Influence of Different Conditioning Treatments on the Bond Integrity of Root Dentin to rGO Infiltrated Dentin Adhesive. SEM, EDX, FTIR and MicroRaman Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of the EA
2.2. Addition of rGO Nanoparticles in The Adhesive
2.3. Characterization of the rGO filler Nanoparticles and Adhesive
2.4. Preparation of Teeth, Bonding Procedure, and Grouping of Specimens
2.5. Push-out Bond Strength Test and Failure Mode Investigation
2.6. SEM Investigation of The Root Dentin Adhesive Interface
2.7. FTIR Spectroscopy, DC Analysis, and Rheometry
2.8. Statistical Analysis
3. Results
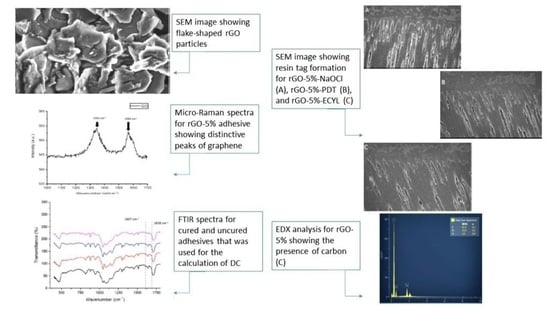
3.1. rGO Filler Nanoparticles Characterization Outcomes
3.2. Adhesive-Dentin Interface, Push-out Bond Strength, and Interfacial Failure Analysis Results
3.3. FTIR Spectroscopy and DC Analysis Outcomes
3.4. Rheological Properties Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AlShehab, A.H.; AlHazoom, A.A.; Alowa, M.H.; AlAli, H.A.; Abdulmohsen, A.A.; Farooq, I. Effect of bristle stiffness of manual toothbrushes on normal and demineralized human enamel-An in vitro profilometric study. Int. J. Dent. Hyg. 2018, 16, e128–e132. [Google Scholar] [CrossRef] [PubMed]
- Thompson, V.; Craig, R.G.; Curro, F.A.; Green, W.S.; Ship, J.A. Treatment of deep carious lesions by complete excavation or partial removal: A critical review. J. Am. Dent. Assoc. 2008, 139, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Resin based restorative dental materials: Characteristics and future perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Ruddock, F.M.; Dowling, A.H.; Byrne, K.; Schmitt, W.; Khalakhan, I.; Nemoto, Y.; Guo, H.; Shrestha, L.K.; Ariga, K.; et al. Graphene composites with dental and biomedical applicability. Beilstein. J. Nanotechnol. 2018, 9, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.M.; Manso, A.P.; Geraldeli, S.; Tay, F.R.; Pashley, D.H. Durability of bonds and clinical success of adhesive restorations. Dent. Mater. 2012, 28, 72–86. [Google Scholar] [CrossRef]
- Stewart, C.A.; Finer, Y. Biostable, antidegradative and antimicrobial restorative systems based on host-biomaterials and microbial interactions. Dent. Mater. 2019, 35, 36–52. [Google Scholar] [CrossRef]
- De Almeida Neves, A.; Coutinho, E.; Cardoso, M.V.; Lambrechts, P.; Van Meerbeek, B. Current concepts and techniques for caries excavation and adhesion to residual dentin. J. Adhes. Dent. 2011, 13, 7–22. [Google Scholar]
- Peumans, M.; Kanumilli, P.; De Munck, J.; Van Landuyt, K.; Lambrechts, P.; Van Meerbeek, B. Clinical effectiveness of contemporary adhesives: A systematic review of current clinical trials. Dent. Mater. 2005, 21, 864–881. [Google Scholar] [CrossRef]
- Huang, B.; Siqueira, W.L.; Cvitkovitch, D.G.; Finer, Y. Esterase from a cariogenic bacterium hydrolyzes dental resins. Acta Biomater. 2018, 71, 330–338. [Google Scholar] [CrossRef]
- Ferracane, J.L. Models of Caries Formation around Dental Composite Restorations. J. Dent. Res. 2017, 96, 364–371. [Google Scholar] [CrossRef]
