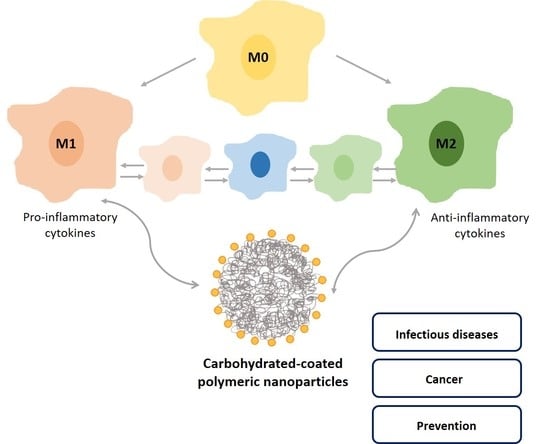
Modulation of Macrophages M1/M2 Polarization Using Carbohydrate-Functionalized Polymeric Nanoparticles
Abstract
1. Introduction
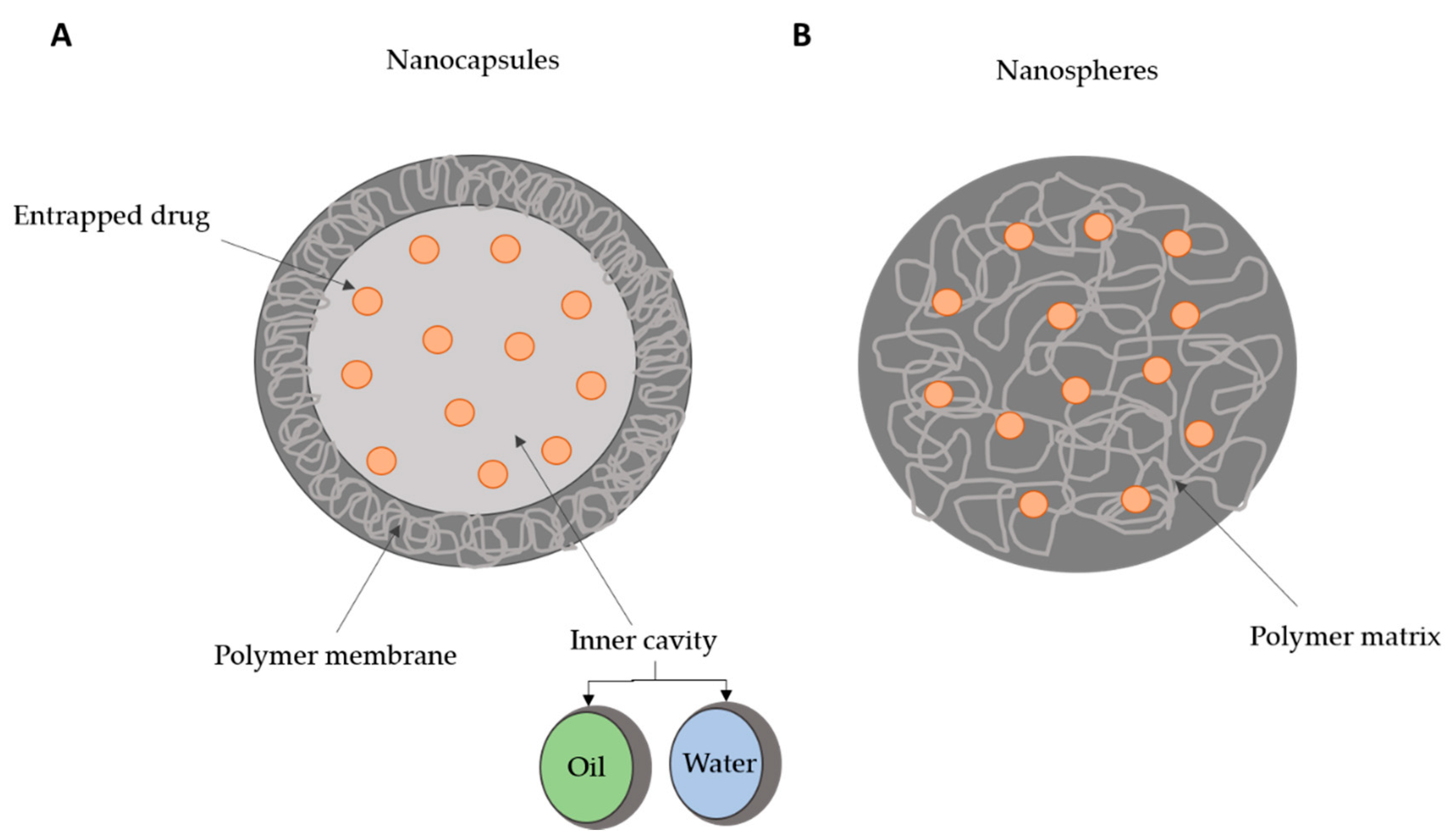
2. Polymeric Nanoparticles as Biomedical Delivery Devices
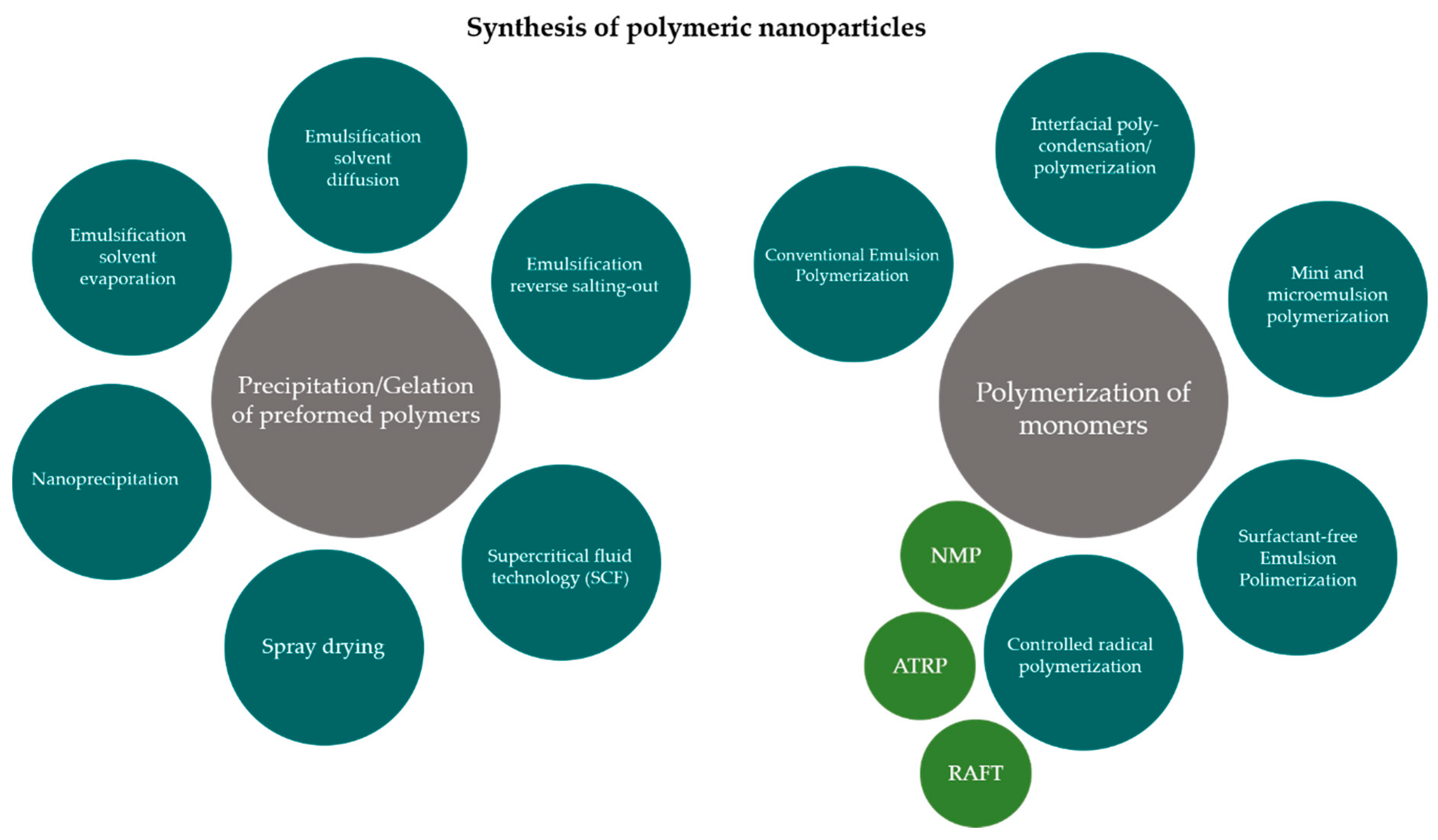
3. Production Methods for Polymeric Nanoparticles and Surface Properties Modifications
4. Carbohydrate-Functionalized Polymeric Nanoparticles
5. Macrophages
5.1. Functions and Polarization State
5.2. Macrophage Polarization Mediated by Nanocarriers
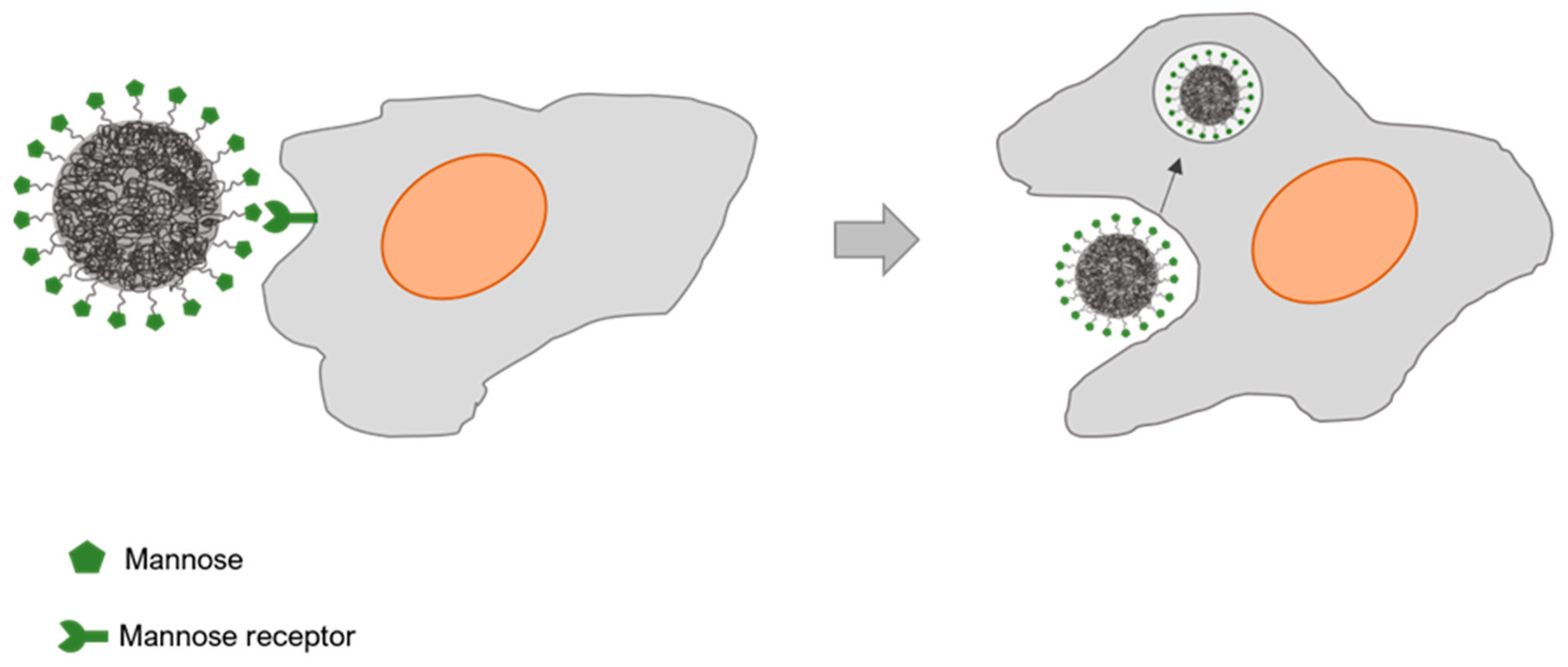
5.3. Mannose Receptor
5.4. Mannose Receptor-Targeted Nanocarriers Interactions with Macrophages
5.4.1. Mannose Receptor-Targeting Nanocarriers towards Infection Resolution
5.4.2. Mannose Receptor-Targeting Nanocarriers towards Tumor-Associated Macrophages
5.4.3. Mannose Receptor-Targeting Nanocarriers towards Prevention Approaches
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Steichen, D.S.; Caldorera-Moore, M.; Peppas, N.A. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur. J. Pharm. Sci. 2013, 48, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, M.; Ali, H.; Ahmed, N.; Munir, R.; Talib, S.; Khan, A.S.; Ambrus, R. Drug delivery to macrophages: A review of nano-therapeutics targeted approach for inflammatory disorders and cancer. Expert Opin. Drug Deliv. 2020, 17, 1239–1257. [Google Scholar] [CrossRef] [PubMed]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef]
- Duncan, R.; Gaspar, R. Nanomedicine(s) under the microscope. Mol. Pharm. 2011, 8, 2101–2141. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Kulkarni, A.; Rao, P. Synthesis of polymeric nanomaterials for biomedical applications. Nanomater. Tissue Eng. 2013, 27–63. [Google Scholar]
- Nagavarma, B.V.N.; Yadav, H.; Ayaz, A.; Vasudha, L.S.; Shivakumar, H.G. Different techniques for preparation of polymeric nanoparticles—A review. Asian J. Pharm. Clin. Res. 2012, 5, 16–23. [Google Scholar]
- Vauthier, C.; Bouchemal, K. Methods for the preparation and manufacture of polymeric nanoparticles. Pharm. Res. 2009, 26, 1025–1058. [Google Scholar] [CrossRef] [PubMed]
- Samrot, A.; Burman, U.; Philip, S.N.S.; Chandrasekaran, K. Synthesis of curcumin loaded polymeric nanoparticles from crab shell derived chitosan for drug delivery. Inform. Med. Unlocked 2018, 10, 159–182. [Google Scholar] [CrossRef]
- Gheorghita Puscaselu, R.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From food industry to biomedical applications and management of metabolic disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef]
- Zhao, X.; Lang, Q.; Yildirimer, L.; Lin, Z.; Cui, W.; Annabi, N.; Ng, K.; Dokmeci, M.; Ghaemmaghami, A.; Khademhosseini, A. Photocrosslinkable gelatin hydrogel for epidermal tissue engineering. Adv. Healthc. Mater. 2015, 5, 108–118. [Google Scholar] [CrossRef]
- Lamprecht, A. Nanotherapeutics: Drug Delivery Concepts in Nanoscience; Pan Stanford Publishing: Singapore, 2009; p. 279, chapter xii. [Google Scholar]
- Panyam, J.; Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef]
- Heinz, H.; Pramanik, C.; Heinz, O.; Ding, Y.; Mishra, R.K.; Marchon, D.; Flatt, R.J.; Estrela-Lopis, I.; Llop, J.; Moya, S.; et al. Nanoparticle decoration with surfactants: Molecular interactions, assembly, and applications. Surf. Sci. Rep. 2017, 72, 1–58. [Google Scholar] [CrossRef]
- Qiu, L.Y.; Bae, Y.H. Polymer architecture and drug delivery. Pharm. Res. 2006, 23, 1–30. [Google Scholar] [CrossRef]
- Edlund, U.; Albertsson, A.C. Polyesters based on diacid monomers. Adv. Drug Deliv. Rev. 2003, 55, 585–609. [Google Scholar] [CrossRef]
