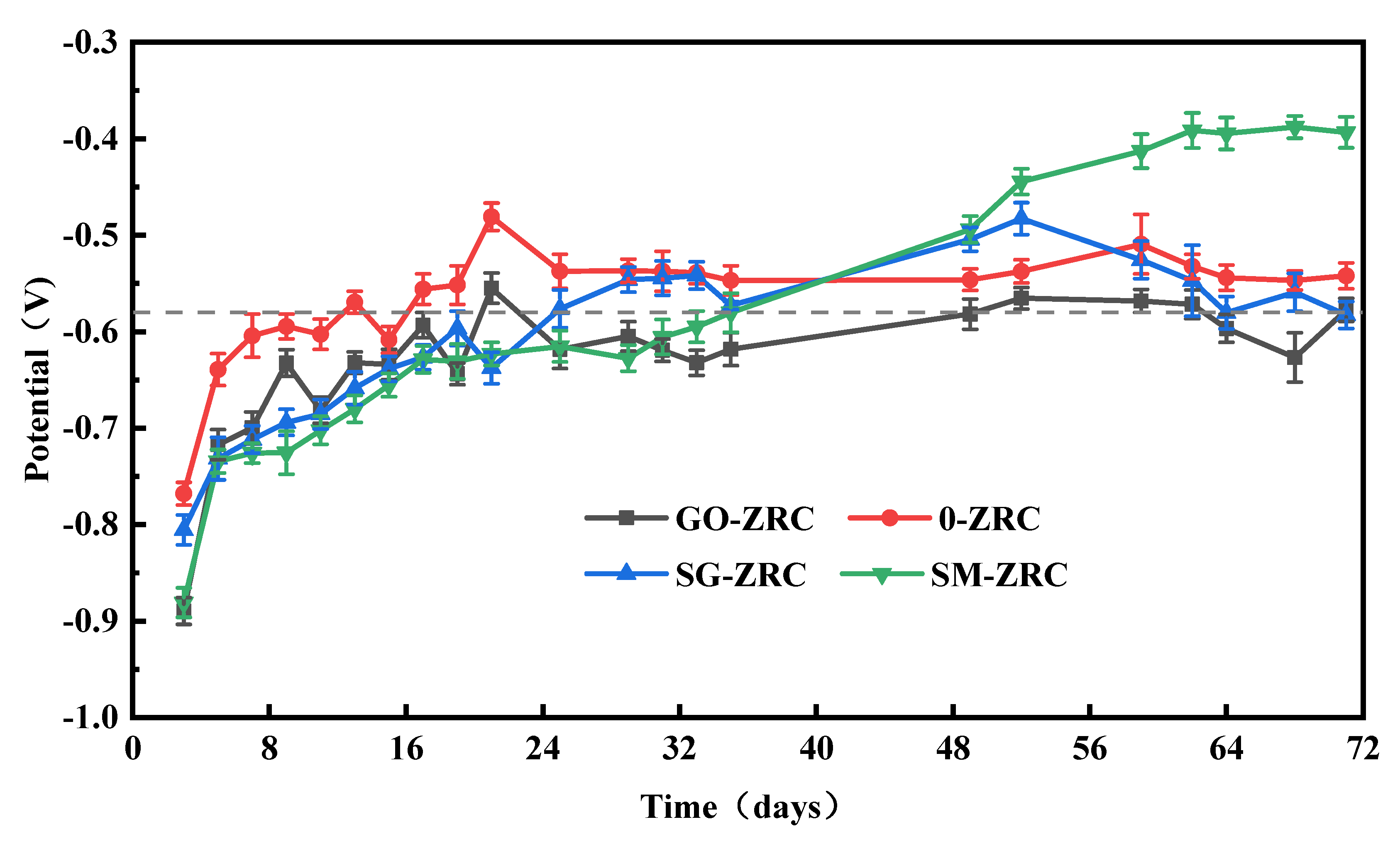
3.2.1. Open Circuit Potential (OCP)
3.2.2. Salt Spray Test
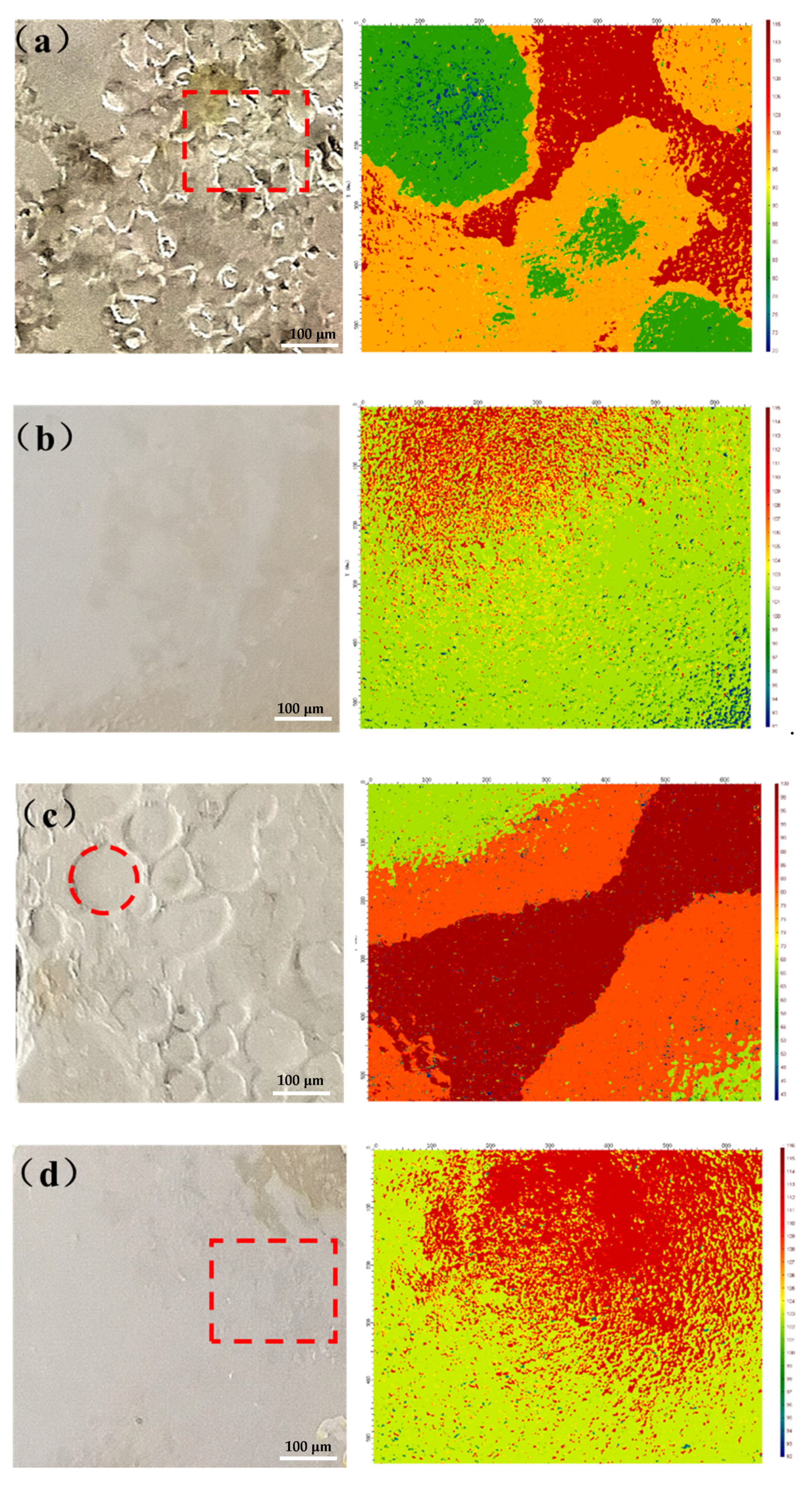
