Dynamics and Structure Formation of Confined Polymer Thin Films Supported on Solid Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
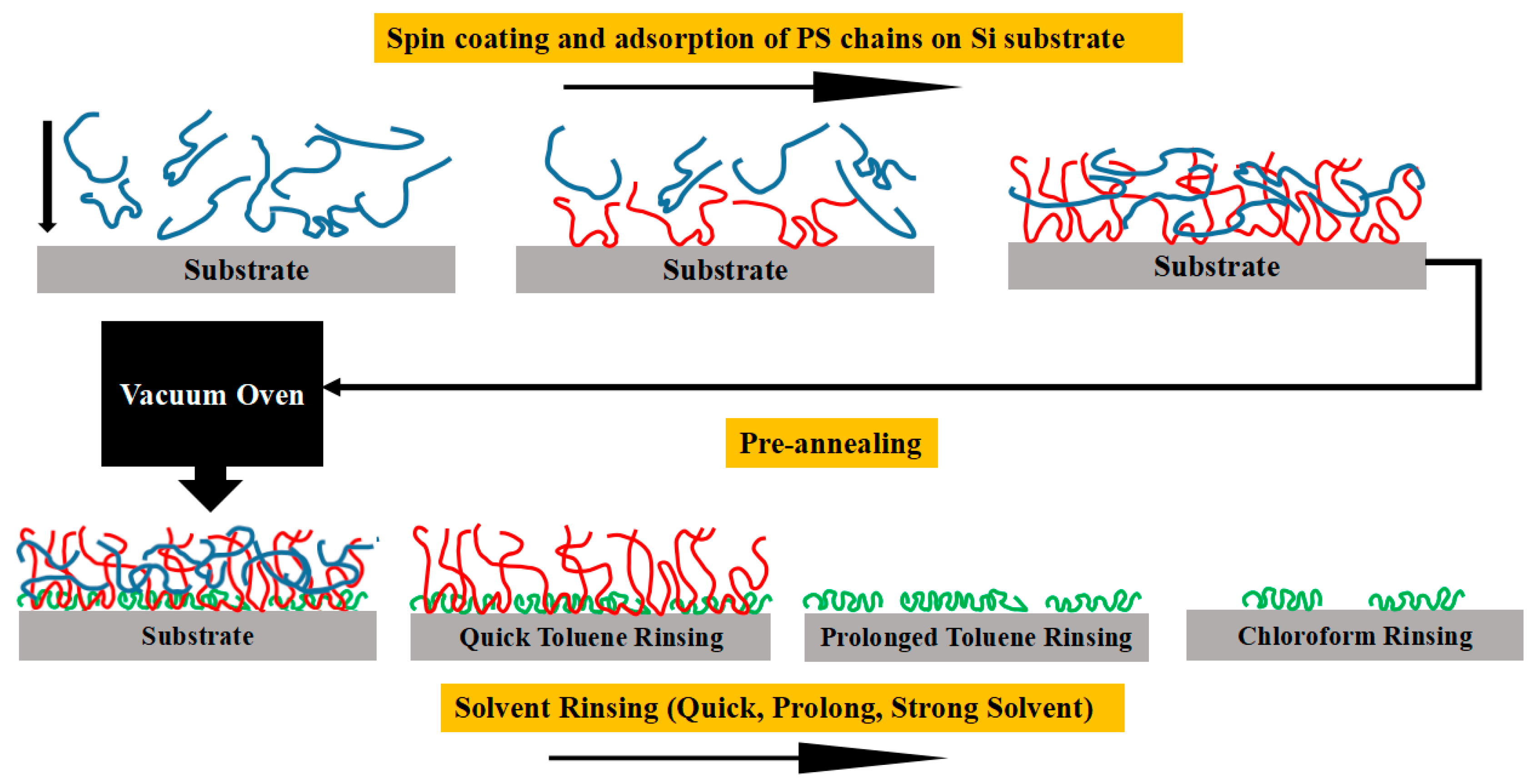
2.2. Sample Preparation
2.2.1. Chemical Treatment for Substrate Cleaning
2.2.2. Spin Coating
2.2.3. Solvent Rinsing
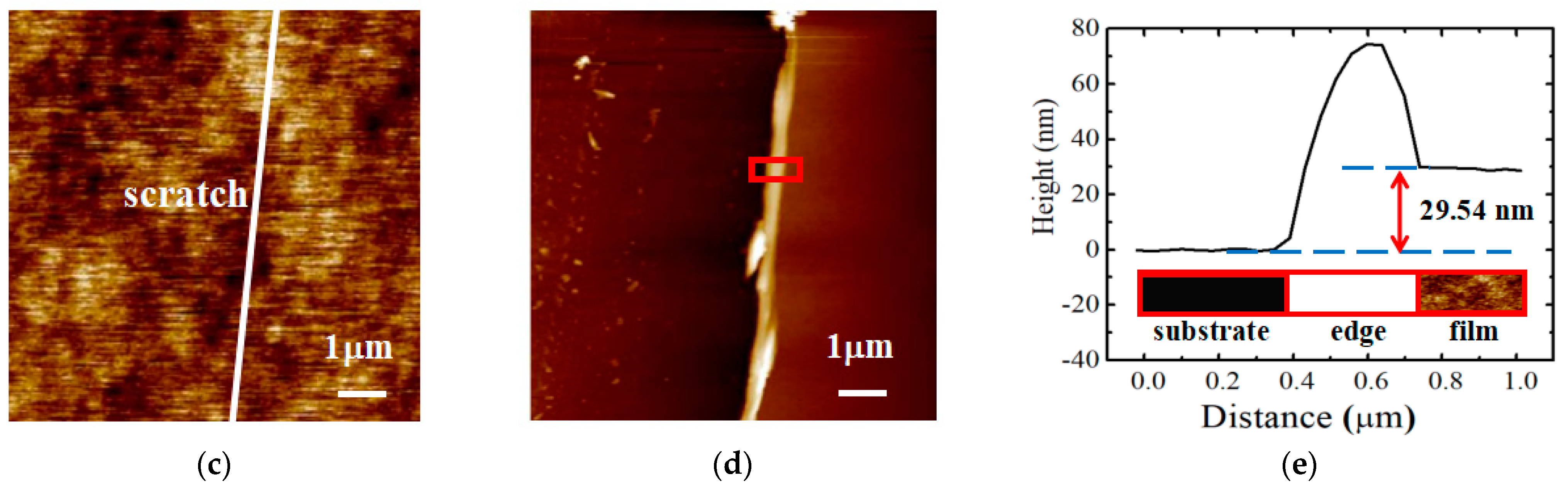
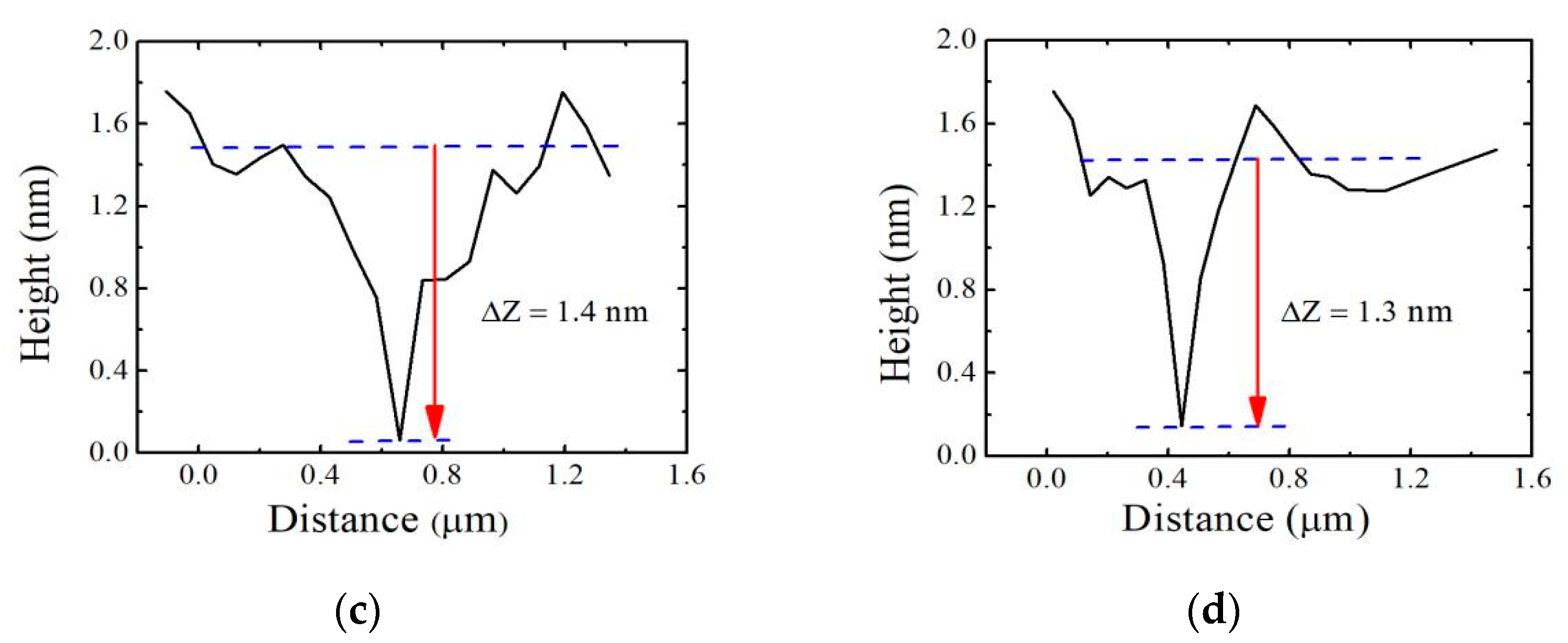
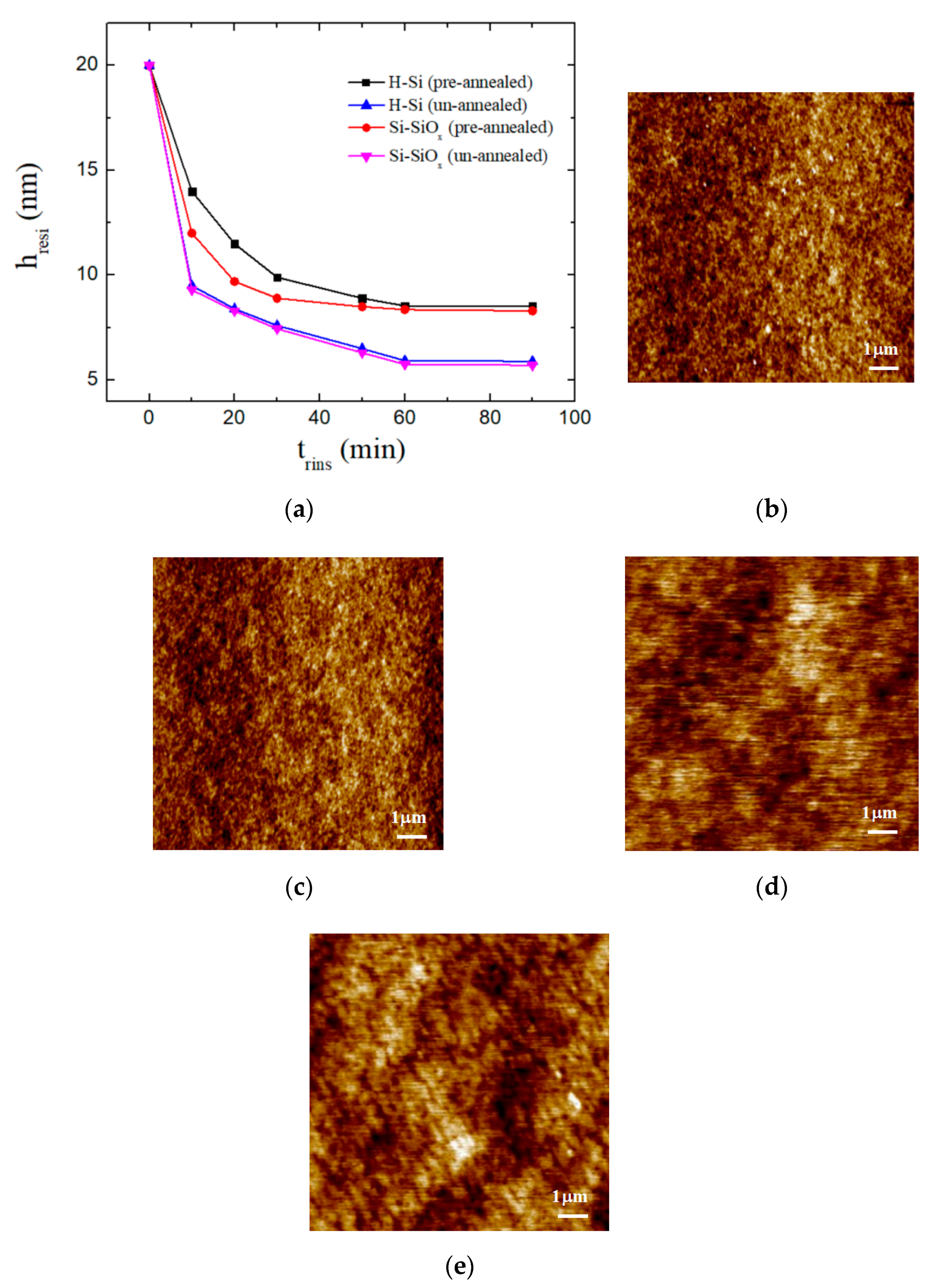
2.3. Characterization
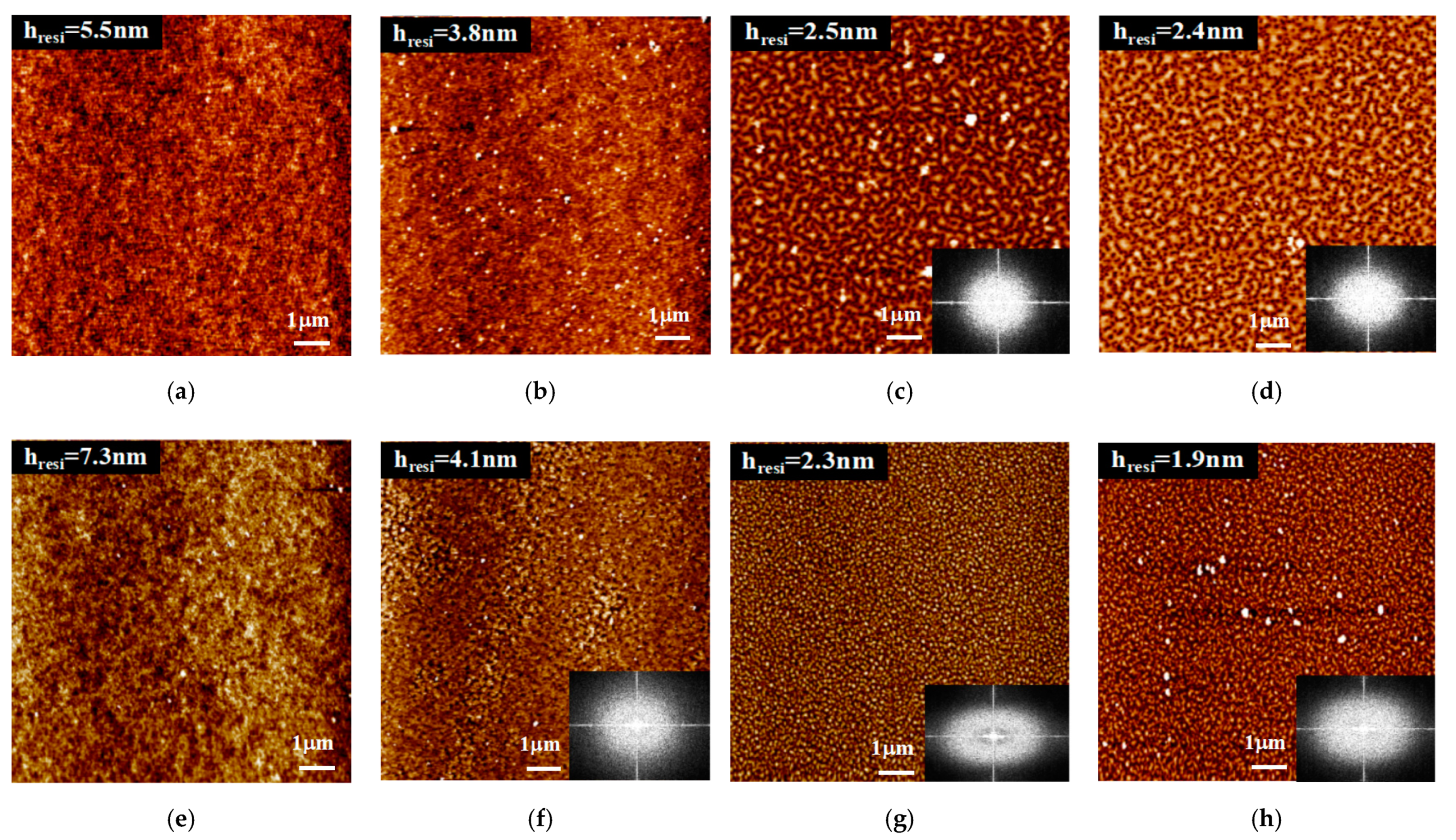
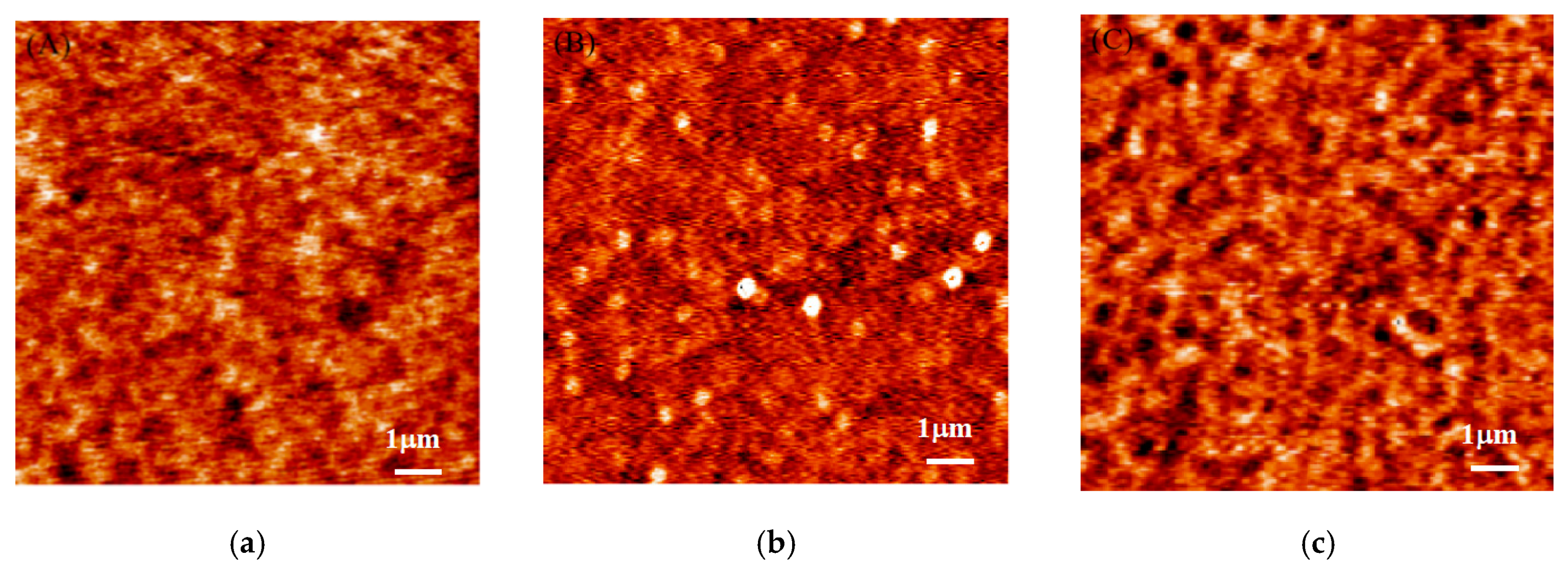
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Forrest, J.A.; Dalnoki-Veress, K.; Dutcher, J.R. Interface and chain confinement effects on the glass transition temperature of thin polymer films. Phys. Rev. E 1997, 56, 5705–5716. [Google Scholar] [CrossRef] [Green Version]
- Malafronte, A.; Auriemma, F.; Santillo, C.; Girolamo, R.D.; Barker, R.; Gerelli, Y.; Rosa, C.D. Block copolymers-based nanoporous thin films with tailored morphology for biomolecules adsorption. Adv. Mater. Interfaces 2020, 7, 1901580. [Google Scholar] [CrossRef]
- Nieswandt, K.; Georgopanos, P.; Aberz, V. Well-defined polyvinylpyridine-block-polystyrene diblock copolymers via RAFT aqueous-alcoholic dispersion polymerization: Synthesis and isoporous thin film morphology. Polym. Chem. 2021, 12, 2210–2221. [Google Scholar] [CrossRef]
- Lu, K.Y.; Lo, T.Y.; Georgopanos, P.; Avgeropoulos, A.; Shi, A.C.; Ho, R.M. Orienting silicon-containing block copolymer films with perpendicular cylinders via entropy and surface plasma treatment. Macromolecules 2017, 50, 9403–9410. [Google Scholar] [CrossRef]
- Segalman, R.A. Patterning with block copolymer thin films. Mater. Sci. Eng. R Rep. 2005, 48, 191–226. [Google Scholar] [CrossRef] [Green Version]
