Iron Species-Supporting Hydrophobic and Nonswellable Polytetrafluoroethylene/Poly(acrylic acid-co-hydroxyethyl methacrylate) Composite Fiber and Its Stable Catalytic Activity for Methylene Blue Oxidative Decolorization
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Polymer Synthesis
2.3. Fiber Formation
2.4. Catalyst Preparation
2.5. Measurement and Characterization
2.5.1. Morphology
2.5.2. Chemical Group
2.5.3. Heat Resistance
2.5.4. Water Contact Angle
2.5.5. Surface Element
2.5.6. Aggregate Structure
2.5.7. Water Resistance
2.5.8. Catalytic Activity
2.5.9. Total Organic Carbon
2.5.10. Free Radical Species
3. Results and Discussion
3.1. The Confirmation of Crosslinking
3.2. The Significance of Crosslinking
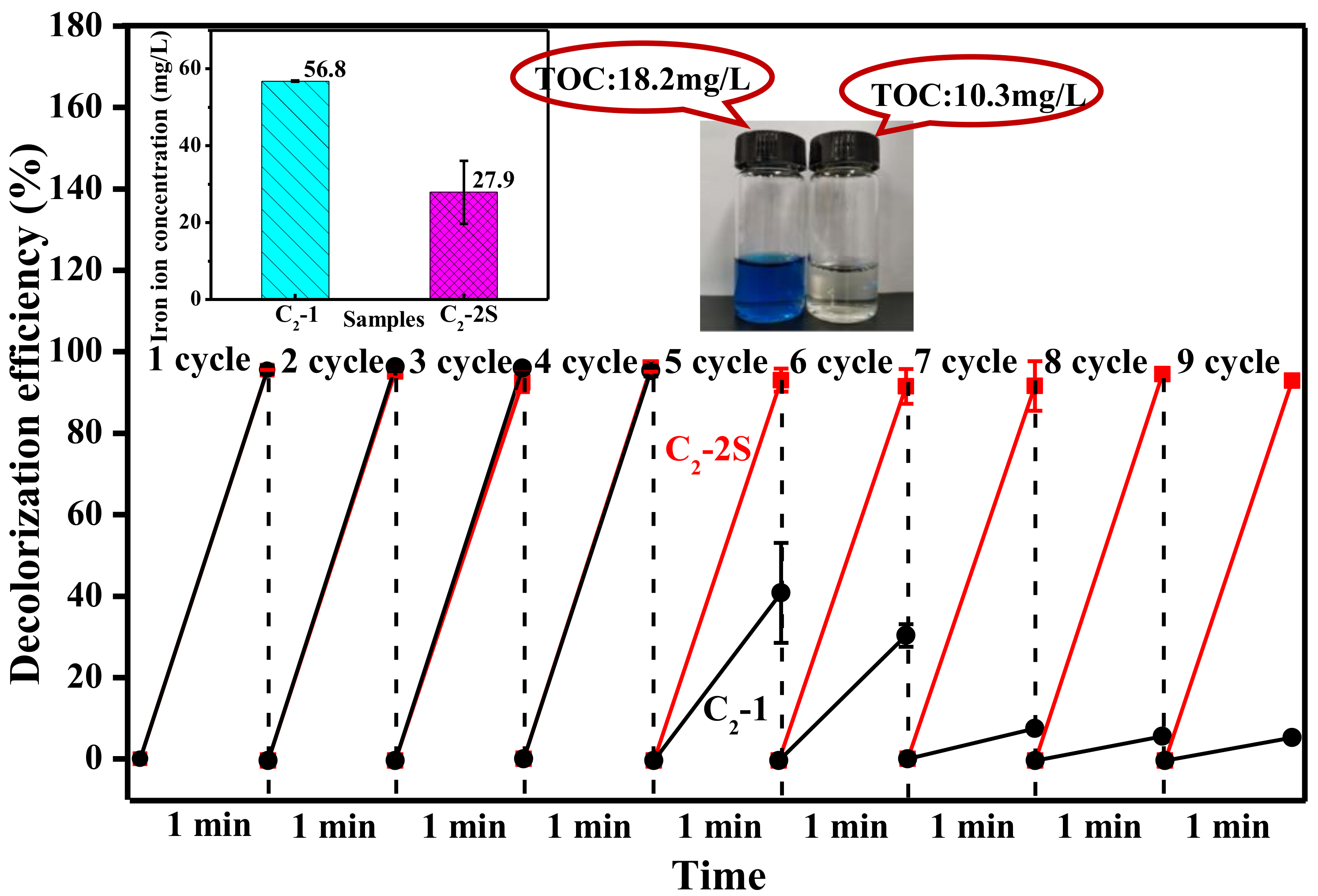
3.3. The Role of PTFE
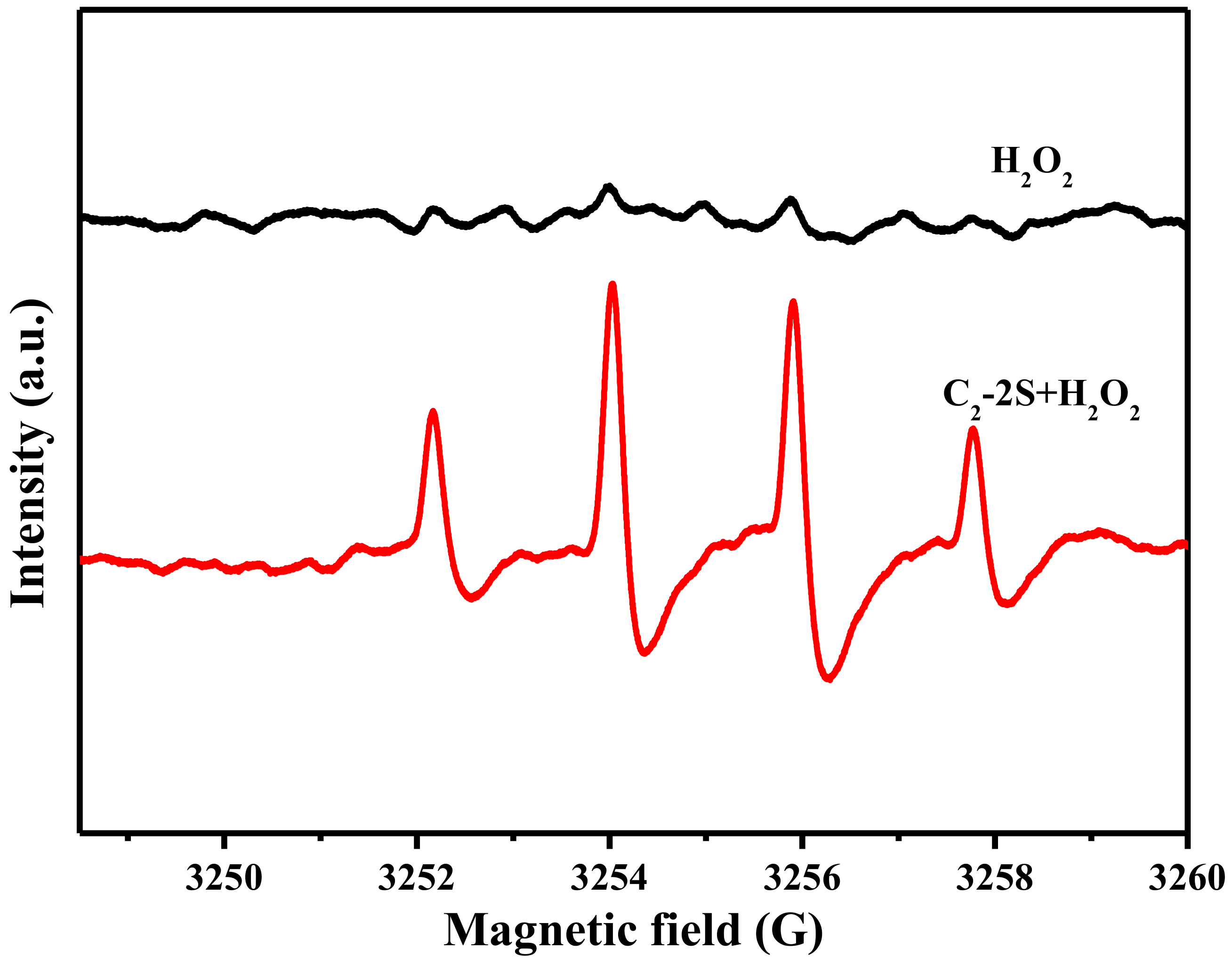
3.4. The Decolorization Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brillas, E.; Martínez-Huitle, C.A. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl. Catal. B Environ. 2015, 166–167, 603–643. [Google Scholar] [CrossRef]
- Khan, H.R.; Murtaza, G.; Choudhary, M.A.; Ahmed, Z.; Malik, M.A. Photocatalytic removal of carcinogenic reactive red S3B dye by using ZnO and Cu doped ZnO nanoparticles synthesized by polyol method: A kinetic study. Sol. Energy 2018, 173, 875–881. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Inchaurrondo, N.; Ramos, C.; Žerjav, G.; Font, J.; Pintar, A.; Haure, P.; Capafons, J.F. Modified diatomites for Fenton-like oxidation of phenol. Microporous Mesoporous Mater. 2017, 239, 396–408. [Google Scholar] [CrossRef]
