On the Effectiveness of Oxygen Plasma and Alkali Surface Treatments to Modify the Properties of Polylactic Acid Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Manufacturing of Scaffolds
2.3. Surface Treatment
2.3.1. Oxygen Plasma Treatments
2.3.2. Alkali Treatments
2.4. Physicochemical Characterization
2.4.1. Evaluation of Carboxyl Groups on the Treated Surface
2.4.2. Energy-Dispersive X-ray (EDX) Spectroscopy Analysis
2.4.3. Water Contact Angle (WCA) Measurements
2.4.4. Differential Scanning Calorimetry (DSC) Analysis
2.5. Enzymatic Degradation Study
2.6. Statistical Analysis
3. Results
3.1. Physicochemical Characterization
3.1.1. Weight Loss Due to the Application of the Surface Treatments
3.1.2. Evaluation of Carboxyl Groups on the Treated Surface
3.1.3. EDX Analysis
3.1.4. WCA Measurements
3.1.5. Differential Scanning Calorimetry Analysis (DSC)
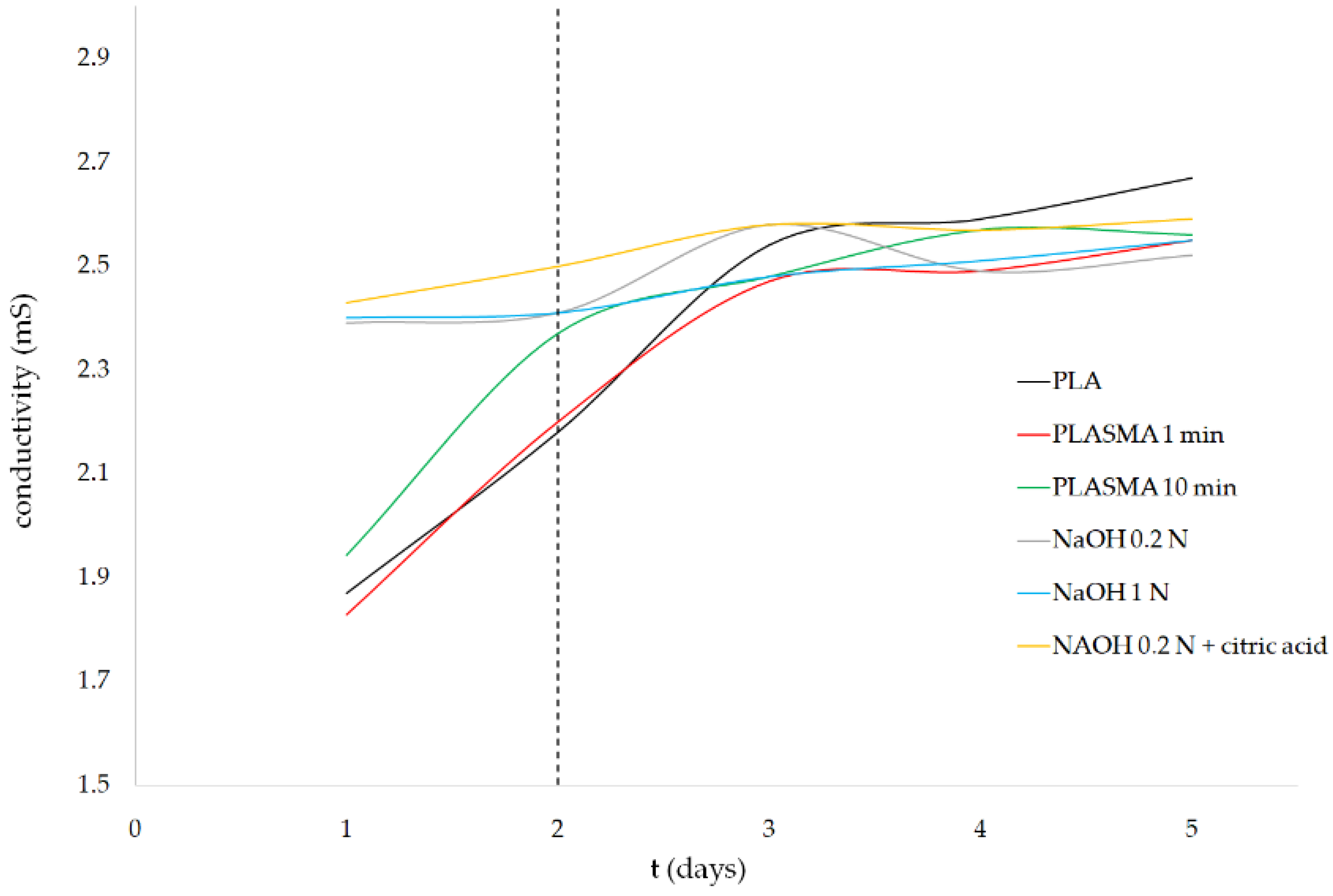
3.2. Enzymatic Degradation Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Group of Samples | Weight Loss (%) | pH | Conductivity (mS) | Initial Porosity (%) | Final Porosity (%) |
|---|---|---|---|---|---|
| PLA | 0.01 ± 0.01 | 7.56 ± 0.01 | 2.00 ± 0.01 | 44.1 ± 3.8 | 43.1 ± 2.2 |
| NaOH 0.2 N + citric acid | 1.37 ± 0.11 1 | 4.57 ± 0.01 1 | 2.41 ± 0.01 1 | 41.0 ± 1.3 | 44.2 ± 2.8 |
References
- Puppi, D.; Chiellini, F.; Piras, A.M.; Chiellini, E. Polymeric materials for bone and cartilage repair. Prog. Polym. Sci. 2010, 35, 403–440. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Ruiz-Hernández, E. Bioceramics: From bone regeneration to cancer nanomedicine. Adv. Mater. 2011, 23, 5177–5218. [Google Scholar] [CrossRef] [PubMed]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, P.X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef]
- Foresti, R.; MacAluso, C.; Rossi, S.; Selleri, S.; Perini, P.; Freyrie, A.; Raposio, E.; Fenaroli, P.; Concari, G.; De Filippo, M.; et al. 3D reconstruction cutting and smart devices for personalized medicine. In Proceedings of the 2020 Italian Conference on Optics and Photonics, Parma, Italy, 9–11 September 2020. [Google Scholar]
- Foresti, R.; Ghezzi, B.; Vettori, M.; Bergonzi, L.; Attolino, S.; Rossi, S.; Tarabella, G.; Vurro, D.; von Zeppelin, D.; Iannotta, S.; et al. 3D printed masks for powders and viruses safety protection using food grade polymers: Empirical tests. Polymers 2021, 13, 617. [Google Scholar] [CrossRef] [PubMed]