- Han, F.Y.; Thurecht, K.J.; Whittaker, A.K.; Smith, M.T. Biodegradable PLGA-based microparticles for producing sustained-release drug formulations and strategies for improving drug loading. Front. Pharmacol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliver. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C. Polymeric Nanomaterials in Nanotherapeutic, 1st ed.; Elsevier: San Diego, CA, USA, 2018. [Google Scholar]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles-based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, Y.; Xie, R.; Gong, S. A review on core-shell structured unimolecular nanoparticles for biomedical applications. Adv. Drug Deliv. Rev. 2018, 130, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wen, H.-F.; Yu, D.-G.; Yang, Y.; Zhang, D.-F. Electrosprayed hydrophilic nanocomposites coated with shellac for colon-specific delayed drug delivery. Mater. Des. 2018, 143, 248–255. [Google Scholar] [CrossRef]
- Huang, W.D.; Xu, X.; Wang, H.L.; Huang, J.X.; Zuo, X.H.; Lu, X.J.; Liu, X.L.; Yu, D.G. Electrosprayed ultra-thin coating of ethyl cellulose on drug nanoparticles for improved sustained release. Nanomaterials 2020, 10, 1758. [Google Scholar] [CrossRef]
- Khan, I.; Gothwal, A.; Sharma, A.K.; Kesharwani, P.; Gupta, L.; Iyer, A.K.; Gupta, U. PLGA nanoparticles and their versatile role in anticancer drug delivery. Crit. Rev. Ther. Drug Carrier Syst. 2016, 33, 159–193. [Google Scholar] [CrossRef]
- Storm, G.; Belliot, S.O.; Daemen, T.; Lasic, D.D. Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv. Drug Deliv. Rev. 1995, 17, 31–48. [Google Scholar] [CrossRef]
- Hans, M.L.; Lowman, A.L. Biodegradable nanoparticles for drug delivery and targeting. Curr. Opin. Solid State Mater. Sci. 2002, 6, 319–327. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Torchilin, V.P.; Trubetskoy, V.S. Which polymers can make nanoparticulate drug carriers long-circulating? Adv. Drug Deliv. Rev. 1995, 16, 141–155. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The importance of poly(ethylene glycol) alternatives for overcoming PEG immunogenicity in drug delivery and bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Gref, R.; Domb, A.; Quellec, P.; Blunk, T.; Muller, R.H.; Verbavatz, J.M.; Langer, R. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Adv. Drug Deliv. Rev. 1995, 16, 215–233. [Google Scholar] [CrossRef]
- Tobio, M.; Sanchez, A.; Vila, A.; Soriano, I.I.; Evora, C.; Vila-Jato, J.L.; Alonso, M.J. The role of PEG on the stability in digestive fluids and in vivo fate of PEG-PLA nanoparticles following oral administration. Colloids Surf. B Biointerfaces 2000, 18, 315–323. [Google Scholar] [CrossRef]
- Calvo, P.; Gouritin, B.; Brigger, I.; Lasmezas, C.; Deslys, J.P.; Williams, A.; Andreux, J.P.; Dormont, D.; Couvreur, P. PEGylated polycyanoacrylate nanoparticles as vector for drug delivery in prion diseases. J. Neurosci. Methods 2001, 111, 151–155. [Google Scholar] [CrossRef]
- Avgoustakis, K.; Beletsi, A.; Panagi, Z.; Klepetsanis, P.; Karydas, A.G.; Ithakissios, D.S. PLGA-mPEG nanoparticles of cisplatin: In vitro nanoparticle degradation, in vitro drug release and in vivo drug residence in blood properties. J. Control. Release 2002, 79, 123–135. [Google Scholar] [CrossRef]
- Srinivasan, M.; Rajabi, M.; Mousa, S.A. Multifunctional nanomaterials and their applications in drug delivery and cancer therapy. Nanomaterials 2015, 5, 1690–1703. [Google Scholar] [CrossRef]
- Ang, C.; Tan, S.; Zhao, Y. Recent advances in biocompatible nanocarriers for delivery of chemotherapeutic cargoes towards cancer therapy. Org. Biomol. Chem. 2014, 12, 4776–4806. [Google Scholar] [CrossRef]
- Karra, N.; Nassar, T.; Ripin, A.; Schwob, O.; Borlak, J.; Benita, S. Antibody conjugated PLGA nanoparticles for targeted delivery of paclitaxel palmitate: Efficacy and biofate in a lung cancer mouse model. Small 2013, 9, 4221–4236. [Google Scholar] [CrossRef]
- Coester, C.; Kreuter, J.; von Briesen, H.; Langer, K. Preparation of avidin-labelled gelatin nanoparticles as carriers for biotinylated peptide nucleic acid (PNA). Int. J. Pharm. 2000, 196, 147–149. [Google Scholar] [CrossRef]
- Hua, S. Advances in oral drug delivery for regional targeting in the gastrointestinal tract—Influence of physiological, pathophysiological and pharmaceutical factors. Front Pharmacol. 2020, 1, 524. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, G.; Becer, C. Glyconanoparticles and their interactions with lectins. Polym. Chem. 2015, 6, 5503–5514. [Google Scholar] [CrossRef]