- Lei, X.; You, M.; Pan, F.; Liu, M.; Yang, P.; Xia, D.; Li, Q.; Wang, Y.; Fu, J. CuFe2O4@GO nanocomposite as an effective and recoverable catalyst of peroxymonosulfate activation for degradation of aqueous dye pollutants. Chin. Chem. Lett. 2019, 30, 2216–2220. [Google Scholar] [CrossRef]
- Paździor, K.; Bilińska, L.; Ledakowicz, S. A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem. Eng. J. 2019, 376, 120597. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2016, 284, 582–598. [Google Scholar] [CrossRef]
- Liu, M.; Yu, Y.; Xiong, S.; Lin, P.; Hu, L.; Chen, S.; Wang, H.; Wang, L. A flexible and efficient electro-Fenton cathode film with aeration function based on polyphenylene sulfide ultra-fine fiber. React. Funct. Polym. 2019, 139, 42–49. [Google Scholar] [CrossRef]
- Segura, Y.; Martínez, F.; Melero, J. Effective pharmaceutical wastewater degradation by Fenton oxidation with zero-valent iron. Appl. Catal. B Environ. 2013, 136–137, 64–69. [Google Scholar] [CrossRef]
- Chen, W.; Yang, X.; Huang, J.; Zhu, Y.; Zhou, Y.; Yao, Y.; Li, C. Iron oxide containing graphene/carbon nanotube based carbon aerogel as an efficient E-Fenton cathode for the degradation of methyl blue. Electrochim. Acta 2016, 200, 75–83. [Google Scholar] [CrossRef]
- Liu, J.; Cui, J.; Zhao, T.; Fan, S.; Zhang, C.; Hu, Q.; Hou, X. Fe3O4-CeO2 loaded on modified activated carbon as efficient heterogeneous catalyst. Colloids Surf. A Physicochem. Eng. Asp. 2019, 565, 59–69. [Google Scholar] [CrossRef]
- Pang, J.; Fu, F.; Li, W.; Zhu, L.; Tang, B. Fe-Mn binary oxide decorated diatomite for rapid decolorization of methylene blue with H2O2. Appl. Surf. Sci. 2019, 478, 54–61. [Google Scholar] [CrossRef]
