Synthesis of a New Polyanion Possessing Dense 1,2,3-Triazole Backbone
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
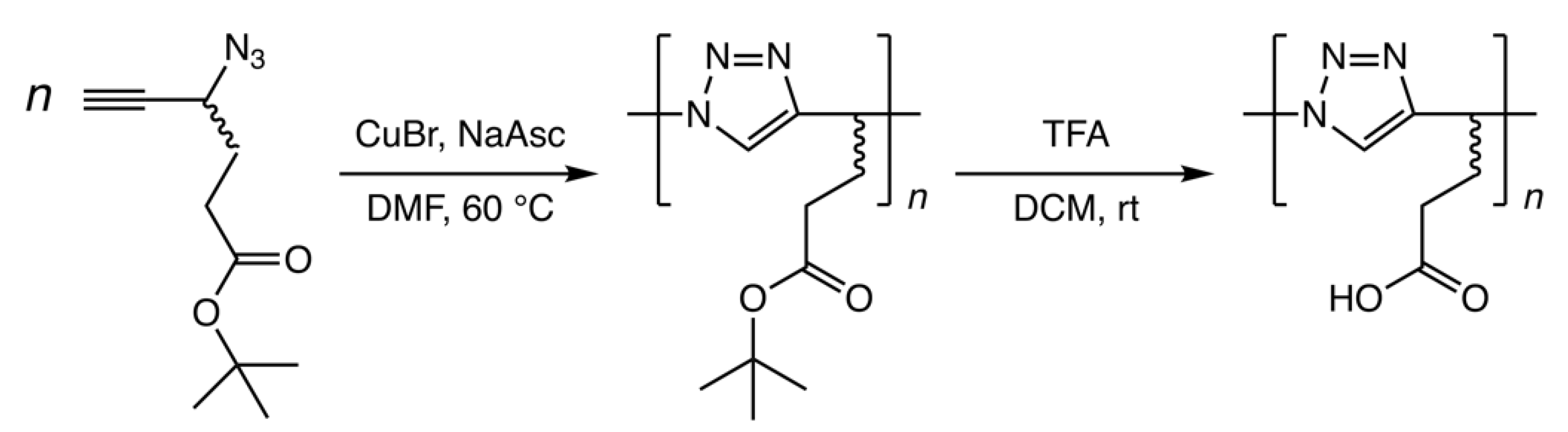
2.3. Preparation of Poly(4-azido-5-hexynoic acid) (Poly(AH))
2.4. Turbidimetric and Potentiometric Titrations
2.5. Adsorption Tests
3. Results and Discussion
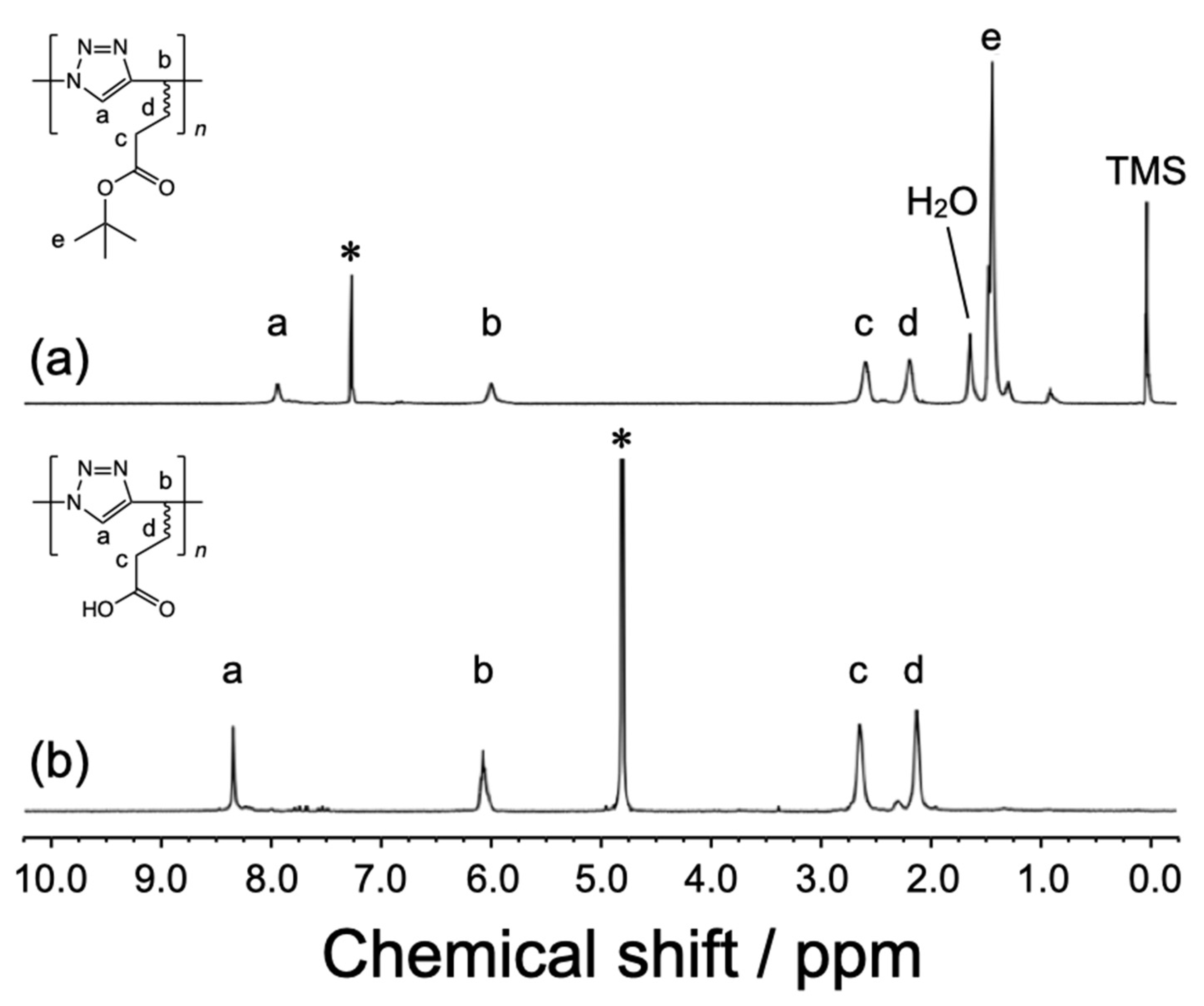
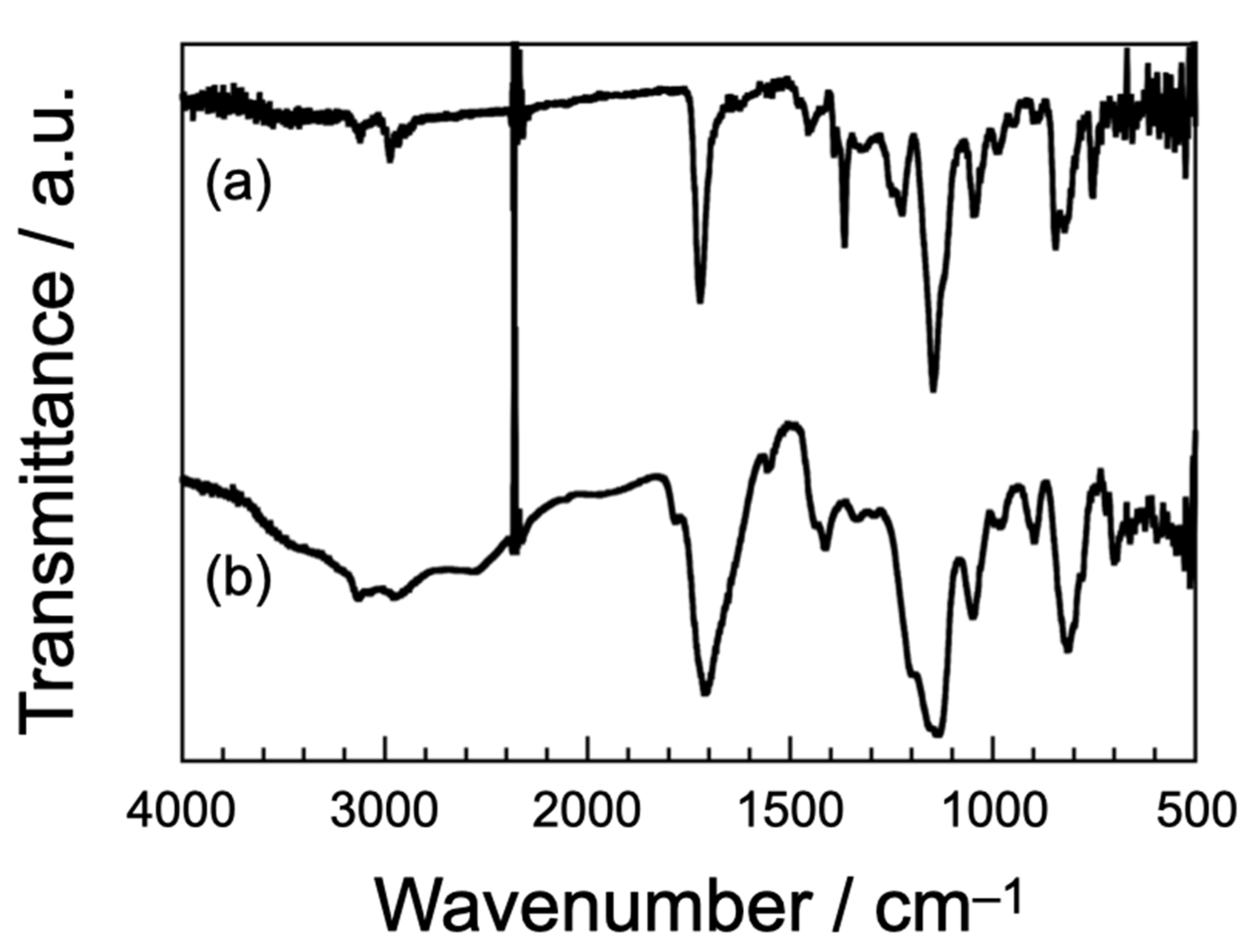
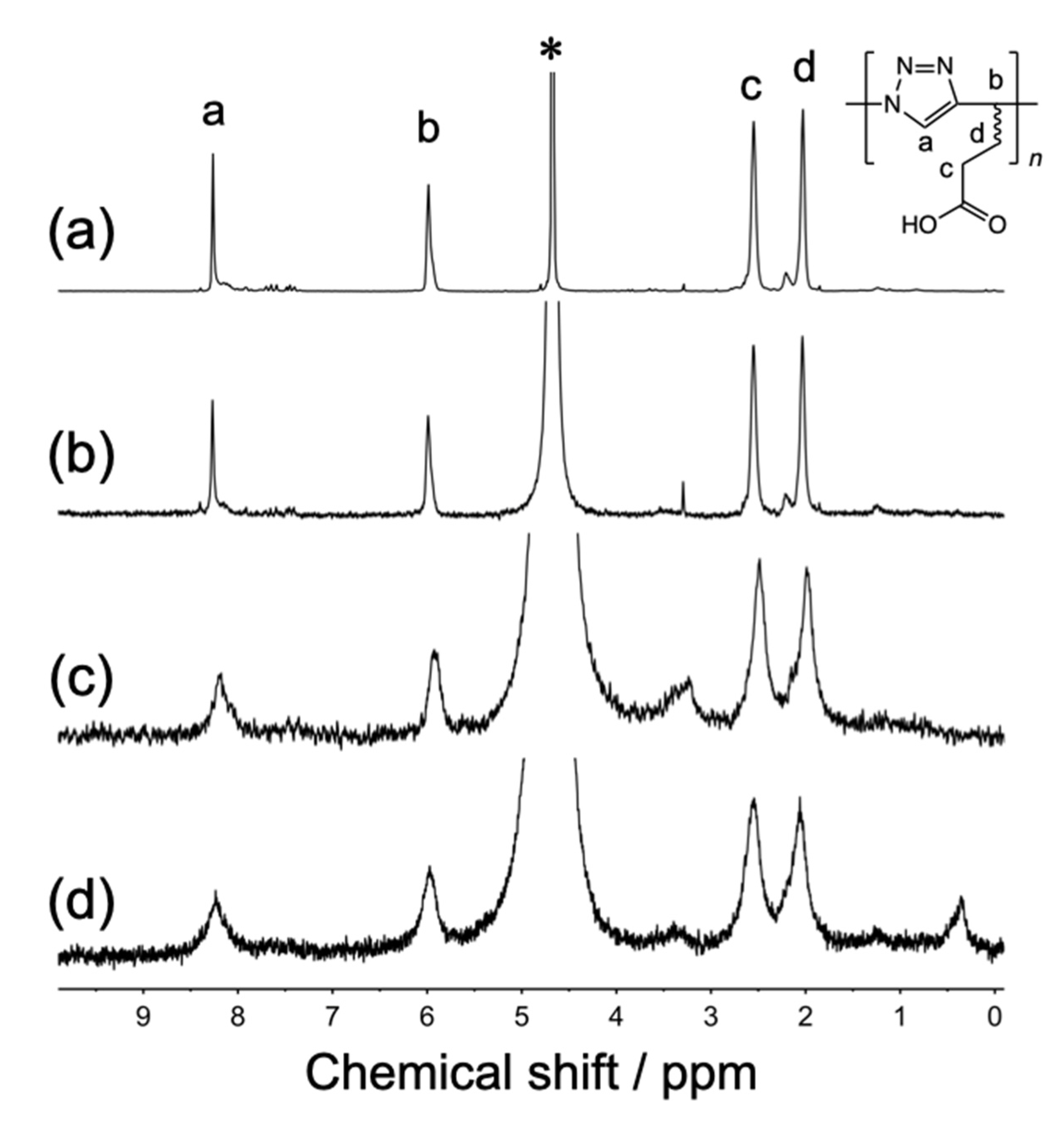
3.1. Synthesis and Structural Characterization of Poly(AH)
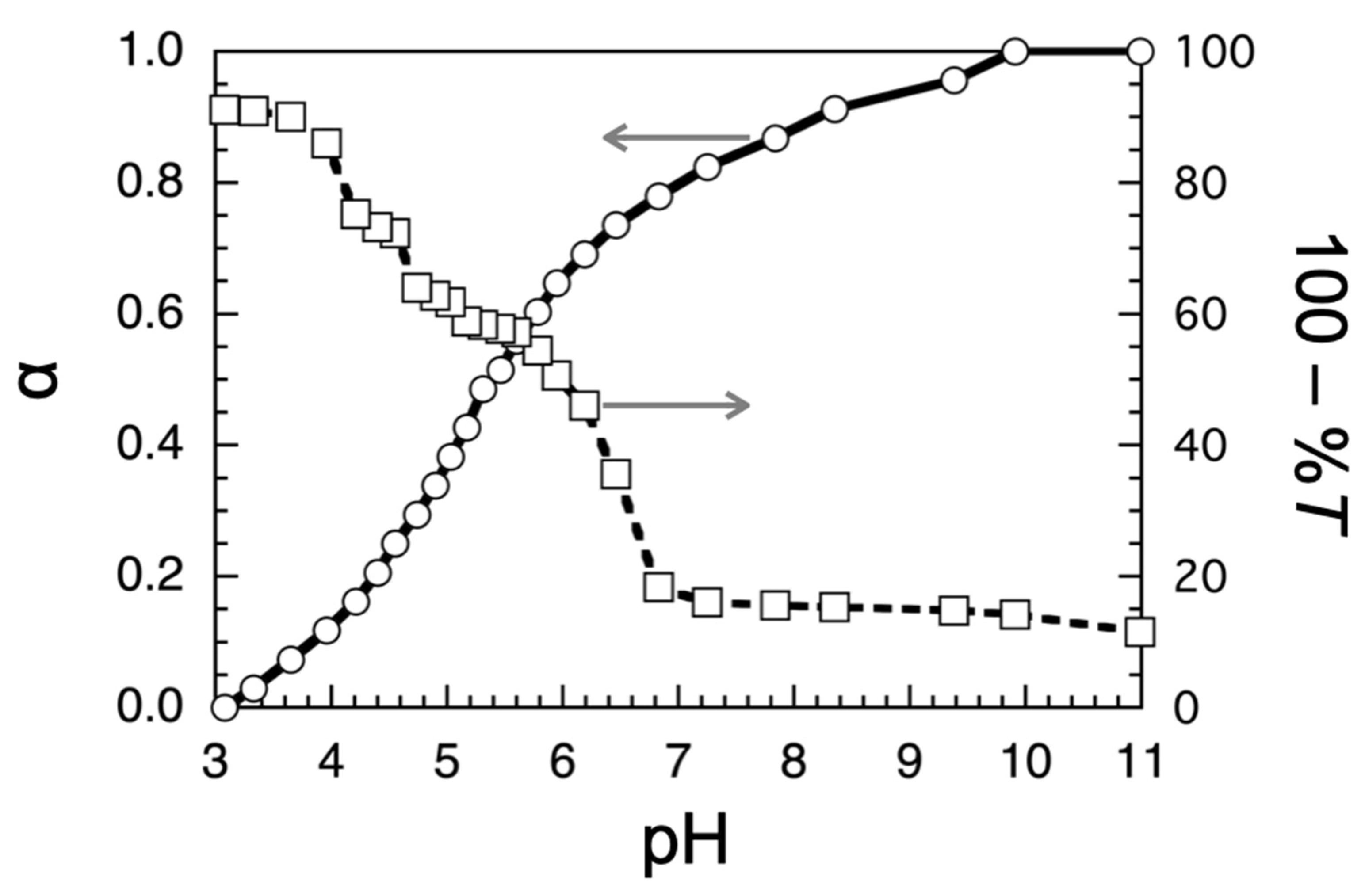
3.2. Turbidimetric and Potentiometric Titrations
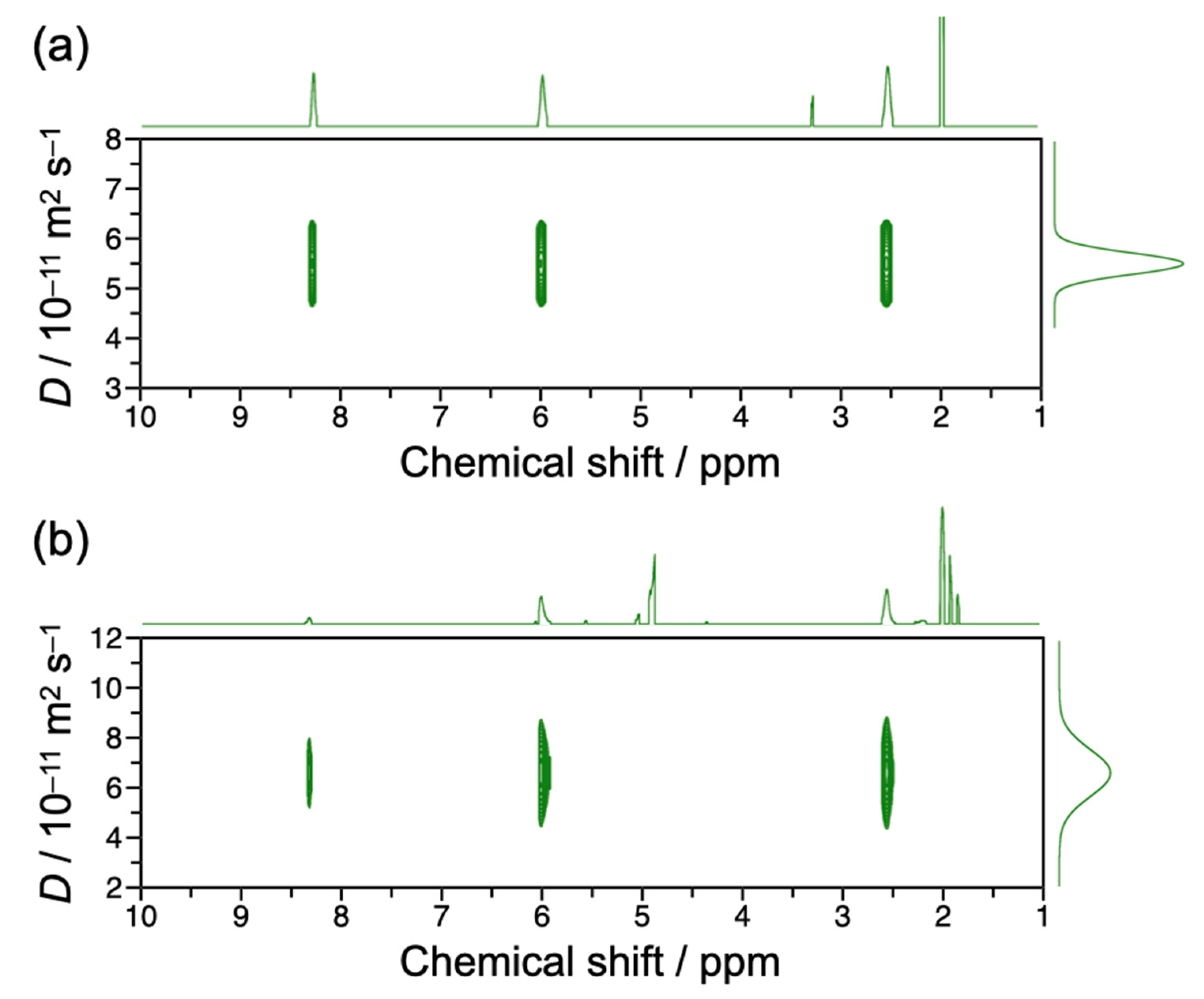
3.3. Pulse-Field-Gradient Spin-Echo NMR
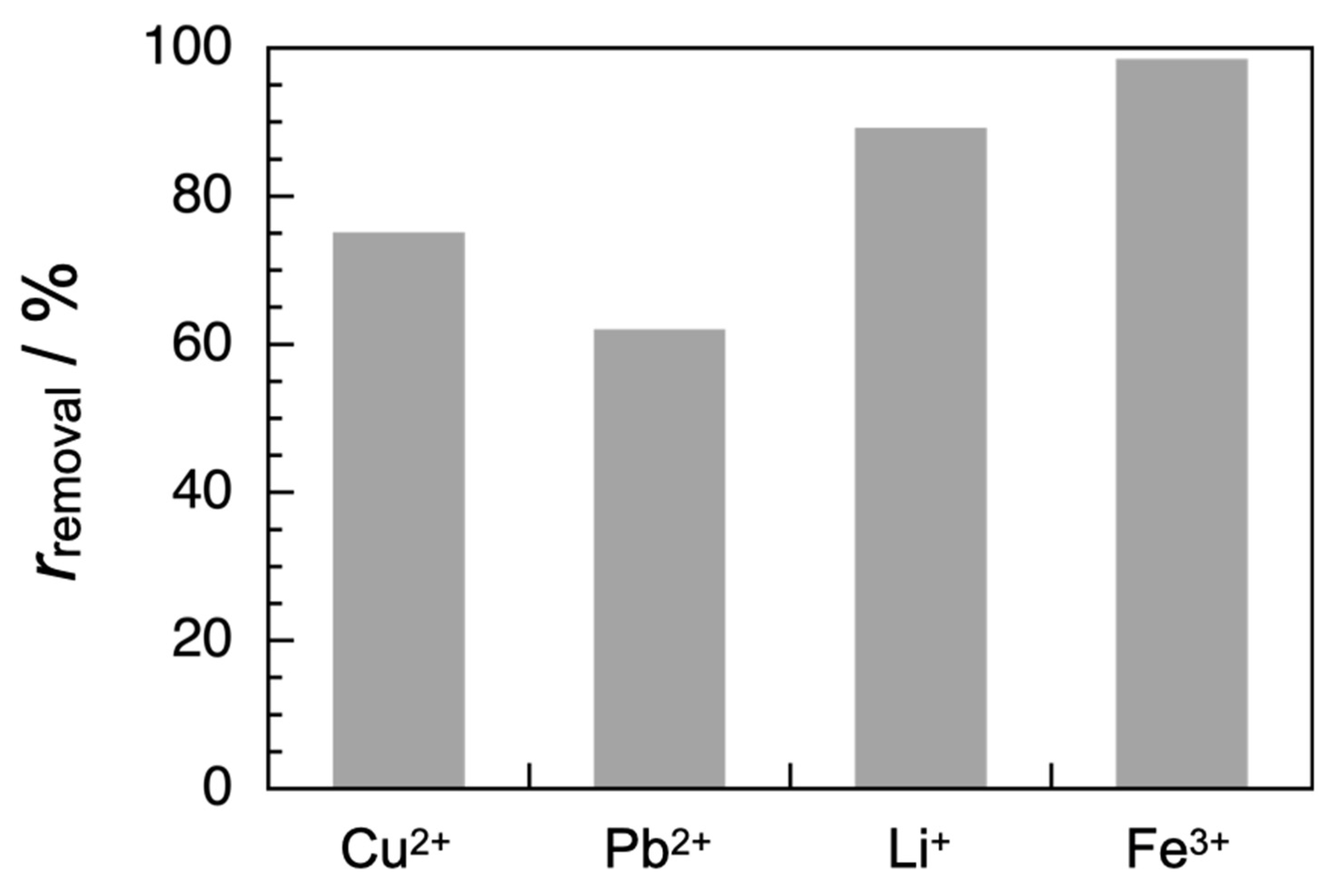
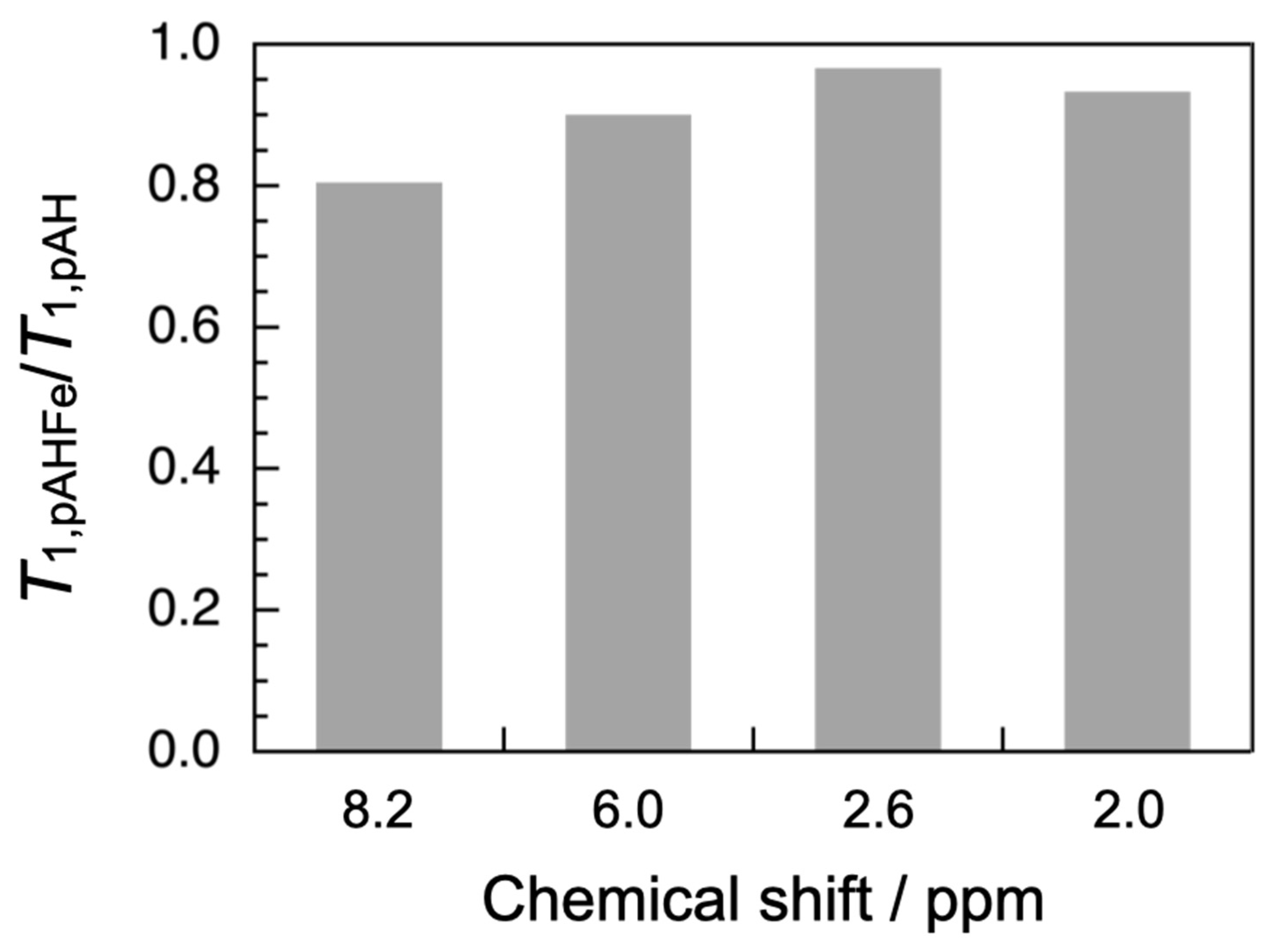
3.4. Adsorption Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tripathy, S.; Kumar, J.; Nalwa, H.S. Applications of polyelectrolytes and theoretical models. In Handbook of Polyelectrolytes and Their Applications; American Scientific Publishers: Stevenson Ranch, CA, USA, 2002. [Google Scholar]
- Nussinovitch, A. Water-Soluble Polymer Applications in Foods; Blackwell Science Ltd.: Oxford, UK, 2003. [Google Scholar]
- Williams, P.A. Handbook of Industrial Water Soluble Polymers; Blackwell Publishing: Oxford, UK, 2007. [Google Scholar]