- Germain, L.; Fuentes, C.A.; van Vuure, A.W.; des Rieux, A.; Dupont-Gillain, C. 3D-printed biodegradable gyroid scaffolds for tissue engineering applications. Mater. Des. 2018, 151, 113–122. [Google Scholar] [CrossRef]
- Zhao, H.; Li, L.; Ding, S.; Liu, C.; Ai, J. Effect of porous structure and pore size on mechanical strength of 3D-printed comby scaffolds. Mater. Lett. 2018, 223, 21–24. [Google Scholar] [CrossRef]
- Richbourg, N.R.; Peppas, N.A.; Sikavitsas, V.I. Tuning the biomimetic behavior of scaffolds for regenerative medicine through surface modifications. J. Tissue Eng. Regen. Med. 2019, 13, 1275–1293. [Google Scholar] [CrossRef]
- Chu, P.K.; Chen, J.Y.; Wang, L.P.; Huang, N. Plasma-surface modification of biomaterials. Mater. Sci. Eng. R Rep. 2002, 36, 143–206. [Google Scholar] [CrossRef]
- Croll, T.I.; O’Connor, A.J.; Stevens, G.W.; Cooper-White, J.J. Controllable surface modification of poly(lactic-co-glycolic acid) (PLGA) by hydrolysis or aminolysis I: Physical, chemical, and theoretical aspects. Biomacromolecules 2004, 5, 463–473. [Google Scholar] [CrossRef]
- De Jong, S.J.; Arias, E.R.; Rijkers, D.T.S.; Van Nostrum, C.F.; Kettenes-Van Den Bosch, J.J.; Hennink, W.E. New insights into the hydrolytic degradation of poly(lactic acid): Participation of the alcohol terminus. Polymers 2001, 42, 2795–2802. [Google Scholar] [CrossRef]
- Guo, C.; Xiang, M.; Dong, Y. Surface modification of poly (lactic acid) with an improved alkali-acid hydrolysis method. Mater. Lett. 2015, 140, 144–147. [Google Scholar] [CrossRef]
- Nam, Y.S.; Yoon, J.J.; Lee, J.G.; Park, T.G. Adhesion behaviours of hepatocytes cultured onto biodegradable polymer surface modified by alkali hydrolysis process. J. Biomater. Sci. Polym. Ed. 1999, 10, 1145–1158. [Google Scholar] [PubMed]
- Shao, J.; Chen, S.; Du, C. Citric acid modification of PLLA nano-fibrous scaffolds to enhance cellular adhesion, proliferation and osteogenic differentiation. J. Mater. Chem. B 2015, 3, 5291. [Google Scholar] [CrossRef]
- Lao, L.; Tan, H.; Wang, Y.; Gao, C. Chitosan modified poly(l-lactide) microspheres as cell microcarriers for cartilage tissue engineering. Colloids Surf. B Biointerfaces 2008, 66, 218–225. [Google Scholar] [CrossRef]