- Sun, T.; Gong, M.; Cai, Y.; Xiao, S.; Zhang, L.; Zhang, Y.; Xu, Z.; Zhang, D.; Liu, Y.; Zhou, C. MCM-41-supported Fe(Mn)/Cu bimetallic heterogeneous catalysis for enhanced and recyclable photo-Fenton degradation of methylene blue. Res. Chem. Intermed. 2020, 46, 459–474. [Google Scholar] [CrossRef]
- Hu, L.; Liu, Z.; He, C.; Wang, P.; Chen, S.; Xu, J.; Wu, J.; Wang, L.; Wang, H. Ferrous-oxalate-decorated polyphenylene sulfide fenton catalytic microfiber for methylene blue degradation. Compos. Part B Eng. 2019, 176, 107220. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, Q.-L.; Liu, H.; Zhang, C.-X.; You, Y.-W.; Li, N.-N.; Xiao, C.-F. Preparation, characterization, and applications of electrospun ultrafine fibrous PTFE porous membranes. J. Membr. Sci. 2017, 523, 317–326. [Google Scholar] [CrossRef]
- Kang, W.; Zhao, H.; Ju, J.; Shi, Z.; Qiao, C.; Cheng, B. Electrospun poly(tetrafluoroethylene) nanofiber membranes from PTFE-PVA-BA-H2O gel-spinning solutions. Fibers Polym. 2016, 17, 1403–1413. [Google Scholar] [CrossRef]
- Chen, S.; Xu, N.; Ren, M.; Xiao, C.; Zhang, X. PEI/GO-codecorated poly(acrylic acid-co-hydroxyethyl methacrylate) fiber as a carrier to support iron ions and its catalytic performance for methylene blue decolorization. J. Macromol. Sci. Part A 2020, 57, 531–543. [Google Scholar] [CrossRef]
