Novel Mangosteen-Leaves-Based Marker Ink: Color Lightness, Viscosity, Optimized Composition, and Microstructural Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Factors and Levels of the Design of Experiment
2.2. Design of Experiment
2.3. Raw Materials and Sample Preparation
2.4. Viscosity Test
2.5. Color Lightness Test
2.6. Thermogravimetric Analysis
2.7. Microstructure of Rice Husk Ash
3. Results and Discussion
3.1. Statistical Analysis of Color Lightness and Viscosity Properties
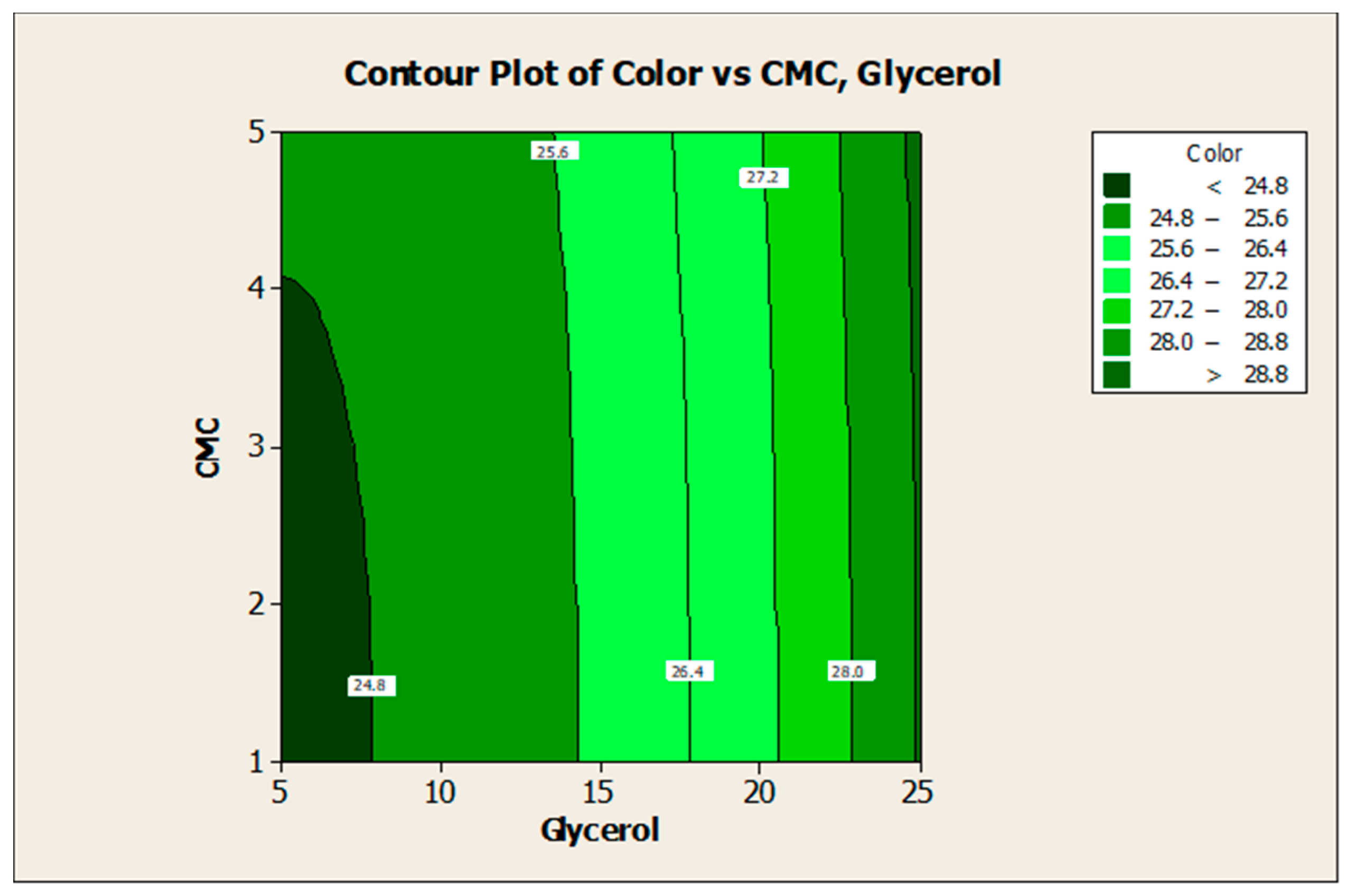
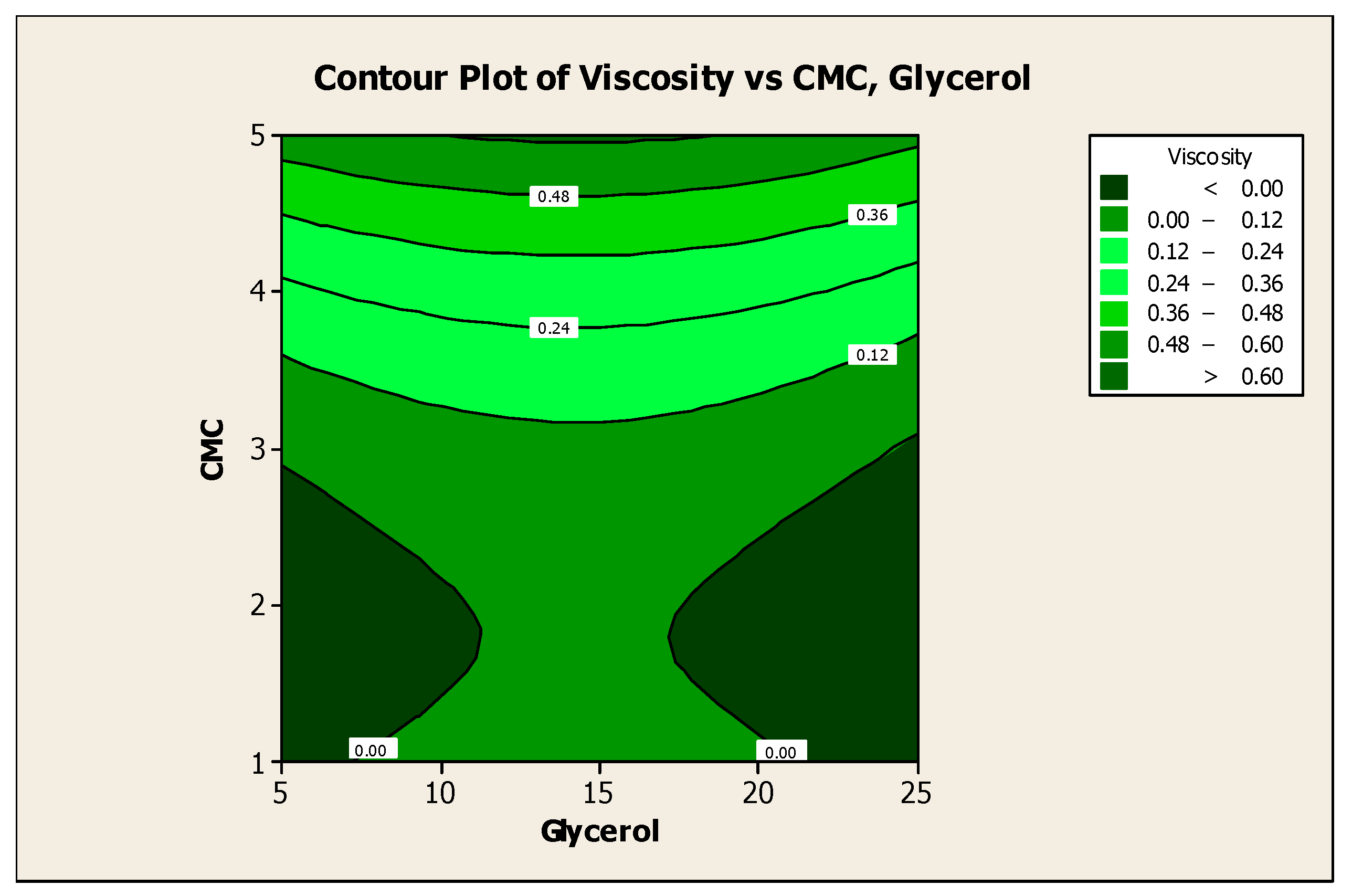
3.2. Effect of Factors on Color Lightness and Viscosity
3.3. Optimization of the Responses
3.4. Experimental Validation
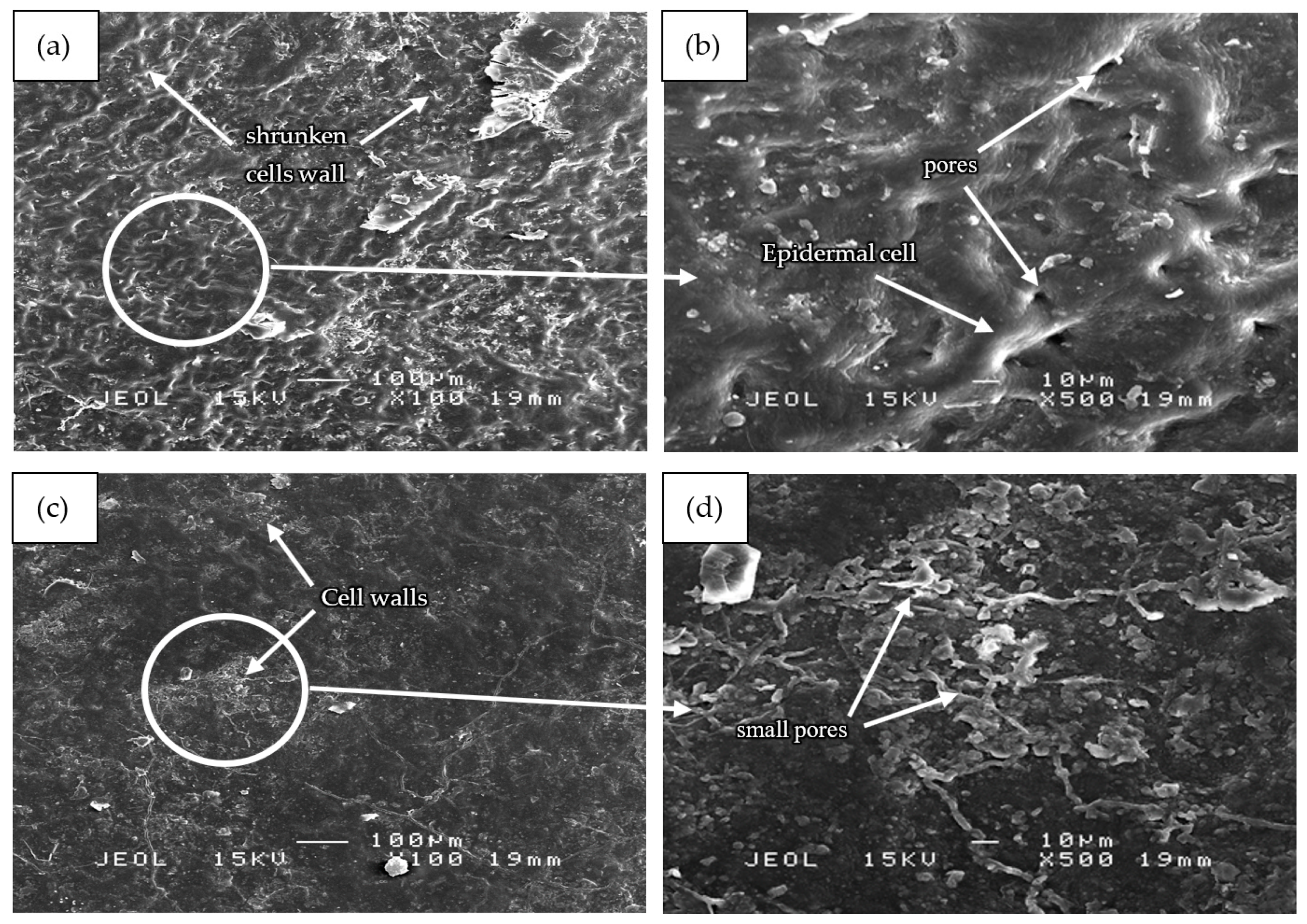
3.5. Microstructure Analysis of Mangosteen Leaves
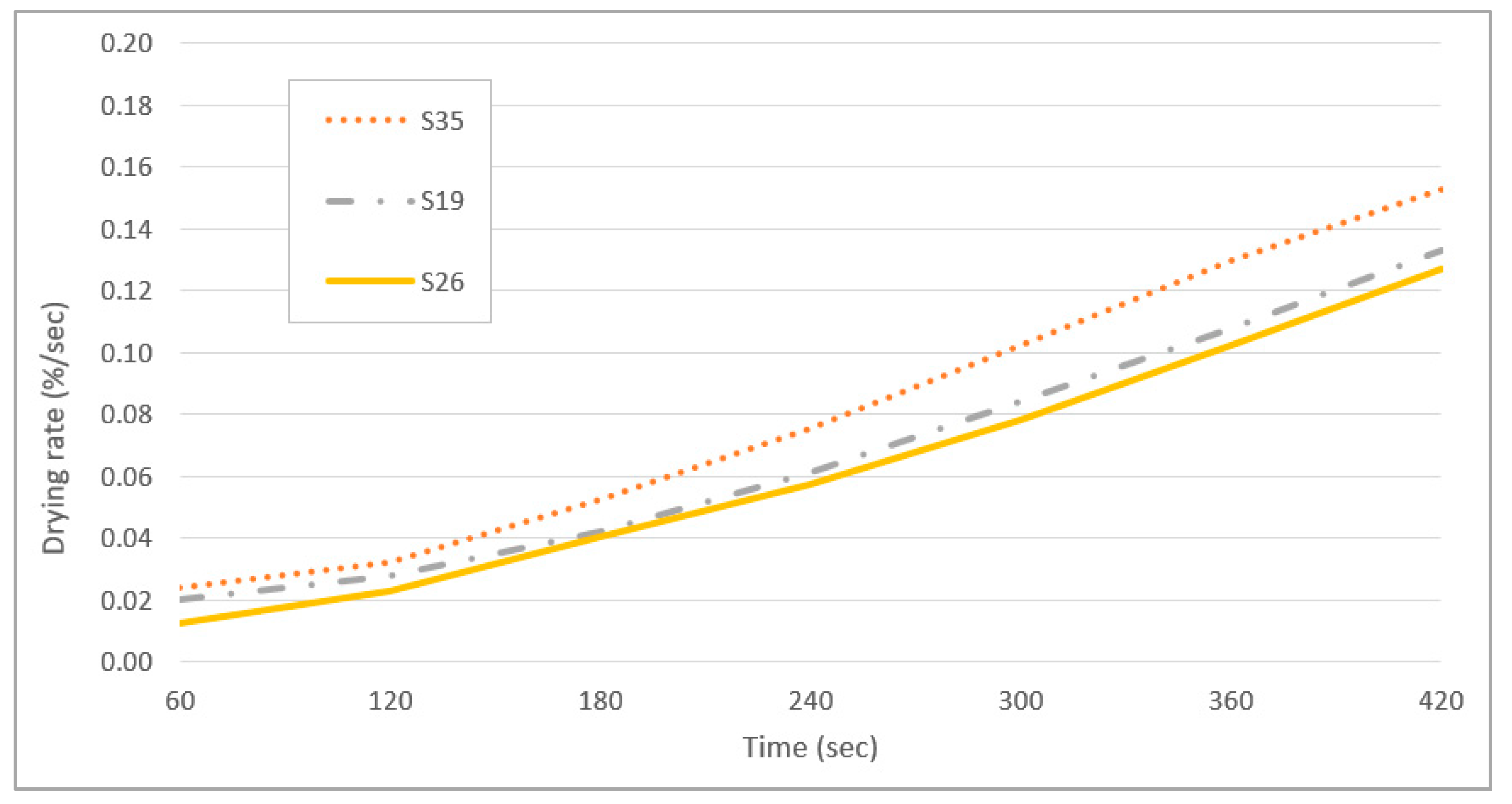
3.6. Drying Properties of Marker Ink
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piero, A.R.L.; Puglisi, I.; Rapisarda, A.P.; Petrone, G. Anthocyanins Accumulation and Related Gene Expression in Red Orange Fruit Induced by Low Temperature Storage. J. Agric. Food Chem. 2005, 53, 9083–9088. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, Y.; Liu, G.; Chen, S.; Zhang, Y.; Chen, C.; Chu, F.; Song, Y. Fabricating High-Resolution Metal Pattern with Inkjet Printed Water-Soluble Sacrificial Layer. ACS Appl. Mater. Interfaces 2020, 12, 22108–22114. [Google Scholar] [CrossRef]
- Sun, J.; Bao, B.; He, M.; Zhou, H.; Song, Y. Recent Advances in Controlling the Depositing Morphologies of Inkjet Droplets. ACS Appl. Mater. Interfaces 2015, 7, 28086–28099. [Google Scholar] [CrossRef] [PubMed]
- Puertas-Bartolomé, M.; Włodarczyk-Biegun, M.K.; Del Campo, A.; Vázquez-Lasa, B.; San Román, J. 3D Printing of a Reactive Hydrogel Bio-Ink Using a Static Mixing Tool. Polymers 2020, 12, 1986. [Google Scholar] [CrossRef] [PubMed]
- Noah, A.; Usman, S.; Alao, O.; Omisakin, O.; Olawale, A. Preliminary Investigation on Production of Brown Ink from Gmelina arborea (ROXB) Fruit Extract. Int. J. Sci. Basic Appl. Res. 2014, 18, 297–303. [Google Scholar]
- Nwafulugo, F.; Samuel, F.; Nyam, T.; Omale, S.; Nwosibe, P.O. Marker Ink Production from Berry Seed Extract. Int. J. Sci. Eng. Res. 2019, 10, 698–704. [Google Scholar]
