1. Introduction
The ratio of energy production coming from renewable sources is constantly increasing and, by its aleatory nature, it requires efficient storage solutions. Another important factor to be considered is the explosion of the electric vehicles market, with a request for performances at least equivalent to the ones of fossil fueled vehicles. From these considerations, the need for batteries with more and more energy and power density arises. To this day, Li-ion batteries are the ones meeting the widest market and range of utilization [
1]. However, this technology is reaching its theoretical values and it will not be able to sustain the challenges of future applications, mainly in terms of energy density. On the other hand, while far from the market, Li-O
2 batteries represent a valid alternative by combining the low density and highly reactive metallic Li to the abundant, low-cost, and environmentally friendly O
2, thus allowing to reach a theoretical specific energy as high as 3582 Wh kg
−1 [
2]. Proof of the reversibility of this system was demonstrated in the early 2000s using an organic liquid electrolyte and a MnO
2-based catalyst [
3]. Since then, many efforts have been made to further understand this chemistry and solve its issues to render it more stable and safer. Indeed, one of the drawbacks of this technology is linked to the use of metallic Li, which, upon battery recharge, tends to be inhomogeneously redeposited at the anode, thus forming so-called dendrites, 3D needles which can either break down creating “dead Li” and deplete the active material, thus causing capacity loss, or grow to pierce the separator and short-circuit the cell, leading to hazards of thermal runaway and even explosion accidents [
4]. Another problematic issue is the O
2 cross-over, meaning that the excess of unreacted O
2 at the cathode dissolves in the liquid electrolyte and goes to react directly with metallic Li, causing the formation of a passivating film on the anode surface, once again hampering the cell safety [
5]. Last but not least, in an ideal Li-O
2 open battery, the use of traditional liquid electrolytes would cause additional issues, such as solvent evaporation, leakage, and flammability [
4].
A common solution to these issues consists in the creation of a physical barrier blocking dendrite growth and penetration as well as O
2 cross-over. Such barrier can either be a functionalized separator or a solid electrolyte [
5,
6,
7]. The replacement of liquid electrolytes with solid ones does not change the fundamental reaction of the Li-O
2 batteries, which is still the well-known formation and decomposition of Li
2O
2. However, only a few works about ceramic-based Li-O
2 batteries have been reported because of the difficulty in establishing a tri-phase (O
2/e
−/Li
+) reaction between the cathode and a ceramic-based electrolyte [
8,
9,
10]. Moreover, solid state electrolytes with low conductivity at room temperature require an elevated operating temperature of the cells, which aggravates the side-reactions and the safety hazards [
4]. Therefore, in the last few years, multifunctional polymer-based electrolytes for quasi-solid-state Li-O
2 batteries have been thoroughly studied [
8]. However, polymer electrolytes share a common issue with ceramic electrolytes, which is their high interfacial resistance and low ionic conductivity, both limiting their practical applications at ambient temperature [
11,
12]. On the other hand, gel polymer electrolytes (GPEs), composed of liquid electrolytes entrapped in polymer matrices, have been successfully used for Li-ion battery applications due to their low interfacial resistances and high ionic conductivity [
11,
13,
14]. GPEs with different polymer-solvent combinations have been developed in Li-O
2 batteries and they have been shown to efficiently protect Li anode from oxygen cross-over, as well as limit electrolyte evaporation [
15,
16,
17]. Furthermore, the incorporation of fillers, organic or inorganic, to both polymer and liquid electrolytes has been shown to improve the Li
+ transport properties, such as Li-ion transference number and ionic conductivity, mainly through their interactions with the polymer, solvent, or salt [
11,
18,
19,
20]. One particularly interesting organic filler is the dextrin-based nanosponge. Dextrin-based nanosponges (NS) are hyper-crosslinked polymers characterized by the ability to encapsulate a great variety of substances in the liquid phase. More importantly, dextrin-based NS are nanostructured within a three-dimensional network. Our group already reported the use of NS encapsulated in polymer matrices for different applications [
21,
22].