- Reis, A.; Carrilho, M.; Breschi, L.; Loguercio, A.D. Overview of clinical alternatives to minimize the degradation of the resin-dentin bonds. Oper. Dent. 2013, 38, E1–E25. [Google Scholar] [CrossRef]
- Kalachandra, S. Influence of fillers on the water sorption of composites. Dent. Mater. 1989, 5, 283–288. [Google Scholar] [CrossRef]
- Kim, J.S.; Cho, B.H.; Lee, I.B.; Um, C.M.; Lim, B.S.; Oh, M.H.; Chang, C.G.; Son, H.H. Effect of the hydrophilic nanofiller loading on the mechanical properties and the microtensile bond strength of an ethanol-based one-bottle dentin adhesive. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 72, 284–291. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-based materials: Synthesis, characterization, properties, and applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef]
- Ge, Z.; Yang, L.; Xiao, F.; Wu, Y.; Yu, T.; Chen, J.; Lin, J.; Zhang, Y. Graphene Family Nanomaterials: Properties and Potential Applications in Dentistry. Int. J. Biomater. 2018, 2018, 1539678. [Google Scholar] [CrossRef]
- Placha, D.; Jampilek, J. Graphenic Materials for Biomedical Applications. Nanomaterials 2019, 9, 1758. [Google Scholar] [CrossRef]
- Seabra, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Duran, N. Nanotoxicity of graphene and graphene oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef]
- Mei, L.; Wei, H.; Wenjing, C.; Xiaokun, H. Graphene Oxide-Silica Composite Fillers into the Experimental Dental Adhesives for Potential Therapy. Med. Res. 2017, 1, 42. [Google Scholar] [CrossRef]
- Haeri, S.; Asghari, M.; Ramezanzadeh, B. Enhancement of the mechanical properties of an epoxy composite through inclusion of graphene oxide nanosheets functionalized with silica nanoparticles through one and two steps sol-gel routes. Prog. Org. Coat. 2017, 111, 1–12. [Google Scholar] [CrossRef]
- Ozcan, U.E.; Karabork, F.; Yazman, S.; Akdemir, A. Effect of Silica/Graphene Nanohybrid Particles on the Mechanical Properties of Epoxy Coatings. Arab. J. Sci. Eng. 2019, 44, 5723–5731. [Google Scholar] [CrossRef]
- Yoon, Y.; Kye, H.; Yang, W.S.; Kang, J.-W. Comparing Graphene Oxide and Reduced Graphene Oxide as Blending Materials for Polysulfone and Polyvinylidene Difluoride Membranes. Appl. Sci. 2020, 10, 2015. [Google Scholar] [CrossRef]
- Alrahlah, A.; Khan, R.; Al-Odayni, A.B.; Saeed, W.S.; Bautista, L.S.; Vohra, F. Evaluation of Synergic Potential of rGO/SiO2 as Hybrid Filler for BisGMA/TEGDMA Dental Composites. Polymers 2020, 12, 3025. [Google Scholar] [CrossRef] [PubMed]
- Radhi, A.; Mohamad, D.; Rahman, F.S.A.; Abdullah, A.M.; Hasan, H. Mechanism and factors influence of graphene-based nanomaterials antimicrobial activities and application in dentistry. J. Mater. Res. Technol. 2021, 11, 1290–1307. [Google Scholar] [CrossRef]
- Ye, Q.; Spencer, P.; Wang, Y.; Misra, A. Relationship of solvent to the photopolymerization process, properties, and structure in model dentin adhesives. J. Biomed. Mater. Res. A 2007, 80, 342–350. [Google Scholar] [CrossRef] [PubMed]