- Nieswandt, K.; Georgopanos, P.; Abetz, C.; Filiz, V.; Abetz, V. Synthesis of poly(3-vinylpyridine)-block-polystyrene diblock copolymers via surfactant-free RAFT emulsion polymerization. Materials 2019, 12, 3145. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, J.; Chu, C.-W.; Szmanda, C.R.; Ma, L.; Yang, Y. Programmable polymer thin film and non-volatile memory device. Nat. Mater. 2004, 3, 918–922. [Google Scholar] [CrossRef]
- Chen, F.; Peng, D.; Ogata, Y.; Tanaka, K.; Yang, Z.; Fujii, Y.; Yamada, N.; Lam, C.-H.; Tsui, O.K. Confinement effect on the effective viscosity of plasticized polymer films. Macromolecules 2015, 48, 7719–7726. [Google Scholar] [CrossRef]
- Huang, Q.; Yoon, I.; Villanueva, J.; Kim, K.; Sirbuly, D.J. Quantitative mechanical analysis of thin compressible polymer monolayers on oxide surfaces. Soft Matter 2014, 10, 8001–8010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Ma, P.X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef]
- Reiter, G.; De Gennes, P. Spin-cast, thin, glassy polymer films: Highly metastable forms of matter. Eur. Phys. J. E 2001, 6, 25–28. [Google Scholar] [CrossRef]
- Seemann, R.; Herminghaus, S.; Neto, C.; Schlagowski, S.; Podzimek, D.; Konrad, R.; Mantz, H.; Jacobs, K. Dynamics and structure formation in thin polymer melt films. J. Phys. Condens. Matter 2005, 17, S267. [Google Scholar] [CrossRef] [Green Version]
- Doi, M.; Edwards, S.F.; Edwards, S.F. The Theory of Polymer Dynamics; Oxford University Press: Oxford, UK, 1988; Volume 73. [Google Scholar]
- Wu, G.; Asai, S.; Zhang, C.; Miura, T.; Sumita, M. A delay of percolation time in carbon-black-filled conductive polymer composites. J. Appl. Phys. 2000, 88, 1480–1487. [Google Scholar] [CrossRef]
- Hub, C.; Harton, S.E.; Hunt, M.A.; Fink, R.; Ade, H. Influence of sample preparation and processing on observed glass transition temperatures of polymer nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 2270–2276. [Google Scholar] [CrossRef]