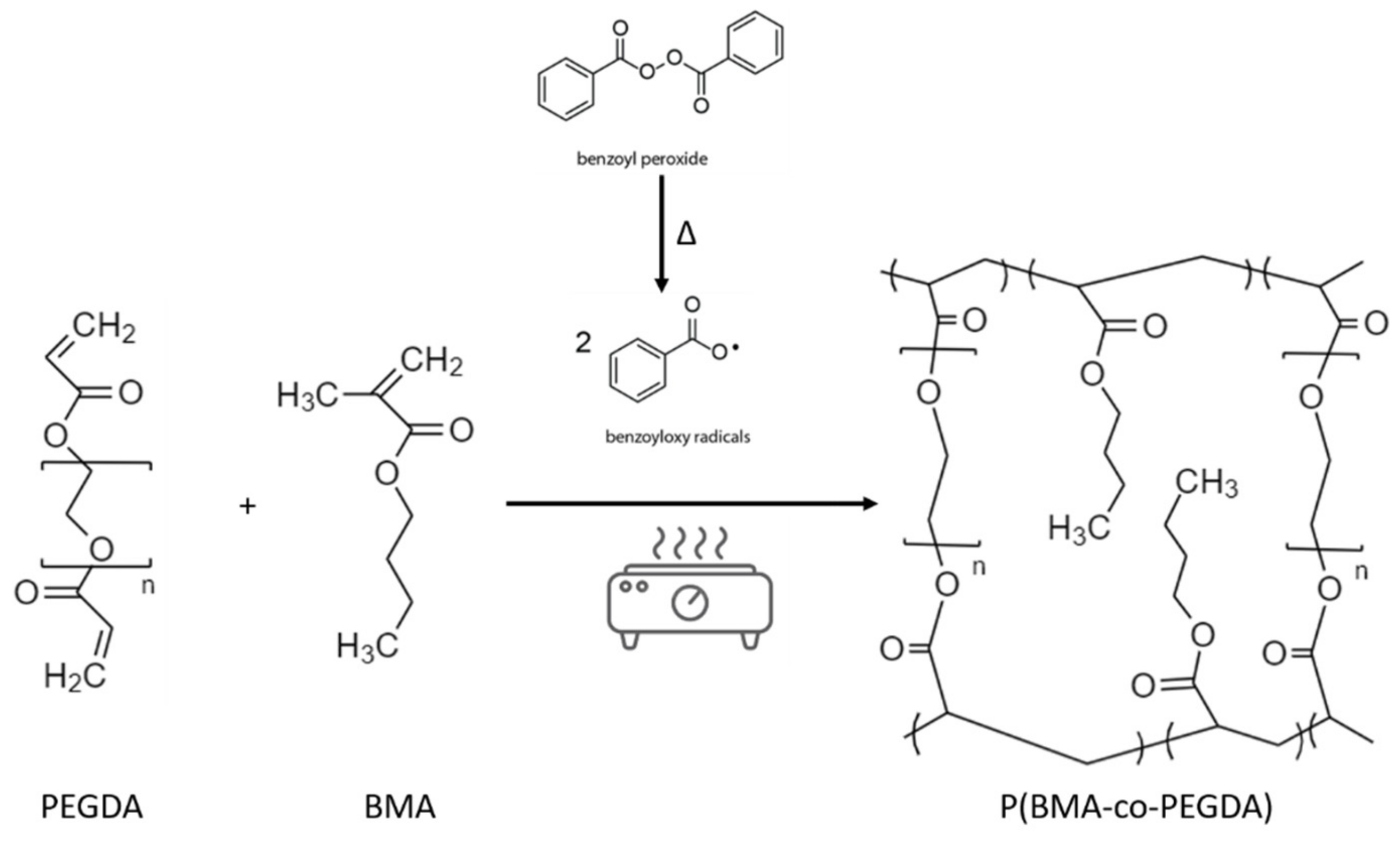
In this work, we report the preparation of a methacrylate-based polymer matrix by a solvent-free, thermally induced, radical polymerization, encompassing NS and swollen in liquid electrolyte in order to obtain a composite gel polymer electrolyte (CGPE). This simple process allowed us to obtain a highly cross-linked CGPE with good mechanical properties and numerous long polymer chains rich of ethoxy groups, favoring Li-ions conduction. Additionally, the integration of NS as an additive permits effectively reducing the O2 cross-over upon the electrolyte, thus stabilizing the metallic Li interface while increasing the O2 content at the cathode side and improving reactions kinetics, allowing better cyclability and greatly improving the cell safety.