- Dagde, K.K.; Nwosa, G.; Ukpaka, C. Formulation of White Board Marker Ink Using Locally Sourced Raw Materials. Eur. J. Eng. Res. Sci. 2019, 4, 107–114. [Google Scholar] [CrossRef]
- Shan, T.; Ma, Q.; Guo, K.; Liu, J.; Li, W.; Wang, F.; Wu, E. Xanthones from Mangosteen Extracts as Natural Chemopreventive Agents: Potential Anticancer Drugs. Curr. Mol. Med. 2011, 11, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Nakasone, H.Y.; Paull, R.E. Tropical Fruits; Cab International: Wallingford, UK, 1998; pp. 132–148. [Google Scholar]
- Yapwattanaphun, C.; Subhadrabandhu, S.; Sugiura, A.; Yonemori, K.; Utsunomiya, N. Utilization of some Garcinia species in Thailand. In Proceedings of the 2000 International Symposium on Tropical and Subtropical Fruits, Cairns, Australia, 25 November–1 December 2000; Volume 575, pp. 563–570. [Google Scholar]
- Abdul-Rahman, A.; Goh, H.-H.; Loke, K.-K.; Noor, N.M.; Aizat, W.M. RNA-seq analysis of mangosteen (Garcinia mangostana L.) fruit ripening. Genom. Data 2017, 12, 159–160. [Google Scholar] [CrossRef]