- Rivas, B.L.; Pereira, E.; Maureira, A. Functional water-soluble polymers: Polymer–metal ion removal and biocide properties. Polym. Int. 2009, 58, 1093–1114. [Google Scholar] [CrossRef]
- Rivas, B.L.; Pereira, E.D.; Palencia, M.; Sánchez, J. Water-soluble functional polymers in conjunction with membranes to remove pollutant ions from aqueous solutions. Prog. Polym. Sci. 2011, 36, 294–322. [Google Scholar] [CrossRef]
- Kadajji, V.G.; Betageri, G.V. Water soluble polymers for pharmaceutical applications. Polymers 2011, 3, 1972. [Google Scholar] [CrossRef]
- Dehaen, W.; Bakulev, V.A. Chemistry of 1,2,3-Triazoles; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Schulze, B.; Schubert, U.S. Beyond click chemistry–supramolecular interactions of 1,2,3-triazoles. Chem. Soc. Rev. 2014, 43, 2522–2571. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huffman, J.C.; Flood, A.H. Can terdentate 2,6-bis(1,2,3-triazol-4-yl)pyridines form stable coordination compounds? Chem. Commun. 2007, 2692–2694. [Google Scholar] [CrossRef] [PubMed]
- Meudtner, R.M.; Hecht, S. Responsive backbones based on alternating triazole-pyridine/benzene copolymers: From helically folding polymers to metallosupramolecularly crosslinked gels. Macromol. Rapid Commun. 2008, 29, 347–351. [Google Scholar] [CrossRef]