- Sharma, A. Relationship of thin film stability and morphology to macroscopic parameters of wetting in the apolar and polar systems. Langmuir 1993, 9, 861–869. [Google Scholar] [CrossRef]
- Reiter, G. Dewetting of thin polymer films. Phys. Rev. Lett. 1992, 68, 75. [Google Scholar] [CrossRef]
- Reiter, G. Unstable thin polymer films: Rupture and dewetting processes. Langmuir 1993, 9, 1344–1351. [Google Scholar] [CrossRef]
- Redon, C.; Brochard-Wyart, F.; Rondelez, F. Dynamics of dewetting. Phys. Rev. Lett. 1991, 66, 715. [Google Scholar] [CrossRef]
- Debrégeas, G.; Martin, P.; Brochard-Wyart, F. Viscous bursting of suspended films. Phys. Rev. Lett. 1995, 75, 3886. [Google Scholar] [CrossRef]
- Chandran, S.; Reiter, G. Segmental Rearrangements Relax Stresses in Non-equilibrated Polymer Films. ACS Macro Lett. 2019, 8, 646–650. [Google Scholar] [CrossRef]
- Xue, L.; Han, Y. Inhibition of Dewetting of Thin Polymer Films. Prog. Mater. Sci. 2012, 57, 947–979. [Google Scholar] [CrossRef]
- Napolitano, S.W. The Lifetime of The Deviations from Bulk Behaviour in Polymers Confined at The Nanoscale. Nat. Commun. 2011, 2, 1–7. [Google Scholar] [CrossRef]
- Koga, T.; Jiang, N.; Gin, P.; Endoh, M.; Narayanan, S.; Lurio, L.; Sinha, S. Impact of an irreversibly adsorbed layer on local viscosity of nanoconfined polymer melts. Phys. Rev. Lett. 2011, 107, 225901. [Google Scholar] [CrossRef]
- Jiang, N.; Endoh, M.K.; Koga, T. Structures and Dynamics of Adsorbed Polymer Nanolayers on Planar Solids. In Non-Equilibrium Phenomena in Confined Soft Matter; Springer: Cham, Switzerland, 2015; pp. 129–160. [Google Scholar]
- Guiselin, O. Irreversible adsorption of a concentrated polymer solution. EPL 1992, 17, 225–230. [Google Scholar] [CrossRef]