- Wrolstad, R. Anthocyanin Pigments—Bioactivity and Coloring Properties. J. Food Sci. 2004, 69, C419–C425. [Google Scholar] [CrossRef]
- Rasmussen, S.E.; Frederiksen, H.; Krogholm, K.S.; Poulsen, L. Dietary proanthocyanidins: Occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. Mol. Nutr. Food Res. 2005, 49, 159–174. [Google Scholar] [CrossRef]
- Kusumawati, N.; Santoso, A.B.; Sianita, M.M.; Muslim, S. Extraction, Characterization and Application of Natural Dyes from the Fresh Mangosteen (Garcinia mangostana L.) Peel. Int. J. Adv. Sci. Eng. Inf. Technol. 2017, 7, 878–885. [Google Scholar] [CrossRef]
- Habib, M.A.; Khoda, B. Development of clay based novel bio-ink for 3D bio-printing process. Procedia Manuf. 2018, 26, 846–856. [Google Scholar] [CrossRef]
- Ghannam, M.T.; Esmail, M.N. Rheological properties of carboxymethyl cellulose. J. Appl. Polym. Sci. 1997, 64, 289–301. [Google Scholar] [CrossRef]
- Pagliaro, M.; Rossi, M. The Future of Glycerol; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Bonnardeaux, J. Glycerin Overview; Western Australia Department of Agriculture and Food: Kensington, Australia, 2006.