- Eddie Ip, W.K.; Takahashi, K.; Alan Ezekowitz, R.; Stuart, L.M. Mannose-binding lectin and innate immunity. Immunol. Rev. 2009, 230, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Cade, D.; Ramus, E.; Rinaudo, M.; Auzély-Velty, R.; Delair, T.; Hamaide, T. Tailoring of bioresorbable polymers for elaboration of sugar-functionalized nanoparticles. Biomacromolecules 2004, 5, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Vela-Ramirez, J.; Goodman, J.; Boggiatto, P.; Roychoudhury, R.; Pohl, N.; Hostetter, J.; Wannemuehler, M.; Narasimhan, B. Safety and biocompatibility of carbohydrate-functionalized polyanhydride nanoparticles. AAPS J. 2014, 17, 256–267. [Google Scholar] [CrossRef]
- Lemarchand, C.; Gref, R.; Passirani, C.; Garcion, E.; Petri, B.; Müller, R.; Costantini, D.; Couvreur, P. Influence of polysaccharide coating on the interactions of nanoparticles with biological systems. Biomaterials 2006, 2, 108–118. [Google Scholar] [CrossRef]
- Morille, M.; Passirani, C.; Letrou-Bonneval, E.; Benoit, J.; Pitard, B. Galactosylated DNA lipid nanocapsules for efficient hepatocyte targeting. Int. J. Pharm. 2009, 379, 293–300. [Google Scholar] [CrossRef]
- Pramudya, I.; Chung, H. Recent progress of glycopolymer synthesis for biomedical applications. Biomater. Sci. 2019, 7, 4848–4872. [Google Scholar] [CrossRef]
- Babiuch, K.; Stenzel, M.H. Synthesis and Application of Glycopolymers; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Zhang, Y.; Chan, J.; Moretti, A.; Uhrich, K. Designing polymers with sugar-based advantages for bioactive delivery applications. J. Control. Release 2015, 219, 355–368. [Google Scholar] [CrossRef]
- Ladmiral, V.; Melia, E.; Haddleton, D. Synthetic glycopolymers: An overview. Eur. Polym. J. 2004, 40, 431–449. [Google Scholar] [CrossRef]
- Miura, Y. Design and synthesis of well-defined glycopolymers for the control of biological functionalities. Polym. J. 2012, 44, 679–689. [Google Scholar] [CrossRef]
- Boehnke, N.; Dolph, K.; Juarez, V.; Lanoha, J.; Hammond, P. Electrostatic conjugation of nanoparticle surfaces with functional peptide motifs. Bioconjugate Chem. 2020, 31, 2211–2219. [Google Scholar] [CrossRef]
- Sidorov, I.; Prabakaran, P.; Dimitrov, D. Non-covalent conjugation of nanoparticles to antibodies via electrostatic interactions—A computational model. J. Comp. Theor. Nanosci. 2007, 46, 1103–1107. [Google Scholar] [CrossRef]
- Hermanson, G. Bioconjugate Techniques, 3rd ed.; Amsterdam University Press: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted drug delivery with polymers and magnetic nanoparticles: Covalent and noncovalent approaches, release control, and clinical studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef]
- Kim, I.-S.; Kim, S.-H.; Cho, C.-S. Preparation of polymeric nanoparticles composed of cholic acid and poly(ethylene glycol) end-capped with a sugar moiety. Macromol. Rapid Commun. 2000, 21, 1272–1275. [Google Scholar] [CrossRef]
- Palmioli, A.; La Ferla, B. Glycofunctionalization of poly(lactic-co-glycolic acid) polymers: Building blocks for the generation of defined sugar-coated nanoparticles. Org. Lett. 2018, 20, 3509–3512. [Google Scholar] [CrossRef]
- Crucho, C.I.C.; Barros, M.T. Formulation of functionalized PLGA polymeric nanoparticles for targeted drug delivery. Polym. Chem. 2015, 68, 41–46. [Google Scholar] [CrossRef]
- Rieger, J.; Freichels, H.; Imberty, A.; Putaux, J.-L.; Delair, T.; Jérôme, C.; Auzély-Velty, R. Polyester nanoparticles presenting mannose residues: Toward the development of new vaccine delivery systems combining biodegradability and targeting properties. Biomacromolecules 2009, 10, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Freichels, H.; Wagner, M.; Okwieka, P.; Meyer, R.G.; Mailänder, V.; Landfester, K.; Musyanovych, A. (Oligo)mannose functionalized hydroxyethyl starch nanocapsules: One route to drug delivery systems with targeting properties. J. Mater. Chem. B 2013, 1, 4338–4348. [Google Scholar] [CrossRef]