- Pourghaz, Y.; Dongare, P.; Thompson, D.W.; Zhao, Y. Click functionalized poly(p-phenylene ethynylene)s as highly selective and sensitive fluorescence turn-on chemosensors for Zn2+ and Cd2+ ions. Chem. Commun. 2011, 47, 11014–11016. [Google Scholar] [CrossRef]
- Mishra, V.; Jung, S.-H.; Park, J.M.; Jeong, H.M.; Lee, H.-I. Triazole-containing hydrogels for time-dependent sustained drug release. Macromol. Rapid Commun. 2014, 35, 442–446. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Han, H.; Sun, R.; Xie, M.; Liao, X. Hyperbranched poly(triazole) with thermal and metal ion dual stimuli-responsiveness. Polym. Chem. 2015, 6, 4801–4808. [Google Scholar] [CrossRef]
- Ghosh, K.; Panja, A.; Panja, S. Cholesterol appended bis-1,2,3-triazoles as simple supramolecular gelators for the naked eye detection of Ag+, Cu2+ and Hg2+ ions. New J. Chem. 2016, 40, 3476–3483. [Google Scholar] [CrossRef]
- Huisgen, R. 1,3-Dipolar cycloadditions. past and future. Angew. Chem. Int. Ed. Engl. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Abboud, J.-L.M.; Foces-Foces, C.; Notario, R.; Trifonov, R.E.; Volovodenko, A.P.; Ostrovskii, V.A.; Alkorta, I.; Elguero, J. Basicity of N-H- and N-methyl-1,2,3-triazoles in the gas phase, in solution, and in the solid state—An experimental and theoretical study. Eur. J. Org. Chem. 2001, 2001, 3013–3024. [Google Scholar] [CrossRef]