3. Results and Discussion
The P(BMA-co-PEGDA) membranes were synthesized by a radical copolymerization of BMA and PEGDA (see
Figure 1), allowing to obtain a highly cross-linked polymer matrix. Both monomer and polymer were chosen first for their characteristics, in particular the presence of numerous ethoxy groups, playing a crucial role in Li-ions conduction and second, for their ability to be cross-linked together. Indeed, a cross-linked polymer matrix confers a more robust character to polymer electrolytes, thus helping reduce Li dendrite permeation and the associated safety hazards [
24]. The as obtained membranes were successively activated in liquid electrolyte and renamed CGPE.
Pictures of the obtained CGPEs with and without NS are reported in
Figure 2a–c. The CGPE without NS was perfectly transparent, while the other two were homogeneously white, demonstrating macroscopically the homogeneous distribution of the NS in the polymer matrix in both cases. All the CGPEs were reticulated onto a glass-slide and then peeled off, being perfectly self-standing. The morphology of the different CGPEs was further studied by FESEM analysis both in top-view and cross-section modes. Pictures of the CGPE without NS (
Figure 2d,g) demonstrate a globular structure, probably due to bubbles forming during the thermal treatment. Indeed, BMA possesses a low evaporation point and probably begins forming gases during the polymerization. Such a phenomenon did not seem to appear in the formulations containing NS; on the contrary, the surfaces of the CGPE containing 5 wt% (
Figure 2e) and 10 wt% (
Figure 2f) of NS were smooth and homogeneous. However, while the cross-section of the CGPE containing 5 wt% (
Figure 2h) of NS appeared homogeneous and regular, the one of the CGPE containing 10 wt% (
Figure 2i) presented a porous structure caused by structural inhomogeneities. The CGPEs’ thicknesses, as measured on cross-sections, were 25 µm without NS (
Figure 2g), 40 µm with 5 wt% of NS (
Figure 2h), and 85 µm with 10 wt% of NS (
Figure 2i).
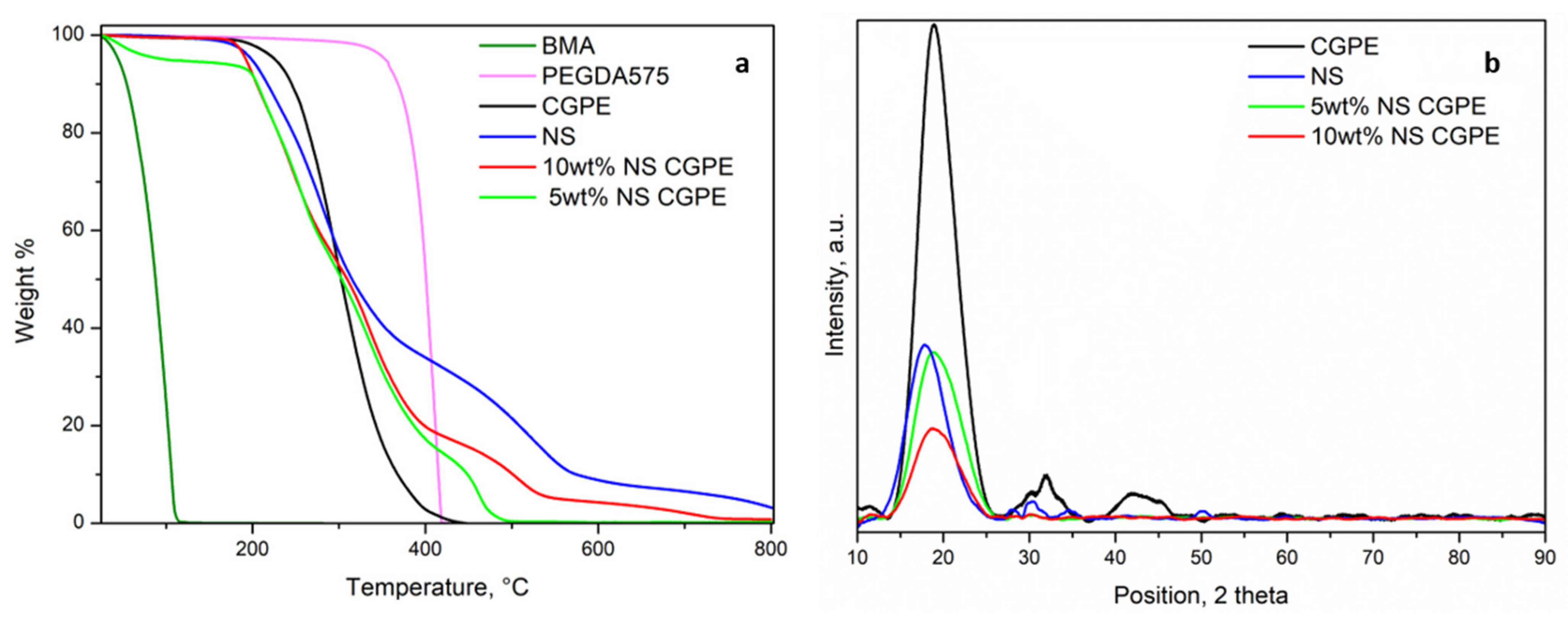
The thermal stability of the CGPEs was investigated by TGA and compared to the analysis of each component of the precursor solution; results are reported in