- Kim, N.; Jiang, D.; Jacobi, A.; Lennox, K.; Rose, S.; Behlke, M.; Salem, A. Synthesis and characterization of mannosylated pegylated polyethylenimine as a carrier for siRNA. Int. J. Pharm. 2012, 427, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Barros, D.; Costa Lima, S.A.; Cordeiro-da-Silva, A. Surface functionalization of polymeric nanospheres modulates macrophage activation. Nanomedicine 2015, 10, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Ganbold, T.; Baigude, H. Design of mannose-functionalized curdlan nanoparticles for macrophage-targeted siRNA delivery. ACS App. Mater. Interfaces 2018, 10, 14463–14474. [Google Scholar] [CrossRef] [PubMed]
- Haniffa, M.; Bigley, V.; Collin, M. Human mononuclear phagocyte system reunited. Semin. Cell Dev. Biol. 2015, 41, 59–69. [Google Scholar] [CrossRef]
- Epelman, S.; Lavine, K.J.; Randolph, G.J. Origin and functions of tissue macrophages. Immunity 2014, 41, 21–35. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000 Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Patil, T.S.; Dehpande, A.S. Mannosylated nanocarriers mediated site-specific drug delivery for the treatment of cancer and other infectious diseases: A state of the art review. J. Control. Release 2020, 320, 239–252. [Google Scholar] [CrossRef]
- Mosaiab, T.; Farr, D.C.; Kiefel, M.J.; Houston, T.A. Carbohydrate-based nanocarriers and their application to target macrophages and deliver antimicrobial agents. Adv. Drug Deliv. Rev. 2019, 152, 94–129. [Google Scholar] [CrossRef]
- Sun, B.; Wang, X.; Ji, Z.; Li, R.; Xia, T. NLRP3 inflammasome activation induced by engineered nanomaterials. Small 2013, 9, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Guo, M.; Xu, J.; Wu, F.; Fan, J.; Huang, Q.; Yang, G.; Lv, Z.; Wang, X.; Jin, Y. Nanoparticles targeting macrophages as potential clinical therapeutic agents against cancer and inflammation. Front. Immunol. 2019, 10, 1998. [Google Scholar] [CrossRef] [PubMed]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A.V. Endocytosis of nanomedicines. J. Control. Release 2010, 145, 182–195. [Google Scholar] [CrossRef]
- Carrillo-Conde, B.; Song, E.H.; Chavez-Santoscoy, A.; Phanse, Y.; Ramer-Tait, A.E.; Pohl, N.L.; Wannemuehler, M.J.; Bellaire, B.H.; Narasimhan, B. Mannose-functionalized “pathogen-like” polyanhydride nanoparticles target C-type lectin receptors on dendritic cells. Mol. Pharm. 2011, 8, 1877–1886. [Google Scholar] [CrossRef]
- Hamdy, S.; Haddadi, A.; Shayeganpour, A.; Samuel, J.; Lavasanifar, A. Activation of antigen-specific T cell-responses by mannan-decorated PLGA nanoparticles. Pharm. Res. 2011, 28, 2288–2301. [Google Scholar] [CrossRef]
- Shrivastavaa, R.; Shukla, N. Attributes of alternatively activated (M2) macrophages. Life Sci. 2019, 224, 222–231. [Google Scholar] [CrossRef]
- Ezekowitz, R.A.B.; Sastry, K.; Bailly, P.; Warner, A. Molecular characterization of the Human macrophage mannose receptor—Demonstration of multiple carbohydrate recognition-like domains and phagocytosis of teasts in cos-1 cells. J. Exp. Med. 1990, 172, 1785–1794. [Google Scholar] [CrossRef]
- East, L.; Isacke, C.M. The mannose receptor family. BBA Gen. Subj. 2002, 1572, 364–386. [Google Scholar] [CrossRef]
- Hu, J.; Wei, P.; Seeberger, P.; Yin, Y. Mannose-functionalized nanoscaffolds for targeted delivery in biomedical applications. Chem. Asian J. 2018, 13, 3448–3459. [Google Scholar] [CrossRef]
- McKenzie, E.J.; Taylor, P.R.; Stillion, R.J.; Lucas, A.D.; Harris, J.; Gordon, S.; Martinez-Pomares, L. Mannose receptor expression and function define a new population of murine dendritic cells. J. Immunol. 2007, 178, 4975–4983. [Google Scholar] [CrossRef]
- Linehan, S.A.; Martinez-Pomares, L.; Stahl, P.D.; Gordon, S. Mannose receptor and its putative ligands in normal murine lymphoid and nonlymphoid organs: In situ expression of mannose receptor by selected macrophages, endothelial cells, perivascular microglia, and mesangial cells, but not dendritic cells. J. Exp. Med. 1999, 189, 1961–1972. [Google Scholar] [CrossRef] [PubMed]