- Medeiros, P.S.G.; Barbosa, C.R.F.; Fontes, F.A.O. Effects of addition glycerol co-product of biodiesel in the thermophysical properties of water-glycerol solution applied as secondary coolant. In Proceedings of the 13th Brazilian Congress of Thermal Sciences and Engineering, Uberlandia, Brazil, 5–10 December 2010. [Google Scholar]
- Cao, H.; Ai, L.; Yang, Z.; Zhu, Y. Application of Xanthan Gum as a Pre-Treatment and Sharpness Evaluation for Inkjet Printing on Polyester. Polymers 2019, 11, 1504. [Google Scholar] [CrossRef] [PubMed]
- Almoiqli, M.; Aldalbahi, A.; Rahaman, M.; Govindasami, P.; Alzahly, S. Influence of Biopolymer Carrageenan and Glycerine on the Properties of Extrusion Printed Inks of Carbon Nanotubes. Polymers 2018, 10, 1148. [Google Scholar] [CrossRef]
- Osemeahon, S.; John, M.; Dimas, B. Evaluation of the Use of Carbon Black from Waste Materials for the Production of Erasable White Board Ink. Chem. Res. J. 2020, 5, 97–104. [Google Scholar]
- Czitrom, V. One-factor-at-a-time versus designed experiments. Am. Stat. 1999, 53, 126–131. [Google Scholar]
- Box, G.E.; Wilson, K.B. On the experimental attainment of optimum conditions. In Breakthroughs in Statistics; Springer: Berlin/Heidelberg, Germany, 1992; pp. 270–310. [Google Scholar]
- Czyrski, A.; Jarzębski, H. Response Surface Methodology as a Useful Tool for Evaluation of the Recovery of the Fluoroquinolones from Plasma—The Study on Applicability of Box-Behnken Design, Central Composite Design and Doehlert Design. Processes 2020, 8, 473. [Google Scholar] [CrossRef]
- Flaifel, M.H. An Approach Towards Optimization Appraisal of Thermal Conductivity of Magnetic Thermoplastic Elastomeric Nanocomposites Using Response Surface Methodology. Polymers 2020, 12, 2030. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.Z.; Roslan, S.A.; Sapuan, S.; Rasid, Z.A.; Mohd Nor, A.F.; Md Daud, M.Y.; Dolah, R.; Yusoff, M.Z.M. Mercerization Optimization of Bamboo (Bambusa vulgaris) Fiber-Reinforced Epoxy Composite Structures Using a Box–Behnken Design. Polymers 2020, 12, 1367. [Google Scholar] [CrossRef]
- Azeem, B.; KuShaari, K.; Naqvi, M.; Keong, L.K.; AlMesfer, M.K.; Al-Qodah, Z.; Naqvi, S.R.; Elboughdiri, N. Production and Characterization of Controlled Release Urea Using Biopolymer and Geopolymer as Coating Materials. Polymers 2020, 12, 400. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, F.; Dong, L.; Li, Z.; Chen, L.; He, X.; Gong, J.; Zhang, J.; Li, Q. Design and Optimization of Flexible Polypyrrole/Bacterial Cellulose Conductive Nanocomposites Using Response Surface Methodology. Polymers 2019, 11, 960. [Google Scholar] [CrossRef] [PubMed]
- Tabaraki, R.; Nateghi, A. Optimization of ultrasonic-assisted extraction of natural antioxidants from rice bran using response surface methodology. Ultrason. Sonochem. 2011, 18, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Hyun, J.C. Drying of Coated Film. In Handbook of Solvents; ChemTec Publishing: Scarborough, ON, Canada, 2001; Volume 386. [Google Scholar]
- Zhou, W.; Apkarian, R.; Wang, Z.L.; Joy, D. Fundamentals of Scanning Electron Microscopy (SEM). In Scanning Microscopy for Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–40. [Google Scholar]
