From Amorphous Silicones to Si-Containing Highly Ordered Polymers: Some Romanian Contributions in the Field †
Abstract
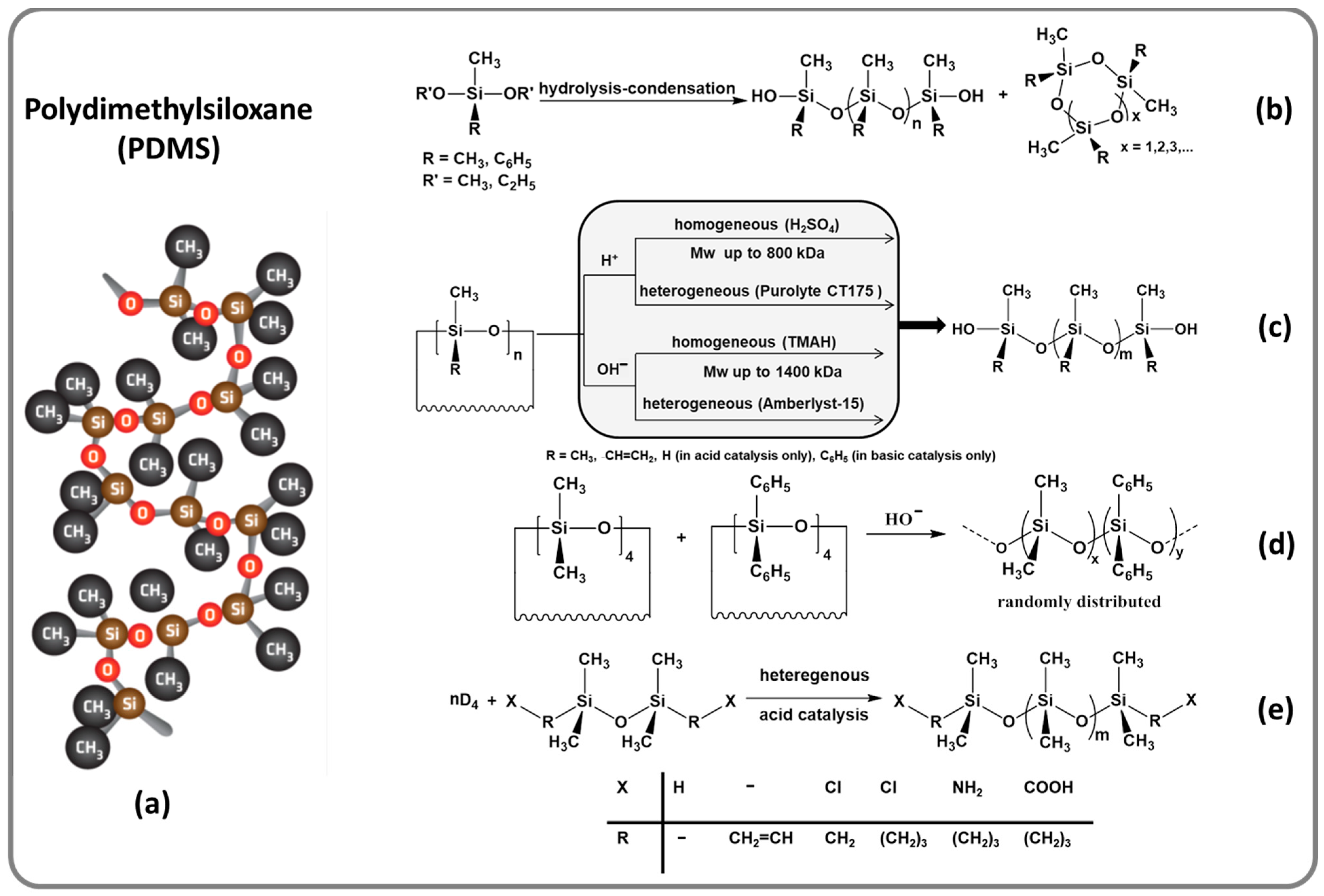
1. Context and Early Romanian Achievements in the Field of Silicones
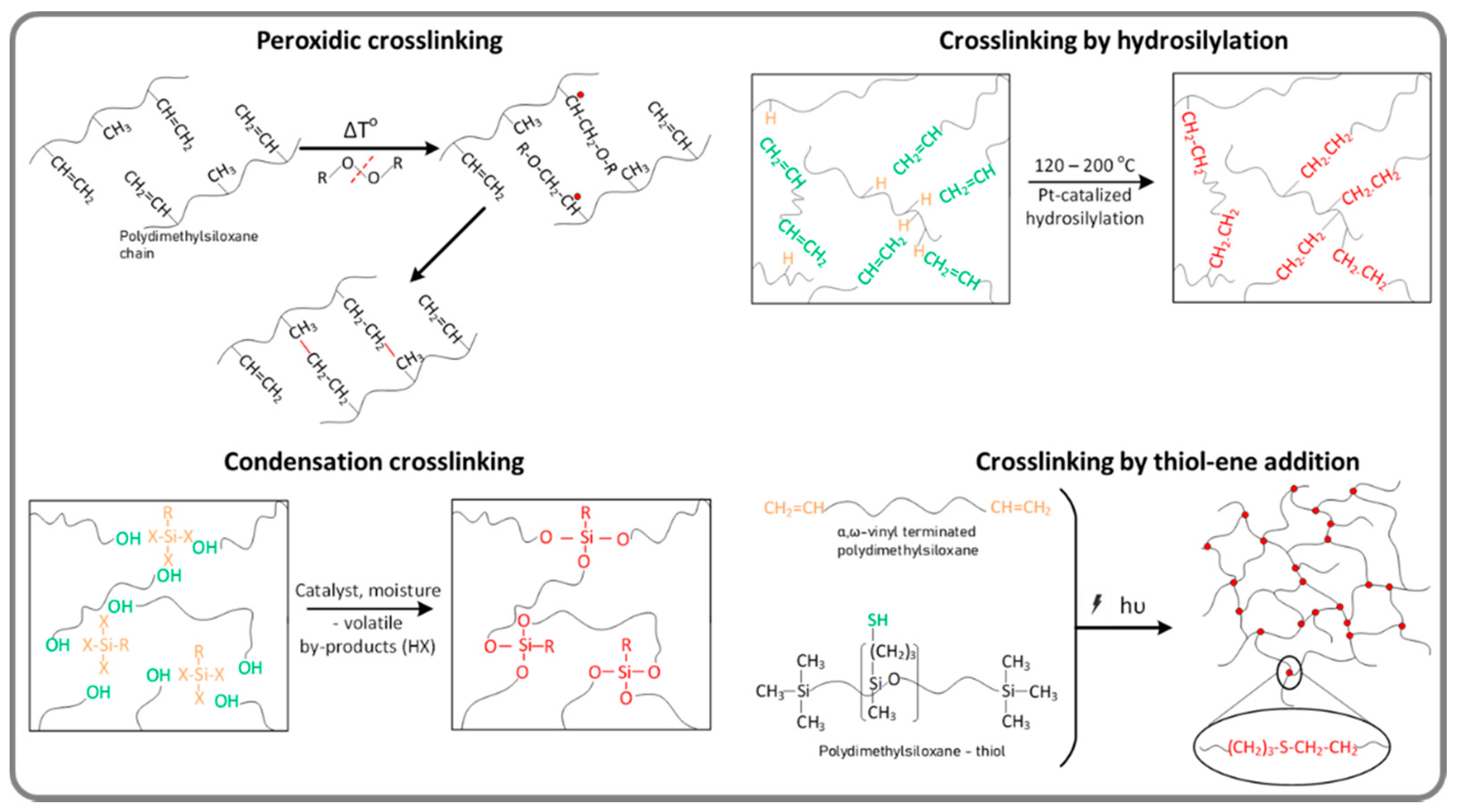
2. Classic Amorphous Silicones Renewed for Emerging Applications
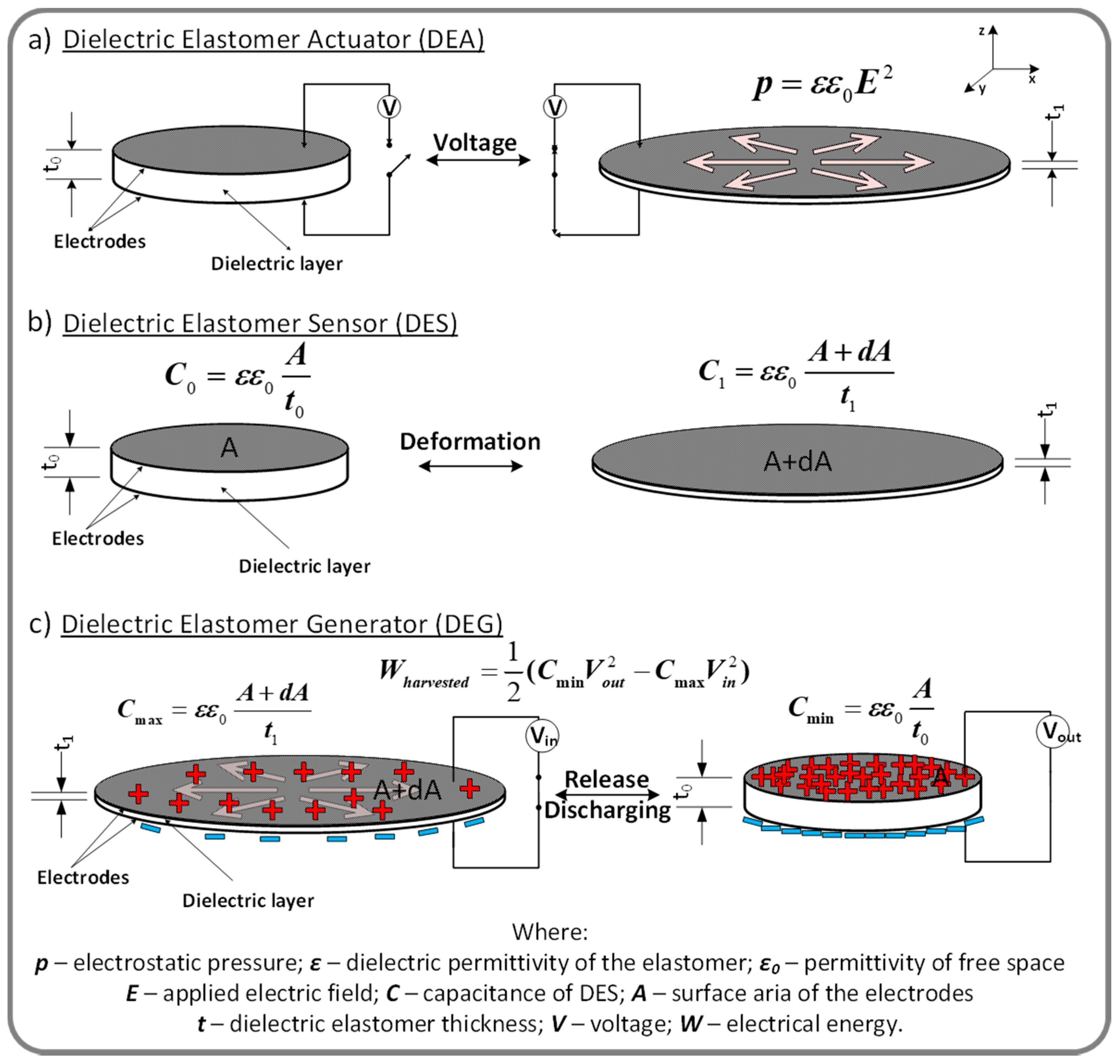
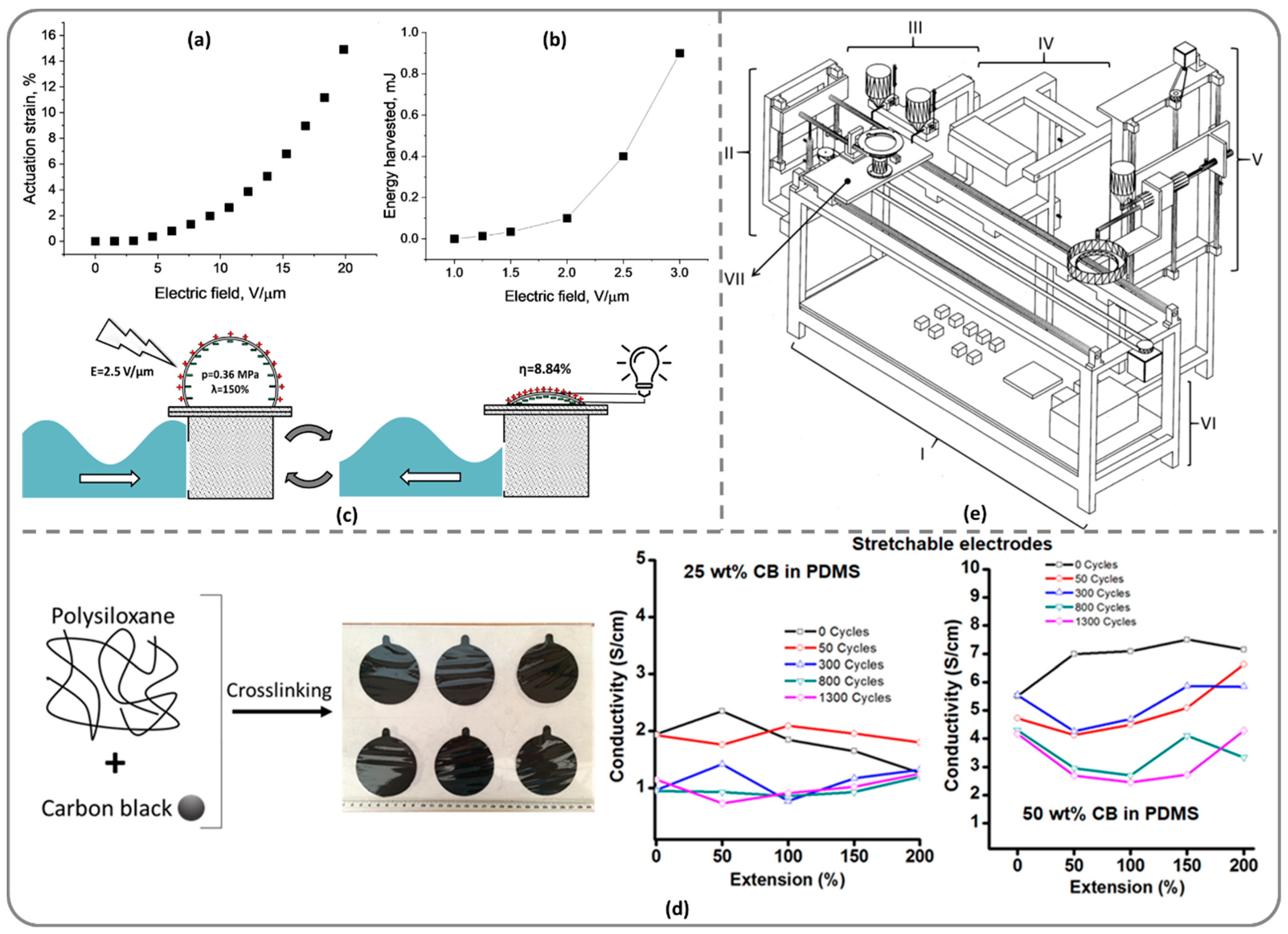
Electromechanically Active Silicone Elastomers
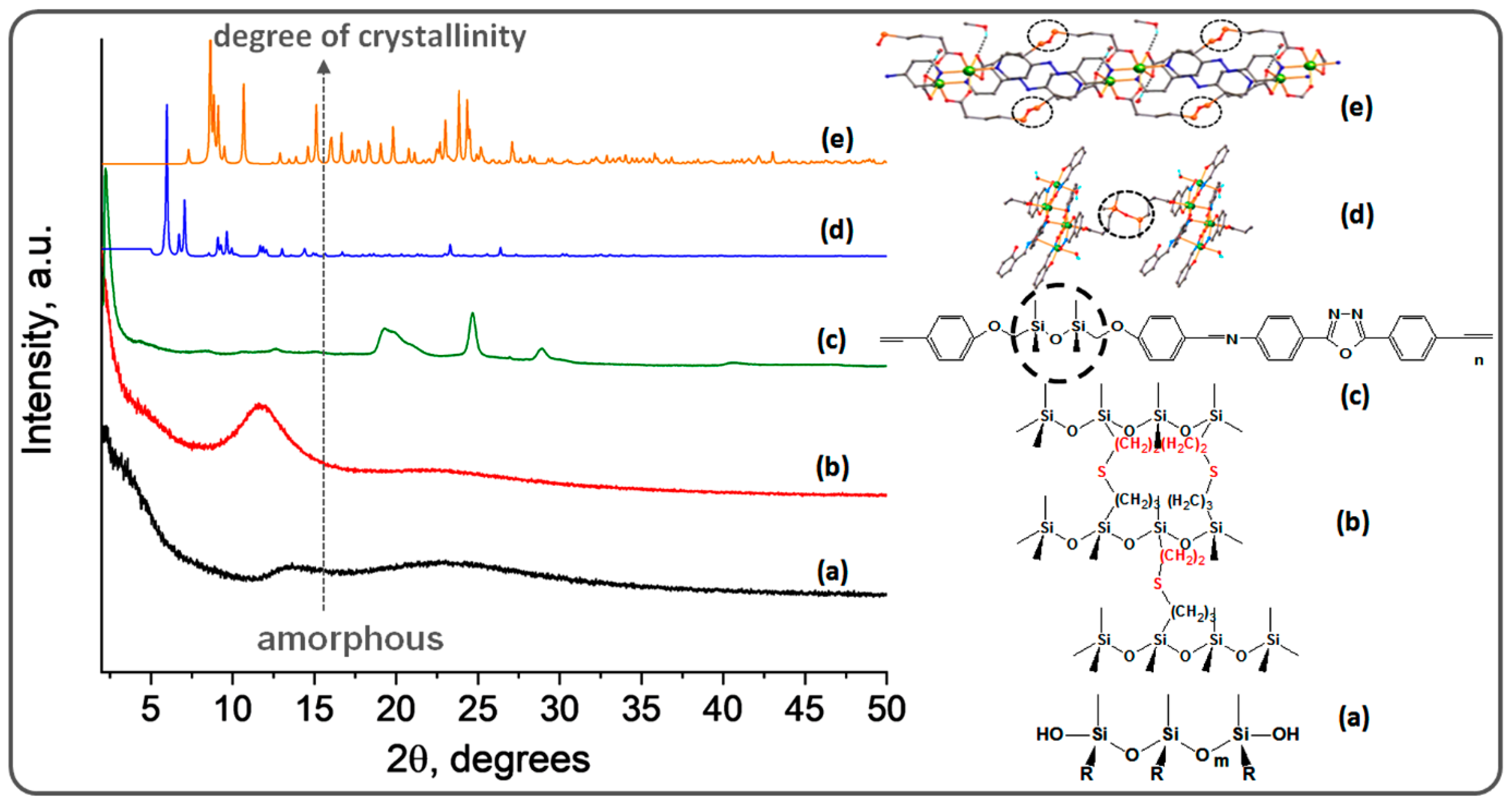
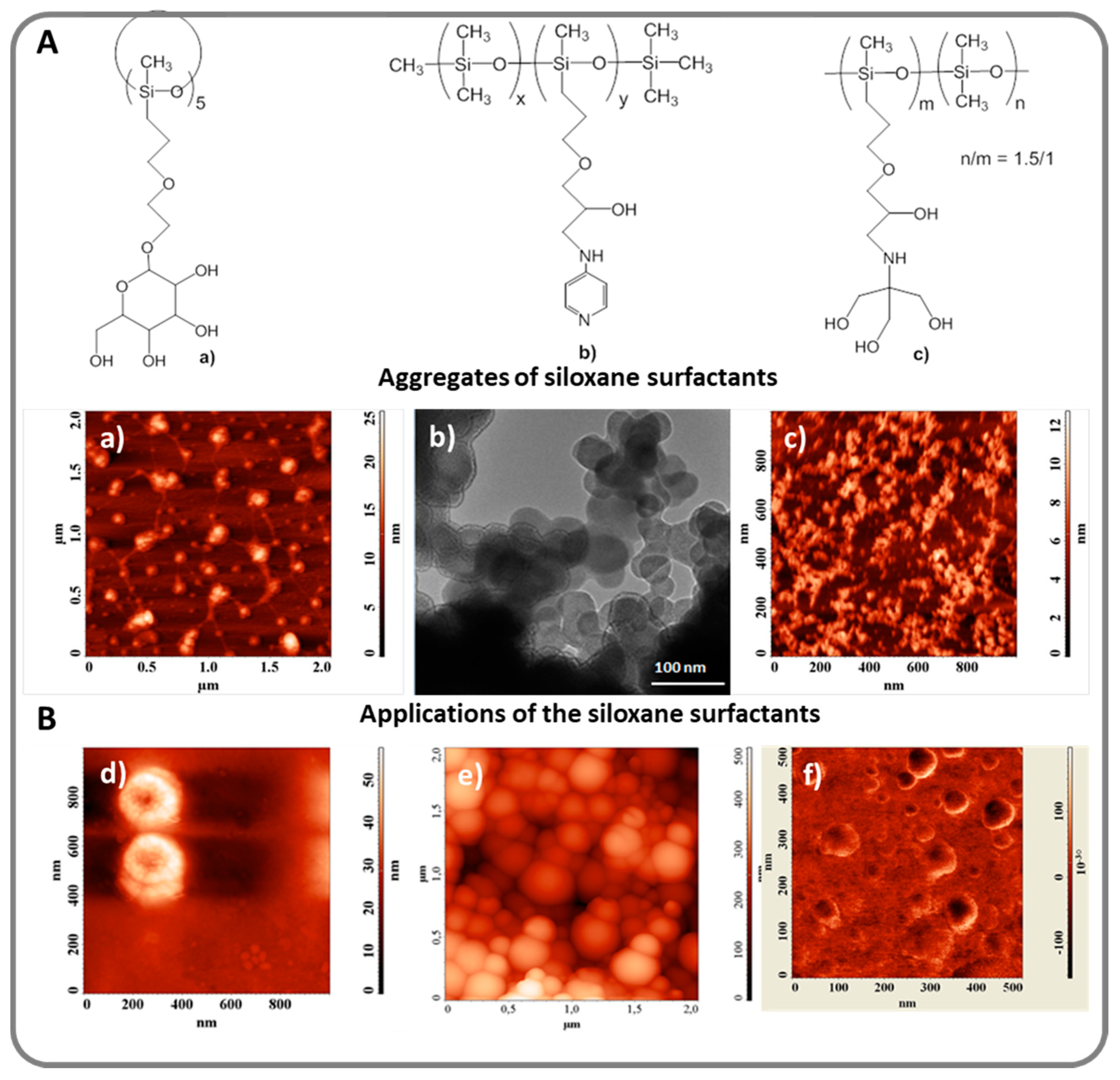
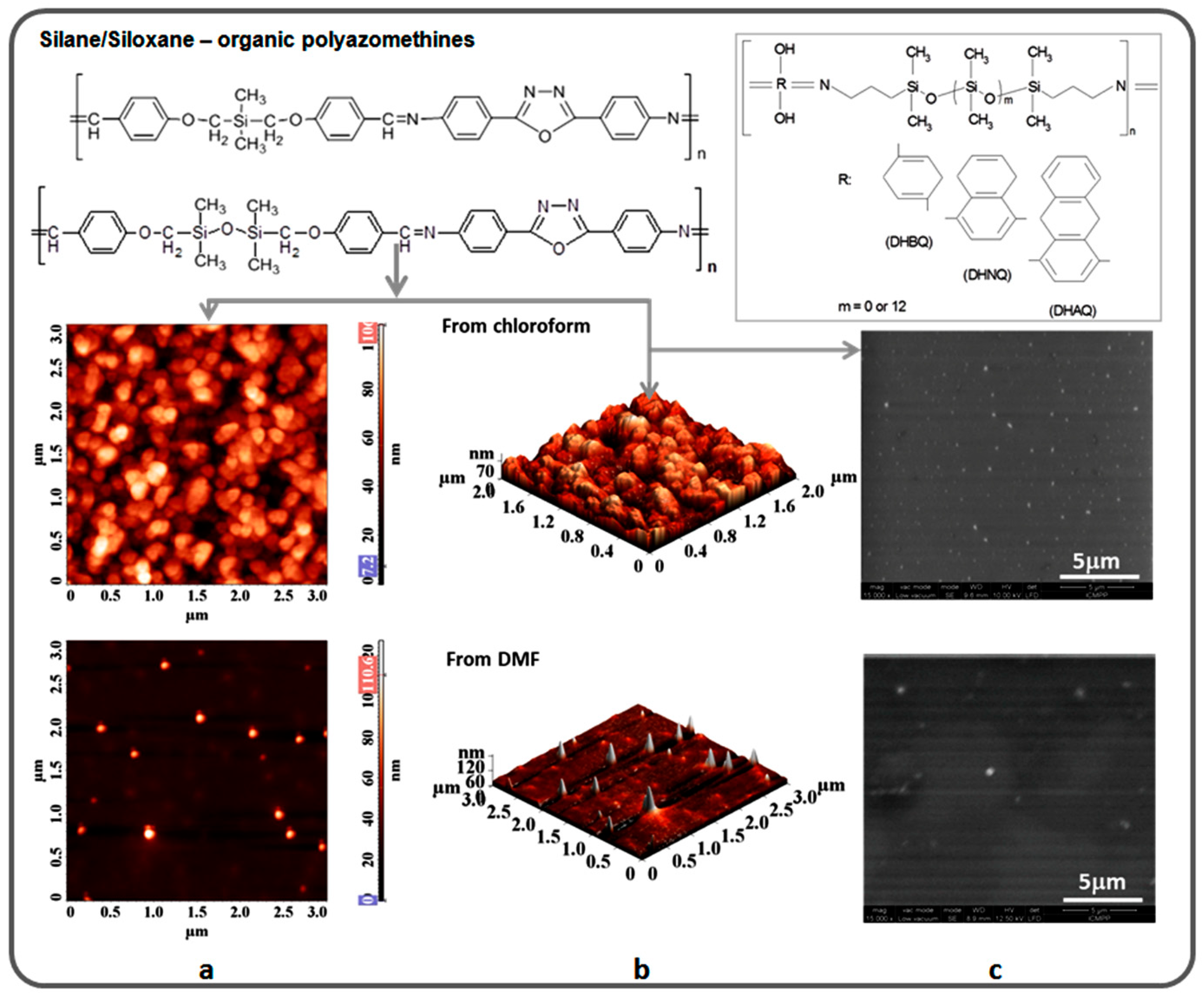
3. Siloxane-Siloxane and Siloxane-Organic Copolymers
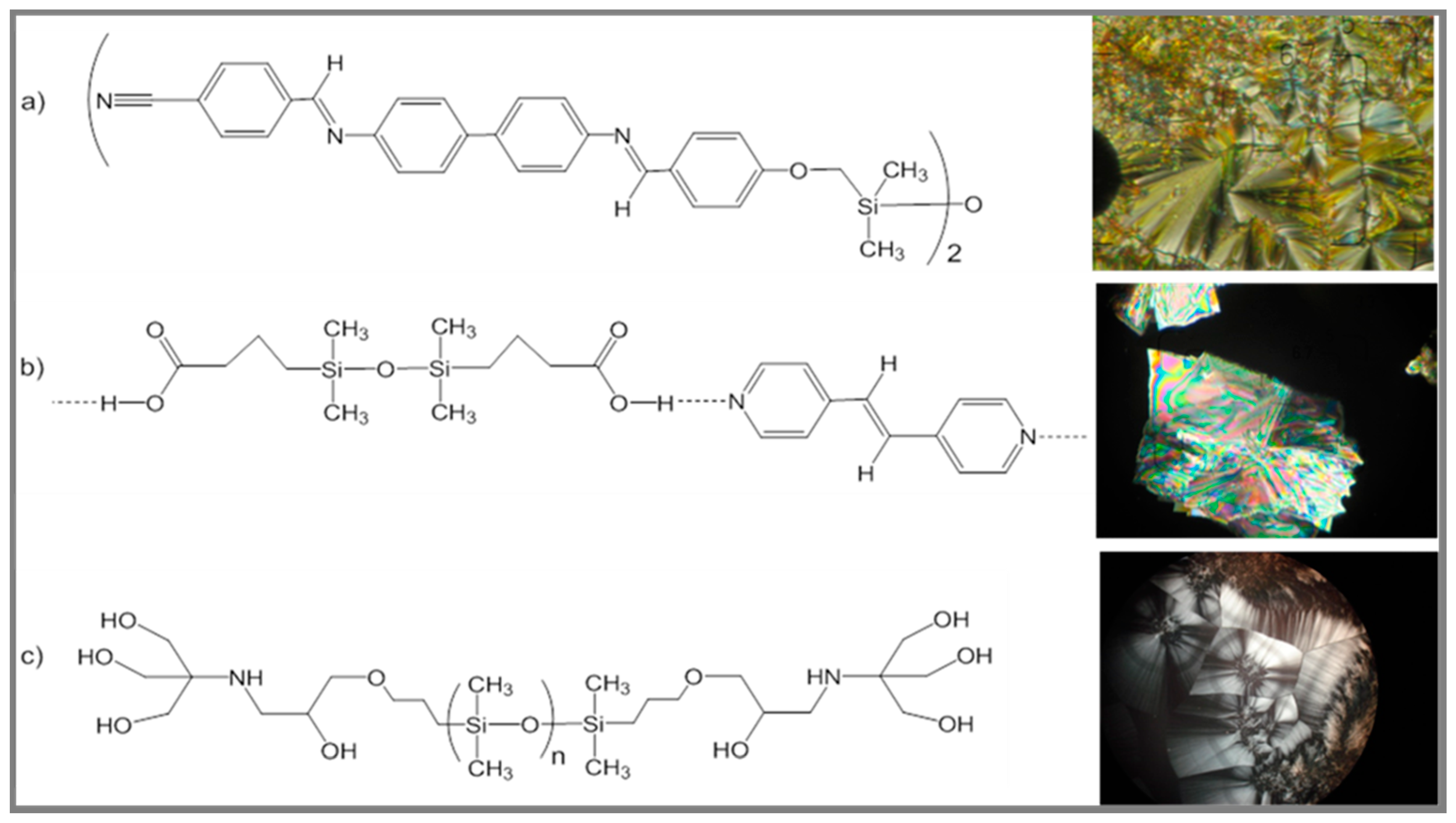
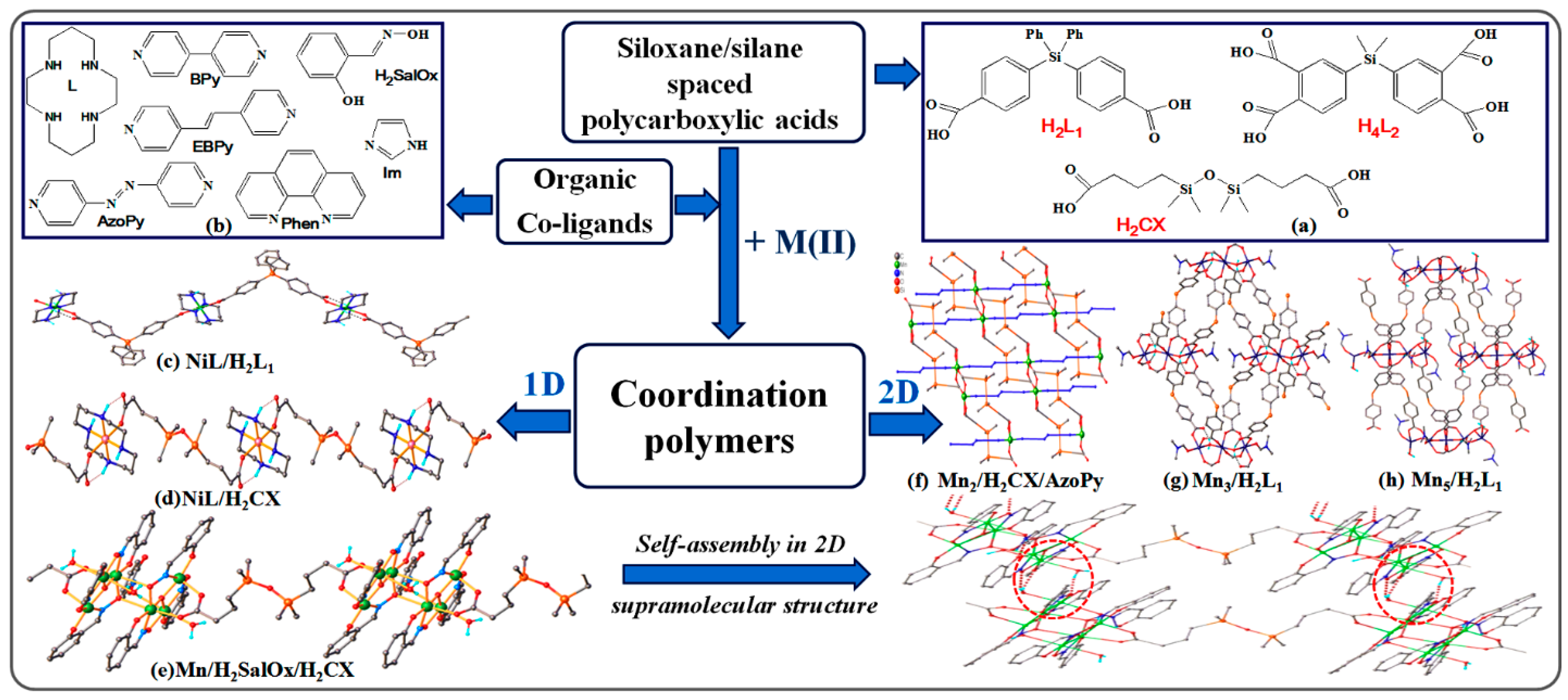
4. Highly Ordered Coordination Polymers Containing Silicon or Silicone Motif
5. Conclusions and Outlooks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marcu, M.; Stiubianu, G.; Vosniuc, I.; Spiratos, M. Procedure to Obtain Dimethylsiloxane Cycles. Romanian Patent 65111, 19 September 1978. [Google Scholar]
- Marcu, M.; Spiratos, M.; Stiubianu, G. Procedure for the Preparation of the Elastomer Polydimethylsiloxane. Romanian Patent 638851, 16 October 1978. [Google Scholar]
- Marcu, M.; Ilie, S.; Stiubianu, G. Procedure for Obtaining Polydimethylsiloxane-α,ω-Diols. Romanian Patent 75237, 30 September 1980. [Google Scholar]
- Marcu, M.; Ilie, S.; Stiubianu, G.; Perjoiu, M.; Roman, L.G.; Pricop, L. Process for Obtaining One-Component Silicone Rubbers with Room Temperature Vulcanizing. Romanian Patent 83044, 30 January 1984. [Google Scholar]
- Marcu, M.; Ilie, S.; Stiubianu, G.; Perjoiu, M.; Roman, G.; Pricop, L.; Streba, E. Process for Obtaining Methylvinylsilicone Rubber. Romanian Patent 86739, 30 April 1985. [Google Scholar]
- Marcu, M.; Ilie, S.; Stiubianu, G. Process for obtaining heptamethylvinylcyclotetrasiloxane. Romanian Patent 86382, 28 April 1985. [Google Scholar]
- Harabagiu, V.; Cotzur, C.; Luchian, N.; Marcu, M.; Bostan, M.; Istrate, M. Procedure for Obtaining a Multifunctional Silicone Lubricant. Romanian Patent 104035, 9 March 1992. [Google Scholar]