- Lew, D.B.; Songumize, E.; Pontow, S.E.; Stahl, P.D.; Rattazzi, M.C. A mannose receptor mediates mannosyl-rich glycoprotein-induced mitogenesis in bovine airway smooth-muscle cells. J. Clin. Invest. 1994, 94, 1855–1863. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stahl, P.D.; Ezekowitz, R.A. The mannose receptor is a pattern recognition receptor involved in host defense. Curr. Opin. Immunol. 1998, 10, 50–55. [Google Scholar] [CrossRef]
- Schreiber, S.; Blum, J.S.; Stenson, W.F.; MacDermott, R.P.; Stahl, P.D.; Teitelbaum, S.L.; Perkins, S.L. Monomeric IgG2a promotes maturation of bone-marrow macrophages and expression of the mannose receptor. Proc. Natl. Acad. Sci. USA 1991, 88, 1616–1620. [Google Scholar] [CrossRef]
- Stein, M.; Keshav, S.; Harris, N.; Gordon, S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: A marker of alternative immunologic macrophage activation. J. Exp. Med. 1992, 176, 287–292. [Google Scholar] [CrossRef]
- Doyle, A.G.; Herbein, G.; Montaner, L.J.; Minty, A.J.; Caput, D.; Ferrara, P.; Gordon, S. Interleukin-13 alters the activation state of murine macrophages in vitro—Comparison with interleukin-4 and interferon-gamma. Eur. J. Immunol. 1994, 24, 1441–1445. [Google Scholar] [CrossRef]
- Martinez-Pomares, L.; Reid, D.M.; Brown, G.D.; Taylor, P.R.; Stillion, R.J.; Linehan, S.A.; Zamze, S.; Gordon, S.; Wong, S.Y. Analysis of mannose receptor regulation by IL-4, IL-10, and proteolytic processing using novel monoclonal antibodies. J. Leukoc. Biol. 2003, 73, 604–613. [Google Scholar] [CrossRef]
- Harris, N.; Super, M.; Rits, M.; Chang, G.; Ezekowitz, R.A. Characterization of the murine macrophage mannose receptor: Demonstration that the downregulation of receptor expression mediated by interferon-gamma occurs at the level of transcription. Blood 1992, 80, 2363–2373. [Google Scholar] [CrossRef]
- Shibata, Y.; Metzger, W.J.; Myrvik, Q.N. Chitin particle-induced cell-mediated immunity is inhibited by soluble mannan: Mannose receptor-mediated phagocytosis initiates IL-12 production. J. Immunol. 1997, 159, 2462–2467. [Google Scholar]
- Engering, A.J.; Cella, M.; Fluitsma, D.; Brockhaus, M.; Hoefsmit, E.C.; Lanzavecchia, A.; Pieters, J. The mannose receptor functions as a high capacity and broad specificity antigen receptor in human dendritic cells. Eur. J. Immunol. 1997, 27, 2417–2425. [Google Scholar] [CrossRef]
- Tan, M.C.; Mommaas, A.M.; Drijfhout, J.W.; Jordens, R.; Onderwater, J.J.; Verwoerd, D.; Mulder, A.A.; van der Heiden, A.N.; Scheidegger, D.; Oomen, L.C.; et al. Mannose receptor-mediated uptake of antigens strongly enhances HLA class II-restricted antigen presentation by cultured dendritic cells. Eur. J. Immunol. 1997, 27, 2426–2435. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Das, P.K. Role of mannose N-acetylglucosamine receptors in blood clearance and cellular attachment of Leishmania donovani. Mol. Biochem. Parasit. 1988, 28, 55–62. [Google Scholar] [CrossRef]
- Kang, P.B.; Azad, A.K.; Schlesinger, L.S. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J. Exp. Med. 2005, 202, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Irache, J.M.; Salman, H.H.; Gamazo, C.; Espuelas, S. Mannose-targeted systems for the delivery of therapeutics. Expert Opin Drug Deliv. 2008, 5, 703–724. [Google Scholar] [CrossRef]
- Song, E.-H.; Manganiello, M.J.; Chow, J.-H.; Ghosn, B.; Convertine, A.J.; Stayton, P.S.; Schnapp, L.M.; Ratner, D.M. In vivo targeting of alveolar macrophages via RAFT-based glycopolymers. Biomaterials 2012, 33, 6889–6897. [Google Scholar] [CrossRef]
- Nycholat, C.M.; Rademacher, C.; Kawasaki, N.; Paulson, J.C. In silico-aided design of a glycan ligand of sialo-adhesin for in vivo targeting of macrophages. J. Am. Chem. Soc. 2012, 134, 15696–15699. [Google Scholar] [CrossRef]
- Adler, A.F.; Leong, K.W. Emerging links between surface nanotechnology and endocytosis: Impact on nonviral gene delivery. Nano Today 2010, 5, 553–569. [Google Scholar] [CrossRef]
- Kaur, I.P.; Singh, H. Nanostructured drug delivery for better management of tuberculosis. J. Control. Release 2014, 184, 36–50. [Google Scholar] [CrossRef]