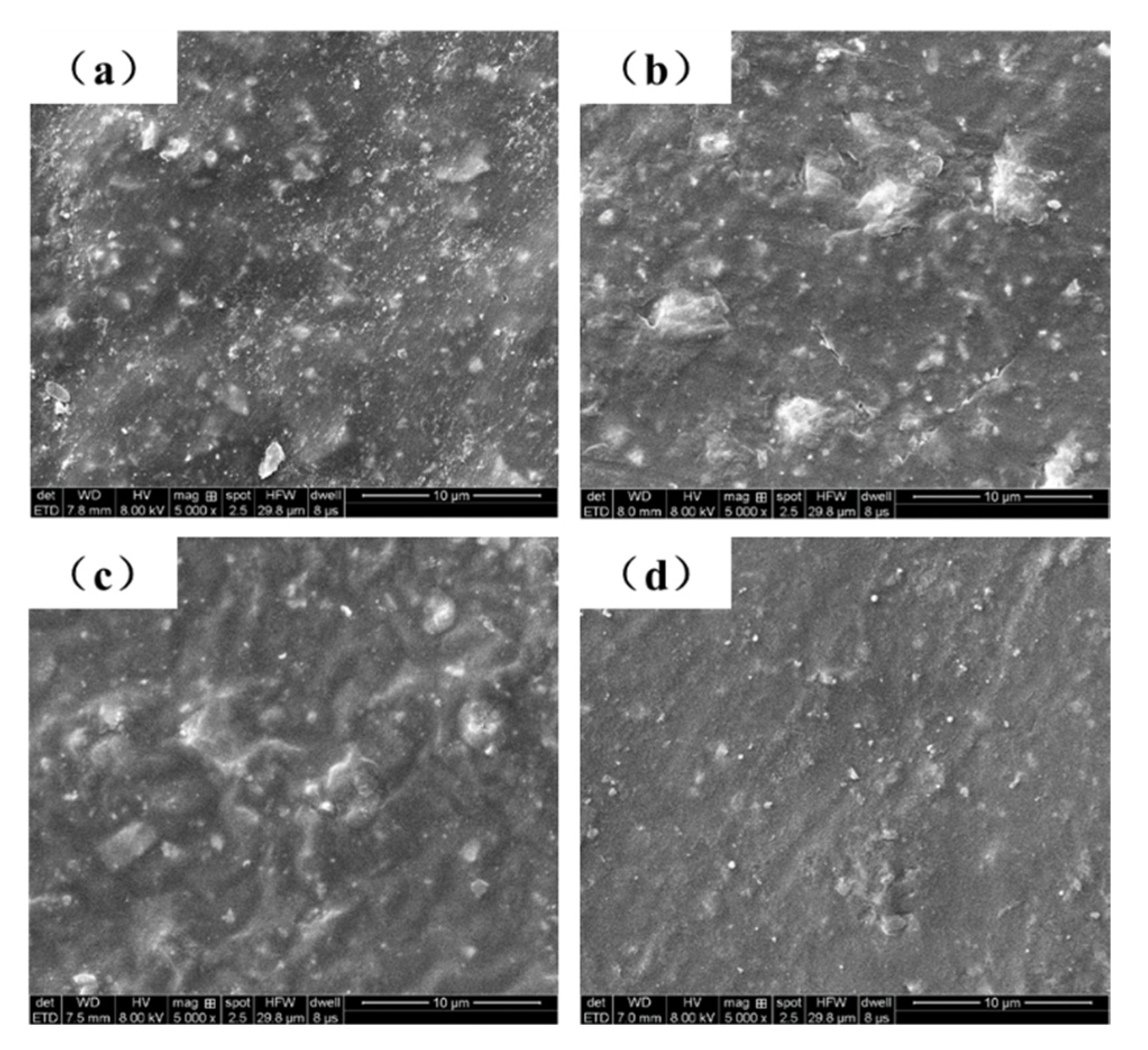
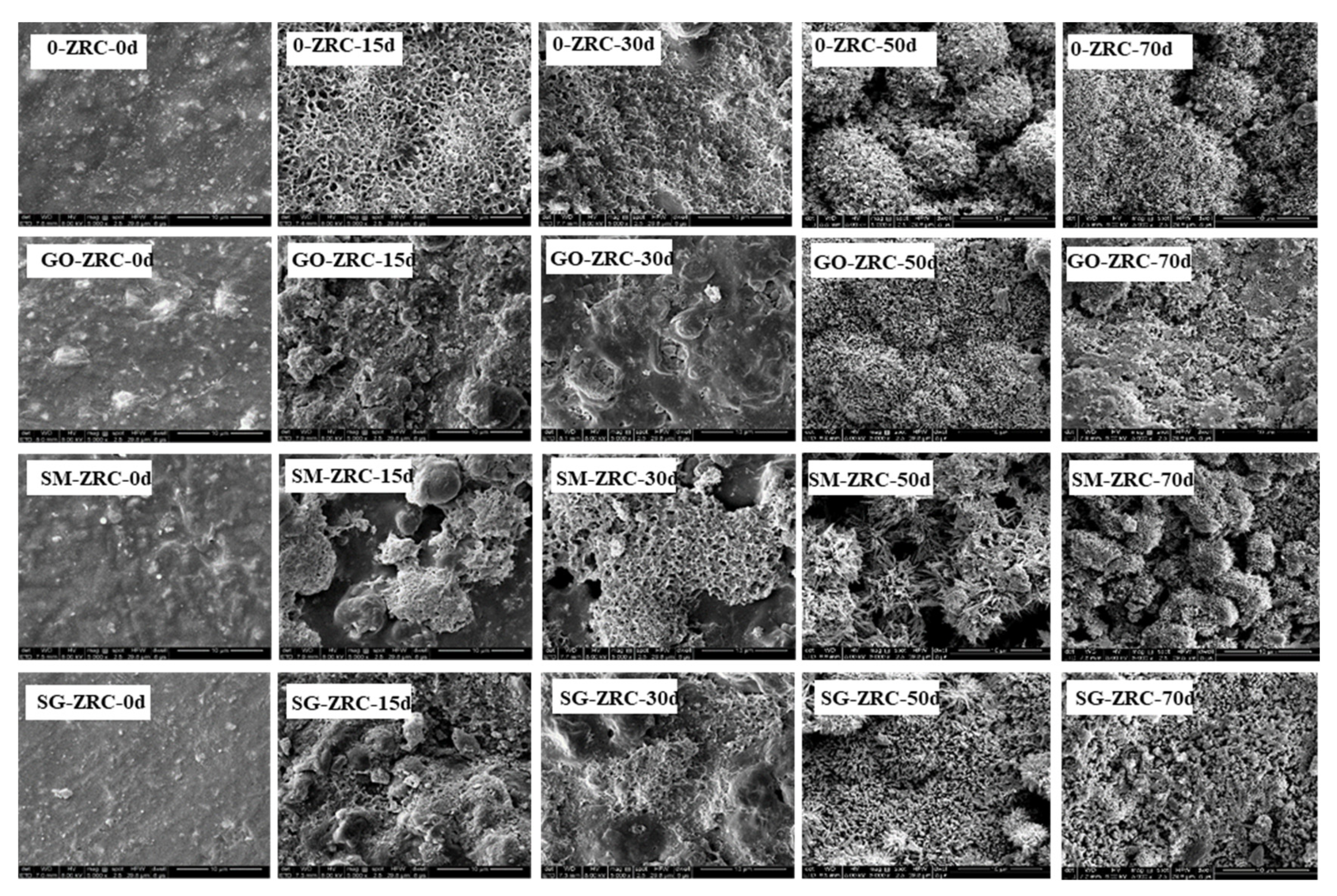
3.2.3. SEM Analysis
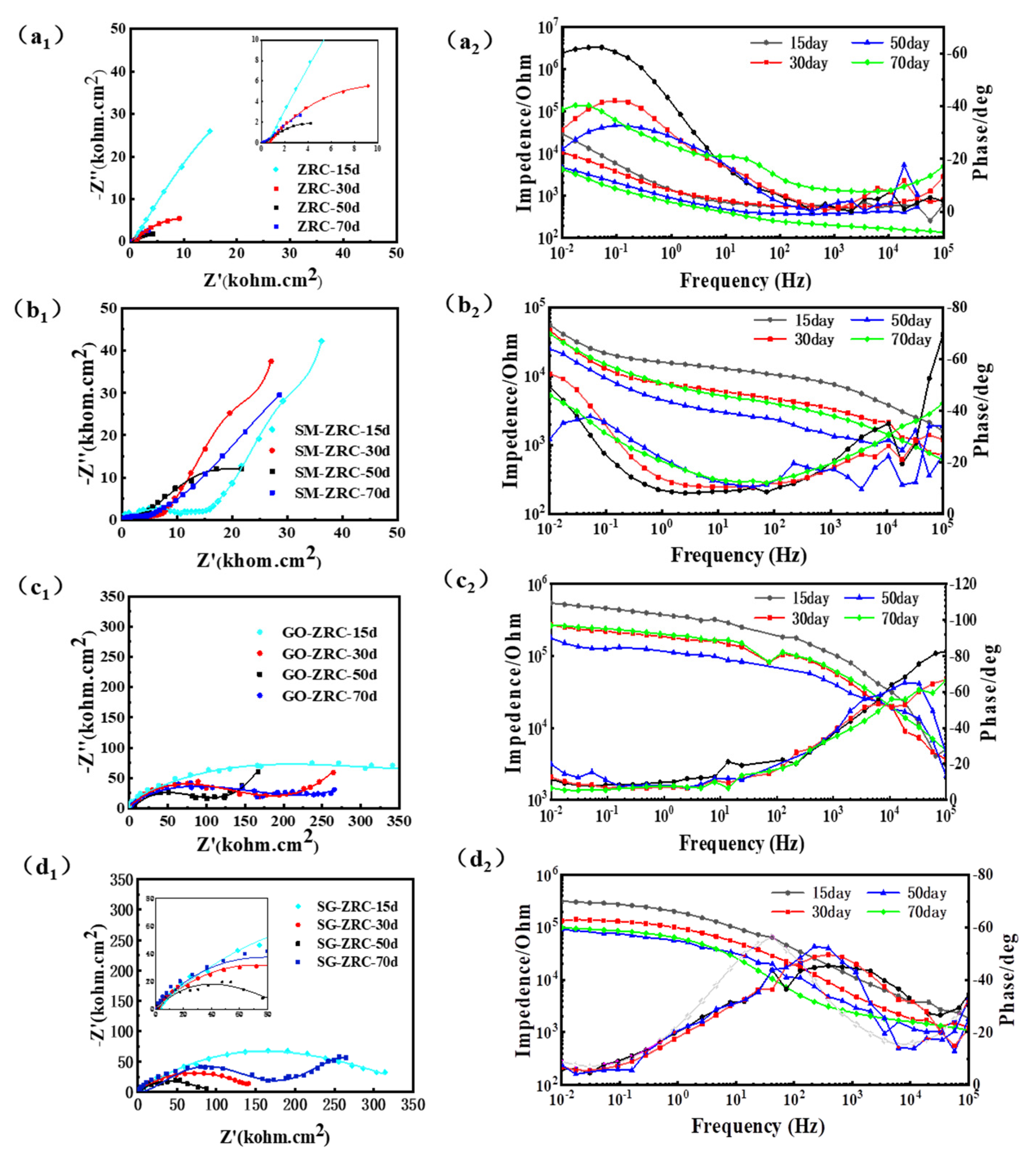