- Fujii, Y.; Yang, Z.; Leach, J.; Atarashi, H.; Tanaka, K.; Tsui, O.K.C. Affinity of polystyrene films to hydrogen-passivated silicon and its relevance to the Tg of the films. Macromolecules 2009, 42, 7418–7422. [Google Scholar] [CrossRef]
- Gin, P.; Jiang, N.; Liang, C.; Taniguchi, T.; Akgun, B.; Satija, S.K.; Endoh, M.K.; Koga, T. Revealed architectures of adsorbed polymer chains at solid-polymer melt interfaces. Phys. Rev. Lett. 2012, 109, 265501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Housmans, C.; Sferrazza, M.; Napolitano, S. Kinetics of irreversible chain adsorption. Macromolecules 2014, 47, 3390–3393. [Google Scholar] [CrossRef]
- Rotella, C.; Napolitano, S.; Vandendriessche, S.; Valev, V.K.; Verbiest, T.; Larkowska, M.; Kucharski, S.; Wubbenhorst, M. Adsorption kinetics of ultrathin polymer films in the melt probed by dielectric spectroscopy and second-harmonic generation. Langmuir 2011, 27, 13533–13538. [Google Scholar] [CrossRef] [PubMed]
- Bal, J.K.; Beuvier, T.; Unni, A.B.; Chavez Panduro, E.A.; Vignaud, G.; Delorme, N.; Chebil, M.S.; Grohens, Y.; Gibaud, A. Stability of polymer ultrathin films (<7 nm) made by a top-down approach. ACS Nano 2015, 9, 8184–8193. [Google Scholar] [CrossRef]
- Jiang, N.; Wang, J.; Di, X.; Cheung, J.; Zeng, W.; Endoh, M.K.; Koga, T.; Satija, S.K. Nanoscale adsorbed structures as a robust approach for tailoring polymer film stability. Soft Matter 2016, 12, 1801–1809. [Google Scholar] [CrossRef]
- Chen, F.; Takatsuji, K.; Zhao, D.; Yu, X.; Kumar, S.K.; Tsui, O.K. Unexpected thermal annealing effects on the viscosity of polymer nanocomposites. Soft Matter 2017, 13, 5341–5354. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Shang, J.; Di, X.; Endoh, M.K.; Koga, T. Formation mechanism of high-density, flattened polymer nanolayers adsorbed on planar solids. Macromolecules 2014, 47, 2682–2689. [Google Scholar] [CrossRef]