- AlFawaz, Y.F.; Almutairi, B.; Kattan, H.F.; Zafar, M.S.; Farooq, I.; Naseem, M.; Vohra, F.; Abduljabbar, T. Dentin Bond Integrity of Hydroxyapatite Containing Resin Adhesive Enhanced with Graphene Oxide Nano-Particles-An SEM, EDX, Micro-Raman, and Microtensile Bond Strength Study. Polymers 2020, 12, 2978. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamdan, R.S.; Almutairi, B.; Kattan, H.F.; Alsuwailem, N.A.; Farooq, I.; Vohra, F.; Abduljabbar, T. Influence of Hydroxyapatite Nanospheres in Dentin Adhesive on the Dentin Bond Integrity and Degree of Conversion: A Scanning Electron Microscopy (SEM), Raman, Fourier Transform-Infrared (FTIR), and Microtensile Study. Polymers 2020, 12, 2948. [Google Scholar] [CrossRef]
- Yoshida, T.; Yamaguchi, K.; Tsubota, K.; Takamizawa, T.; Kurokawa, H.; Rikuta, A.; Ando, S.; Miyazaki, M. Effect of metal conditioners on polymerization behavior of bonding agents. J. Oral Sci. 2005, 47, 171–175. [Google Scholar] [CrossRef][Green Version]
- Zhou, W.; Liu, S.; Zhou, X.; Hannig, M.; Rupf, S.; Feng, J.; Peng, X.; Cheng, L. Modifying Adhesive Materials to Improve the Longevity of Resinous Restorations. Int. J. Mol. Sci. 2019, 20, 723. [Google Scholar] [CrossRef]
- Felemban, N.H.; Ebrahim, M.I. The influence of adding modified zirconium oxide-titanium dioxide nano-particles on mechanical properties of orthodontic adhesive: An in vitro study. BMC Oral Health 2017, 17, 43. [Google Scholar] [CrossRef]
- Jun, S.K.; Yang, S.A.; Kim, Y.J.; El-Fiqi, A.; Mandakhbayar, N.; Kim, D.S.; Roh, J.; Sauro, S.; Kim, H.W.; Lee, J.H.; et al. Multi-functional nano-adhesive releasing therapeutic ions for MMP-deactivation and remineralization. Sci. Rep. 2018, 8, 5663. [Google Scholar] [CrossRef]
- Wagner, A.; Belli, R.; Stotzel, C.; Hilpert, A.; Muller, F.A.; Lohbauer, U. Biomimetically- and hydrothermally-grown HAp nanoparticles as reinforcing fillers for dental adhesives. J. Adhes. Dent. 2013, 15, 413–422. [Google Scholar]
- Bin-Shuwaish, M.S.; Maawadh, A.M.; Al-Hamdan, R.S.; Alresayes, S.; Almohareb, T.; Almutairi, B.; Vohra, F.; Abduljabbar, T. Influence of graphene oxide filler content on the dentin bond integrity, degree of conversion and bond strength of experimental adhesive. A SEM, micro-Raman, FTIR and microtensile study. Mater. Res. Express 2020, 7, 115403. [Google Scholar] [CrossRef]
- Soares, C.P.P.; de Lacerda Baptista, R.; Cesar, D.V. Solvothermal Reduction of Graphite Oxide Using Alcohols. Mater. Res. 2017, 21, e20170726. [Google Scholar] [CrossRef]
- Munther, M.; Shaygan, M.; Centeno, A.; Neumaier, D.; Zurutuza, A.; Momeni, K.; Davami, K. Probing the mechanical properties of vertically-stacked ultrathin graphene/Al2O3 heterostructures. Nanotechnology 2019, 30, 185703. [Google Scholar] [CrossRef]
- Wang, J.H.; Yang, K.; Zhang, B.Z.; Zhou, Z.F.; Wang, Z.R.; Ge, X.; Wang, L.L.; Chen, Y.J.; Wang, X.J. Effects of Er:YAG laser pre-treatment on dentin structure and bonding strength of primary teeth: An in vitro study. BMC Oral Health 2020, 20, 316. [Google Scholar] [CrossRef]