- Kolb, H.C.; Sharpless, K.B. The growing impact of click chemistry on drug discovery. Drug Discov. Today 2003, 8, 1128–1137. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-Triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Fazio, F.; Bryan, M.C.; Blixt, O.; Paulson, J.C.; Wong, C.-H. Synthesis of sugar arrays in microtiter plate. J. Am. Chem. Soc. 2002, 124, 14397–14402. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Lahann, J. Click Chemistry for Biotechnology and Materials Science; Wiley & Sons: Chichester, UK, 2009. [Google Scholar]
- Qin, A.; Lam, J.W.Y.; Tang, B.Z. Click polymerization. Chem. Soc. Rev. 2010, 39, 2522–2544. [Google Scholar] [CrossRef]
- Huang, D.; Liu, Y.; Qin, A.; Tang, B.Z. Recent advances in alkyne-based click polymerizations. Polym. Chem. 2018, 9, 2853–2867. [Google Scholar] [CrossRef]
- Atanase, L.I.; Glaied, O.; Riess, G. Crystallization kinetics of PCL tagged with well-defined positional triazole defects generated by click chemistry. Polymer 2011, 52, 3074–3081. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, J.; Shen, X.; Zhang, X.A.; Sun, J.Z.; Qin, A.; Tang, B.Z. Self-healing hyperbranched poly(aroyltriazole)s. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef]