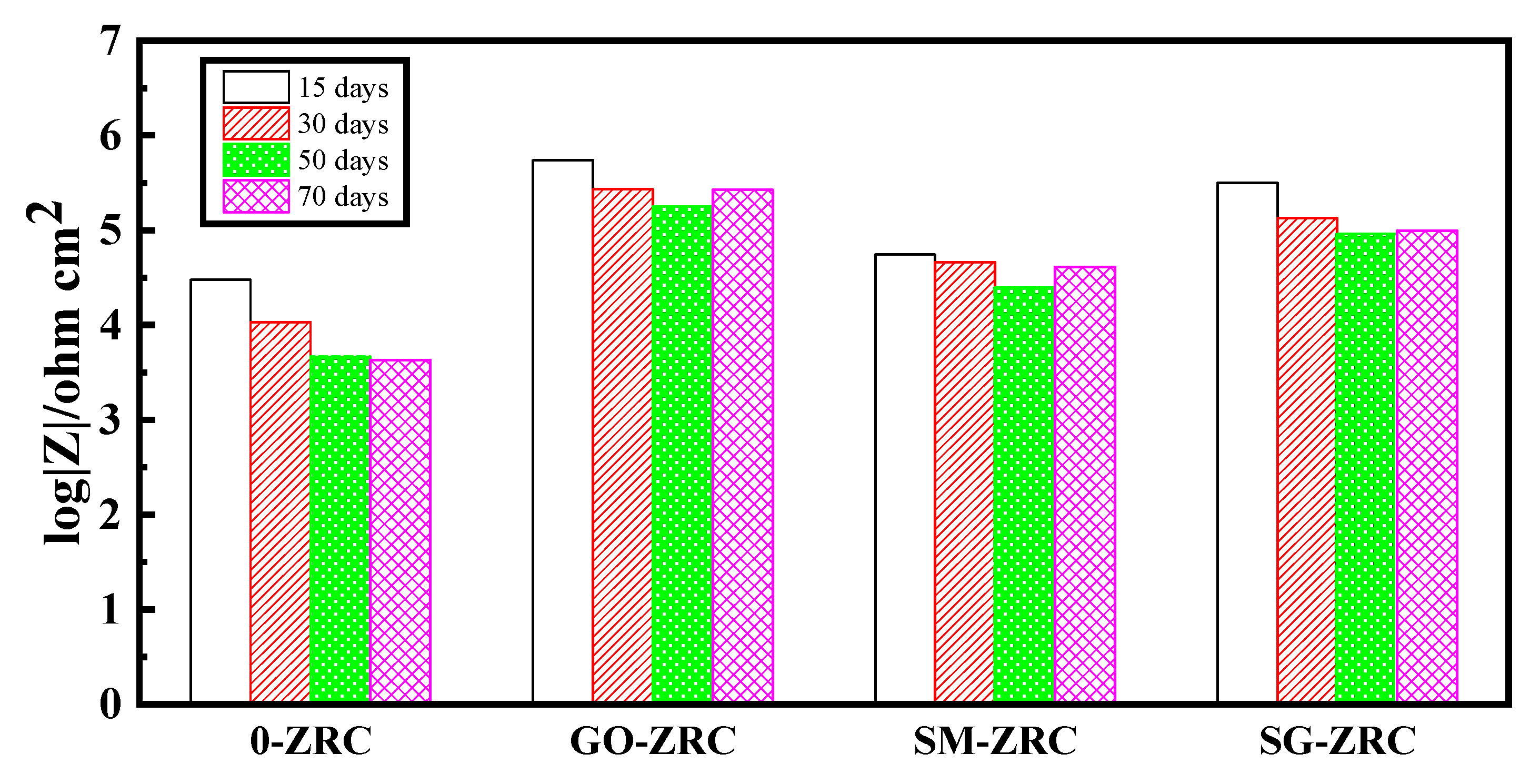
3.2.4. EIS Analysis
4. Anti-Corrosion Mechanism
5. Conclusions
- (1)
- GO is successfully non-covalently modified by SMWCNTs to form SM-GO, of which the interlayer spacing increases, and the dispersion in the coating matrix is improved.