- Wells, O.C.; Joy, D.C. The early history and future of the SEM. Surf. Interface Anal. 2006, 38, 1738–1742. [Google Scholar] [CrossRef]
- Kowalska, G.; Wyrostek, J.; Kowalski, R.; Pankiewicz, U. Evaluation of glycerol usage for the extraction of anthocyanins from black chokeberry and elderberry fruits. J. Appl. Res. Med. Aromat. Plants 2021, 22, 100296. [Google Scholar] [CrossRef]
- Teszlák, P.; Gaál, K.; Pour Nikfardjam, M.S. Influence of grapevine flower treatment with gibberellic acid (GA3) on polyphenol content of Vitis vinifera L. wine. Anal. Chim. Acta 2005, 543, 275–281. [Google Scholar] [CrossRef]
- Edali, M.; Esmail, M.N.; Vatistas, G.H. Rheological properties of high concentrations of carboxymethyl cellulose solutions. J. Appl. Polym. Sci. 2001, 79, 1787–1801. [Google Scholar] [CrossRef]
- Benchabane, A.; Bekkour, K. Rheological properties of carboxymethyl cellulose (CMC) solutions. Colloid Polym. Sci. 2008, 286, 1173–1180. [Google Scholar] [CrossRef]
- Lewicki, P.P. Effect of pre-drying treatment, drying and rehydration on plant tissue properties: A review. Int. J. Food Prop. 1998, 1, 1–22. [Google Scholar] [CrossRef]
- Ben Salem-Fnayou, A.; Bouamama, B.; Ghorbel, A.; Mliki, A. Investigations on the leaf anatomy and ultrastructure of grapevine (Vitis vinifera) under heat stress. Microsc. Res. Tech. 2011, 74, 756–762. [Google Scholar] [CrossRef]
- Baniwal, S.K.; Bharti, K.; Chan, K.Y.; Fauth, M.; Ganguli, A.; Kotak, S.; Mishra, S.K.; Nover, L.; Port, M.; Scharf, K.-D.; et al. Heat stress response in plants: A complex game with chaperones and more than twenty heat stress transcription factors. J. Biosci. 2004, 29, 471–487. [Google Scholar] [CrossRef]
- Hou, X.; Chen, G.; Xing, T.; Wei, Z. Reactive ink formulated with various alcohols for improved properties and printing quality onto cotton fabrics. J. Eng. Fibers Fabr. 2019, 14, 1558925019849242. [Google Scholar] [CrossRef]
- Jouault, N.; Vallat, P.; Dalmas, F.; Said, S.; Jestin, J.; Boué, F. Well-Dispersed Fractal Aggregates as Filler in Polymer−Silica Nanocomposites: Long-Range Effects in Rheology. Macromolecules 2009, 42, 2031–2040. [Google Scholar] [CrossRef]
- Esbati, A.H.; Irani, S. Effect of functionalized process and CNTs aggregation on fracture mechanism and mechanical properties of polymer nanocomposite. Mech. Mater. 2018, 118, 106–119. [Google Scholar] [CrossRef]
- Ben Amor, B.; Allaf, K. Impact of texturing using instant pressure drop treatment prior to solvent extraction of anthocyanins from Malaysian Roselle (Hibiscus sabdariffa). Food Chem. 2009, 115, 820–825. [Google Scholar] [CrossRef]
- Nabli, R.; Achour, S.; Jourdes, M.; Helal, A.N.; Ezzili, B.; Teissedre, P.-L. Anthocyanin composition and extraction from Grenache noir (Vitis vinifera L.) vine leaf using an experimental design. I—By ethanol or sulfur dioxide. OENO One 2012, 46, 295. [Google Scholar] [CrossRef]
- Munekata, T.; Suzuki, T.; Yamakawa, S.; Asahi, R. Effects of viscosity, surface tension, and evaporation rate of solvent on dry colloidal structures: A lattice Boltzmann study. Phys. Rev. E 2013, 88, 052314. [Google Scholar] [CrossRef] [PubMed]