- Seemann, R.; Herminghaus, S.; Jacobs, K. Dewetting patterns and molecular forces: A reconciliation. Phys. Rev. Lett. 2001, 86, 5534–5537. [Google Scholar] [CrossRef]
- Yin, H.; Napolitano, S.; Schönhals, A. Molecular mobility and glass transition of thin films of poly (bisphenol a carbonate). Macromolecules 2012, 45, 1652–1662. [Google Scholar] [CrossRef]
- Braatz, M.-L.; Infantas Meléndez, L.; Sferrazza, M.; Napolitano, S. Unexpected impact of irreversible adsorption on thermal expansion: Adsorbed layers are not that dead. J. Chem. Phys. 2017, 146, 203304. [Google Scholar] [CrossRef]
- Vanroy, B.; Wübbenhorst, M.; Napolitano, S. Crystallization of thin polymer layers confined between two adsorbing walls. ACS Macro Lett. 2013, 2, 168–172. [Google Scholar] [CrossRef]
- Asada, M.; Jiang, N.; Sendogdular, L.; Sokolov, J.; Endoh, M.K.; Koga, T.; Fukuto, M.; Yang, L.; Akgun, B.; Dimitriou, M. Melt crystallization/dewetting of ultrathin PEO films via carbon dioxide annealing: The effects of polymer adsorbed layers. Soft Matter 2014, 10, 6392–6403. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Tong, D.E.; Cui, J.; Soccio, M.; García, C.; Ezquerra, T.A.; Nogales, A. Does the glass transition of polymers change upon 3D confinement? Macromol. Chem. Phys. 2014, 215, 1620–1624. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Peng, D.; Lam, C.-H.; Tsui, O.K. Viscosity and surface-promoted slippage of thin polymer films supported by a solid substrate. Macromolecules 2015, 48, 5034–5039. [Google Scholar] [CrossRef]
- Yang, Z.; Fujii, Y.; Lee, F.K.; Lam, C.-H.; Tsui, O.K. Glass transition dynamics and surface layer mobility in unentangled polystyrene films. Science 2010, 328, 1676–1679. [Google Scholar] [CrossRef] [Green Version]