- (2)
- Due to the conductivity and physical shielding of the nanoparticles, the zinc-rich coatings with conductive nanoparticles have a longer cathodic protection time and a larger impedance modulus than pure zinc-rich coatings. That is, the corrosion resistance of the composite coating is enhanced.
- (3)
- SMWCNTs have strong galvanic corrosion with zinc particles and metal substrates. Due to the presence of oxygen-containing functional groups, the galvanic corrosion of GO is relatively weak. Thus, the galvanic corrosion effect of SM-GO lies between them. Therefore, the regularity of the corrosion resistance of the coatings is recorded as GO-ZRC > SG-ZRC > SM-ZRC.
- (4)
- It is proved that carbon nanotubes should not be used to modify GO in zinc-rich coatings. When modifying GO, attention should be paid to eliminating its cathodic behavior in order to reduce the galvanic corrosion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marchebois, H.; Savall, C.; Bernard, J.; Touzain, S. Electrochemical behavior of zinc-rich powder coatings in artificial sea water. Electrochim. Acta 2004, 49, 2945–2954. [Google Scholar] [CrossRef]
- Zhou, S.; Wu, Y.; Zhao, W.; Yu, J.; Jiang, F.; Wu, Y.; Ma, L. Designing reduced graphene oxide/zinc rich epoxy composite coatings for improving the anticorrosion performance of carbon steel substrate. Mater. Des. 2019, 169, 107694. [Google Scholar] [CrossRef]
- Teng, S.; Gao, Y.; Cao, F.; Kong, D.; Zheng, X.; Ma, X.; Zhi, L. Zinc-reduced graphene oxide for enhanced corrosion protection of zinc-rich epoxy coatings. Prog. Org. Coat. 2018, 123, 185–189. [Google Scholar] [CrossRef]
- Shirehjini, F.T.; Danaee, I.; Eskandari, H.; Zarei, D. Effect of Nano Clay on Corrosion Protection of Zinc-rich Epoxy Coatings on Steel 37. J. Mater. Sci. Technol. 2016, 32, 1152–1160. [Google Scholar] [CrossRef]
- Ding, R.; Zheng, Y.; Yu, H.; Li, W.; Wang, X.; Gui, T. Study of water permeation dynamics and anti-corrosion mechanism of graphene/zinc coatings. J. Alloy. Compd. 2018, 748, 481–495. [Google Scholar] [CrossRef]
- Luo, X.; Yin, H.; Ren, J.; Yan, H.; Huang, X.; Yang, D.; He, L. Enhanced mixing of binary droplets induced by capillary pressure. J. Colloid Interface Sci. 2019, 545, 35–42. [Google Scholar] [CrossRef]
- Berry, V. Impermeability of graphene and its applications. Carbon N. Y. 2013, 62, 1–10. [Google Scholar] [CrossRef]
- Kowalczyk, K.; Spychaj, T. Zinc-free varnishes and zinc-rich paints modified with ionic liquids. Corros. Sci. 2014, 78, 111–120. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Mohamadzadeh Moghadam, M.H.; Shohani, N.; Mahdavian, M. Effects of highly crystalline and conductive polyaniline/graphene oxide composites on the corrosion protection performance of a zinc-rich epoxy coating. Chem. Eng. J. 2017, 320, 363–375. [Google Scholar] [CrossRef]
- Hayatdavoudi, H.; Rahsepar, M. Smart inhibition action of layered double hydroxide nanocontainers in zinc-rich epoxy coating for active corrosion protection of carbon steel substrate. J. Alloy. Compd. 2017, 711, 560–567. [Google Scholar] [CrossRef]
- Shreepathi, S.; Bajaj, P.; Mallik, B.P. Electrochemical impedance spectroscopy investigations of epoxy zinc rich coatings: Role of Zn content on corrosion protection mechanism. Electrochim. Acta 2010, 55, 5129–5134. [Google Scholar] [CrossRef]
- Feng, Y.; Yin, J.; Liu, S.; Wang, Y.; Li, B.; Jiao, T. Facile Synthesis of Ag/Pd Nanoparticle–Loaded Poly(ethylene imine) Composite Hydrogels with Highly Efficient Catalytic Reduction of 4-Nitrophenol. ACS Omega 2020, 5, 3725–3733. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Bi, Z.; Zhang, R.; Liu, J.; Yu, X.; Li, Z. A comprehensive review on graphene-based anti-corrosive coatings. Chem. Eng. J. 2019, 373, 104–121. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Ang, P.K.; Yang, J. The chemistry of graphene. J. Mater. Chem. 2010, 20, 2277–2289. [Google Scholar] [CrossRef]
- Yang, S.Y.; Lin, W.N.; Huang, Y.L.; Tien, H.W.; Wang, J.Y.; Ma, C.C.M.; Li, S.M.; Wang, Y.S. Synergetic effects of graphene platelets and carbon nanotubes on the mechanical and thermal properties of epoxy composites. Carbon N. Y. 2011, 49, 793–803. [Google Scholar] [CrossRef]