- Martin, V.; Ribeiro, I.A.; Alves, M.M.; Gonçalves, L.; Claudio, R.A.; Grenho, L.; Fernandes, M.H.; Gomes, P.; Santos, C.F.; Bettencourt, A.F. Engineering a multifunctional 3D-printed PLA-collagen-minocycline-nanoHydroxyapatite scaffold with combined antimicrobial and osteogenic effects for bone regeneration. Mater. Sci. Eng. C 2019, 101, 15–26. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Preferential adsorption of cell adhesive proteins from complex media on self-assembled monolayers and its effect on subsequent cell adhesion. Acta Biomater. 2015, 26, 72–81. [Google Scholar] [CrossRef]
- Li, B.; Ma, Y.; Wang, S.; Moran, P.M. Influence of carboxyl group density on neuron cell attachment and differentiation behavior: Gradient-guided neurite outgrowth. Biomaterials 2005, 26, 4956–4963. [Google Scholar] [CrossRef]
- Chen, W.; Nichols, L.; Brinkley, F.; Bohna, K.; Tian, W.; Priddy, M.W.; Priddy, L.B. Alkali treatment facilitates functional nano-hydroxyapatite coating of 3D printed polylactic acid scaffolds. Mater. Sci. Eng. C 2021, 120, 111686. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Sutera, A.; Botta, L.; Fontana, R.M.; Gallo, G. Plasma modified PLA electrospun membranes for actinorhodin production intensification in Streptomyces coelicolor immobilized-cell cultivations. Colloids Surf. B Biointerfaces 2017, 157, 233–241. [Google Scholar] [CrossRef]
- Nakagawa, M.; Teraoka, F.; Fujimoto, S.; Hamada, Y.; Kibayashi, H.; Takahashi, J. Improvement of cell adhesion on poly(L-lactide) by atmospheric plasma treatment. J. Biomed. Mater. Res. Part A 2006, 77, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Jordá-Vilaplana, A.; Fombuena, V.; García-García, D.; Samper, M.D.; Sánchez-Nácher, L. Surface modification of polylactic acid (PLA) by air atmospheric plasma treatment. Eur. Polym. J. 2014, 58, 23–33. [Google Scholar] [CrossRef]
- Wang, M.; Favi, P.; Cheng, X.; Golshan, N.H.; Ziemer, K.S.; Keidar, M.; Webster, T.J. Cold atmospheric plasma (CAP) surface nanomodified 3D printed polylactic acid (PLA) scaffolds for bone regeneration. Acta Biomaterialia 2016, 46, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Shinbo, T.; Kanamori, T.; Wang, P.C.; Niwa, M.; Kawakami, H.; Nagaoka, S.; Hirakawa, K.; Kamiya, M. Surface modification of poly(L-lactic acid) affects initial cell attachment, cell morphology, and cell growth. J. Artif. Organs 2004, 7, 187–193. [Google Scholar] [CrossRef]