- Ceballo, L.; Toledano, M.; Osorio, R.; Tay, F.R.; Marshall, G.W. Bonding to Er-YAG-laser-treated dentin. J. Dent. Res. 2002, 81, 119–122. [Google Scholar] [CrossRef]
- Anchieta, R.B.; Oliveira, F.G.; Sundfeld, R.H.; Rahal, V.; Machado, L.S.; Alexandre, R.S.; Sundefeld, M.L.; Rocha, E.P. Analysis of hybrid layer thickness, resin tag length and their correlation with microtensile bond strength using a total etch adhesive to intact dentin. Acta Odontol. Latinoam. 2011, 24, 272–278. [Google Scholar]
- Majeed, A.; AlShwaimi, E. Push-Out Bond Strength and Surface Microhardness of Calcium Silicate-Based Biomaterials: An in vitro Study. Med. Princ. Pract. 2017, 26, 139–145. [Google Scholar] [CrossRef]
- Al Jeaidi, Z.A. Influence of resin cements and root canal disinfection techniques on the adhesive bond strength of fibre reinforced composite post to radicular dentin. Photodiagnosis Photodyn. Ther. 2021, 33, 102108. [Google Scholar] [CrossRef]
- Strazzi Sahyon, H.B.; Pereira da Silva, P.; Silva de Oliveira, M.; Angelo Cintra, L.T.; Gomes-Filho, J.E.; Henrique Dos Santos, P.; Sivieri-Araujo, G. Effect of photodynamic therapy on the mechanical properties and bond strength of glass-fiber posts to endodontically treated intraradicular dentin. J. Prosthet. Dent. 2018, 120, 317.e1–317.e7. [Google Scholar] [CrossRef]
- Alonaizan, F.A.; Alofi, R.S.; AlFawaz, Y.F.; Alsahhaf, A.; Al-Aali, K.A.; Vohra, F.; Abduljabbar, T. Effect of Photodynamic Therapy, Er,Cr:YSGG, and Nd:YAG Laser on the Push-Out Bond Strength of Fiber Post to Root Dentin. Photobiomodul. Photomed. Laser Surg. 2020, 38, 24–29. [Google Scholar] [CrossRef] [PubMed]
- De Munck, J.; Vargas, M.; Van Landuyt, K.; Hikita, K.; Lambrechts, P.; Van Meerbeek, B. Bonding of an auto-adhesive luting material to enamel and dentin. Dent. Mater. 2004, 20, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, B.; Alkahtany, M.F.; Almadi, K.H.; Hussain, M.; Abduljabbar, T. Adhesive strength of bioactive resin for endodontic tooth build up to dentin treated with conventional and contemporary treatments. Photodiagnosis Photodyn. Ther. 2021, 33, 102159. [Google Scholar] [CrossRef] [PubMed]
- Vohra, F.; Bukhari, I.A.; Sheikh, S.A.; Naseem, M.; Hussain, M. Photodynamic activation of irrigation (using different laser prototypes) on push out bond strength of fiber posts. Photodiagnosis Photodyn. Ther. 2020, 30, 101716. [Google Scholar] [CrossRef]
- Zorba, Y.O.; Erdemir, A.; Turkyilmaz, A.; Eldeniz, A.U. Effects of different curing units and luting agents on push-out bond strength of translucent posts. J. Endod. 2010, 36, 1521–1525. [Google Scholar] [CrossRef]
- Alofi, R.S.; Alshiddi, I.F.; AlFawaz, Y.F.; Alsahhaf, A.; Al-Aali, K.A.; Abduljabbar, T.; Vohra, F. Influence of Er,Cr:YSGG Laser Irradiation on the Push-Out Bond Strength of Zirconia and Glass Fiber Posts with Radicular Dentin. Int. J. Biomater. 2019, 2019, 4869853. [Google Scholar] [CrossRef]