- Hu, H.; He, Y.; Long, Z.; Zhan, Y. Synergistic effect of functional carbon nanotubes and graphene oxide on the anti-corrosion performance of epoxy coating. Polym. Adv. Technol. 2017, 28, 754–762. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, W.; Qiang, Y.; Chen, Z.; Wang, L.; Gao, X.; Fang, Z. π-π interaction between fluorinated reduced graphene oxide and acridizinium ionic liquid: Synthesis and anti-corrosion application. Carbon N. Y. 2020, 159, 292–302. [Google Scholar] [CrossRef]
- Li, J.; Cui, J.; Yang, J.; Ma, Y.; Qiu, H.; Yang, J. Silanized graphene oxide reinforced organofunctional silane composite coatings for corrosion protection. Prog. Org. Coat. 2016, 99, 443–451. [Google Scholar] [CrossRef]
- Cui, M.; Ren, S.; Zhao, H.; Xue, Q.; Wang, L. Polydopamine coated graphene oxide for anticorrosive reinforcement of water-borne epoxy coating. Chem. Eng. J. 2018, 335, 255–266. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.; Zheng, Z.; He, H.; Wei, M.; Huang, X. Fabrication of graphene oxide/multi-walled carbon nanotube/urushiol formaldehyde polymer composite coatings and evaluation of their physico-mechanical properties and corrosion resistance. Prog. Org. Coat. 2019, 127, 131–139. [Google Scholar] [CrossRef]
- Jing, L.-C.; Wang, T.; Cao, W.-W.; Wen, J.-G.; Zhao, H.; Ning, Y.-J.; Yuan, X.-T.; Tian, Y.; Teng, L.-H.; Geng, H.-Z. Water-based polyurethane composite anticorrosive barrier coating via enhanced dispersion of functionalized graphene oxide in the presence of acidified multi-walled carbon nanotubes. Prog. Org. Coat. 2020, 146, 105734. [Google Scholar] [CrossRef]
- Turhan, M.C.; Li, Q.; Jha, H.; Singer, R.F.; Virtanen, S. Corrosion behaviour of multiwall carbon nanotube/magnesium composites in 3.5% NaCl. Electrochim. Acta 2011, 56, 7141–7148. [Google Scholar] [CrossRef]
- Gergely, A.; Pászti, Z.; Mihály, J.; Drotár, E.; Török, T. Galvanic function of zinc-rich coatings facilitated by percolating structure of the carbon nanotubes. Part I: Characterization of the nano-size particles. Prog. Org. Coat. 2015, 78, 437–445. [Google Scholar] [CrossRef]
- Gergely, A.; Pászti, Z.; Mihály, J.; Drotár, E.; Török, T. Galvanic function of zinc-rich coatings facilitated by percolating structure of the carbon nanotubes. Part II: Protection properties and mechanism of the hybrid coatings. Prog. Org. Coat. 2014, 77, 412–424. [Google Scholar] [CrossRef]
- El-Khodary, S.A.; El-Enany, G.M.; El-Okr, M.; Ibrahim, M. Modified iron doped polyaniline/sulfonated carbon nanotubes for all symmetric solid-state supercapacitor. Synth. Met. 2017, 233, 41–51. [Google Scholar] [CrossRef]
- Morsy, M.; Helal, M.; El-Okr, M.; Ibrahim, M. Preparation, purification and characterization of high purity multi-wall carbon nanotube. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2014, 132, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Dhall, S.; Jaggi, N.; Nathawat, R. Functionalized multiwalled carbon nanotubes based hydrogen gas sensor. Sens. Actuators A Phys. 2013, 201, 321–327. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon N. Y. 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Bhaskaran; Pancharatna, P.D.; Lata, S.; Singh, G. Imidazolium based ionic liquid as an efficient and green corrosion constraint for mild steel at acidic pH levels. J. Mol. Liq. 2019, 278, 467–476. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Zhang, Y.; Jing, C. Enhancing the Corrosion Resistance of Epoxy Coatings by Impregnation with a Reduced Graphene Oxide-Hydrophobic Ionic Liquid Composite. ChemElectroChem 2018, 5, 3300–3306. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, C.; Han, D.; Ma, S.; Guo, W.; Cai, H.; Wang, X. Effect of graphene on corrosion resistance of waterborne inorganic zinc-rich coatings. J. Alloy. Compd. 2019, 774, 255–264. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, D.; Liu, T.; Liu, Z.; Pu, J.; Liu, W.; Zhao, H.; Li, X.; Wang, L. Superior corrosion resistance and self-healable epoxy coating pigmented with silanzied trianiline-intercalated graphene. Carbon N. Y. 2019, 142, 164–176. [Google Scholar] [CrossRef]