- Lv, Y.; Xu, N.; Feng, Y.; Lu, Y. Fiber prepared via swelling and thermal cross-linking as reusable Fenton catalyst for methylene blue decolorization. Adv. Polym. Technol. 2018, 37, 3186–3198. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, N.; Lv, Y.; Feng, Y. Fibrous material based on a combination of poly(acrylic acid-co-hydroxyethyl methacrylate) with iron ions as a heterogeneous Fenton catalyst for dye oxidative decomposition. J. Appl. Polym. Sci. 2017, 134, 44875. [Google Scholar] [CrossRef]
- Stevanic, J.S.; Salmén, L. Molecular origin of mechano-sorptive creep in cellulosic fibres. Carbohydr. Polym. 2020, 230, 115615. [Google Scholar] [CrossRef]
- Amariei, G.; Boltes, K.; Letón, P.; Iriepa, I.; Moraleda, I.; Rosal, R. Poly(amidoamine) dendrimers grafted on electrospun poly(acrylic acid)/poly(vinyl alcohol) membranes for host–guest encapsulation of antioxidant thymol. J. Mater. Chem. B 2017, 5, 6776–6785. [Google Scholar] [CrossRef]
- Iqbal, D.N.; Tariq, M.; Khan, S.M.; Gull, N.; Iqbal, S.S.; Aziz, A.; Nazir, A.; Iqbal, M. Synthesis and characterization of chitosan and guar gum based ternary blends with polyvinyl alcohol. Int. J. Biol. Macromol. 2020, 143, 546–554. [Google Scholar] [CrossRef]
- Guo, S.; Liu, Q.; Zhao, J.; Jin, G.; Wang, X.; Lang, Z.; He, W.; Gong, Z. Evaluation and comparison of erythritol-based composites with addition of expanded graphite and carbon nanotubes. Appl. Energy 2017, 205, 703–709. [Google Scholar] [CrossRef]
- Mahendraprabu, A.; Sangeetha, T.; Kannan, P.; Karthick, N.; Kumbharkhane, A.; Arivazhagan, G. Hydrogen bond interactions of ethyl acetate with methyl Cellosolve: FTIR spectroscopic and dielectric relaxation studies. J. Mol. Liq. 2020, 301, 112490. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Shaw, S.; Alla, S.; Meena, S.; Mandal, R.; Prasad, N. Stabilization of temperature during magnetic hyperthermia by Ce substituted magnetite nanoparticles. J. Magn. Magn. Mater. 2017, 434, 181–186. [Google Scholar] [CrossRef]