- Lu, E.; Franzblau, S.; Onyuksel, H.; Popescu, C. Preparation of aminoglycoside-loaded chitosan nanoparticles using dextran sulphate as a counterion. J. Microencapsul. 2009, 26, 346–354. [Google Scholar] [CrossRef]
- Nimje, N.; Agarwal, A.; Saraogi, G.K.; Lariya, N.; Rai, G.; Agrawal, H.; Agrawal, G.P. Mannosylated nanoparticulate carriers of rifabutin for alveolar targeting. J. Drug Target. 2009, 17, 777–787. [Google Scholar] [CrossRef]
- Kumar, P.V.; Asthana, A.; Dutta, T.; Jain, N.K. Intracellular macrophage uptake of rifampicin loaded mannosylated dendrimers. J. Drug Target. 2006, 14, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Moretton, M.A.; Chiappetta, D.A.; Andrade, F.; das Neves, J.; Ferreira, D.; Sarmento, B.; Sosnik, A. Hydrolyzed galactomannan-modified nanoparticles and flower-like polymeric micelles for the active targeting of rifampicin to macrophages. J. Biomed. Nanotechnol. 2013, 9, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Wagner, V.; Minguez-Menendez, A.; Pena, J.; Fernández-Prada, C. Innovative solutions for the control of leishmaniases: Nanoscale drug delivery systems. Curr. Pharm. Des. 2019, 25, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Santoscoy, A.V.; Roychoudhury, R.; Pohl, N.L.; Wannemuehler, M.J.; Narasimhan, B.; Ramer-Tait, A.E. Tailoring the immune response by targeting C-type lectin receptors on alveolar macrophages using “pathogen-like” amphiphilic polyanhydride nanoparticles. Biomaterials 2012, 33, 4762–4772. [Google Scholar] [CrossRef]
- Chaubey, P.; Patel, R.R.; Mishra, B. Development and optimization of curcumin-loaded mannosylated chitosan nanoparticles using response surface methodology in the treatment of visceral leishmaniasis. Expert Opin. Drug Deliv. 2014, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Gupta, Y.; Jain, A.; Saxena, A.R.; Khare, P.; Jain, A. Mannosylated gelatin nanoparticles bearing an anti-HIV drug didanosine for site-specific delivery. Nanomedicine 2008, 41–48. [Google Scholar] [CrossRef]
- Li, Q.; Du, Y.-Z.; Yuan, H.; Zhang, X.-G.; Miao, J.; Cui, F.-D.; Hu, F.-Q. Synthesis of Lamivudine stearate and antiviral activity of stearic acid-g-chitosan oligosaccharide polymeric micelles delivery system. Eur. J. Pharm. Sci. 2010, 41, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Gajbhiye, V.; Ganesh, N.; Barve, J.; Jain, N.K. Synthesis, characterization and targeting potential of zidovudine loaded sialic acid conjugated-mannosylated poly(propyleneimine) dendrimers. Eur. J. Pharm. Sci. 2013, 48, 668–679. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P. The interaction of anticancer therapies with tumor-associated macrophages. J. Exp. Med. 2015, 212, 435–445. [Google Scholar] [CrossRef]
- Rodell, C.B.; Arlauckas, S.P.; Cuccarese, M.F.; Garris, C.S.; Li, R.; Ahmed, M.S.; Kohler, R.H.; Pittet, M.J.; Weissleder, R. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat. Biomed. Eng. 2018, 2, 578–588. [Google Scholar] [CrossRef]
- Ryan, R.A.; Ortega, A.; Whitney, J.B.; Kumar, B.; Tikhomirov, O.; McFadden, I.D.; Yull, F.E.; Giorgio, T.D. Biocompatible mannosylated endosomal-escape nanoparticles enhance selective delivery of short nucleotide sequences to tumor associated macrophages. Nanoscale 2014, 7, 500–510. [Google Scholar]
- Zhang, M.; Yan, L.; Kim, J.A. Modulating mammary tumor growth, metastasis and immunosuppression by siRNA-induced MIF reduction in tumor microenvironment. Cancer Gene Ther. 2015, 22, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Nah, J.W.; Cho, M.H.; Park, T.G.; Cho, C.S. Receptor-mediated gene delivery into antigen presenting cells using mannosylated chitosan/DNA nanoparticles. J. Nanosci. Nanotechnol. 2006, 6, 2796–2803. [Google Scholar] [CrossRef]
- Xia, W.; Hilgenbrink, A.R.; Matteson, E.L.; Lockwood, M.B.; Cheng, J.X.; Low, P.S. A functional folate receptor is induced during macrophage activation and can be used to target drugs to activated macrophages. Blood 2009, 113, 438–446. [Google Scholar] [CrossRef]