- Lv, X.; Li, X.; Li, N.; Zhang, H.; Zheng, Y.; Wu, J.; Tao, X. ZrO2 nanoparticle encapsulation of graphene microsheets for enhancing anticorrosion performance of epoxy coatings. Surf. Coat. Technol. 2019, 358, 443–451. [Google Scholar] [CrossRef]
- Cao, X.; Huang, F.; Huang, C.; Liu, J.; Cheng, Y.F. Preparation of graphene nanoplate added zinc-rich epoxy coatings for enhanced sacrificial anode-based corrosion protection. Corros. Sci. 2019, 159, 108120. [Google Scholar] [CrossRef]
- Jalili, M.; Rostami, M.; Ramezanzadeh, B. An investigation of the electrochemical action of the epoxy zinc-rich coatings containing surface modified aluminum nanoparticle. Appl. Surf. Sci. 2015, 328, 95–108. [Google Scholar] [CrossRef]
- Li, W.; Fan, Z.; Li, X.; Jiang, B.; Yan, F.; Zhang, Z.; Wang, X. Improved anti-corrosion performance of epoxy zinc rich coating on rusted steel surface with aluminum triphosphate as rust converter. Prog. Org. Coat. 2019, 135, 483–489. [Google Scholar] [CrossRef]
- Mahato, P.; Mishra, S.K.; Murmu, M.; Murmu, N.C.; Hirani, H.; Banerjee, P. A prolonged exposure of Ti-Si-B-C nanocomposite coating in 3.5 wt.% NaCl solution: Electrochemical and morphological analysis. Surf. Coat. Technol. 2019, 375, 477–488. [Google Scholar] [CrossRef]
- Murmu, M.; Saha, S.K.; Murmu, N.C.; Banerjee, P. Amine cured double Schiff base epoxy as efficient anticorrosive coating materials for protection of mild steel in 3.5% NaCl medium. J. Mol. Liq. 2019, 278, 521–535. [Google Scholar] [CrossRef]
- Murmu, M.; Saha, S.K.; Murmu, N.C.; Banerjee, P. Effect of stereochemical conformation into the corrosion inhibitive behaviour of double azomethine based Schiff bases on mild steel surface in 1 mol L−1 HCl medium: An experimental, density functional theory and molecular dynamics simulation study. Corros. Sci. 2019, 146, 134–151. [Google Scholar] [CrossRef]
- Shen, L.; Li, Y.; Zhao, W.; Miao, L.; Xie, W.; Lu, H.; Wang, K. Corrosion Protection of Graphene-Modified Zinc-Rich Epoxy Coatings in Dilute NaCl Solution. ACS Appl. Nano Mater. 2019, 2, 180–190. [Google Scholar] [CrossRef]
- Xu, X.; Yi, D.; Wang, Z.; Yu, J.; Zhang, Z.; Qiao, R.; Sun, Z.; Hu, Z.; Gao, P.; Peng, H.; et al. Greatly Enhanced Anticorrosion of Cu by Commensurate Graphene Coating. Adv. Mater. 2018, 30, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hayatdavoudi, H.; Rahsepar, M. A mechanistic study of the enhanced cathodic protection performance of graphene-reinforced zinc rich nanocomposite coating for corrosion protection of carbon steel substrate. J. Alloy. Compd. 2017, 727, 1148–1156. [Google Scholar] [CrossRef]
- Du, P.; Wang, J.; Liu, G.; Zhao, H.; Wang, L. Facile synthesis of intelligent nanocomposites as encapsulation for materials protection. Mater. Chem. Front. 2019, 3, 321–330. [Google Scholar] [CrossRef]
- Dhall, S.N.; Jaggi, R. Nathawat. Sens. Actuators A Phys. 2013, 201, 321–332. [Google Scholar] [CrossRef]
- Ding, R.; Li, W.; Wang, X.; Gui, T.; Li, B.; Han, P.; Tian, H.; Liu, A.; Wang, X.; Liu, X.; et al. A brief review of corrosion protective films and coatings based on graphene and graphene oxide. J. Alloy. Compd. 2018, 764, 1039–1055. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, X.; Zhao, W.; Wang, Y.; Wang, C.; Xue, Q. Corrosion mechanism of graphene coating with different defect levels. J. Alloy. Compd. 2019, 777, 135–144. [Google Scholar] [CrossRef]




| Additives | Coating | Descriptions | Ref. |
|---|---|---|---|
| Polyaniline/ graphene oxide (GO-PANI) | Epoxy zinc-rich coating | Both the cathodic protection properties and barrier performance of the ZRC are improved after addition of 0.1 wt.% GO and GO-PANI nanosheets to the ZRC sample. | [9] |
| Fluorinated reduced graphene oxide (FrGO-IL) | Waterborne epoxy coating | It improves the corrosion resistance performance of the coating by exerting the superior shielding effect and inhibiting the ability for micro-galvanic corrosion. | [17] |
| Silanized graphene oxide (SGO) | Silane coating | The composite coatings exhibit the highest protective properties with the addition of 0.2 wt.% of SGO. | [18] |
| Polydopamine-coated graphene oxide (GO-PDA) | water-borne epoxy coating | The results demonstrate that the inclusion of well-dispersed GO-PDA nanosheets leads to a remarkable improvement in the corrosion protection performance of water-borne EP coating. | [19] |