- Teixeira, B.N.; Aprile, P.; Mendonça, R.H.; Kelly, D.J.; da Silva Moreira Thiré, R.M. Evaluation of bone marrow stem cell response to PLA scaffolds manufactured by 3D printing and coated with polydopamine and type I collagen. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 37–49. [Google Scholar] [CrossRef]
- Moraczewski, K.; Stepczyńska, M.; Malinowski, R.; Rytlewski, P.; Jagodziński, B.; Zenkiewicz, M. Stability studies of plasma modification effects of polylactide and polycaprolactone surface layers. Appl. Surf. Sci. 2016, 377, 228–237. [Google Scholar] [CrossRef]
- Li, J.; Oh, K.; Yu, H. Surface rearrangements of oxygen plasma treated polystyrene: Surface dynamics and humidity effect. Chin. J. Polym. Sci. 2005, 23, 187–196. [Google Scholar] [CrossRef]
- Canal, C.; Molina, R.; Bertran, E.; Erra, P. Wettability, ageing and recovery process of plasma-treated polyamide 6. J. Adhes. Sci. Technol. 2004, 18, 1077–1089. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Wang, Y.K.; Chen, P.; Deng, S.B.; Ruan, R. Non-thermal plasma assisted polymer surface modification and synthesis: A review. Int. J. Agric. Biol. Eng. 2014, 7, 1–9. [Google Scholar]
- Durán, I.R.; Vanslambrouck, S.; Chevallier, P.; Hoesli, C.A.; Laroche, G. Atmospheric pressure cold plasma versus wet-chemical surface treatments for carboxyl functionalization of polylactic acid: A first step toward covalent immobilization of bioactive molecules. Colloids Surf. B Biointerfaces 2020, 189, 110847. [Google Scholar] [CrossRef] [PubMed]
- Gibeop, N.; Lee, D.W.; Prasad, C.V.; Toru, F.; Kim, B.S.; Song, J. Il Effect of plasma treatment on mechanical properties of jute fiber/poly (lactic acid) biodegradable composites. Adv. Compos. Mater. 2013, 22, 389–399. [Google Scholar] [CrossRef]
- Denes, F.S.; Manolache, S. Macromolecular plasma-chemistry: An emerging field of polymer science. Prog. Polym. Sci. 2004, 29, 815–885. [Google Scholar] [CrossRef]
- Uchida, E.; Uyama, Y.; Ikada, Y. Sorption of low-molecular-weight anions into thin polycation layers grafted onto a film. Langmuir 1993, 9, 1121–1124. [Google Scholar] [CrossRef]