- Chelaru, N.; Bancila, M.; Marcu, M.; Luchian, N.; Angheluta, A.; Onofrei, M. Process and Installation for Obtaining Continuous Flow of Demoulding Polydimethylsiloxane Oils. Romanian Patent 109853, 30 June 1995. [Google Scholar]
- Ionescu, C.; Marcu, M.; Giurgiu, D. Reactor for the Synthesis of Metilclorsilanilor. Romanian Patent 109823, 30 June 1995. [Google Scholar]
- Luchian, N.; Marcu, M.; Sacarescu, L.; Rugina, T. Procedure for Obtaining Silicone Greases. Romanian Patent 111202, 30 September 1998. [Google Scholar]
- Cazacu, M.; Marcu, M. Silicone Rubbers. Ix. Contributions to polydimethylsiloxane-α,ω-diols synthesis by heterogeneous catalysis. J. Macromol. Sci. A 1995, 32 (Suppl. 7), 1019–1029. [Google Scholar] [CrossRef]
- Cazacu, M.; Marcu, M.; Holerca, M.N.; Petrovan, S.; Lazarescu, S. Heterogeneous catalyzed copolymerization of octamethylcyclotetrasiloxane with 1,3,5,7-tetravinyl-1,3,5,7-tetramethylcyclo-tetrasiloxane. J. Macromol. Sci. A 1996, 33, 65–76. [Google Scholar] [CrossRef]
- Cazacu, M.; Marcu, M.; Ibanescu, C.; Petrovan, S.; Holerca, M.; Simionescu, M. Cationic heterogeneous copolymerization of octamethylcyclotetrasiloxane with 1,3,5,7-tetramethyl-1,3,5,7-tetravinylcyclotetra-siloxane: Optimization of reaction conditions. Polym. Plast. Technol. Eng. 1996, 35, 327–347. [Google Scholar] [CrossRef]
- Cazacu, M.; Marcu, M.; Dragan, S.; Matricala, C. Anionic polymerization of cyclosiloxanes in heterogeneous medium. J. Appl. Polym. Sci. 1996, 60, 731–734. [Google Scholar] [CrossRef]
- Cazacu, M.; Marcu, M.; Vlad, A.; Caraiman, D.; Racles, C. Synthesis of functional telechelic polydimethylsiloxanes by ion-exchangers catalysis. Eur. Polym. J. 1999, 35, 1629–1635. [Google Scholar] [CrossRef]
- Cazacu, M.; Marcu, M.; Dragan, S.; Matricala, C.; Simionescu, M.; Holerca, M. Dimethyldiphenylsiloxane copolymers synthesis by ion exchanger catalysis. Polymer 1997, 38, 3967–3971. [Google Scholar] [CrossRef]