Figure 3. While the BMA monomer starts evaporating at around 100 °C (T
eb = 163 °C), PEGDA was thermally stable up to 400 °C. NS showed a more complicated profile with different weight losses (200 °C, 400 °C, and 600 °C) (see
Table S1). The CGPE without NS started degrading around 230 °C, therefore between BMA and PEGDA, and without any intermediary plateau, thus demonstrating a complete polymerization. Both CGPEs containing NS started degrading a little earlier (around 200 °C), precisely following the degradation profile of pure NS. This phenomenon can be explained by their effect on the increase of amorphous fraction in the polymer matrix, as discussed in the following paragraph [
25]. These results indicate that CGPEs containing NS can be used as a safe and reliable solid electrolyte separating the anode and cathode even at elevated temperatures, which could greatly improve the safety of the Li-O
2 battery [
26].
Crystalline phases in polymer electrolytes represent an obstacle to Li-ions conduction as they block the segmental motion of polymer chains. Therefore, XRD analysis was performed on the different CGPEs and on the NS additive to assess this characteristic (
Figure 3b). The CGPE without additive showed a pronounced peak at 2θ = 20° and two smaller peaks at 30° and 45°, indicating a semi-crystalline nature. In particular, the shape of the first most intense peak reflects the ordered packing of polymer chains while the second and third peaks denote the ordering inside the main chains, at different ranges [
27]. The NS powder showed a single peak around 2θ = 20°, which was therefore superposed to the one of the polymer matrix. This superposition does not allow us to draw any definitive conclusion regarding the presence of crystalline regions in the CGPEs containing NS additives. However, the intensity of the peak in the samples with 5 wt% and 10 wt% of NS, being lower than that of the polymer matrix without additive, suggests an overall decrease of crystallinity in the samples containing NS. In fact, the absence of crystalline phases, hinting to a highly amorphous matrix, has been demonstrated to not only be beneficial to ionic conductivity, as previously explained, but also to a more homogeneous Li plating [
24].