- Liu, Y.; He, T.; Gao, C. Surface modification of poly(ethylene terephthalate) via hydrolysis and layer-by-layer assembly of chitosan and chondroitin sulfate to construct cytocompatible layer for human endothelial cells. Colloids Surf. B Biointerfaces 2005, 46, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Garlotta, D. A literature review of poly(lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Tsuji, H.; Muramatsu, H. Blends of aliphatic polyesters: V. Non-enzymatic and enzymatic hydrolysis of blends from hydrophobic poly(L-lactide) and hydrophilic poly(vinyl alcohol). Polym. Degrad. Stab. 2001, 71, 403–413. [Google Scholar] [CrossRef]
- Yang, G.H.; Kim, M.; Kim, G. Additive-manufactured polycaprolactone scaffold consisting of innovatively designed microsized spiral struts for hard tissue regeneration. Biofabrication 2017, 9, 015005. [Google Scholar] [CrossRef]
- Vesel, A.; Mozetic, M. New developments in surface functionalization of polymers using controlled plasma treatments. J. Phys. D Appl. Phys. 2017, 50, 293001. [Google Scholar] [CrossRef]
- Ouellette, R.J.; Rawn, J.D. Nucleophilic Substitution and Elimination Reactions. In Organic Chemistry Study Guide; Elsevier: Amsterdam, The Netherlands, 2015; pp. 169–182. [Google Scholar]
- Yip, J.; Chan, K.; Sin, K.M.; Lau, K.S. Comprehensive study of polymer fiber surface modifications Part 2: Low-temperature oxygen-plasma treatment. Polym. Int. 2004, 53, 634–639. [Google Scholar] [CrossRef]
- Ye, P.; Wan, R.B.; Wang, X.P. Quantitative enzyme immobilization: Control of the carboxyl group density on support surface. J. Mol. Catal. B Enzym. 2009, 61, 296–302. [Google Scholar] [CrossRef]
- Hegyesi, N.; Zhang, Y.; Kohári, A.; Polyák, P.; Sui, X.; Pukánszky, B. Enzymatic degradation of PLA/cellulose nanocrystal composites. Ind. Crop. Prod. 2019, 141, 111799. [Google Scholar] [CrossRef]
- Tsuji, H.; Ishida, T. Poly(l-lactide). X. Enhanced surface hydrophilicity and chain-scission mechanisms of poly(l-lactide) film in enzymatic, alkaline, and phosphate-buffered solutions. J. Appl. Polym. Sci. 2002, 87, 1628–1633. [Google Scholar] [CrossRef]
- Araque-Monrós, M.C.; Vidaurre, A.; Gil-Santos, L.; Gironés Bernabé, S.; Monleón-Pradas, M.; Más-Estellés, J. Study of the degradation of a new PLA braided biomaterial in buffer phosphate saline, basic and acid media, intended for the regeneration of tendons and ligaments. Polym. Degrad. Stab. 2013, 98, 1563–1570. [Google Scholar] [CrossRef]