- Bhargava, S.; Makowiec, M.E.; Blanchet, T.A. Wear reduction mechanisms within highly wear-resistant graphene- and other carbon-filled PTFE nanocomposites. Wear 2020, 444–445, 203163. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Huang, X.; Zou, X.; Shi, J.; Xu, Y.; Hu, X.; Sun, Y.; Zhai, X. Hypha-templated synthesis of carbon/ZnO microfiber for dopamine sensing in pork. Food Chem. 2021, 335, 127646. [Google Scholar] [CrossRef]
- Somsesta, N.; Sricharoenchaikul, V.; Aht-Ong, D. Adsorption removal of methylene blue onto activated carbon/cellulose biocomposite films: Equilibrium and kinetic studies. Mater. Chem. Phys. 2020, 240, 122221. [Google Scholar] [CrossRef]
- Smolyanskii, A.S.; Arsentyev, M.A.; Rashkovskii, A.Y.; Politova, E.D. Radiation-Induced Changes in the Degree of Crystallinity of Powdered Polytetrafluoroethylene. Crystallogr. Rep. 2019, 64, 553–558. [Google Scholar] [CrossRef]
- Guo, Y.; Li, C.; Gong, Z.; Guo, Y.; Wang, X.; Gao, B.; Qin, W.; Wang, G. Photocatalytic decontamination of tetracycline and Cr(VI) by a novel α-FeOOH/FeS2 photocatalyst: One-pot hydrothermal synthesis and Z-scheme reaction mechanism insight. J. Hazard. Mater. 2020, 397, 122580. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, F.; Lei, T.; Ren, Z.; Wang, S.; Yang, X. Phosphorus removal by in situ sprayed ferric chloride in Dianchi Lake: Efficiency, stability, and mechanism. Process. Saf. Environ. Prot. 2019, 131, 320–328. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, K.; Cai, W.; Qiao, L.; Ying, Y.; Li, W.; Yu, J.; Lin, M.; Che, S. Effect of chloride ion on crystalline phase transition of iron oxide produced by ultrasonic spray pyrolysis. Adv. Powder Technol. 2018, 29, 1953–1959. [Google Scholar] [CrossRef]
- Wang, X.; Liu, P.; Fu, M.; Ma, J.; Ning, P. Novel sequential process for enhanced dye synergistic degradation based on nano zero-valent iron and potassium permanganate. Chemosphere 2016, 155, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cui, K.; Guo, Z.; Yang, T.; Cao, Y.; Xiang, Y.; Chen, H.; Xi, M. Heterogeneous Fenton-like degradation of tetracyclines using porous magnetic chitosan microspheres as an efficient catalyst compared with two preparation methods. Chem. Eng. J. 2020, 379, 122324. [Google Scholar] [CrossRef]
- Hong, P.; Li, Y.; He, J.; Saeed, A.; Zhang, K.; Wang, C.; Kong, L.; Liu, J. Rapid degradation of aqueous doxycycline by surface CoFe2O4/H2O2 system: Behaviors, mechanisms, pathways and DFT calculation. Appl. Surf. Sci. 2020, 526, 146557. [Google Scholar] [CrossRef]
- Cai, J.; Li, H. Electrospun polymer nanofibers coated with TiO2 hollow spheres catalyze for high synergistic photo-conversion of Cr(VI) and As(III) using visible light. Chem. Eng. J. 2020, 398, 125644. [Google Scholar] [CrossRef]
- Ma, C.; Feng, S.; Zhou, J.; Chen, R.; Wei, Y.; Liu, H.; Wang, S. Enhancement of H2O2 decomposition efficiency by the co-catalytic effect of iron phosphide on the Fenton reaction for the degradation of methylene blue. Appl. Catal. B Environ. 2019, 259, 118015. [Google Scholar] [CrossRef]