Additionally to the crystalline nature of the polymer matrix, the liquid electrolyte uptake is another very important factor to ensure a good ionic conductivity inside the CGPE. The results obtained (see
Table 1) show a much higher uptake using NS additives, demonstrating their good compatibility with the liquid electrolyte, in our case LiTFSI 0.5 M in DMSO. Moreover, as previously demonstrated, cross-linked polymer matrices containing additives show a better capability in retaining liquid electrolyte over time when compared to traditional glass fiber separators [
28].
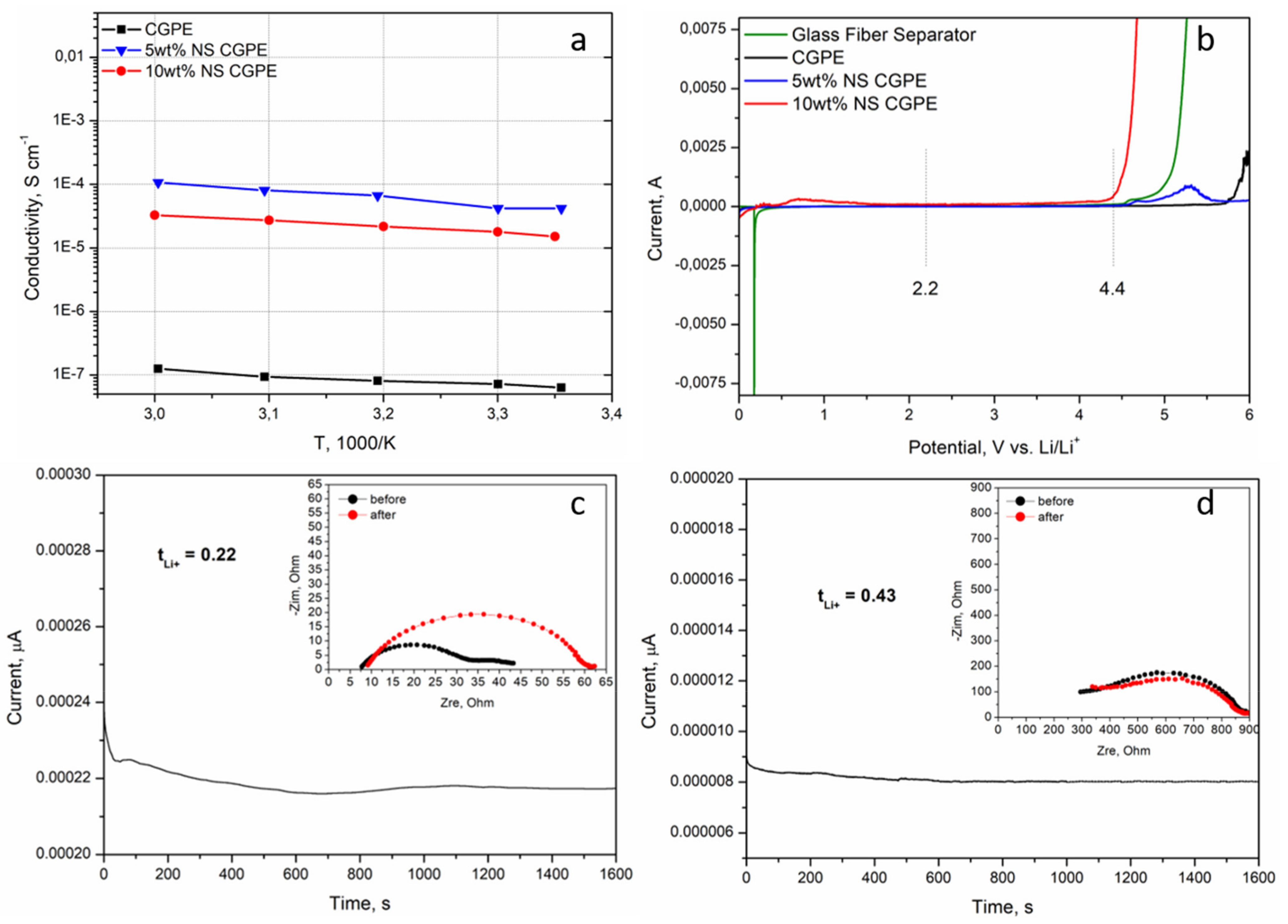
In order to experimentally verify the influence of the two previously discussed parameters, ionic conductivities of the CGPE without NS and of the two CGPEs containing NS were assessed by EIS from room temperature to 60 °C. The results are reported in
Figure 4a. In all cases, the conductivity increased with temperature, demonstrating that the increase of temperature leads to a faster movement of the polymer chains and, therefore, easier Li-ion transport [
26]. The polymer matrix without additive had the lowest conductivity as could be predicted from the XRD (
Figure 3b); indeed crystalline portions partially block the movement of polymer chains, hampering Li-ion transport. The highest ionic conductivity of the CGPE containing 5 wt% of NS can be attributed to two reasons. Firstly, BMA was cross-linked with PEGDA and modified with NS, which significantly reduced the crystallinity of the cross-linked polymer (
Figure 3b), thus accelerating the transport of Li-ions inside the electrolyte [
28]. Secondly, the as-modified CGPE was able to retain far more liquid electrolyte (
Table 1), helping Li-ion conduction through a mixed mechanism between polymer chain motions and classic Li-ion diffusion in a liquid electrolyte.
The decomposition of the electrolyte at upper voltage is a serious issue affecting both the performance and the safety of the Li-O
2 battery. Hence, an electrolyte with stable electrochemical window has great significance for the stable operation of such system [
26]. The electrochemical windows of the CGPE without additive and of the CGPEs containing different NS contents were tested by LSV and compared to a glass fiber separator containing liquid electrolyte; the results are reported in