- Fakhraai, Z.; Forrest, J. Measuring the surface dynamics of glassy polymers. Science 2008, 319, 600–604. [Google Scholar] [CrossRef]
- Delorme, N.; Chebil, M.S.; Vignaud, G.; Le Houerou, V.; Bardeau, J.F.; Busselez, R.; Gibaud, A.; Grohens, Y. Experimental evidence of ultrathin polymer film stratification by AFM force spectroscopy. Eur. Phys. J. E 2015, 38, 1–8. [Google Scholar] [CrossRef]
- Shin, K.; Hu, X.; Zheng, X.; Rafailovich, M.; Sokolov, J.; Zaitsev, V.; Schwarz, S. Silicon oxide surface as a substrate of polymer thin films. Macromolecules 2001, 34, 4993–4998. [Google Scholar] [CrossRef]
- O’Shaughnessy, B.; Vavylonis, D. Irreversible adsorption from dilute polymer solutions. Eur. Phys. J. E 2003, 11, 213–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcon, V.; van der Vegt, N.F. How does low-molecular-weight polystyrene dissolve: Osmotic swelling vs. surface dissolution. Soft Matter 2014, 10, 9059–9064. [Google Scholar] [CrossRef] [PubMed]
- Miller-Chou, B.A.; Koenig, J.L. A review of polymer dissolution. Prog. Polym. Sci. 2003, 28, 1223–1270. [Google Scholar] [CrossRef] [Green Version]
- You, J.; Zhang, S.; Huang, G.; Shi, T.; Li, Y. Solvent annealing induced phase separation and dewetting in PMMA/SAN blend film: Film thickness and solvent dependence. J. Chem. Phys. 2013, 138, 244907. [Google Scholar] [CrossRef]
- Wolterink, J.K.; Stuart, M.C.; Barkema, G. Desorption of polymers: Role of the stagnant layer. Mol. Phys. 2006, 104, 639–645. [Google Scholar] [CrossRef]
- García, M.T.; Gracia, I.; Duque, G.; de Lucas, A.; Rodríguez, J.F. Study of the solubility and stability of polystyrene wastes in a dissolution recycling process. Waste Manag. 2009, 29, 1814–1818. [Google Scholar] [CrossRef]