- Zhang, M.; Dong, H.; Zhao, L.; Wang, D.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total. Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Morshed, M.N.; Bouazizi, N.; Behary, N.; Guan, J.; Nierstrasz, V. Stabilization of zero valent iron (Fe0) on plasma/dendrimer functionalized polyester fabrics for Fenton-like removal of hazardous water pollutants. Chem. Eng. J. 2019, 374, 658–673. [Google Scholar] [CrossRef]


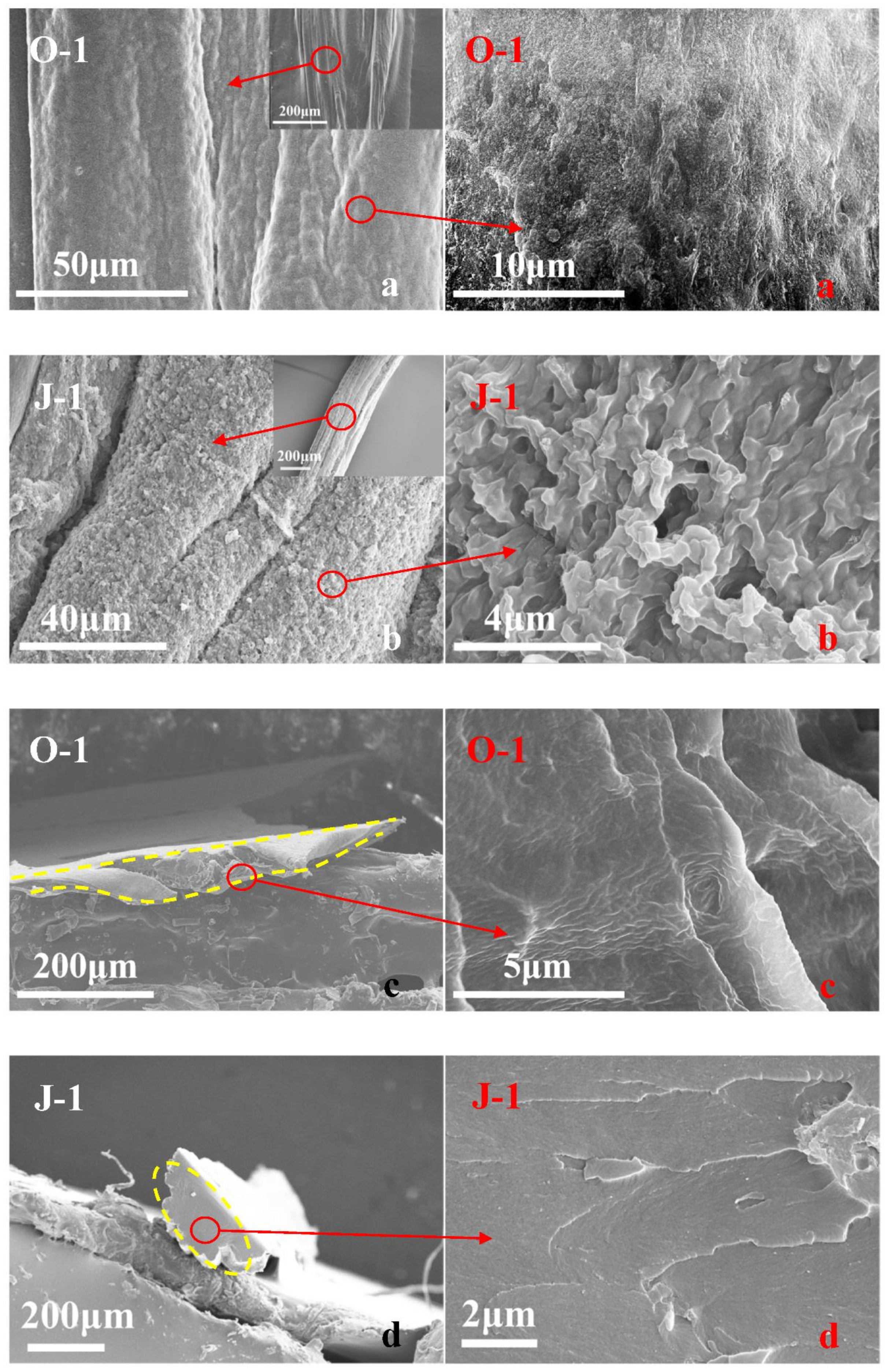
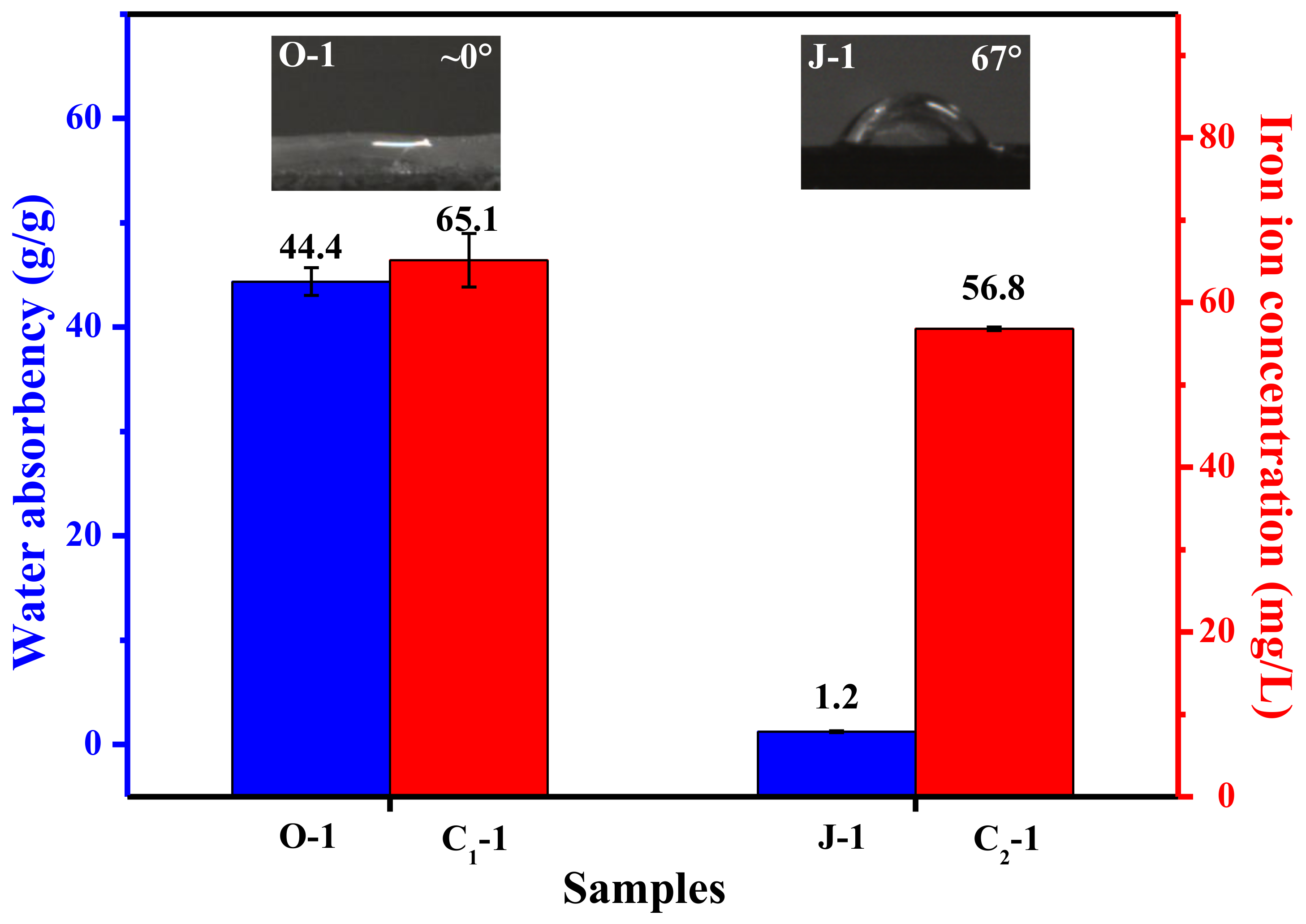
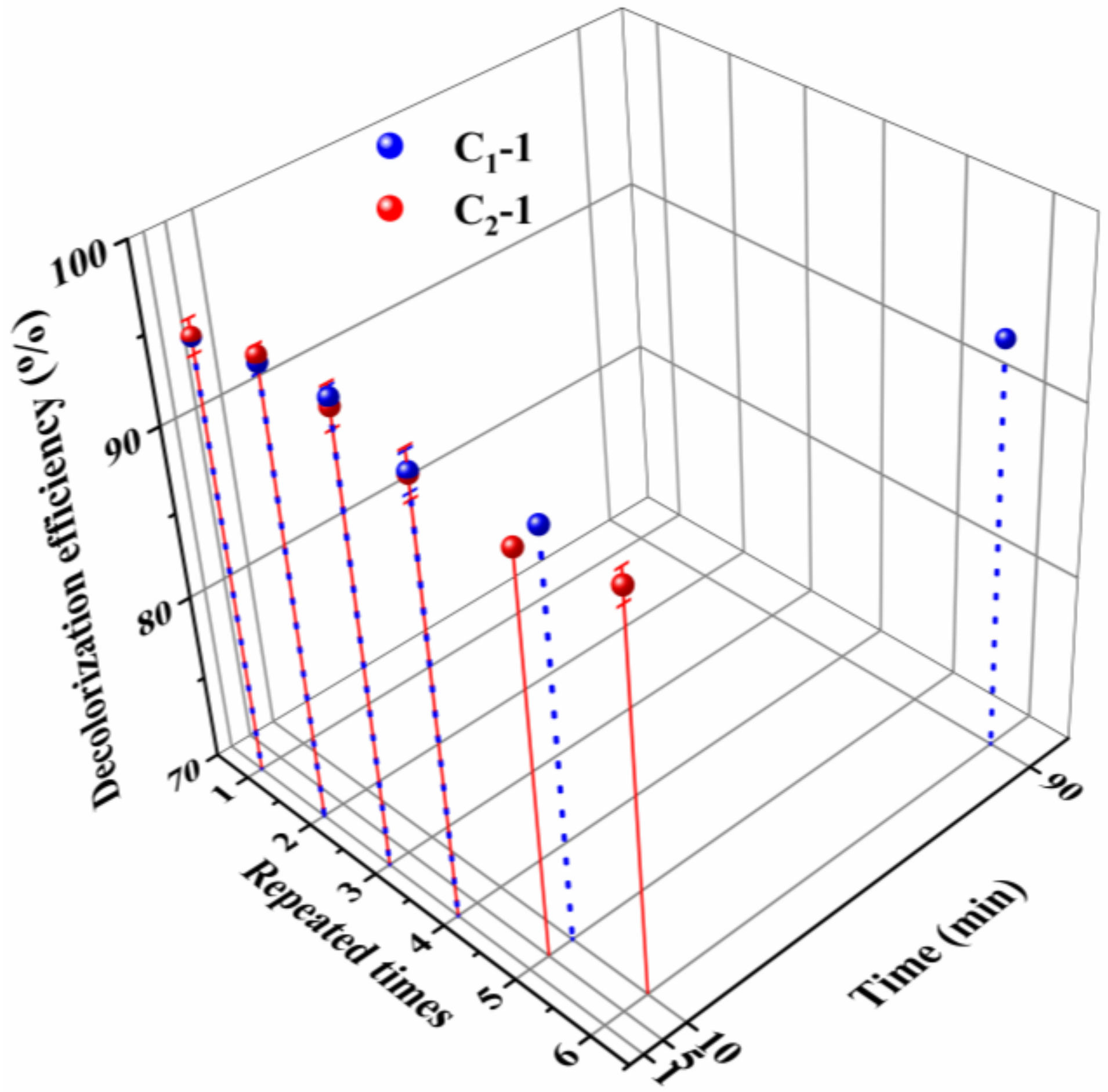
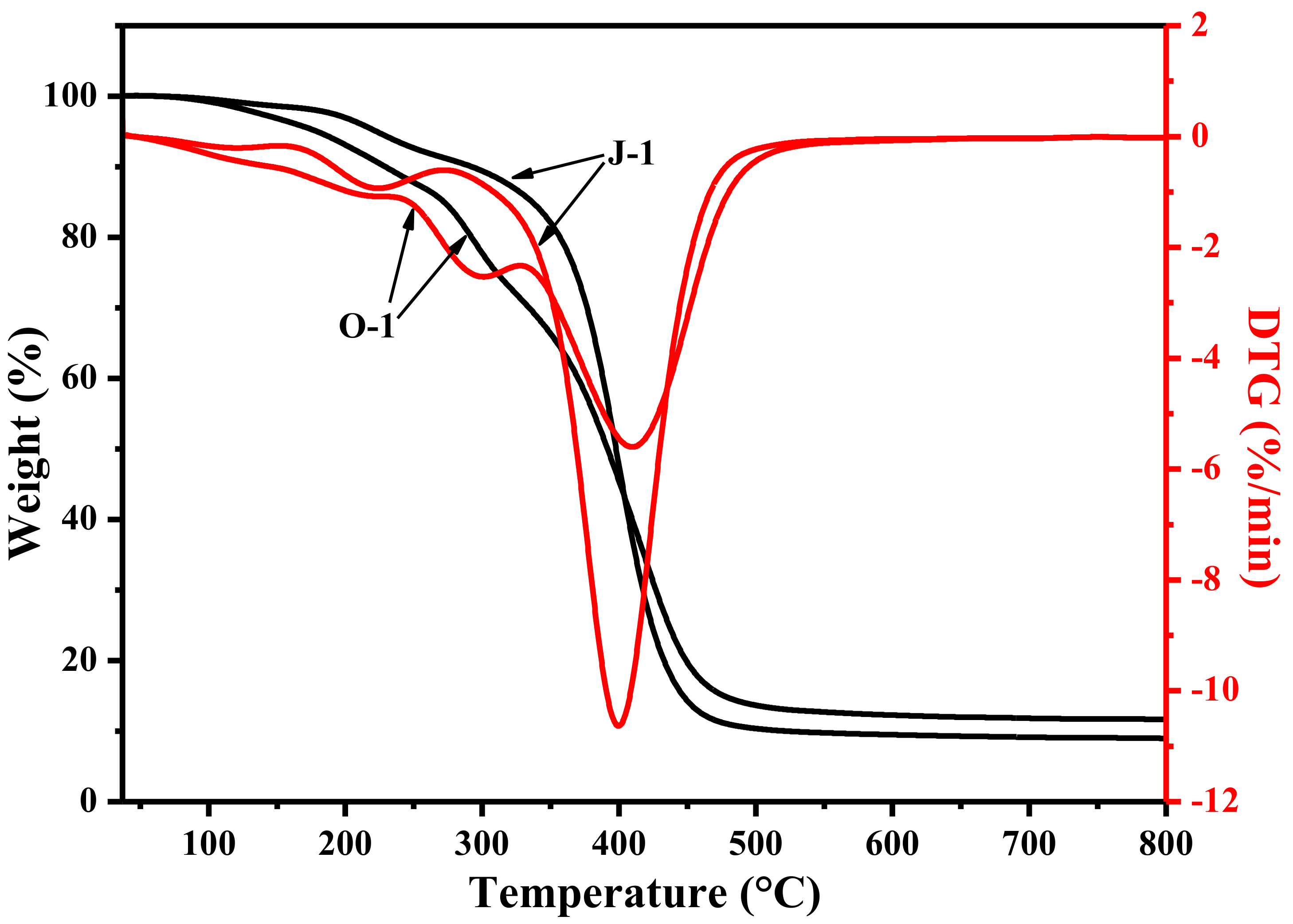




| Raw Material | Solvent | Coagulant | As-Spun Fiber | Dry As-Spun Fiber | Treatment Reagent | Treatment Condition | Sample |
|---|---|---|---|---|---|---|---|
| P(AA-co-HEMA) | NaOH aqueous solution | H2SO4 aqueous solution | F-1 | O-1 | - | - | - |
| P(AA-co-HEMA) PTFE | NaOH aqueous solution | H2SO4 aqueous solution | F-2 | O-2 | - | - | - |
| P(AA-co-HEMA) | NaOH aqueous solution | H2SO4 aqueous solution | F-1 | - | Erythritol water | 200 °C, 30 min vacuum crosslinking | J-1 |
| P(AA-co-HEMA) | NaOH aqueous solution | H2SO4 aqueous solution | F-1 | - | Concentrated H2SO4 FeCl2·4H2O water | 60 °C, 2.5 h vacuum drying | C1-1 |
| P(AA-co-HEMA) | NaOH aqueous solution | H2SO4 aqueous solution | F-1 | - | Concentrated H2SO4 FeCl2·4H2O erythritol water | 200 °C, 30 min vacuum crosslinking | C2-1 |
| P(AA-co-HEMA) PTFE | NaOH aqueous solution | H2SO4 aqueous solution | F-2 | - | Concentrated H2SO4 FeCl2·4H2O erythritol water | 200 °C, 30 min vacuum crosslinking | C2-2 |