- Cazacu, M. Polymers containing Si, O and other elements within backbone. In Advances in Functional Heterochain Polymers; Cazacu, M., Ed.; Chapter 1; Nova Science Publishers: New York, NY, USA, 2008; pp. 1–32. [Google Scholar]
- Marcu, M.; Cazacu, M.; Lazarescu, S.; Matricala, C.; Simionescu, M.; Bolohan, S. Procedure to Obtain Tetramethyl-tetravinylcyclotetrasiloxane. Romanian Patent 114329, 30 January 2001. [Google Scholar]
- Cazacu, M. Possibilities to develop functional materials on silicone/silica backbones. In Recent Developments in Silicone-Based Materials; Chapter 1; Nova Science Publishers: New York, NY, USA, 2010; pp. 1–34. [Google Scholar]
- Cazacu, M. Polysiloxanes bearing ionic groups. In Focus on Ionic Polymers; Dragan, S., Ed.; Research Signpost: Thiruvananthapuram, India, 2005; pp. 261–292. [Google Scholar]
- Iojoiu, C.; Abadie, M.J.M.; Harabagiu, V.; Pinteala, M.; Simionescu, B.C. Synthesis and photocrosslinking of benzyl acrylate substituted polydimethylsiloxanes. Eur. Polym. J. 2000, 36, 2115–2123. [Google Scholar] [CrossRef]
- Simionescu, C.I.; Rusa, M.; David, G.; Pinteala, M.; Harabagiu, V.; Simionescu, B.C. Block and graft copolymers with polysiloxane and poly(N-acyliminoethylene) sequences. Angew. Makromolek. Chem. 1997, 253, 139–149. [Google Scholar] [CrossRef]
- Rosati, D.; Perrin, M.; Navard, P.; Harabagiu, V.; Pinteala, M.; Simionescu, B.C. Synthesis of poly(styrene-dimethylsiloxane) block copolymers: Influence of the phase-separated morphologies on the thermal behaviors. Macromolecules 1998, 31, 4301–4308. [Google Scholar] [CrossRef]
- Cazacu, M.; Racles, C. Recent developments in siloxane-based polymers and copolymers. In New Trends in Nonionic (co)Polymers and Hybrids; Dragan, S., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2006; pp. 167–213. [Google Scholar]
- Cazacu, M.; Vlad, A.; Racles, C.; Marcu, M. Incorporation of siloxanes in hydrolytically degradable structures. Eur. Polym. J. 2003, 39, 527–535. [Google Scholar] [CrossRef]
- Cazacu, M.; Vlad, A.; Simionescu, M.; Racles, C.; Marcu, M. Incorporation of the siloxanes in hydrolytically degradable organic structures. II. Segmented siloxaneimide poly(anhydride)s. J. Macromol. Sci. A 2002, 39, 1487–1499. [Google Scholar] [CrossRef]