- Hashidzume, A.; Nakamura, T.; Sato, T. Copper-catalyzed azide-alkyne cycloaddition oligomerization of 3-azido-1-propyne derivatives. Polymer 2013, 54, 3448–3451. [Google Scholar] [CrossRef]
- Nakano, S.; Hashidzume, A.; Sato, T. Quarternization of 3-azido-1-propyne oligomers obtained by copper(I)-catalyzed azide–alkyne cycloaddition polymerization. Beilstein J. Org. Chem. 2015, 11, 1037–1042. [Google Scholar] [CrossRef]
- Yang, Y.; Mori, A.; Hashidzume, A. Emission properties of diblock copolymers composed of poly(ethylene glycol) and dense 1,2,3-triazole blocks. Polymers 2019, 11, 1086. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hashidzume, A. A new associative diblock copolymer of poly(ethylene glycol) and dense 1,2,3-triazole blocks: Self-association behavior and thermoresponsiveness in water. Macromol. Chem. Phys. 2019, 220, 1900317. [Google Scholar] [CrossRef]
- Yang, Y.; Kamon, Y.; Lynd, N.A.; Hashidzume, A. Self-healing thermoplastic elastomers formed from triblock copolymers with dense 1,2,3-triazole blocks. Macromolecules 2020, 53, 10323–10329. [Google Scholar] [CrossRef]
- Yamasaki, S.; Kamon, Y.; Xu, L.; Hashidzume, A. Synthesis of dense 1,2,3-triazole polymers soluble in common organic solvents. Polymers 2021, accepted. [Google Scholar]