| P(AA-co-HEMA) PTFE | NaOH aqueous solution | H2SO4 aqueous solution | F-2 | - | Concentrated H2SO4 FeCl2·4H2O erythritol water | 200 °C, 30 min vacuum crosslinking 380 °C, 7 min sintering | C2-2S |
| P(AA-co-HEMA) PTFE | NaOH aqueous solution | H2SO4 aqueous solution | F-2 | - | Concentrated H2SO4 erythritol water | 200 °C, 30 min vacuum crosslinking 380 °C, 7 min sintering | J-2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, N.; Ren, M. Iron Species-Supporting Hydrophobic and Nonswellable Polytetrafluoroethylene/Poly(acrylic acid-co-hydroxyethyl methacrylate) Composite Fiber and Its Stable Catalytic Activity for Methylene Blue Oxidative Decolorization. Polymers 2021, 13, 1570. https://doi.org/10.3390/polym13101570
Xu N, Ren M. Iron Species-Supporting Hydrophobic and Nonswellable Polytetrafluoroethylene/Poly(acrylic acid-co-hydroxyethyl methacrylate) Composite Fiber and Its Stable Catalytic Activity for Methylene Blue Oxidative Decolorization. Polymers. 2021; 13(10):1570. https://doi.org/10.3390/polym13101570
Chicago/Turabian StyleXu, Naiku, and Mengru Ren. 2021. "Iron Species-Supporting Hydrophobic and Nonswellable Polytetrafluoroethylene/Poly(acrylic acid-co-hydroxyethyl methacrylate) Composite Fiber and Its Stable Catalytic Activity for Methylene Blue Oxidative Decolorization" Polymers 13, no. 10: 1570. https://doi.org/10.3390/polym13101570
APA StyleXu, N., & Ren, M. (2021). Iron Species-Supporting Hydrophobic and Nonswellable Polytetrafluoroethylene/Poly(acrylic acid-co-hydroxyethyl methacrylate) Composite Fiber and Its Stable Catalytic Activity for Methylene Blue Oxidative Decolorization. Polymers, 13(10), 1570. https://doi.org/10.3390/polym13101570