- Pislaru-Danescu, L.; Telipan, G.; Racles, C. CO2 Concentration Sensor with Sensitive Element Based on Supramolecular Organo-Siloxane Polymer. Romanian Patent 130871, 30 April 2020. [Google Scholar]
- Cazacu, M.; Racles, C.; Vlad, A.; Antohe, M.; Forna, N. Silicone-based Composite for Relining of Removable Dental Prosthesis. J. Compos. Mater. 2009, 43, 2045–2055. [Google Scholar] [CrossRef]
- Cazacu, M.; Ignat, M.; Racles, C.; Vlad, A.; Alexandru, M.; Zarnescu, G. Polydimethylsiloxane/silica composites incorporating pyrite powders for actuation elements. Polym. Int. 2009, 58, 745–751. [Google Scholar] [CrossRef]
- Alexandru, M.; Cristea, M.; Cazacu, M.; Ioanid, A.; Simionescu, B.C. Composite materials based on polydimethylsiloxane andin situgenerated silica by using the sol-gel technique. Polym. Compos. 2009, 30, 751–759. [Google Scholar] [CrossRef]
- Sacarescu, L.; Kostromin, S.; Bronnikov, S. Synthesis and properties of polydiphenylsilane/fullerene C60 nanocomposites. Mater. Chem. Phys. 2015, 149–150, 430–436. [Google Scholar] [CrossRef]
- Sacarescu, L.; Mangalagiu, I.; Simionescu, M.; Sacarescu, G.; Ardeleanu, R. Polysilanes. a new route toward high performance EL devices. Macromol. Symp. 2008, 267, 123–128. [Google Scholar]
- Stiubianu, G.; Racles, C.; Nistor, A.; Cazacu, M.; Simionescu, B.C. Cellulose modification by crosslinking with siloxane diacids. Cellul. Chem. Technol. 2011, 45, 157–162. [Google Scholar]
- Stiubianu, G.; Cazacu, M.; Nicolescu, A.; Hamciuc, V.; Vlad, S. Silicone-modified cellulose. Crosslinking of the cellulose acetate with 1,1,3,3-tetramethyldisiloxane by Pt-catalyzed dehydrogenative coupling. J. Polym. Res. 2009, 17, 837–845. [Google Scholar] [CrossRef]
- Stiubianu, G.; Racles, C.; Cazacu, M.; Simionescu, B.C. Silicone-modified cellulose. Crosslinking of cellulose acetate with poly[dimethyl(methyl-H)siloxane] by Pt-catalyzed dehydrogenative coupling. J. Mater. Sci. 2010, 45, 4141–4150. [Google Scholar] [CrossRef]