- Kim, D.-H.; Park, S.-J.; Choi, Y.-W.; Lee, S.-K. Optimization of solvent for the determination of polybrominated diphenyl ethers in high-impact polystyrene by GC/ECD. Bull. Korean Chem. Soc. 2012, 33, 3485–3488. [Google Scholar] [CrossRef] [Green Version]
- Sherrington, D.C. Preparation, structure and morphology of polymer supports. Chem. Commun. 1998, 21, 2275–2286. [Google Scholar] [CrossRef]
- O’Shaughnessy, B.; Vavylonis, D. Irreversibility and polymer adsorption. Phys. Rev. Lett. 2003, 90, 056103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unni, A.B.; Vignaud, G.; Chapel, J.; Giermanska, J.; Bal, J.; Delorme, N.; Beuvier, T.; Thomas, S.; Grohens, Y.; Gibaud, A. Probing the density variation of confined polymer thin films via simple model-independent nanoparticle adsorption. Macromolecules 2017, 50, 1027–1036. [Google Scholar] [CrossRef]
- Ton-That, C.; Shard, A.; Bradley, R. Thickness of spin-cast polymer thin films determined by angle-resolved XPS and AFM tip-scratch methods. Langmuir 2000, 16, 2281–2284. [Google Scholar] [CrossRef]
- Akabori, K.-I.; Tanaka, K.; Takahara, A.; Kajiyama, T.; Nagamura, T. Substrate effect on mechanical relaxation of polystyrene in ultrathin films. Eur. Phys. J. Spec. Top. 2007, 141, 173–180. [Google Scholar] [CrossRef]
- Xu, L.; Sharma, A.; Joo, S.W. Dewetting of stable thin polymer films induced by a poor solvent: Role of polar interactions. Macromolecules 2012, 45, 6628–6633. [Google Scholar] [CrossRef]
- Lee, S.H.; Yoo, P.J.; Kwon, S.J.; Lee, H.H. Solvent-driven dewetting and rim instability. J. Chem. Phys. 2004, 121, 4346–4351. [Google Scholar] [CrossRef]
- Williams, M.B.; Davis, S.H. Nonlinear theory of film rupture. J. Colloid Interface Sci. 1982, 90, 220–228. [Google Scholar] [CrossRef]
- Verma, A.; Sharma, A. Submicrometer pattern fabrication by intensification of instability in ultrathin polymer films under a water–solvent mix. Macromolecules 2011, 44, 4928–4935. [Google Scholar] [CrossRef] [Green Version]