- Stejskal, E.O.; Tanner, J.E. Spin diffusion measurements: Spin echoes in the presence of a time-dependent field gradient. J. Chem. Phys. 1965, 42, 288–292. [Google Scholar] [CrossRef]
- Tanner, J.E.; Stejskal, E.O. Restricted self-diffusion of proteins in colloidal systems by the pulse-gradient, spin-echo method. J. Chem. Phys. 1968, 49, 1768–1777. [Google Scholar] [CrossRef]
- Stilbs, P. Fourier transform pulsed-gradient spin-echo studies of molecular diffusion. Prog. Nucl. Magn. Reson. Spectrosc. 1987, 19, 1–45. [Google Scholar] [CrossRef]
- Morris, K.F.; Stilbs, P.; Johnson, C.S. Analysis of mixtures based on molecular size and hydrophobicity by means of diffusion-ordered 2D NMR. Anal. Chem. 1994, 66, 211–215. [Google Scholar] [CrossRef]
- Huo, R.; Wehrens, R.; Van Duynhoven, J.; Buydens, L.M.C. Assessment of techniques for DOSY NMR data processing. Anal. Chim. Acta 2003, 490, 231–251. [Google Scholar] [CrossRef]
- Erdmann, K.; Gutsze, A. Model of proton longitudinal magnetic relaxation in hydrated crosslinked poly(methacrylic acid). Colloid Polym. Sci. 1987, 265, 667–673. [Google Scholar] [CrossRef]
- Raby, P.; Budd, P.M.; Heatley, F.; Price, C. Nuclear magnetic relaxation of α-13C nuclei of helical poly(γ-hexyl-L-glutamate) and poly(γ-benzyl-L-glutamate). J. Polym. Sci. Part B Polym. Phys. 1991, 29, 451–456. [Google Scholar] [CrossRef]