- Enescu, D.; Hamciuc, V.; Pricop, L.; Hamaide, T.; Harabagiu, V.; Simionescu, B.C. Polydimethylsiloxane-modified chitosan I. Synthesis and structural characterisation of graft and crosslinked copolymers. J. Polym. Res. 2009, 16, 73–80. [Google Scholar]
- Enescu, D.; Hamciuc, V.; Ardeleanu, R.; Cristea, M.; Ioanid, A.; Harabagiu, V.; Simionescu, B.C. Polydimethylsiloxane modified chitosan. Part III: Preparation and characterization of hybrid membranes. Carbohydr. Polym. 2009, 76, 268–278. [Google Scholar]
- Stiubianu, G.; Cazacu, M.; Cristea, M.; Vlad, A. Polysiloxane-lignin composites. J. Appl. Polym. Sci. 2009, 113, 2313–2321. [Google Scholar] [CrossRef]
- Cazacu, M.; Stiubianu, G. Process for Obtaining a Room Temperature Vulcanization Silicone Rubber. Romanian Patent 126477, 29 March 2013. [Google Scholar]
- Marcu, M.; Vlad, A. Procedure to Obtain Diphenylsilanediol in Heterogeneous Catalysis with Anion Exchangers. Romanian Patent 22779, 29 January 2010. [Google Scholar]
- Tugui, C.; Tiron, V.; Dascalu, M.; Sacarescu, L.; Cazacu, M. From ultra-high molecular weight polydimethylsiloxane to super-soft elastomer. Eur. Polym. J. 2019, 120, 109243. [Google Scholar] [CrossRef]
- Cazacu, M.; Vlad, A.; Alexandru, M.; Budrugeac, P.; Racles, C.; Iacomi, F. Polydimethyldiphenylsiloxanes/silica interconnected networks: Preparation and properties evaluation. Polym. Bull. 2010, 64, 421–434. [Google Scholar] [CrossRef]
- Brunchi, C.-E.; Morariu, S.; Cazacu, M.; Bercea, M. Influence of the solvent quality on the thermodynamic behavior of polymethylphenylsiloxane solutions. Ind. Eng. Chem. Res. 2010, 49, 12740–12746. [Google Scholar] [CrossRef]
- Andriot, M.; Chao, S.H.; Colas, A.; Cray, S.; de Buyl, F.; DeGroot, J.V.; Dupont, A.; Easton, T.; Garaud, J.L.; Gerlach, E.; et al. Silicones in industrial applications. In Inorganic Polymers; De Jaeger, R., Gleria, M., Eds.; Nova Science: Hauppauge, NY, USA, 2007; pp. 61–161. [Google Scholar]
- Functional Groups. 27 April 2019. Available online: https://bio.libretexts.org/@go/page/8390 (accessed on 7 May 2021).