- Jiang, N.; Cheung, J.; Guo, Y.; Endoh, M.K.; Koga, T.; Yuan, G.; Satija, S.K. Stability of adsorbed polystyrene nanolayers on silicon substrates. Macromol. Chem. Phys. 2018, 219, 1700326. [Google Scholar] [CrossRef]
- Thomas, K.R.; Chenneviere, A.; Reiter, G.; Steiner, U. Nonequilibrium behavior of thin polymer films. Phys. Rev. E 2011, 83, 021804. [Google Scholar] [CrossRef]
- Reiter, G. Dewetting as a probe of polymer mobility in thin films. Macromolecules 1994, 27, 3046–3052. [Google Scholar] [CrossRef]
- Wyart, F.B.; Martin, P.; Redon, C. Liquid/liquid dewetting. Langmuir 1993, 9, 3682–3690. [Google Scholar] [CrossRef]
- Masson, J.-L.; Green, P.F. Viscosity of entangled polystyrene thin film melts: Film thickness dependence. Phys. Rev. E 2002, 65, 031806. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.U.; Xi, Y.; Li, H.; Chen, F.; Liu, D.; Wei, J. Dynamics and Structure Formation of Confined Polymer Thin Films Supported on Solid Substrates. Polymers 2021, 13, 1621. https://doi.org/10.3390/polym13101621
Rahman MU, Xi Y, Li H, Chen F, Liu D, Wei J. Dynamics and Structure Formation of Confined Polymer Thin Films Supported on Solid Substrates. Polymers. 2021; 13(10):1621. https://doi.org/10.3390/polym13101621
Chicago/Turabian StyleRahman, Mujib Ur, Yonghao Xi, Haipeng Li, Fei Chen, Dongjie Liu, and Jinjia Wei. 2021. "Dynamics and Structure Formation of Confined Polymer Thin Films Supported on Solid Substrates" Polymers 13, no. 10: 1621. https://doi.org/10.3390/polym13101621
APA StyleRahman, M. U., Xi, Y., Li, H., Chen, F., Liu, D., & Wei, J. (2021). Dynamics and Structure Formation of Confined Polymer Thin Films Supported on Solid Substrates. Polymers, 13(10), 1621. https://doi.org/10.3390/polym13101621