- Berk, Z. Food Process Engineering and Technology; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Varadarajan, P. To Determine the Drying Rate and the Rewettability Characteristics of Water Based Flexographic News Inks. Master’s Thesis, Rochester Institute of Technology, Rochester, NY, USA, 1990. [Google Scholar]
- Vaden, T.D.; Imre, D.; Beránek, J.; Shrivastava, M.; Zelenyuk, A. Evaporation kinetics and phase of laboratory and ambient secondary organic aerosol. Proc. Natl. Acad. Sci. USA 2011, 108, 2190–2195. [Google Scholar] [CrossRef]
- Roldin, P.; Eriksson, A.; Nordin, E.; Hermansson, E.; Mogensen, D.; Rusanen, A.; Boy, M.; Swietlicki, E.; Svenningsson, B.; Zelenyuk, A.; et al. Modelling non-equilibrium secondary organic aerosol formation and evaporation with the aerosol dynamics, gas- and particle-phase chemistry kinetic multilayer model ADCHAM. Atmos. Chem. Phys. 2014, 14, 7953–7993. [Google Scholar] [CrossRef]





| Factor | Unit | Notation | Levels | ||||
|---|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | |||
| CMC | wt % | V1 | 1 | 2 | 3 | 4 | 5 |
| Glycerol | wt % | V2 | 5 | 10 | 15 | 20 | 25 |
| Factors | Levels | Total Number of Experimental Runs | |
|---|---|---|---|
| Full Factorial Design | RSM | ||
| 2 | 5 | 25 | 14 |
| 3 | 5 | 125 | 20 |
| 4 | 5 | 625 | 30 |
| 5 | 5 | 3125 | 54 |
| Sample | Coded Factor | Uncoded Factor | ||
|---|---|---|---|---|
| V1 | V2 | V1 | V2 | |
| S1 | −2 | 0 | 5 | 3 |
| S2 | 0 | 2 | 15 | 5 |
| S3 | 1 | −1 | 20 | 2 |
| S4 | −1 | 1 | 10 | 4 |
| S5 | −2 | 0 | 5 | 3 |
| S6 | 0 | 0 | 15 | 3 |
| S7 | −1 | −1 | 10 | 2 |
| S8 | 0 | 0 | 15 | 3 |
| S9 | 2 | 0 | 25 | 3 |
| S10 | 0 | 0 | 15 | 3 |
| S11 | 0 | 0 | 15 | 3 |
| S12 | 0 | −2 | 15 | 1 |
| S13 | 1 | −1 | 20 | 2 |
| S14 | 0 | 0 | 15 | 3 |
| S15 | −2 | 0 | 5 | 3 |
| S16 | −1 | −1 | 10 | 2 |
| S17 | −1 | −1 | 10 | 2 |
| S18 | 0 | 0 | 15 | 3 |
| S19 | 1 | 1 | 20 | 4 |
| S20 | 0 | 0 | 15 | 3 |
| S21 | 0 | 0 | 15 | 3 |
| S22 | 0 | 0 | 15 | 3 |
| S23 | 1 | −1 | 20 | 2 |
| S24 | 1 | 1 | 20 | 4 |
| S25 | 0 | 0 | 15 | 3 |
| S26 | −1 | 1 | 10 | 4 |
| S27 | 0 | −2 | 15 | 1 |
| S28 | 0 | 2 | 15 | 5 |
| S29 | 0 | 0 | 15 | 3 |
| S30 | 0 | 0 | 15 | 3 |
| S31 | 2 | 0 | 25 | 3 |
| S32 | 0 | −2 | 15 | 1 |
| S33 | −1 | 1 | 10 | 4 |
| S34 | 0 | 0 | 15 | 3 |
| S35 | 2 | 0 | 25 | 3 |
| S36 | 1 | 1 | 20 | 4 |
| S37 | 0 | 0 | 15 | 3 |
| S38 | 0 | 2 | 15 | 5 |
| S39 | 0 | 0 | 15 | 3 |
| Sample | Glycerol (V1) | CMC (V2) | Glycerol (V1) | CMC (V2) | Viscosity (Pa.s) | Color lightness (L*) |
|---|---|---|---|---|---|---|
| S1 | −2 | 0 | 5 | 3 | 0.0221 | 24.70 |
| S2 | 0 | 2 | 15 | 5 | 0.7278 | 25.83 |
| S3 | 1 | −1 | 20 | 2 | 0.0132 | 27.20 |
| S4 | −1 | 1 | 10 | 4 | 0.2320 | 25.11 |
| S5 | −2 | 0 | 5 | 3 | 0.0163 | 24.73 |
| S6 | 0 | 0 | 15 | 3 | 0.1137 | 25.79 |
| S7 | −1 | −1 | 10 | 2 | 0.0076 | 24.93 |
| S8 | 0 | 0 | 15 | 3 | 0.1090 | 25.78 |
| S9 | 2 | 0 | 25 | 3 | 0.0091 | 28.80 |
| S10 | 0 | 0 | 15 | 3 | 0.1117 | 25.62 |
| S11 | 0 | 0 | 15 | 3 | 0.1098 | 25.58 |
| S12 | 0 | −2 | 15 | 1 | 0.0045 | 25.83 |
| S13 | 1 | −1 | 20 | 2 | 0.0111 | 27.16 |
| S14 | 0 | 0 | 15 | 3 | 0.1083 | 25.80 |
| S15 | −2 | 0 | 5 | 3 | 0.0203 | 24.78 |
| S16 | −1 | −1 | 10 | 2 | 0.0109 | 25.14 |
| S17 | −1 | −1 | 10 | 2 | 0.0084 | 24.99 |
| S18 | 0 | 0 | 15 | 3 | 0.1042 | 25.61 |