- Noll, W. Chemistry and Technology of Silicones; Academic Press Inc.: New York, NY, USA, 1968; pp. 287–317. [Google Scholar]
- Voronkov, M.G.; Mileshkevich, V.P.; Yuzhelevskii, Y.A. The Siloxane Bond.: Physical Properties and Chemical Transformations; Voronkov, M.G., Mileshkevich, V.P., Yuzhelevskii, Y.A., Eds.; Springer: New York, NY, USA, 1978; pp. 159–212. [Google Scholar]
- Kuo, A.C.M. Poly(dimethylsiloxane) Polymer Data Handbook; Oxford University Press, Inc.: Oxford, UK, 1999; pp. 411–435. [Google Scholar]
- Surface Tension and Its Measurement. In Adhesives Technology Handbook; Elsevier: Amsterdam, The Netherlands, 2009; pp. 21–36. [CrossRef]
- Klykken, P.; Servinski, M.; Thomas, X. Silicone Film-Forming Technologies for Health Care Applications, Dow Corning White Paper, Literature no. 2-1068A-01 2009. Available online: http://citeseerx.ist.psu.edu/viewdoc/download;jsessionid=C84383C5BB2BBA983BF41F08CE6476D6?doi=10.1.1.578.7746&rep=rep1&type=pdf (accessed on 19 March 2021).
- Shankar, R.; Ghosh, T.K.; Spontak, R.J. Dielectric elastomers as next-generation polymeric actuators. Soft Matter 2007, 3, 1116. [Google Scholar] [CrossRef]
- Wang, N.; Cui, C.; Guo, H.; Chen, B.; Zhang, X. Advances in dielectric elastomer actuation technology. Sci. China Technol. Sci. 2018, 61, 1512–1527. [Google Scholar] [CrossRef]
- Tugui, C.; Stiubianu, G.T.; Serbulea, M.S.; Cazacu, M. Silicone dielectric elastomers optimized by crosslinking pattern—A simple approach to high-performance actuators. Polym. Chem. 2020, 11, 3271–3284. [Google Scholar] [CrossRef]
- Zaltariov, M.-F.; Cazacu, M.; Racles, C.; Musteata, V.; Vlad, A.; Airinei, A. Metallopolymers based on a polyazomethine ligand containing rigid oxadiazole and flexible tetramethyldisiloxane units. J. Appl. Polym. Sci. 2015, 132, 41631–41642. [Google Scholar] [CrossRef]
- Zaltariov, M.-F.; Cazacu, M.; Sacarescu, L.; Vlad, A.; Novitchi, G.; Train, C.; Shova, S.; Arion, V.B. Oxime-bridged Mn6 clusters inserted in one-dimensional coordination polymer. Macromolecules 2016, 49, 6163–6172. [Google Scholar] [CrossRef]
- Shova, S.; Vlad, A.; Damoc, M.; Tiron, V.; Dascalu, M.; Novitchi, G.; Ursu, C.; Cazacu, M. Nanoscale coordination polymer of dimanganese(II) as infinite, flexible nano-sheets with photo-switchable morphology. Eur. J. Inorg. Chem. 2020, 2020, 2043–2054. [Google Scholar] [CrossRef]
- Carpi, F.; De Rossi, D.; Kornbluh, R.; Pelrine, R.E.; Sommer-Larsen, P. (Eds.) Dielectric Elastomers as Electromechanical Transducers. Fundamentals, Materials, Devices, Models and Applications of an Emerging Electroactive Polymer Technology; Elsevier Science: Amsterdam, The Netherlands, 24 March 2008. [Google Scholar]
- Shankar, R.; Ghosh, T.K.; Spontak, R.J. Electroactive Nanostructured Polymers as Tunable Actuators. Adv. Mater. 2007, 19, 2218–2223. [Google Scholar] [CrossRef]
- Koo, C.M. Electroactive thermoplastic dielectric elastomers as a new generation polymer actuators. In Thermoplastic Elastomers; El-Sonbati, A., Ed.; InTech: London, UK, 2012; Volume 19, pp. 399–416. [Google Scholar]
- Poplavko, Y.M. Polar dielectrics in electronics. In Electronic Materials: Principles and Applied Science; Elsevier: Amsterdam, The Netherlands, 2018; pp. 509–600. [Google Scholar] [CrossRef]
- Ni, N.; Zhang, L. Dielectric Elastomer Sensors. In Elastomers; Çankaya, N., Ed.; Chapter 11; Intechopen Publication: London, UK, 2017; pp. 231–253. [Google Scholar]
- Ignat, M.; Zarnescu, G.; Hamciuc, E.; Hamciuc, C.; Cazacu, M.; Sava, I. Microactuator Based on Polymer. Romanian Patent 127096 A2, 29 January 2016. [Google Scholar]
- Pelrine, R.; Kornbluh, R.D.; Eckerle, J.; Jeuck, P.; Oh, S.; Pei, Q.; Stanford, S. Dielectric elastomers: Generator mode fundamentals and applications. In Smart Structures and Materials 2001: Electroactive Polymer Actuators and Devices; Bar-Cohen, Y., Ed.; Society for Optics and Photonics: Bellingham, WA, USA, 2001; Volume 4329, pp. 148–156. [Google Scholar]
- Baumgartner, R.; Keplinger, C.; Kaltseis, R.; Schwödiauer, R.; Bauer, S. Dielectric elastomers: From the beginning of modern science to applications in actuators and energy harvesters. Electroactive Polymer Actuators and Devices (EAPAD) 2011. Proc. SPIE 2011, 7976, 1–6. [Google Scholar] [CrossRef]
- Zhao, H.; Hussain, A.M.; Israr, A.; Vogt, D.M.; Duduta, M.; Clarke, D.R.; Wood, R.J. A wearable soft haptic communicator based on dielectric elastomer actuators. Soft Robot. 2020, 7, 451–461. [Google Scholar] [CrossRef]
- Moretti, G.; Rosset, S.; Vertechy, R.; Anderson, I.; Fontana, M. A review of dielectric elastomer generator systems. Adv. Intell. Syst. 2020, 2, 2000125. [Google Scholar] [CrossRef]
- Park, B.J.; Park, S.; Choi, M.; Park, S.K.; Yun, S.; Shin, E.; Woong, J. Monolithic focus-tunable lens technology enabled by disk-type dielectric-elastomer actuators. Sci. Rep. 2020, 10, 16937. [Google Scholar] [CrossRef]
- Wang, J.; Gao, D.; Lee, P.S. Recent Progress in Artificial Muscles for Interactive Soft Robotics. Adv. Mater. 2021, 33, 2003088. [Google Scholar] [CrossRef] [PubMed]
- Bahl, S.; Nagar, H.; Singh, I.; Sehgal, S. Smart materials types, properties and applications: A review. Mater. Today Proc. 2020, 28, 1302–1306. [Google Scholar] [CrossRef]
- Van de Voorde, M.; de Gruyter, W. (Eds.) Nanoscience and Nanotechnology: Advances and Developments in Nano-sized Materials. GmbH & Co KG: Berlin, Germany; Boston, MA, USA, 11 June 2018. [Google Scholar]
- Zhu, F.; Zhang, C.; Qian, J.; Chen, W.Q. Mechanics of dielectric elastomers: Materials, structures, and devices. J. Zhejiang Univ. Sci. A 2016, 17, 1–21. [Google Scholar]
- Clarson, S.J.; Semlyen, J.A. Siloxane Polymers; Englewood Cliffs, N.J., Ed.; Prentice Hall: Hoboken, NJ, USA, 1993. [Google Scholar]
- Lötters, J.C.; Olthuis, W.; Veltink, P.H.; Bergveld, P. The mechanical properties of the rubber elastic polymer polydimethylsiloxane for sensor applications. J. Micromech. Microeng 1997, 7, 145–147. [Google Scholar] [CrossRef]
- Mata, A.; Fleischman, A.J.; Roy, S. Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems. Biomed. Microdevices 2005, 7, 281–293. [Google Scholar] [CrossRef]
- Tugui, C.; Cazacu, M.; Sacarescu, L.; Bele, A.; Stiubianu, G.; Ursu, C.; Racles, C. Full silicone interpenetrating bi-networks with different organic groups attached to the silicon atoms. Polymer 2015, 77, 312–322. [Google Scholar] [CrossRef]
- Bricout, N. Advantages and disadvantages of silicones. In Breast Surgery; Springer: Paris, France, 1996. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A.; Zaborski, M. Polymer-based sensors: A review. Polym. Test. 2018, 67, 342–348. [Google Scholar] [CrossRef]
- Xu, F.; Li, X.; Shi, Y.; Li, L.; Wang, W.; He, L.; Liu, R. Recent developments for flexible pressure sensors: A review. Micromachines 2018, 9, 580. [Google Scholar] [CrossRef]
- Tugui, C.; Ursu, C.; Sacarescu, L.; Asandulesa, M.; Stoian, G.; Ababei, G.; Cazacu, M. Stretchable energy harvesting devices: Attempts to produce high performance electrodes. ACS Sustain. Chem. Eng. 2017, 5, 7851–7858. [Google Scholar] [CrossRef]
- Bele, A.; Tugui, C.; Sacarescu, L.; Iacob, M.; Stiubianu, G.; Dascalu, M.; Racles, C.; Cazacu, M. Ceramic nanotubes-based elastomer composites for applications in electromechanical transducers. Mater. Des. 2018, 141, 120–131. [Google Scholar] [CrossRef]