Figure 4b. Interestingly, the CGPE without additive was the one demonstrating the largest stability window, up to 5.5 V. This can be explained by its semi-crystalline nature and low electrolyte uptake, both reducing the possibilities of side reactions with Li. Even if slightly narrower, the stable electrochemical window of the CGPE containing 5 wt% NS can ensure its integrity during battery operation for this specific application (namely between 2.25 V and 4.4 V). Given its limited stability window, the CGPE containing 10 wt% NS was not further considered in this work.
t
Li+ is an important parameter for electrolytes, indeed the higher the t
Li+, the better it can mitigate the anion accumulation around the electrode/electrolyte interface by alleviating the concentration polarization [
29]. Indeed, such polarization causes concentration gradients at the electrode/electrolyte interface, which have been demonstrated in literature to be one of the principal causes of Li dendrite nucleation and growth, thus causing capacity fading and, more importantly, high instability and low safety of the cells [
30,
31]. The t
Li+ value of the 5 wt% NS CGPE was assessed by a combination of potential polarization and EIS at room temperature (
Figure 4d) and compared to the one of a commercial glass fiber separator impregnated with liquid electrolyte (
Figure 4c). The results show that the t
Li+ value of the 5 wt% NS CGPE was almost twice that of the liquid electrolyte on the glass fiber separator. This phenomenon is attributed to the synergy between the composite polymer matrix and the liquid electrolyte, which have a very positive effect on Li-ion migration. The compatibility of the 5 wt% NS CGPE with metallic Li negative electrode was enhanced; furthermore, the transition of the crystalline phase of the polymer matrix alone, leading to the increase of the amorphous region, greatly reduces the Li-ion migration resistance [
26]. In addition, such t
Li+ values are in line with previously reported ones for GPEs [
11,
28].
One important issue of the Li-O
2 technology is the so-called O
2 cross-over. In particular, O
2 gets dissolved in the liquid electrolyte and comes into contact with the Li metal anode, thus causing its oxidation and successive passivation, leading to capacity fading and an eventual safety hazard. To assess the effect of NS additive on blocking O
2, permeation measurements were performed on the CGPEs with and without NS. The results are reported in
Table 2 and show an O
2 permeation reduced by 80% in the membrane containing NS, indeed confirming the blocking role of NS and its benefit in enhancing cell safety.