- Schiller, C.; Epple, M. Carbonated calcium phosphates are suitable pH-stabilising fillers for biodegradable polyesters. Biomaterials 2003, 24, 2037–2043. [Google Scholar] [CrossRef]
- Niaza, K.V.; Senatov, F.S.; Kaloshkin, S.D.; Maksimkin, A.V.; Chukov, D.I. 3D-printed scaffolds based on PLA/HA nanocomposites for trabecular bone reconstruction. J. Phys. Conf. Ser. 2016, 741, 012068. [Google Scholar] [CrossRef]
- Rafiee, M.; Farahani, R.D.; Therriault, D. Multi-Material 3D and 4D Printing: A Survey. Adv. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Foresti, R.; Rossi, S.; Selleri, S. Bio composite materials: Nano functionalization of 4D bio engineered scaffold. In Proceedings of the 2019 IEEE International Conference on BioPhotonics, Taipei, Taiwan, 15–18 September 2019. [Google Scholar]
- Chen, S.; Guo, Y.; Liu, R.; Wu, S.; Fang, J.; Huang, B.; Li, Z.; Chen, Z.; Chen, Z. Tuning surface properties of bone biomaterials to manipulate osteoblastic cell adhesion and the signaling pathways for the enhancement of early osseointegration. Colloids Surf. B Biointerfaces 2018, 164, 58–69. [Google Scholar] [CrossRef] [PubMed]



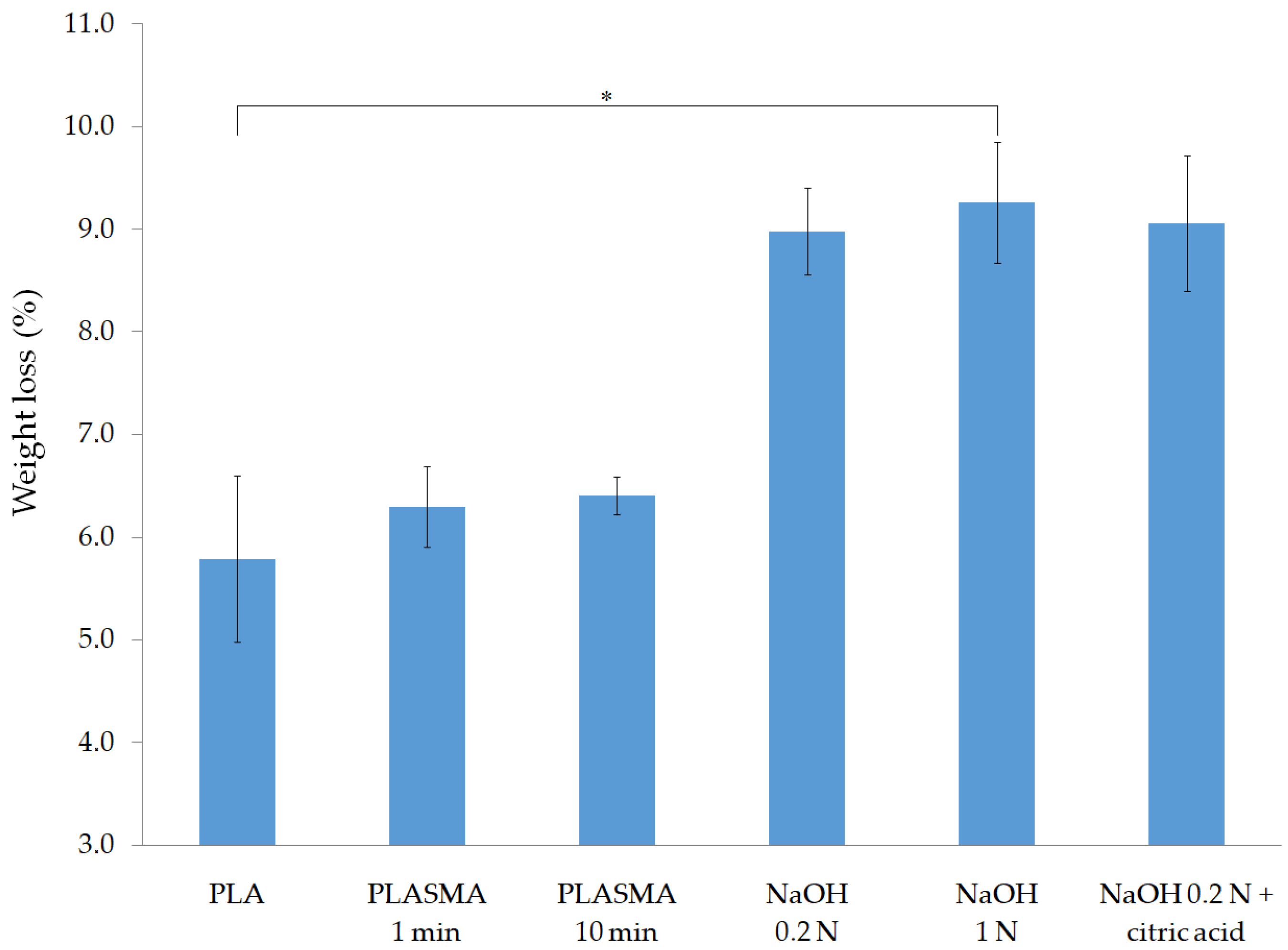

| Group of Samples | %Weight Loss |
|---|---|
| PLASMA 1 min | 0.01 ± 0.02 |
| PLASMA 10 min | 0.21 ± 0.02 |
| 0.2 N NaOH | 1.93 ± 0.11 |
| 1 N NaOH | 5.85 ± 0.25 1 |
| 0.2 N NaOH + citric acid | 2.08 ± 0.12 2 |
| (a) | |||||
| Group of Samples | Tg (°C) | Tonset (°C) | Tpeak (°C) | ΔH (J/g) | %Xc |
| PLA | 63.5 ± 1.2 | 167.0 ± 3.2 | 176.0 ± 0.1 | 47.0 ± 1.5 | 50.1 ± 1.6 |
| PLASMA 1 min | 64.2 ± 0.6 | 169.0 ± 0.1 | 175.6 ± 0.2 | 46.8 ± 1.5 | 50.0 ± 1.6 |
| PLASMA 10 min | 64.4 ± 0.5 | 169.3 ± 0.2 | 175.6 ± 0.1 2 | 49.5 ± 1.1 | 52.9 ± 1.2 |
| 0.2 N NaOH | 61.5 ± 0.6 | 169.7 ± 1.2 | 175.9 ± 0.1 | 50.5 ± 1.4 3 | 53.9 ± 2.4 4 |
| 1 N NaOH | 60.3 ± 0.6 1 | 169.5 ± 1.2 | 175.7 ± 0.1 | 50.3 ± 1.4 | 53.6 ± 2.7 |
| 0.2 NaOH + citric acid | 63.4 ± 0.6 | 164.8 ± 1.2 | 175.7 ± 0.1 | 48.8 ± 1.4 | 52.1 ± 2.1 |
| (b) | |||||
| Group of Samples | Tg (°C) | Tonset (°C) | Tpeak (°C) | ΔH (J/g) | %Xc |
| PLA | 63.5 ± 1.2 | 167.0 ± 3.2 | 176.0 ± 0.1 | 47.0 ± 1.5 | 50.1 ± 1.6 |