- Bele, A.; Tugui, C.; Asandulesa, M.; Ionita, D.; Vasiliu, L.; Stiubianu, G.; Iacob, M.; Racles, C.; Cazacu, M. Conductive stretchable composites properly engineered to develop highly compliant electrodes for dielectric elastomer actuators. Smart Mater. Struct. 2018, 27, 105005. [Google Scholar] [CrossRef]
- Bele, A.; Cazacu, M.; Neagu, M.; Popescu, M.; Racles, C.; Ioanid, G.I. Modular Installation and Procedure for Obtaining Stratified Polymeric Generators. Romanian Patent 132642, 30 March 2020. [Google Scholar]
- Zhang, J.; Sheng, J.; ONeill, C.T.; Walsh, C.J.; Wood, R.J.; Ryu, J.H.; Desai, J.P.; Yip, M.C. Robotic Artificial Muscles: Current Progress and Future Perspectives. IEEE Trans. Robot. 2019, 35, 761–781. [Google Scholar] [CrossRef]
- Kussmaul, B.; Risse, S.; Wegener, M.; Bluemke, M.; Krause, J.; Wagner, J.; Feller, T.; Clauberg, K.; Hitzbleck, J.; Gerhard, R.; et al. New DEA materials by organic modification of silicone and polyurethane networks. Electroact. Polym. Actuators Devices 2013, 8687. [Google Scholar] [CrossRef]
- Biedermann, M.; Blümke, M.; Wegener, M.; Krüger, H. Improved actuation strain of PDMS-based DEA materials chemically modified with softening agents. Electroact. Polym. Actuators Devices 2015, 9430. [Google Scholar] [CrossRef]
- Borayek, R.; Zhang, P.; Willy, H.J.; Zedan, M.; Zhu, J.; Ding, J. Programmable, UV-printable dielectric elastomers actuate at low voltage without prestretch and supporting frames. ACS Appl. Electron. Mater. 2020, 2, 4042–4053. [Google Scholar] [CrossRef]
- Opris, D.M. Polar Elastomers as novel materials for electromechanical actuator applications. Adv. Mater. 2018, 30, 1703678. [Google Scholar] [CrossRef] [PubMed]
- Racles, C.; Cazacu, M.; Fischer, B.; Opris, D.M. Synthesis and characterization of silicones containing cyanopropyl groups and their use in dielectric elastomer actuators. Smart Mater. Struct. 2013, 22, 104004. [Google Scholar] [CrossRef]
- Racles, C.; Cozan, V.; Bele, A.; Dascalu, M. Polar silicones: Structure-dielectric properties relationship. Des. Monomers Polym. 2016, 19, 496–507. [Google Scholar] [CrossRef]
- Racles, C.; Dascalu, M.; Bele, A.; Tiron, V.; Asandulesa, M.; Tugui, C.; Vasiliu, A.L.; Cazacu, M. All-silicone elastic composites with counter-intuitive piezoelectric response, designed for electromechanical applications. J. Mater. Chem. C 2017, 5, 6997–7010. [Google Scholar] [CrossRef]
- Racles, C.; Bele, A.; Dascalu, M.; Musteata, V.E.; Varganici, C.D.; Ionita, D.; Vlad, S.; Cazacu, M.; Dünki, S.J.; Opris, D.M. Polar-nonpolar interconnected elastic networks with increased permittivity and high breakdown fields for dielectric elastomer transducers. RSC Adv. 2015, 5, 58428–58438. [Google Scholar] [CrossRef]
- Racles, C.; Musteata, V.E.; Bele, A.; Dascalu, M.; Tugui, C.; Matricala, A.L. Highly stretchable composites from PDMS and polyazomethine fine particles. RSC Adv. 2015, 5, 102599–102609. [Google Scholar] [CrossRef]
- Bele, A.; Cazacu, M.; Stiubianu, G.; Vlad, S.; Ignat, M. Polydimethylsiloxane–barium titanate composites: Preparation and evaluation of the morphology, moisture, thermal, mechanical and dielectric behavior. Compos. B Eng. 2015, 68, 237–245. [Google Scholar] [CrossRef]
- Bele, A.; Cazacu, M.; Stiubianu, G.; Vlad, S. Silicone-barium titanate composites with increased electromechanical sensitivity. The effects of the filler morphology. RSC Adv. 2014, 4, 58522–58529. [Google Scholar] [CrossRef]
- Bele, A.; Stiubianu, G.; Varganici, C.-D.; Ignat, M.; Cazacu, M. Silicone dielectric elastomers based on radical crosslinked high molecular weight polydimethylsiloxane co-filled with silica and barium titanate. J. Mater. Sci. 2015, 50, 6822–6832. [Google Scholar] [CrossRef]
- Bele, A.; Dascalu, M.; Tugui, C.; Iacob, M.; Racles, C.; Sacarescu, L.; Cazacu, M. Dielectric silicone elastomers filled with in situ generated polar silsesquioxanes: Preparation, characterization and evaluation of electromechanical performance. Mater. Des. 2016, 106, 454–462. [Google Scholar] [CrossRef]
- Ştiubianu, G.; Soroceanu, A.; Varganici, C.-D.; Tugui, C.; Cazacu, M. Dielectric elastomers based on silicones filled with transitional metal complexes. Compos. B Eng. 2016, 93, 236–243. [Google Scholar] [CrossRef][Green Version]
- Stiubianu, G.; Dumitriu, A.-M.C.; Varganici, C.-D.; Tugui, C.; Iacob, M.; Bele, A.; Cazacu, M. Changes induced in the properties of dielectric silicone elastomers by the incorporation of transition metal complexes. High Perform. Polym. 2016, 28, 915–926. [Google Scholar] [CrossRef]
- Cazacu, M.; Racles, C.; Zaltariov, M.-F.; Dumitriu, A.-M.C.; Ignat, M.; Ovezea, D.; Stiubianu, G. Electroactive composites based on polydimethylsiloxane and some new metal complexes. Smart Mater. Struct. 2013, 22, 104008. [Google Scholar] [CrossRef]
- Iacob, M.; Sirbu, D.; Tugui, C.; Stiubianu, G.; Sacarescu, L.; Cozan, V.; Zeleňáková, A.; Čižmár, E.; Feher, A.; Cazacu, M. Superparamagnetic amorphous iron oxide nanowires self-assembled into ordered layered structures. RSC Adv. 2015, 5, 62563–62570. [Google Scholar] [CrossRef]
- Iacob, M.; Tugui, C.; Tiron, V.; Bele, A.; Vlad, S.; Vasiliu, T.; Cazacu, M.; Vasiliu, A.L.; Racles, C. Iron oxide nanoparticles as dielectric and piezoelectric enhancers for silicone elastomers. Smart Mater. Struct. 2017, 26, 105046. [Google Scholar] [CrossRef]
- Dvornic, P.R.; Jovanovic, J.D.; Govedarica, M.N. On the critical molecular chain length of polydimethylsiloxane. J. Appl. Polym. Sci. 1993, 49, 1497–1507. [Google Scholar] [CrossRef]
- Ryan, K.J.; Lupton, K.E.; Pape, P.; John, V.B. Ultra‐high‐molecular‐weight functional siloxane additives in polymers. Effects on processing and properties. J. Vynil Addit. Technol. 2000, 6, 7–19. [Google Scholar]
- Racles, C.; Dascalu, M.; Bele, A.; Cazacu, M. Reactive and functional silicones for special applications. In Reactive and Functional Polymers Volume One; Gutiérrez, T.J., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2020; Volume 11, pp. 235–291. [Google Scholar]
- Guoyong, W.; Zhiping, D.; Qiuxiao, L.; Wei, Z. Carbohydrate-modified siloxane surfactants and their adsorption and aggregation behavior in aqueous solution. J. Phys. Chem. B 2010, 114, 6872–6877. [Google Scholar] [CrossRef] [PubMed]
- Racles, C. Siloxane-based surfactants containing tromethamol units. Soft Mater 2010, 8, 263–273. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, D. Transition from Micelle to Vesicle of a Novel Sugar-Based Surfactant Containing Trisiloxane. Tenside Surfactants Deterg. 2016, 53, 273–277. [Google Scholar] [CrossRef]
- Racles, C.; Hamaide, T.; Ioanid, A. Siloxane surfactants in polymer nanoparticles formulation. Appl. Organomet. Chem. 2006, 20, 235–245. [Google Scholar] [CrossRef]
- Racles, C.; Silion, M.; Sacarescu, L. Multi-tasking pyridyl-functionalized siloxanes. Colloids Surf. A Physicochem. Eng. Asp. 2018, 547, 102–110. [Google Scholar] [CrossRef]
- Racles, C.; Mares, M.; Sacarescu, L. A Polysiloxane Surfactant Dissolves a Poorly Soluble Drug (Nystatin) in Water. Colloids Surf. A Physicochem. Eng. Asp. 2014, 443, 233–239. [Google Scholar] [CrossRef]
- Racles, C.; Cazacu, M.; Hitruc, G.; Hamaide, T. On the feasibility of chemical reactions in the presence of siloxane-based surfactants. Colloid Polym. Sci. 2009, 287, 461–470. [Google Scholar] [CrossRef]