- Brereton, M.G.; Ward, I.M.; Boden, N.; Wright, P. Nature of the proton NMR transverse relaxation function of polyethylene melts. 1. Monodispersed polyethylenes. Macromolecules 1991, 24, 2068–2074. [Google Scholar] [CrossRef]
- Das, K.K.; Somasundaran, P. Ultra-low dosage flocculation of alumina using polyacrylic acid. Colloids Surf. A 2001, 182, 25–33. [Google Scholar] [CrossRef]
- Hashidzume, A.; Morishima, Y.; Szczubiałka, K. Amphiphilic polyelectrolytes. In Handbook of Polyelectrolytes and Their Applications; Tripathy, S.K., Kumar, J., Nalwa, H.S., Eds.; American Scientific Publishers: Stevenson Ranch, CA, USA, 2002; Volume 2, pp. 1–63. [Google Scholar]
- Saravanan, N.; Yusuff, K.K.M. New complexes of iron(III), cobalt(II), nickel(II) and copper(II) with 2-phenyl-1,2,3-triazole-4-carboxalidene-2-aminophenol. Transit. Met. Chem. 1996, 21, 464–468. [Google Scholar] [CrossRef]
- You, X.; Wei, Z. Two multidentate ligands utilizing triazolyl, pyridinyl and phenolate groups as donors for constructing dinuclear copper(II) and iron(III) complexes: Syntheses, structures, and electrochemistry. Inorg. Chim. Acta 2014, 423, 332–339. [Google Scholar] [CrossRef]
| Sample | Mw/103 1 | Mw/Mn 1 |
|---|---|---|
| poly(tBuAH) 2 | 7.9 | 1.5 |
| poly(tBuAH) 3 | 8.8 | 1.4 |