The interfacial stability of the electrolyte with Li anode was further elucidated by galvanostatic Li plating/stripping on symmetric Li|5 wt% NS CGPE|Li cells. For comparison, symmetric Li|LE|Li cells were simultaneously tested, where LE indicates the liquid electrolyte (LiTFSI 0.5 M in DMSO) in the commercial glass fiber separator, to assess LE compatibility with Li metal at different current densities. A first test was carried out at 0.3 mA cm
−2 with a limited capacity of 0.3 mAh cm
−2 on symmetric cells containing LE, CGPE without NS, and CGPE with 5 wt% NS, respectively. The first five cycles are reported in
Figure S2 and show that, while the cell containing the LE and the one containing the CGPE with 5 wt% NS presented a stable profile with relatively low polarization, the cell containing the CGPE without NS presented severe fluctuations with a large voltage polarization, implying Li dendrite growth from the first cycles on the Li metal surface [
26]. The test was repeated on cells containing the LE and the 5 wt% NS-based CGPE at a higher current density of 0.5 mA cm
−2 and a limited capacity of 0.5 mAh cm
−2; the obtained profiles are shown in
Figure 5.
The symmetric cell containing the 5 wt% NS CGPE shows high overpotential in the first cycles, which is speculated to be attributed to the preconditioning of the cell [
32]; then, the voltage feedback becomes smooth and stable at a relatively low polarization voltage, showing excellent cycling stability and demonstrating that the growth of Li dendrites can be inhibited to a certain extent. This improvement can be attributed to the stabilization of the electrolyte-electrode interface [
11]. In contrast, the interfacial resistance of the liquid electrolyte-based cell kept increasing from the first cycles on, which indicates both the decomposition of liquid electrolyte and the constant formation of a SEI layer, and the accumulation of dead Li, up to the 14th cycle where the cell was failing [
4,
8]. The reason for the lower polarization of the cell containing the 5 wt% NS-based CGPE after 35 cycles compared to the one of the LE cell at the 14th cycle can be attributed to the improved t
Li+ (see
Figure 4c,d), contributing to a lowered concentration polarization in the 5 wt% NS-based CGPE [
28]. This result demonstrates that high ionic conductivity and t
Li+ improvement effectively stabilized the Li interface by promoting uniform Li plating/stripping and stable interfacial layers, hence enhancing the cell safety.
Galvanostatic discharge tests (
Figure 6a), from OCV to 2.25 V vs. Li/Li
+, were performed to evaluate the full discharge capacity of a cell containing the 5 wt% NS CGPE compared to a cell containing liquid electrolyte in a commercial glass fiber separator (referred to as STD), at a current intensity of 0.025 mA cm
−2. The full cells were assembled using a simple, uncatalyzed, commercial GDL as a cathode. Prior to measurements, Li-O
2 cells rested 6 h at OCV under O
2 flow. Results show that the cell containing the 5 wt% NS CGPE was able to discharge for a longer time compared to the STD one, reaching an areal capacity as high as 5.05 mAh cm
−2 in the first case, against 4.44 mAh cm
−2 in the second one. As shown in
Figure 6a, the potential of the STD cell abruptly plummeted at around 4.4 mAh cm
−2, probably because of extended Li passivation due to O
2 cross-over and irregular Li plating and striping. In particular, in literature, noticeable amounts of Li
2CO
3 have been associated to the oxidation of DMSO in long discharge conditions, and excess of O
2, in particular at the anode side, has been considered a critical factor in driving such chemical and electrochemical side reactions [
33,
34]. Hence, in these conditions, both anode and electrolyte consumption could be responsible for the potential drop in the STD cell.
To investigate the cyclability of cells, galvanostatic cycling tests were carried out by limiting the initial full discharge capacity (based on the STD cell performance) to 20% and the potential to 2.25 V in discharge and 4.4 V (vs. Li/Li+) in charge, at the constant current density of 0.025 mA cm−2. During the tests, cells were continuously purged with dry O2 at a flow rate of 3.0 mL min−1. Prior to measurements, the Li-O2 cells rested 6 h at OCV under O2 flow.