| PLASMA 1 min | 63.9 ± 0.2 | 169.2 ± 0.2 | 176.3 ± 0.1 * | 47.1 ± 3.0 | 49.6 ± 2.1 |
| PLASMA 10 min | 63.8 ± 0.4 | 169.3 ± 0.3 | 176.0 ± 0.1 * | 47.8 ± 2.9 | 51.0 ± 3.1 |
| 0.2 N NaOH | 63.4 ± 0.4 * | 167.8 ± 2.3 | 176.1 ± 0.1 * | 50.9 ± 0.9 | 54.3 ± 0.9 |
| 1 N NaOH | 63.2 ± 0.9 * | 168.0 ± 2.4 | 176.0 ± 0.1 * | 46.4 ± 2.0 * | 49.6 ± 2.1 |
| 0.2 NaOH + citric acid | 63.5 ± 0.2 | 166.0 ± 2.3 | 175.8 ± 0.1 1 | 49.2 ± 2.8 | 52.5 ± 3.0 |
| Group of Samples | Initial Porosity (%) | Final Porosity (%) | Elastic Modulus (MPa) | Compressive Yield Strength (MPa) | Compression Strength (MPa) | Strain at Maximum Strength |
|---|---|---|---|---|---|---|
| RC (compression test) | 83.6 ± 7.9 | 7.2 ± 1.0 | - | - | ||
| PLA | 55.5 ± 2.9 | 58.0 ± 3.0 | 81.0 ± 10.7 | 7.0 ± 1.5 | 9.7 ± 2.0 | 0.24 ± 0.07 |
| PLASMA 1 min | 56.0 ± 2.5 | 59.0 ± 2.1 | 72.7 ± 4.9 | 6.5 ± 1.7 | 9.9 ± 2.9 | 0.27 ± 0.04 |
| PLASMA 10 min | 56.1 ± 1.6 | 58.8 ± 1.5 | 73.7 ± 13.3 | 6.6 ± 1.4 | 9.2 ± 1.9 | 0.24 ± 0.03 |
| NaOH 0.2 N | 55.9 ± 1.8 | 60.1 ± 1.7 * | 69.2 ± 13.5 | 6.9 ± 1.5 | 8.9 ± 0.9 | 0.23 ± 0.04 |
| NaOH 1 N | 58.8 ± 0.9 | 61.7 ± 1.6 * | 67.7 ± 8.4 | 6.0 ± 0.8 | 8.4 ± 1.1 | 0.25 ± 0.01 |
| NaOH 0.2 N + citric acid | 56.4 ± 2.4 | 60.8 ± 2.3 | 63.2 ± 7.5 | 5.2 ± 0.8 | 8.2 ± 1.8 | 0.26 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donate, R.; Alemán-Domínguez, M.E.; Monzón, M. On the Effectiveness of Oxygen Plasma and Alkali Surface Treatments to Modify the Properties of Polylactic Acid Scaffolds. Polymers 2021, 13, 1643. https://doi.org/10.3390/polym13101643
Donate R, Alemán-Domínguez ME, Monzón M. On the Effectiveness of Oxygen Plasma and Alkali Surface Treatments to Modify the Properties of Polylactic Acid Scaffolds. Polymers. 2021; 13(10):1643. https://doi.org/10.3390/polym13101643
Chicago/Turabian StyleDonate, Ricardo, María Elena Alemán-Domínguez, and Mario Monzón. 2021. "On the Effectiveness of Oxygen Plasma and Alkali Surface Treatments to Modify the Properties of Polylactic Acid Scaffolds" Polymers 13, no. 10: 1643. https://doi.org/10.3390/polym13101643
APA StyleDonate, R., Alemán-Domínguez, M. E., & Monzón, M. (2021). On the Effectiveness of Oxygen Plasma and Alkali Surface Treatments to Modify the Properties of Polylactic Acid Scaffolds. Polymers, 13(10), 1643. https://doi.org/10.3390/polym13101643