| Metal Ion | ci/mg L−1 | ce/mg L−1 | rremoval/% | qe/mg g−1 |
|---|---|---|---|---|
| Cu2+ | 100 | 24.90 ± 0.02 | 75.10 ± 0.02 | 75.12 ± 0.02 |
| Pb2+ | 100 | 38.0 ± 0.2 | 62.0 ± 0.2 | 62.0 ± 0.2 |
| Li+ | 100 | 10.80 ± 0.02 | 89.20 ± 0.02 | 89.22 ± 0.02 |
| Fe3+ | 100 | 1.46 ± 0.03 | 98.54 ± 0.03 | 98.57 ± 0.03 |
| δ/ppm | T1,pAH 1/s | T1,pAHFe 2/s | T1,pAHFe/T1,pAH |
|---|---|---|---|
| 8.2 | 1.47 | 1.18 | 0.804 |
| 6.0 | 1.22 | 1.10 | 0.900 |
| 2.6 | 0.376 | 0.363 | 0.966 |
| 2.0 | 0.485 | 0.452 | 0.932 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Kamon, Y.; Hashidzume, A. Synthesis of a New Polyanion Possessing Dense 1,2,3-Triazole Backbone. Polymers 2021, 13, 1614. https://doi.org/10.3390/polym13101614
Xu L, Kamon Y, Hashidzume A. Synthesis of a New Polyanion Possessing Dense 1,2,3-Triazole Backbone. Polymers. 2021; 13(10):1614. https://doi.org/10.3390/polym13101614
Chicago/Turabian StyleXu, Linlin, Yuri Kamon, and Akihito Hashidzume. 2021. "Synthesis of a New Polyanion Possessing Dense 1,2,3-Triazole Backbone" Polymers 13, no. 10: 1614. https://doi.org/10.3390/polym13101614
APA StyleXu, L., Kamon, Y., & Hashidzume, A. (2021). Synthesis of a New Polyanion Possessing Dense 1,2,3-Triazole Backbone. Polymers, 13(10), 1614. https://doi.org/10.3390/polym13101614