Figure 6b reports the voltage vs. capacity plot of the second cycle in the STD cell (solid black line) and in the cell containing the 5 wt% NS CGPE (dotted red line). In the last one, the decrease of the charging voltage plateau indicates that lower amounts and more reversible side products were formed at the cathode during the previous discharge, thus allowing an easier conversion during the next recharge, which therefore took place at lower potentials. Such results support the assumption that DMSO decomposition occurs at both electrodes, but mainly at the Li/electrolyte interphase [
35]. This seems to confirm that the 5 wt% NS CGPE can effectively relieve, to some extent, such side reactions. This fact is further verified looking at
Figure 6c,d, respectively, reporting charge/discharge capacities and Coulombic efficiency for the STD cell and the cell containing the 5 wt% NS CGPE. Indeed, after the third cycle, in the STD cell, the charge capacity drops under the discharge capacity, meaning that the system was not able to fully re-convert the discharge products under the cut-off potential of 4.4 V, thus provoking an accumulation at the surface of the electrodes. Such a phenomenon was not verified in the cell containing the 5 wt% NS CGPE, where the discharge and charge capacity values were very stable over the first 10 cycles. Another important fact to keep in mind is that constant O
2 flow leads to the intense evaporation of LE in the system [
36]. Compared to LE, the 5 wt% NS CGPE almost doubles the t
Li+ value and decreases the recharge potential, thus enhancing the cycle stability.
These observations agree with previous studies, showing that electrolytic properties in Li-O
2 batteries, such as ionic conductivity and t
Li+, play a significant role in Li-O
2 battery behavior both in terms of cyclability and safety [
11].
DMSO has been widely studied as an electrolyte solvent for Li-O
2 cells and some papers report that it promotes the formation of flake-like agglomerates, apart from toroids, on the cathode surface. Such agglomerates are considered to be mixed LiOH and Li
2O
2 nanocrystallites [
37] and they were particularly visible on the surface of the cathode cycled in the cell containing the 5 wt% NS CGPE (see
Figure 7b). As a matter of fact, the surface of the STD cell cathode seemed to be covered by a mainly amorphous layer (see
Figure 7a), while the deposit on the membrane cell cathode seemed much more crystalline (see
Figure 7b). It is important to keep in mind that the cycling time used for this study was very long (50 h/cycle), thus allowing the degradation of some byproducts and the formation of amorphous species.
This result was confirmed by the XRD analysis of the same cathodes (see
Figure 7c). Indeed, the spectrum of the cathode from the cell containing 5 wt% NS CGPE showed the typical peaks of crystalline Li
2O
2, while they could not be seen on the spectrum of the STD cell cathode. In particular, the diffraction peaks at 32.9° and 35.0° are attributed to the (100) and (101) crystal planes of Li
2O
2 [
11,
38].
Interestingly, crystalline LiOH could be found on both cathodes; this can be explained by the fact that, while the use of DMSO solvent in Li-O
2 cells electrolytes can stabilize the soluble superoxide intermediates, such process is usually accompanied by side reactions, resulting in the formation of additional discharge byproducts aside from Li
2O
2 [
39]. Possible reactions provoked by O
2− are illustrated in Equations (4) and (5) [
37]:
Comparing the morphology of both cathodes’ surfaces, we can conclude that the accumulation of mainly amorphous and poorly reversible side products onto the STD cell cathode surface provoked pore clogging, limiting O2 diffusion, and poor electronic contact between active material and carbon matrix, thus explaining poorer rechargeability. In contrast, crystalline deposits onto the 5 wt% NS CGPE cell cathode seemed to maintain the porosity of the cathode, allowing better oxygen flow and easier electron exchange. A hypothesis concerning morphology difference could regard the better O2 retention at the cathode surface, thanks to the blocking action of the 5 wt% NS CGPE, thus allowing better reaction kinetics and therefore better cyclability and higher safety.