- Alhenaki, A.M.; Attar, E.A.; Alshahrani, A.; Farooq, I.; Vohra, F.; Abduljabbar, T. Dentin Bond Integrity of Filled and Unfilled Resin Adhesive Enhanced with Silica Nanoparticles-An SEM, EDX, Micro-Raman, FTIR and Micro-Tensile Bond Strength Study. Polymers 2021, 13, 1093. [Google Scholar] [CrossRef]
- Utneja, S.; Talwar, S.; Nawal, R.R.; Sapra, S.; Mittal, M.; Rajain, A.; Verma, M. Evaluation of remineralization potential and mechanical properties of pit and fissure sealants fortified with nano-hydroxyapatite and nano-amorphous calcium phosphate fillers: An in vitro study. J. Conserv. Dent. 2018, 21, 681–690. [Google Scholar] [CrossRef]
- Marchezi, P.E.; Sonai, G.G.; Hirata, M.K.; Schiavon, M.A.; Nogueira, A.F. Understanding the Role of Reduced Graphene Oxide in the Electrolyte of Dye-Sensitized Solar Cells. J. Phys. Chem. C 2016, 120, 23368–23376. [Google Scholar] [CrossRef]







| Adhesive | NaOCl | PDT | ECYL | p Value |
|---|---|---|---|---|
| EA-rGO 0% | 9.40 ± 1.31 A a | 8.77 ± 1.30 A a | 6.63 ± 1.26 A b | <0.01 |
| rGO-5% | 9.13 ± 1.78 A a | 8.26 ± 1.25 A a | 7.14 ± 1.30 A b | <0.01 |
| Type of Failure | ||||
|---|---|---|---|---|
| Study Group | Root Segment | Adhesive-Post | Adhesive-Dentin | Mixed |
| NaOCl | Cervical | 0 | 80 | 20 |
| Apical | 0 | 100 | 0 | |
| PDT | Cervical | 10 | 80 | 10 |
| Apical | 0 | 80 | 20 | |
| ECYL | Cervical | 10 | 80 | 10 |
| Apical | 0 | 100 | 0 | |
| Group | DC (Mean ± SD) | Tukey (p < 0.01) |
|---|---|---|
| EA-rGO-0% | 57.8 ± 8.7 | A |
| rGO-5% | 51.3 ± 6.6 | A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqarawi, F.K.; Alkahtany, M.F.; Almadi, K.H.; Ben Gassem, A.A.; Alshahrani, F.A.; AlRefeai, M.H.; Farooq, I.; Vohra, F.; Abduljabbar, T. Influence of Different Conditioning Treatments on the Bond Integrity of Root Dentin to rGO Infiltrated Dentin Adhesive. SEM, EDX, FTIR and MicroRaman Study. Polymers 2021, 13, 1555. https://doi.org/10.3390/polym13101555
Alqarawi FK, Alkahtany MF, Almadi KH, Ben Gassem AA, Alshahrani FA, AlRefeai MH, Farooq I, Vohra F, Abduljabbar T. Influence of Different Conditioning Treatments on the Bond Integrity of Root Dentin to rGO Infiltrated Dentin Adhesive. SEM, EDX, FTIR and MicroRaman Study. Polymers. 2021; 13(10):1555. https://doi.org/10.3390/polym13101555
Chicago/Turabian StyleAlqarawi, Firas K., Mazen F. Alkahtany, Khalid H. Almadi, Afnan A. Ben Gassem, Faris A. Alshahrani, Mohammad H. AlRefeai, Imran Farooq, Fahim Vohra, and Tariq Abduljabbar. 2021. "Influence of Different Conditioning Treatments on the Bond Integrity of Root Dentin to rGO Infiltrated Dentin Adhesive. SEM, EDX, FTIR and MicroRaman Study" Polymers 13, no. 10: 1555. https://doi.org/10.3390/polym13101555
APA StyleAlqarawi, F. K., Alkahtany, M. F., Almadi, K. H., Ben Gassem, A. A., Alshahrani, F. A., AlRefeai, M. H., Farooq, I., Vohra, F., & Abduljabbar, T. (2021). Influence of Different Conditioning Treatments on the Bond Integrity of Root Dentin to rGO Infiltrated Dentin Adhesive. SEM, EDX, FTIR and MicroRaman Study. Polymers, 13(10), 1555. https://doi.org/10.3390/polym13101555