- Hu, Q.; Bae, M.; Fleming, E.; Lee, J.-Y.; Luo, Y. Biocompatible polymeric nanoparticles with exceptional gastrointestinal stability as oral delivery vehicles for lipophilic bioactives. Food Hydrocoll. 2019, 89, 386–395. [Google Scholar] [CrossRef]
- Thakur, A.; Foged, C. Nanoparticles for mucosal vaccine delivery. Nanoeng. Biomater. Adv. Drug Deliv. 2020, 603, 603–646. [Google Scholar]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, I.; Wu, H.; Yu, G. Chitosan-based nanomaterials for drug delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Rodríguez-Felix, F.; Juárez-Onofre, J.E.; Ruiz-Cruz, S.; Robles-García, M.A.; Borboa-Flores, J.; Wong-Corral, F.J.; Cinco-Moroyoqui, F.J.; Castro-Enríquez, D.D.; Del-Toro-Sánchez, C.L. Zein-polysaccharide nanoparticles as matrices for antioxidant compounds: A strategy for prevention of chronic degenerative diseases. Food Res. Int. 2018, 111, 451–471. [Google Scholar] [CrossRef]
- Jiménez-Fernández, E.; Ruyra, A.; Roher, N.; Zuasti, E.; Infante, C.; Fernández-Díaz, C. Nanoparticles as a novel delivery system for vitamin C administration in aquaculture. Aquaculture 2014, 432, 426–433. [Google Scholar] [CrossRef]
- Li, Y.; Yang, B.; Zhang, X. Oral delivery of imatinib through galactosylated polymeric nanoparticles to explore the contribution of a saccharide ligand to absorption. Int. J. Pharm. 2019, 568, 118508. [Google Scholar] [CrossRef]
- Nair, A.B.; Sreeharsha, N.; Al-Dhubiab, B.E.; Hiremath, J.G.; Shinu, P.; Attimarad, M.; Venugopala, K.N.; Mutahar, M. HPMC-and PLGA-based nanoparticles for the mucoadhesive delivery of Sitagliptin: Optimization and in vivo evaluation in rats. Materials 2019, 12, 4239. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Rashidpour, A.; Almajano, M.P.; Metón, I. Chitosan-based drug delivery system: Applications in fish biotechnology. Polymers 2020, 12, 1177. [Google Scholar] [CrossRef]
- Akhtar, B.; Muhammad, F.; Aslam, B.; Saleemi, M.K.; Sharif, A. Pharmacokinetic profile of chitosan modified poly lactic co-glycolic acid biodegradable nanoparticles following oral delivery of gentamicin in rabbits. Int. J. Biol. Macromol. 2020, 164, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, R.V.; da Cunha Santo, A.M.; de Sousa, V.B.; de Araújo Neves, G.; Navarro de Lima Santana, L.; Rpdrigues Menezes, R. A review on chitosan’s uses as biomaterial: Tissue engineering, drug delivery systems and cancer treatment. Materials 2020, 13, 4995. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Polylactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Song, S.K.; Beck, B.R.; Kim, D.; Park, J.; Kim, J.; Kim, H.D.; Ringø, E. Prebiotics as immunostimulants in aquaculture: A review. Fish Shellfish Immunol. 2014, 40, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Koshio, S.; Esteban, M.Á. Beneficial roles of feed additives as immunostimulants in aquaculture: A review. Rev. Aquac. 2018, 10, 950–974. [Google Scholar] [CrossRef]
- Nawaz, A.; Javaid Bakhsh, A.; Irshad, S.; Hoseinifar, H.S.; Xiong, H. The functionality of prebiotics as immunostimulant: Evidences from trials on terrestrial and aquatic animals. Fish Shellfish Immunol. 2018, 76, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Piazzon, M.C.; Zarra, I.; Leiro, J.; Noya, M.; Lamas, J. Stimulation of turbot phagocytes by Ulva rigida C. Agardh polysaccharides. Aquaculture 2006, 254, 9–20. [Google Scholar] [CrossRef]
- Fernández-Díaz, C.; Coste, O.; Malta, E.-J. Polymer chitosan nanoparticles functionalized with Ulva ohnoi extracts boost in vitro ulvan immunostimulant effect in Solea senegalensis macrophages. Algal Res. 2017, 26, 135–142. [Google Scholar] [CrossRef]
- Tian, J.; Yu, J. Poly(lactic-co-glycolic acid) nanoparticles as candidate DNA vaccine carrier for oral immunization of Japanese flounder (Paralichthys olivaceus) against lymphocystis disease virus. Fish Shellfish Immunol. 2011, 30, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Shilakari Asthana, G.; Asthana, A.; Kohli, D.V.; Vyas, S.P. Mannosylated chitosan nanoparticles for delivery of antisense oligonucleotides for macrophage targeting. BioMed Res. Int. 2014, 2014, 526391. [Google Scholar] [CrossRef] [PubMed]