- Racles, C.; Iacob, M.; Butnaru, M.; Sacarescu, L.; Cazacu, M. Aqueous dispersion of metal oxide nanoparticles, using siloxane surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2014, 448, 160–168. [Google Scholar] [CrossRef]
- Riess, G. Micellization of block copolymers. Prog. Polym. Sci. 2003, 28, 1107–1170. [Google Scholar] [CrossRef]
- Cazacu, M.; Vlad, A.; Munteanu, G.; Airinei, A. Multifunctional materials based on polyazomethines derived from 2,5-dihydroxy-1,4-benzoquinone and siloxane diamines. J. Polym. Sci. A Polym. Chem. 2008, 46, 1862–1872. [Google Scholar] [CrossRef]
- Vlad, A.; Cazacu, M.; Munteanu, G.; Airinei, A.; Budrugeac, P. Polyazomethines derived from polynuclear dihydroxyquinones and siloxane diamines. Eur. Polym. J. 2008, 44, 2668–2677. [Google Scholar] [CrossRef]
- Zaltariov, M.-F.; Cazacu, M.; Shova, S.; Varganici, C.-D.; Vacareanu, L.; Musteata, V.; Airinei, A. A silicon-containing polyazomethine and derived metal complexes: Synthesis, characterization, and evaluation of the properties. Des. Monomers Polym. 2014, 17, 668–683. [Google Scholar] [CrossRef][Green Version]
- Demus, D.; Goodby, J.; Gray, G.W.; Spiess, H.-W.; Vill, V. Handbook of Liquid Crystals; Wiley-VCH: Weinheim, Germany, 1998. [Google Scholar] [CrossRef]
- Teyssier, D.; Boileau, S. Liquid crystalline silicon-containing polymers. In Silicon-Containing Polymers; Jones, R.G., Ando, W., Chojnowski, J., Eds.; Kluver Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 2000; pp. 593–613. [Google Scholar]
- Racles, C. Siloxane-containing liquid crystalline polymers. In Advances in Functional Heterochain Polymers; Cazacu, M., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2008; pp. 33–66. [Google Scholar]
- Zhang, L.; Yao, W.; Gao, Y.; Zhang, C.; Yang, H. Polysiloxane-based side chain liquid crystal polymers: From synthesis to structure-phase transition behavior relationships. Polymers 2018, 10, 794. [Google Scholar] [CrossRef] [PubMed]
- Racles, C.; Cazacu, M. Siloxane-containing liquid-crystalline supramolecular polymers: Preparation and study of thermotropic behavior. J. Appl. Polym. Sci. 2008, 109, 4000–4009. [Google Scholar] [CrossRef]
- Racles, C.; Cozan, V. New siloxane copolymers with pendant azomethine mesogenic units. Rev. Roum. Chim. 2014, 59, 467–474. [Google Scholar]
- Curteanu, S.; Racles, C.; Cozan, V. Prediction of the liquid crystalline property for polyazomethines using modular neural networks. J. Optoelec. Adv. Mat. 2008, 10, 3382–3391. [Google Scholar]
- Racles, C. Phase separation and H-bonding trigger LC behavior in siloxane-containing compounds—Unpuplished work (manuscript in preparation).
- Zaltariov, M.-F.; Cazacu, M. Coordination compounds with siloxane/silane-containing ligands capable of self-assembly at nano/micro scale in solid state and in solution. In Advances in Inorganic Chemistry (Nanoscale Coordination Chemistry); Ruiz Molina, D., van Eldik, R., Eds.; Academic Press: London, UK, 2020; Volume 76, pp. 155–196. [Google Scholar]
- Cazacu, M.; Vlad, A.; Zaltariov, M.-F.; Shova, S.; Novitchi, G.; Train, C. Di- and tetracarboxylic aromatic acids with silane spacers and their copper complexes: Synthesis, structural characterization and properties evaluation. J. Organomet. Chem. 2014, 774, 70–78. [Google Scholar] [CrossRef]
- Vlad, A.; Zaltariov, M.-F.; Shova, S.; Novitchi, G.; Train, C.; Cazacu, M. Metal–organic frameworks based on tri- and penta-nuclear manganese(II) secondary building units self-assembled by a V-shaped silicon containing dicarboxylate. RSC Adv. 2016, 6, 37412–37423. [Google Scholar] [CrossRef]
- Turcan-Trofin, G.-O.; Avadanei, M.; Shova, S.; Vlad, A.; Cazacu, M.; Zaltariov, M.-F. Metallo-supramolecular assemblies of dinuclear Zn(II) and Mn(II) secondary building units (SBUs) and a bent silicon dicarboxylate ligand. Inorg. Chim. Acta 2018, 483, 454–463. [Google Scholar] [CrossRef]
- Cazacu, M.; Turcan-Trofin, G.-O.; Vlad, A.; Bele, A.; Shova, S.; Nicolescu, A.; Bargan, A. Hydrophobic, amorphous metal-organic network readily prepared by complexing the aluminum ion with a siloxane spaced dicarboxylic acid in aqueous medium. J. Appl Polym Sci. 2019, 136, 47144–47155. [Google Scholar] [CrossRef]
- Gavrish, S.P.; Shova, S.; Cazacu, M.; Dascalu, M.; Lampeka, Y.D. Syntheses and crystal structures of the one-dimensional coordination polymers formed by [Ni(cyclam)]2+ cations and 1,3-bis (3-carb oxy prop yl)tetra methyl disiloxane anions in different degrees of deprotonation. Acta Cryst. E 2020, E76, 446–451. [Google Scholar] [CrossRef]
- Gavrish, S.P.; Shova, S.; Cazacu, M.; Lampeka, Y.D. Crystal structure of the one-dimensional coordination polymer formed by the macrocyclic [Ni(cyclam)]2+ cation and the dianion of diphenylsilanediylbis(4-benzoic acid). Acta Cryst. E 2020, E76, 929–932. [Google Scholar] [CrossRef]
- Vlad, A.; Cazacu, M.; Zaltariov, M.-F.; Shova, S.; Turta, C.; Airinei, A. Metallopolymeric structures containing highly flexible siloxane sequence. Polymer 2013, 54, 43–53. [Google Scholar] [CrossRef]
- Vlad, A.; Zaltariov, M.F.; Shova, S.; Novitchi, G.; Varganici, C.D.; Train, C.; Cazacu, M. Flexible linkers and dinuclear metallic nodes build up an original metalorganic framework. CrystEngComm 2013, 15, 5368–5375. [Google Scholar] [CrossRef]
- Racles, C.; Shova, S.; Cazacu, M.; Timpu, D. New highly ordered hydrophobic siloxane-based coordination polymers. Polymer 2013, 54, 6096–6104. [Google Scholar] [CrossRef]
- Vlad, A.; Cazacu, M.; Zaltariov, M.-F.; Bargan, A.; Shova, S.; Turta, C. A 2D metal–organic framework based on dizinc coordination units bridged through both flexible and rigid ligands. J. Mol. Struct. 2014, 1060, 94–101. [Google Scholar] [CrossRef]
- Robin, A.Y.; Fromm, K.M. Coordination polymer networks with O- and N-donors: What they are, why and how they are made. Coord. Chem. Rev. 2006, 250, 2127–2157. [Google Scholar] [CrossRef]
| Properties | Elastomer Type | |||
|---|---|---|---|---|
| Silicone | Acrylic | Polyurethane | Natural Rubber | |
| Atmospheric ageing resistance | E | E | F | P |
| Oxidation resistance | E | E | E | G |
| Heat resistance | E | E | F–G | F–G |
| Low temperature flexibility | E | P | F | E |
| Moisture resistance | E | F | G | G–E |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazacu, M.; Racles, C.; Zaltariov, M.-F.; Dascalu, M.; Bele, A.; Tugui, C.; Bargan, A.; Stiubianu, G. From Amorphous Silicones to Si-Containing Highly Ordered Polymers: Some Romanian Contributions in the Field. Polymers 2021, 13, 1605. https://doi.org/10.3390/polym13101605
Cazacu M, Racles C, Zaltariov M-F, Dascalu M, Bele A, Tugui C, Bargan A, Stiubianu G. From Amorphous Silicones to Si-Containing Highly Ordered Polymers: Some Romanian Contributions in the Field. Polymers. 2021; 13(10):1605. https://doi.org/10.3390/polym13101605
Chicago/Turabian StyleCazacu, Maria, Carmen Racles, Mirela-Fernanda Zaltariov, Mihaela Dascalu, Adrian Bele, Codrin Tugui, Alexandra Bargan, and George Stiubianu. 2021. "From Amorphous Silicones to Si-Containing Highly Ordered Polymers: Some Romanian Contributions in the Field" Polymers 13, no. 10: 1605. https://doi.org/10.3390/polym13101605
APA StyleCazacu, M., Racles, C., Zaltariov, M.-F., Dascalu, M., Bele, A., Tugui, C., Bargan, A., & Stiubianu, G. (2021). From Amorphous Silicones to Si-Containing Highly Ordered Polymers: Some Romanian Contributions in the Field. Polymers, 13(10), 1605. https://doi.org/10.3390/polym13101605