| Graphene oxide/multi-walled carbon nanotubes (GO-MWCNTs) | Urushiol formaldehyde polymer coating | The resulting GO/MWCNTs/UFP films exhibit outstanding properties in terms of electrochemical corrosion, alkali-resistance, hardness and adhesion. | [20] |
| Acidified multi-walled carbon nanotubes and gallic acid modified graphene oxide flakes (FMWCNT-FGO) | Polyurethane coating | The composite coatings simultaneously underwent no corrosion after immersion for 96 h, and the corrosion resistance was better than that of the other coatings. | [21] |
| Coating | 0-ZRC-15d | 0-ZRC-30 d | 0-ZRC-50 d | 0-ZRC-70 d | SG-ZRC-15 d | SG-ZRC-30 d | SG-ZRC-50 d | SG-ZRC-70 d | |
|---|---|---|---|---|---|---|---|---|---|
| Parameter | |||||||||
| Rs (Ω·cm2) | 5.58 × 103 | 5.37 × 103 | 3.75 × 103 | 1.53 × 103 | 1.63 × 103 | 1.13 × 103 | 0.97 × 103 | 1.36 × 103 | |
| YQC (Ω−1·cm−2·sn) | 2.51 × 10−4 | 6.95 × 10−5 | 8.07 × 10−5 | 8.09 × 10−4 | 5.98 × 10−7 | 6.39 × 10−7 | 5.53 × 10−7 | 2.19 × 10−6 | |
| nQC | 0.716 | 0.812 | 0.961 | 0.387 | 0.573 | 0.661 | 0.753 | 0.679 | |
| Rpo (Ω·cm2) | 1.18 × 104 | 4.55 × 103 | 2.41 × 102 | 4.45 × 102 | 1.93 × 105 | 6.47 × 104 | 2.48 × 104 | 8.80 × 103 | |
| Rt,Zn (Ω·cm2) | 1.29 × 104 | 1.91 × 104 | 8.22 × 103 | 4.45 × 103 | - | - | - | - | |
| Ydl,Zn (Ω−1·cm−2·sn) | 2.78 × 10−4 | 3.37 × 10−4 | 6.52 × 10−4 | 8.09 × 10−4 | - | - | - | - | |
| ndl,Zn | 0.747 | 0.644 | 0.543 | 0.397 | - | - | - | - | |
| Rt,Fe (Ω·cm2) | - | - | - | - | 1.30 × 105 | 8.18 × 104 | 6.70 × 104 | 5.40 × 103 | |
| Ydl,Fe (Ω−1·cm−2·sn) | - | - | - | - | 3.53 × 10−6 | 4.04 × 10−6 | 7.62 × 10−6 | 4.80 × 10−6 | |
| ndl,Fe | - | - | - | - | 0.535 | 0.513 | 0.459 | 0.789 | |
| Chi-Square | 1.3 × 10−4 | 3.1 × 10−4 | 2.0 × 10−4 | 2.3 × 10−4 | 1.4 × 10−4 | 5.7 × 10−4 | 2.8 × 10−4 | 3.0 × 10−4 | |
| Coating | GO-ZRC-15 d | GO-ZRC-30 d | GO-ZRC-50 d | GO-ZRC-70 d | SM-ZRC-15 d | SM-ZRC-30 d | SM-ZRC-50 d | SM-ZRC-70 d | |
|---|---|---|---|---|---|---|---|---|---|
| Parameter | |||||||||
| Rs (Ω·cm2) | 1.00 × 10−7 | 6.63 × 10−7 | 1.20 × 10−4 | 7.50 × 10−3 | 1.24 × 10−5 | 6.52 × 10−3 | 1.00 × 10−7 | 1.00 × 10−3 | |
| YQC (Ω−1·cm−2·sn) | 2.19 × 10−6 | 1.88 × 10−8 | 2.76 × 10−8 | 4.32 × 10−6 | 9.82 × 10−7 | 3.30 × 10−22 | 5.05 × 10−5 | 5.96 × 10−6 | |
| nQC | 0.072 | 0.721 | 1.000 | 0.062 | 0.477 | 0.038 | 0.226 | 0.403 | |
| Rpo (Ω·cm2) | 1.00 × 105 | 9.60 × 104 | 5.04 × 104 | 6.19 × 104 | 1.39 × 104 | 2.41 × 103 | 4.35 × 103 | 1 × 102 | |
| Rt,Zn (Ω·cm2) | 5.53 × 105 | 1.29 × 104 | 3.35 × 104 | 9.34 × 103 | - | - | - | - | |
| Ydl,Zn (Ω−1·cm−2·sn) | 3.83 × 10−10 | 1.79 × 10−6 | 1.25 × 10−6 | 2.30 × 10−9 | - | - | - | - | |
| ndl,Zn | 1.000 | 0.401 | 0.586 | 0.843 | - | - | - | - | |
| ZW,Zn | 7.17 × 10−8 | 4.91 × 10−5 | 5.34 × 10−5 | 8.67 × 10−8 | - | - | - | - | |
| Rt,Fe (Ω·cm2) | - | - | - | - | 9.95 × 104 | 4.97 × 103 | 1.10 × 103 | 5.30 × 103 | |
| Ydl,Fe (Ω−1·cm−2·sn) | - | - | - | - | 1.23 × 10−4 | 1.58 × 10−6 | 9.74 × 10−5 | 5.87 × 10−10 | |
| ndl,Fe | - | - | - | - | 0.635 | 0.515 | 0.638 | 1.000 | |
| ZW,Fe(Ω−1·s−0.5) | - | - | - | - | 1.79 × 10−10 | 1.12 × 10−4 | 2.30 × 10−4 | 1.03 × 10−4 | |
| Chi-Square | 7.0 × 10−4 | 2.1 × 10−4 | 3.5 × 10−4 | 6.4 × 10−4 | 3.2 × 10−4 | 3.7 × 10−4 | 5.4 × 10−4 | 4.4 × 10−4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Bi, Z.; Cui, G. Study on the Corrosion Resistance of Graphene Oxide-Based Epoxy Zinc-Rich Coatings. Polymers 2021, 13, 1657. https://doi.org/10.3390/polym13101657
Tian Y, Bi Z, Cui G. Study on the Corrosion Resistance of Graphene Oxide-Based Epoxy Zinc-Rich Coatings. Polymers. 2021; 13(10):1657. https://doi.org/10.3390/polym13101657
Chicago/Turabian StyleTian, Yong, Zhenxiao Bi, and Gan Cui. 2021. "Study on the Corrosion Resistance of Graphene Oxide-Based Epoxy Zinc-Rich Coatings" Polymers 13, no. 10: 1657. https://doi.org/10.3390/polym13101657
APA StyleTian, Y., Bi, Z., & Cui, G. (2021). Study on the Corrosion Resistance of Graphene Oxide-Based Epoxy Zinc-Rich Coatings. Polymers, 13(10), 1657. https://doi.org/10.3390/polym13101657