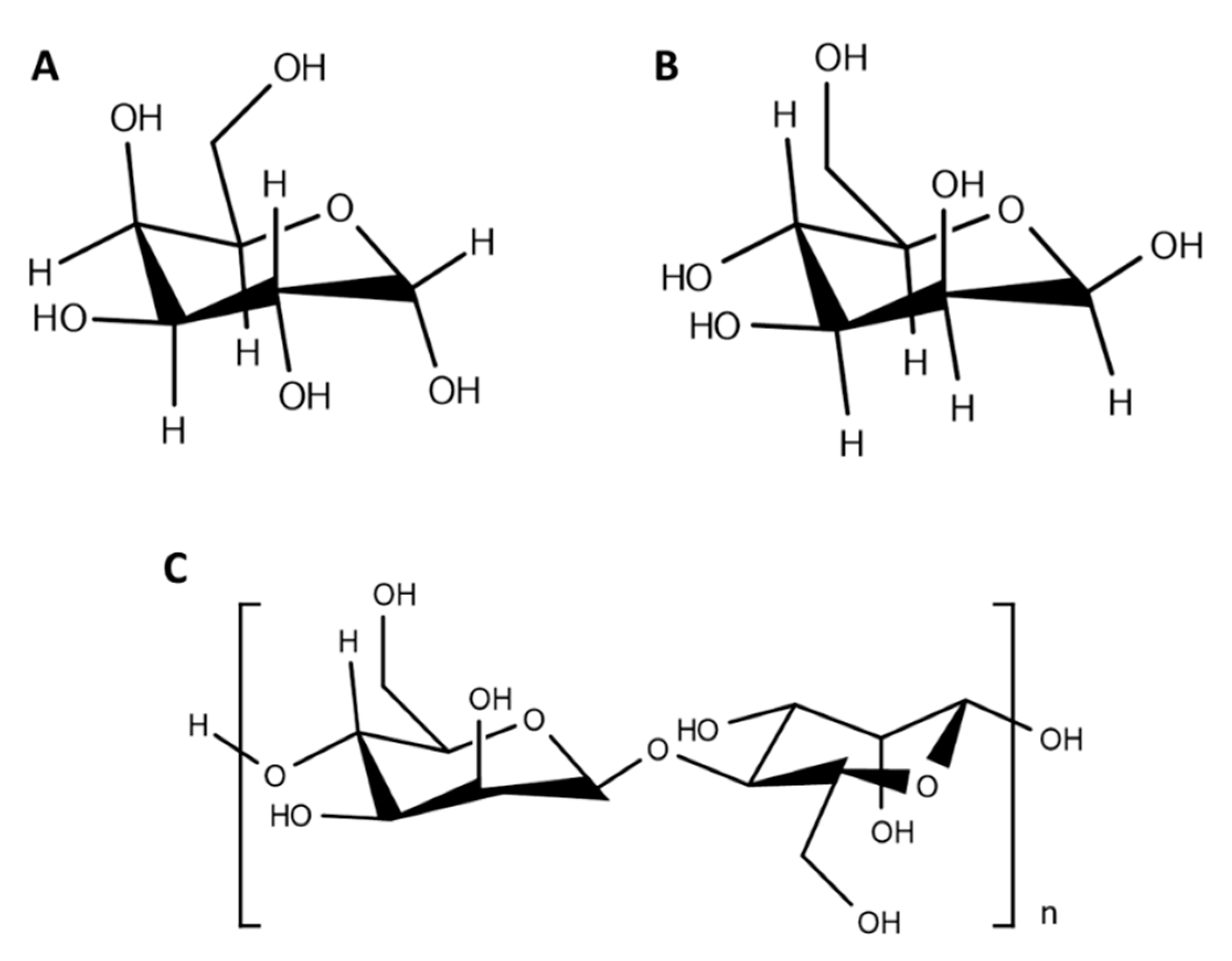
| Polymeric Nanocarrier Composition | Carbohydrate Ligand | Functionalization Strategy | Target Tissue/Cells | Ref |
|---|---|---|---|---|
| Cholic acid and PEG | Galactose | N,N′-dicyclohexyl carbodiimide reaction | Liver-specific delivery | [65] |
| PLGA NPs | Galactose Glucose Mannose | N,N′-diisopropylcarbodiimide/NHS reaction | - | [61] |
| PLGA NPs | Sucrose Cholic acid | DCC/NHS reactions | - | [62] |
| PLA and PEO-b-PCL diblock copolymer NPs | Mannose | Nanoprecipitation-evaporation approach | Mannose receptors | [63] |
| Hydroxyethyl starch nanocapsules | MannoseDimannose Trimannose | Mannose: -Amine to isothiocyanate group reaction Dimannose and trimannose: -Reductive amination | Agglutinin (mannose receptor) | [64] |
| PEI-PEG NPs | Mannose | Binding of mannose to PEI-PEG NPsBinding of mannose to PEI NPs via a PEG spacer | Macrophage cells | [65] |
| PLGA NPs | Mannose Mannan Mannoseamine | DCC/NHS/EDA reaction | Macrophages Leishmania-infected mice | [66] |
| 6-Amino-6-deoxy-curdlan | Mannose | Amine to isothiocyanate group reaction | Mouse peritoneal macrophages | [67] |
| Composition | Carbohydrate | Cargo | Advantages | Ref |
|---|---|---|---|---|
| Chitosan, dextran sulphate | - | Aminoglycoside | Oral administration allowed effective killing of intracellular M. tuberculosis | [103] |
| Gelatin | Mannose | Isoniazid | Effective targeting of macrophages | [104] |
| Poly(epsilon-caprolactone)-b-poly(ethylene-glycol)-b-poly(epsilon-caprolactone) and chitosan | Galactomannan | Rifampicin | Improved cellular internalization in murine macrophages | [106] |
| PLGA | Mannose, mannan and mannosamine | Amphotericin B | Improved in vivo efficacy against visceral leishmaniasis | [66] |
| Polyanhydride | Galactose and di-mannose | - | Increase production of pro-inflammatory cytokines | [108] |
| Chitosan | Mannose | Curcumin | Enhanced the drug residence time within infected macrophages | [109] |
| Gelatin | Mannose | Didanosine | Improved uptake by alveolar macrophages, and in vivo distribution mainly in the lungs, spleen and lymph nodes | [110] |
| Stearate-g-chitosan | Oligosaccharide | Lamiduvine | High cellular uptake with low toxicity in viral infected cells | [111] |
| Sialic acid and poly(propyleneimine) | Mannose | Zidovudine | Low cell toxicity and in vivo biodistribution on the lymph nodes | [112] |
| Advantages | Limitations |
|---|---|
| Surface chemistry can be controlled to reduce impact in the nanoparticles toxicity, immunogenicity, and biodistribution | Production of heterogeneous populations |
| Improved pharmacokinetics/pharmacodynamics profile | Nanoparticles stability during storage, in contact with blood and tissues |
| Effective internalization in targeted cells | Scale up and time of production, particularly for functionalized nanoparticles |
| Site-specific delivery with reduced side-effects | |
| High binding affinity for targeted cells |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, R.G.D.; Reis, B.; Costas, B.; Lima, S.A.C.; Reis, S. Modulation of Macrophages M1/M2 Polarization Using Carbohydrate-Functionalized Polymeric Nanoparticles. Polymers 2021, 13, 88. https://doi.org/10.3390/polym13010088
Andrade RGD, Reis B, Costas B, Lima SAC, Reis S. Modulation of Macrophages M1/M2 Polarization Using Carbohydrate-Functionalized Polymeric Nanoparticles. Polymers. 2021; 13(1):88. https://doi.org/10.3390/polym13010088
Chicago/Turabian StyleAndrade, Raquel G. D., Bruno Reis, Benjamin Costas, Sofia A. Costa Lima, and Salette Reis. 2021. "Modulation of Macrophages M1/M2 Polarization Using Carbohydrate-Functionalized Polymeric Nanoparticles" Polymers 13, no. 1: 88. https://doi.org/10.3390/polym13010088
APA StyleAndrade, R. G. D., Reis, B., Costas, B., Lima, S. A. C., & Reis, S. (2021). Modulation of Macrophages M1/M2 Polarization Using Carbohydrate-Functionalized Polymeric Nanoparticles. Polymers, 13(1), 88. https://doi.org/10.3390/polym13010088