| S19 | 1 | 1 | 20 | 4 | 0.1595 | 27.40 |
| S20 | 0 | 0 | 15 | 3 | 0.0948 | 25.78 |
| S21 | 0 | 0 | 15 | 3 | 0.1077 | 25.84 |
| S22 | 0 | 0 | 15 | 3 | 0.1064 | 25.85 |
| S23 | 1 | −1 | 20 | 2 | 0.0211 | 27.13 |
| S24 | 1 | 1 | 20 | 4 | 0.1571 | 27.48 |
| S25 | 0 | 0 | 15 | 3 | 0.1063 | 25.74 |
| S26 | −1 | 1 | 10 | 4 | 0.2245 | 25.09 |
| S27 | 0 | −2 | 15 | 1 | 0.0045 | 25.70 |
| S28 | 0 | 2 | 15 | 5 | 0.7082 | 25.85 |
| S29 | 0 | 0 | 15 | 3 | 0.1037 | 25.63 |
| S30 | 0 | 0 | 15 | 3 | 0.1083 | 25.72 |
| S31 | 2 | 0 | 25 | 3 | 0.0096 | 28.78 |
| S32 | 0 | −2 | 15 | 1 | 0.0045 | 25.62 |
| S33 | −1 | 1 | 10 | 4 | 0.2173 | 25.22 |
| S34 | 0 | 0 | 15 | 3 | 0.0973 | 25.72 |
| S35 | 2 | 0 | 25 | 3 | 0.0100 | 28.78 |
| S36 | 1 | 1 | 20 | 4 | 0.1344 | 27.00 |
| S37 | 0 | 0 | 15 | 3 | 0.0960 | 25.73 |
| S38 | 0 | 2 | 15 | 5 | 0.6262 | 25.72 |
| S39 | 0 | 0 | 15 | 3 | 0.1083 | 25.76 |
| Term | Notation | Coefficient | Std. Error of Coefficient | p |
|---|---|---|---|---|
| Constant | 25.7739 | 0.03071 | 0.000 | |
| Glycerol | V1 | 1.0331 | 0.02135 | 0.000 |
| CMC | V2 | 0.0347 | 0.02135 | 0.113 |
| Glycerol*Glycerol | V1* V1 | 0.2607 | 0.01545 | 0.000 |
| CMC*CMC | V2* V2 | 0.0098 | 0.01545 | 0.529 |
| R2 = 0.9873 R2 (adj) = 0.9858 | ||||
| Term | Notation | Coefficient | Std. Error of Coefficient | p |
|---|---|---|---|---|
| Constant | 0.0938 | 0.01191 | 0.000 | |
| Glycerol | V1 | −0.0073 | 0.00828 | 0.382 |
| CMC | V2 | 0.1431 | 0.00828 | 0.000 |
| Glycerol*Glycerol | V1* V1 | −0.0235 | 0.00599 | 0.000 |
| CMC*CMC | V2* V2 | 0.0593 | 0.00599 | 0.000 |
| R2 = 0.9292 R2 (adj) = 0.9209 | ||||
| Sample | Color Lightness (L*) | Viscosity (Pa.s) | ||||
|---|---|---|---|---|---|---|
| Experimental Value | Predicted Value | Error (%) | Experimental Value | Predicted Value | Error (%) | |
| SV1 | 24.99 | 24.75 | 0.97 | 0.0144 | 0.0143 | 0.70 |
| SV2 | 24.80 | 24.75 | 0.20 | 0.0152 | 0.0143 | 6.29 |
| SV3 | 24.83 | 24.75 | 0.32 | 0.0172 | 0.0143 | 20.28 |
| Error | 0.50 | Error | 9.09 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Basri, M.S.; Liew Min Ren, B.; A. Talib, R.; Zakaria, R.; Kamarudin, S.H. Novel Mangosteen-Leaves-Based Marker Ink: Color Lightness, Viscosity, Optimized Composition, and Microstructural Analysis. Polymers 2021, 13, 1581. https://doi.org/10.3390/polym13101581
Mohd Basri MS, Liew Min Ren B, A. Talib R, Zakaria R, Kamarudin SH. Novel Mangosteen-Leaves-Based Marker Ink: Color Lightness, Viscosity, Optimized Composition, and Microstructural Analysis. Polymers. 2021; 13(10):1581. https://doi.org/10.3390/polym13101581
Chicago/Turabian StyleMohd Basri, Mohd Salahuddin, Brenda Liew Min Ren, Rosnita A. Talib, Rabitah Zakaria, and Siti Hasnah Kamarudin. 2021. "Novel Mangosteen-Leaves-Based Marker Ink: Color Lightness, Viscosity, Optimized Composition, and Microstructural Analysis" Polymers 13, no. 10: 1581. https://doi.org/10.3390/polym13101581
APA StyleMohd Basri, M. S., Liew Min Ren, B., A. Talib, R., Zakaria, R., & Kamarudin, S. H. (2021). Novel Mangosteen-Leaves-Based Marker Ink: Color Lightness, Viscosity, Optimized Composition, and Microstructural Analysis. Polymers, 13(10), 1581. https://doi.org/10.3390/polym13101581








